Abstract
Background and Aims:
Alpha (α-2) adrenergic agonists have both analgesic and sedative properties when used as an adjuvant in regional anesthesia. A prospective randomized double-blind study was carried out to evaluate the efficacy of epidural route and to compare the efficacy and clinical profile of dexmedetomidine and clonidine as an adjuvant to bupivacaine with special emphasis on their quality of analgesia, sedation and the ability to provide the smooth intra-operative and postoperative course.
Material and Methods:
The study was conducted in prospective, randomized and double-blind manner. It included 60 American Society of Anesthesiologists Class I and II patients undergoing lower limb surgery under epidural anesthesia. Patients were randomly divided into Group A receiving 0.5% isobaric bupivacaine 15 ml with dexmedetomidine 1 μg/kg and Group B receiving 0.5% isobaric bupivacaine 15 ml with clonidine 2 μg/kg epidurally. Onset and duration of sensory and motor blocks, duration of analgesia, sedation, and adverse effects were assessed.
Results:
Demographic data, surgical characteristics cardio-respiratory parameters, side-effect profile were comparable and statistically not significant in both the groups. However, sedation scores with dexmedetomidine were better than clonidine and turned out to be statistically significant. The onset times for sensory and motor blocks were significantly shorter in Group A as compared to Group B. The duration of analgesia and motor block was significantly longer in A Group as compared to Group B.
Conclusion:
Dexmedetomidine is a superior neuraxial adjuvant to bupivacaine when compared to clonidine for early onset of analgesia, superior intra-operative analgesia, stable cardio-respiratory parameters, prolonged postoperative analgesia and providing patient comfort.
Keywords: Alpha 2-adrenergic agonists, bupivacaine and sedation, clonidine, dexmedetomidine, epidural
Introduction
Epidural anesthesia is the most commonly used technique for providing not only peri-operative surgical anesthesia but postoperative analgesia in lower abdominal and limb surgeries.[1] Many techniques and drug regimens, with partial or greater success, have been tried from time to time to calm the patients and to eliminate the anxiety component during regional anesthesia.[2,3,4] Many a time for achieving desired effect, invariably large volumes of local anesthetics are used with deleterious consequences or the impulsive use of large doses of sedation or even general anesthesia defeats the novel purpose of regional anesthesia.[5] To overcome these problems there is an ongoing effort to find a better adjuvant in regional anesthesia.
Alpha 2-adrenergic receptor agonists have been the focus of interest for their sedative, analgesic, peri-operative sympatholytic, anesthetic-sparing, and hemodynamic-stabilizing properties.[6] Dexmedetomidine is a highly selective α-2 adrenergic agonist with an affinity eight times greater than that of clonidine.[7,8,9,10,11,12]
The present double-blind prospective randomized study aims at comparing the hemodynamic, sedative, and analgesia potentiating effects of epidurally administered clonidine and dexmedetomidine when combined with bupivacaine.
Material and Methods
After obtaining permission from the appropriate authority of the institute (KIMS/498/10/2011) and written consent from patients, 60 patients of American Society of Anaesthesiologists Class I and II between the age of 18 and 60 years who were scheduled for elective lower limb orthopedic procedures were enrolled for the study. The patients with heart blocks, significant bradyarrthymias, left ventricular failure, hematological disease, bleeding or coagulation test abnormalities, psychiatric diseases, diabetes, history of drug abuse, allergy to local anesthetics of the amide type and pregnant and lactating women were excluded from the study. Patients were randomly allocated to one of the following two groups in a double blinded fashion using a computer-generated code: Bupivacaine + dexmedetomidine (A), Bupivacaine + clonidine (B) and were administered tablet ranitidine 150 mg as premedicant a night before and on the morning of the surgery.
In the operation theater, an intravenous (IV) access was secured and monitoring devices were attached which included electrocardiograph, pulse oximetry (SpO2), noninvasive blood pressure (BP) and the baseline parameters were recorded. Patients were administered epidural block with 18 gauge Tuohy Needle - Portex Continious Epidural (Smith Med. Inc.) and catheter was secured 3-4 cm into the epidural space. The catheter was then anchored in place on the back of the patient using adhesive tape and a test dose of 3 ml of 2% lignocaine hydrochloride solution containing adrenaline 1:200,000 was injected. After 4-6 min of administering the test dose, patients in Group A received 15 ml of 0.5% bupivacaine and 1 μg/kg of dexmedetomidine. Patients in Group B were administered 15 ml solution of 0.5% bupivacaine and 2 μg/kg of clonidine. The drug preparation was done by an anesthesia technician who was unaware of the randomization The sensory level was assessed by response to pin-prick while the motor weakness was evaluated using a modified Bromage scale (0 = No block, 1 = Inability to raise extended leg, 2 = Inability to flex knee and 3 = Inability to flex ankle and foot). This was assessed every 5 min for 30 min and then every 30 min. The patient was positioned for surgery after 25-30 min of epidural administration of the drugs and ensuring effective sensory and motor block. Surgery was performed by one of four consultant surgeons of similar clinical experience; they were blinded to the allocation group. The following variables were observed for and recorded: The time taken for onset of sensory block at T10; the highest dermatomal level of sensory analgesia; the complete establishment of motor blockade (Bromage 3), the time to two segment regression of analgesic level, regression of analgesic level to S1 dermatome using pin-prick method. Grading of sedation was evaluated by a five-point scale:
Alert and wide awake,
Arousable to verbal command,
Arousable with gentle tactile stimulation,
Arousable with vigorous shaking and
Unarousable.
Sedation scores were recorded just before the initiation of surgery and thereafter every 20 min during the surgical procedure.
Cardio-respiratory parameters were monitored continuously, and recordings were made every 5-30 min and at 10 min interval, thereafter up to 60 min and then at 15 min interval for the next hour and finally at 30 min in the 3rd h. Hypotension, defined as 20% fall in BP from preinduction levels or a systolic BP lower than 100 mmHg, was treated immediately with IV injection of 3-6 mg mephenteramine and heart rate <50 beats/min was treated with 0.3 mg of injection. Atropine intra-operatively and postoperatively. Intravenous fluids were given as per body weight and operative loss requirement. During the surgical procedure and postoperative period adverse event like anxiety, nausea, vomiting, pruritis, shivering, dry mouth, respiratory depression etc., were recorded. Nausea and vomiting were treated with 0.1 mg/kg of IV ondansetron. Shivering was treated with injection tramadol 50 mg IV. All the vital and hemodynamic parameters were recorded in the recovery room also at 1, 5, 10, 20 and 30 min interval. Postoperatively block characteristics were assessed at 30 min intervals till 6 h.
Postoperative pain were assessed by 10-point verbal rating scale (VRS), in which 0 represented no pain and 10 represented worst possible pain. VRS was measured every 30 min postoperatively by an anesthesiologist who was unaware of the patient allocation group. If patient complained of pain (defined as VRS >4), injection diclofenac intramuscular 75 mg was administered. Duration of analgesia (starting from epidural drug administration to once the patient asks for additional epidural analgesia with VRS >4. The group allocation of the patient was revealed after the end of the study.
Statistical analysis
Sample size was determined taking into consideration that a sample size of 30 patients per group was required to produce a difference of 35% between the two groups for the duration of analgesia and would give a power of 80% at an α-level of 0.05. At the end of the study, all data were compiled systematically and analyzed using unpaired Student's t-test and Chi-square test. Statistical Package for Social Science (SPSS) Version 20.0 (IBM SPSS Statistics) was used to compare the continuous variables between the two groups. Data are expressed as mean ± standard deviation. Value of P < 0.05 is considered as significant.
Results
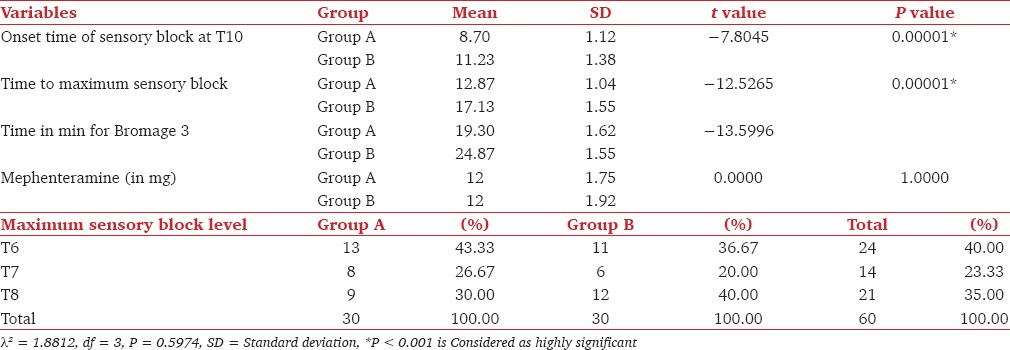
The demographic profiles of the patients in the two groups were comparable [Table 1]. Addition of dexmedetomidine as an adjuvant to epidural bupivacaine resulted in an earlier onset of sensory analgesia at T10 when compared to the addition of clonidine. Dexmedetomidine not only provided earlier onset, but also helped in achieving the maximum analgesic level in a shorter period compared to clonidine group. There was no statistically significant difference in the dermatomal spread among two groups. Motor block of Bromage 3 was achieved earlier in patients from the dexmedetomidine group than of the clonidine group [Table 2].
Table 1.
The demographic profile of patients of both the groups
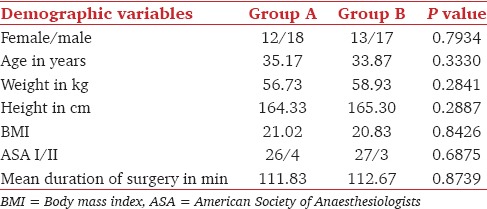
Table 2.
Comparison of initial block characteristics in both the groups
Patients in both the groups remained calm (mean sedation score for Group B was 1.2, and that of Group A was 2.8) throughout surgery but mean sedation scores were significantly higher in the dexmedetomidine group compared to clonidine group (P < 0.0001). Sedation scores were statistically significant at 20 min (P = 0.00001), 40 min (P = 0.00001), 60 min (P = 0.0093) in Group A compared to Group B [Figure 1]. More patients Group A achieved sedation scores of 3 when compared to Group B.
Figure 1.
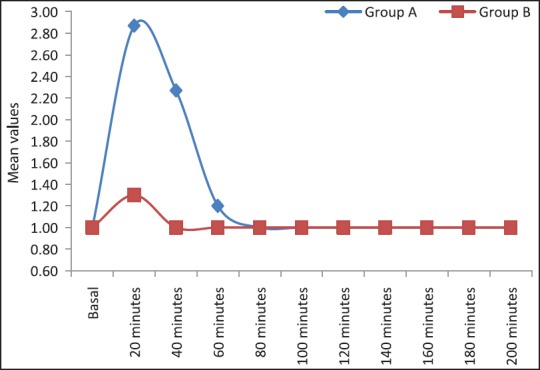
Comparison of intra-operative sedation scores in patients of Group A and Group B
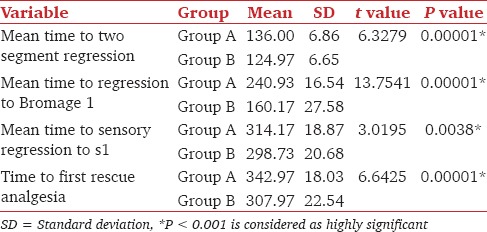
In dexmedetomidine group, all postoperative block and analgesic properties like, time for two segment regression, regression of the sensory block to S1, return of motor power to Bromage 1 was prolonged when compared to clonidine group [Table 3].
Table 3.
Comparison of postoperative block characteristics in both the group
In Group A time to “rescue analgesia” was prolonged compared to Group B. In both groups, the VRS followed a decreasing trend from 0 to 15 min after epidural administration. From 15 to 220 min (4 h) scores were stable and this period totally pain free. The mean VRS score was higher in the clonidine group at each time interval after 220 min (P = 0.0001). In Group A 13% patients needed rescue analgesia at 310 min, 40% at 340 min and 47% at 370 min (P = 0.0057). In Group B, 3% patients needed analgesia at 220 min, 3% at 250 min, 67% at 310 min and 27% patients at 340 min. The duration of analgesia also prolonged in the dexmedetomidine group compared to clonidine group [Figure 2].
Figure 2.
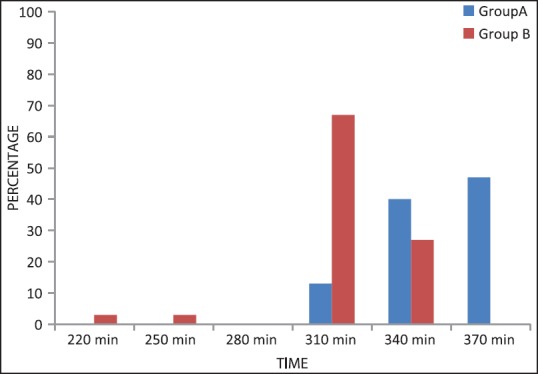
Rescue analgesia requirement among Groups A and B at different time intervals
The cardio-respiratory parameters remained stable throughout the study period. We did not observe any significant difference of heart rate and mean arterial BP in both the groups at the time of administration of drugs [Figure 3]. There was a decreasing trend of heart rate and mean arterial pressure postinjection in both groups and this decrease at 20 min postinjection was not statistically significant. None of the patient showed significant bradycardia or hypotension at any time [Figure 4]. The requirement of mephenteramine was not significant on statistical comparison. Mean respiratory rate in both the groups decreased after giving the drug, the difference between the groups was statistically not significant at different time intervals. Respiratory depression (<10/min) was not observed any group [Figure 5].
Figure 3.
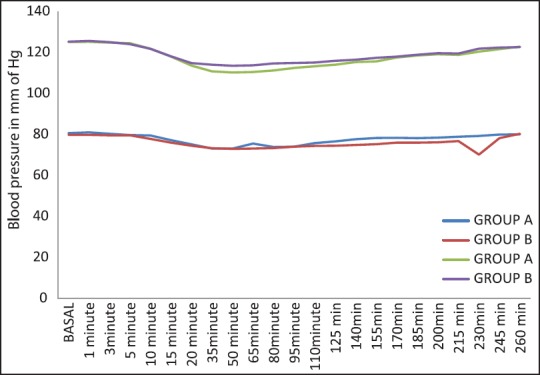
Comparison of Group A and Group B with respect to systolic and diastolic blood pressure (mm Hg)
Figure 4.
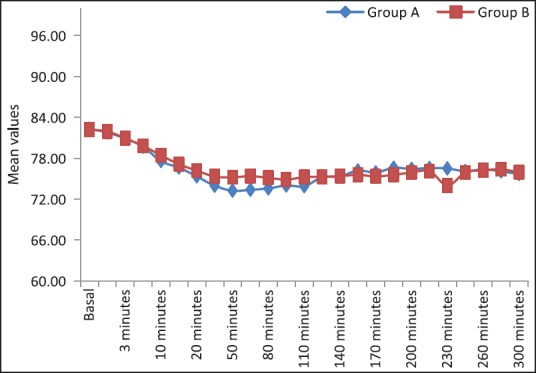
Comparison of Group A and Group B with respect to pulse rate beats/min
Figure 5.
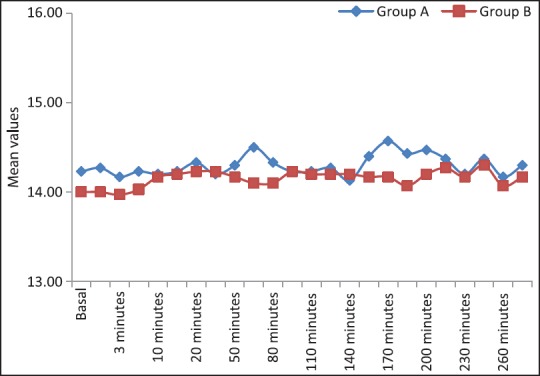
Comparison of Group A and Group B with respect to respiratory rate (breaths/min)
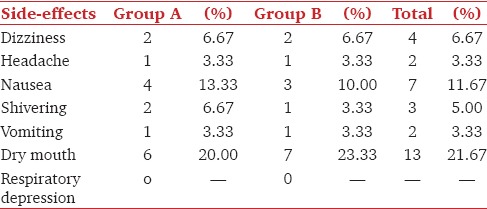
The comparative incidence of various side-effects in both groups was observed in the intra-operative and postoperative period. The incidence of side-effects such as nausea, vomiting, headache and shivering were comparable in both groups. The most common side-effect in both the groups was dryness of the mouth [Table 4].
Table 4.
Comparison of side-effects observed in both the groups during and after the operative period
Discussion
Epidural analgesia offers superior pain relief and early mobilization especially when local anesthetic dose is combined with an adjuvant.[1] Epidural anesthesia is popular and offers several benefits to the patients but at the same time it is linked with drawbacks like pain at the puncture site, fear of needles, and recall of the procedure.[13,14,15,16]. These factors stress the importance of sedation that offers analgesia, anxiolysis, and amnesia. Sedation is known to increase patient's acceptance of regional anesthesia and to greatly improve patient wellbeing during the surgical procedure.[17]
Alpha 2-agonists have evolved as a panacea for various applications/procedures with multiple promising delivery routes. Epidural administration of these drugs is associated with sedation, analgesia, anxiolysis, hypnosis and sympatholysis.[18,19] α-2 agonists may provide an attractive alternative to anesthetic adjunctive agents now in use because of their anesthetic-sparing and hemodynamic-stabilizing effects.[20,21] α-2 adrenoreceptor agonists produce analgesia by depressing release of C - Fiber transmitters and by hyperpolarization of postsynaptic dorsal horn neurons.[22,23,24] The complementary action of local anesthetics and α-2 adrenoreceptor agonists accounts for their profound analgesic properties. The prolongation of the motor block of local anesthetics may be the result of binding of α-2 adrenoreceptor agonists to the motor neurons in the dorsal horn.[4,5] Dexmedetomidine is eight times more specific and highly selective α-2 adrenoreceptor agonist compared to clonidine.[20,25]
This study was undertaken to compare the analgesic efficacy, and sedative effects of two α-2 agonists when administered epidurally along with bupivacaine.
Dexmedetomidine provided a smooth intra-operative analgesia as compared to clonidine which is evident from the results. Addition of either 1 μg/kg dexmedetomidine or 2 μg/kg clonidine as adjuvant to epidural bupivacaine leads to early[3,5,26,27] onset of analgesia, faster achievement of maximum sensory level and motor blockade. It not only prolonged the duration of analgesia but also provided a good sedation level during the surgical procedure without significant hemodynamic effects. Our data support previous studies that used dexmedetomidine and clonidine as additive to regional anesthetics.[5,26,27]
We found no statistical significance in the peak levels of analgesia provided by both drugs. Our findings were in concordance with Salgado et al.[27] Unlike our study Bajwa et al.[5] found that dexmedetomidine provided a significantly higher dermatomal spread compared to clonidine when added as adjuvant to epidural ropivacaine. This is probably due to the lesser amount of dexmedetomidine (1 μg/kg) used in our study.
The hypnotic and supraspinal analgesic effects of dexmedetomidine are mediated by the hyperpolarization of noradrenergic neurons, which suppresses neuronal firing in the locus coeruleus along with inhibition of norepinephrine release and activity in the descending medullospinal noradrenergic pathway.[28,29,30]
The results of our study clearly indicate the effectiveness of epidural dexmedetomidine as adjuvant to bupivacaine in providing sedation, more patients in Group A had sedation score 3 and were arousable by gentle tactile stimulation as compared to Group B. Similar results were seen in study done by.[5,11,26]
The cardio-respiratory parameters, as evident from [Figures 3–5] remained stable throughout the study period which reaffirms the established effects of α-2 agonists in providing a hemodynamically stable peri-operative and postoperative period. The requirement of vasopressors for the maintenance of stable hemodynamic parameters did not reveal significant differences between the both groups on statistical comparison. Similarly, comparable cardio-respiratory parameters were also observed by.[26,27,31]
Avoidance of respiratory depression in the patients who were administered dexmedetomidine and clonidine was one of the most remarkable observation in our study [Figure 5] and the evidence is similar to the earlier studies where researchers have found complete absence of clinically detectable respiratory depression in the previous multiple human studies.[32,33,34]
The dexmedetomidine group showed visible superiority over clonidine group in various postoperative block characteristics like the weaning of sensory and motor block, prolonged postoperative analgesia. Similar to this study, Bajwa et al. found significant prolongation of time to two segmental dermatomal regression and regression to Bromage 1 in dexmedetomidine group when compared to clonidine group. Salgado et al. found the duration of motor block was significantly higher in the dexmedetomidine group (P > 0.05), being on average 30% higher than that observed in the control bupivacaine group.
Intensity of postoperative pain and quality of relief of pain was assessed using VRS and analgesia was provided when VRS was >4. We found significantly higher verbal analogue scores in clonidine group at 220, 250, 310 and 340 min. Our results were similar to studies conducted by Saravana Babu et al., Schnaider et al., El-Hennawy et al. who found significant differences in the visual analog scores in clonidine group compared to dexmedetomidine group. Unlike this study, Salgado et al. found no difference in the scores of pain, assessed in the postanesthesia care unit.
The incidence of side-effects like vomiting, headache, shivering and dizziness were comparable in both the groups and statistically nonsignificant. The incidence of nausea (four patients in Group A and three patients in Group B) and dry mouth (six patients in Group A and seven patients in Group B) was significantly higher in both the groups but it was statistically nonsignificant on comparison. Prevention of shivering in patients with dexmedetomidine (1 of 30) and clonidine (2 of 30) was seen. Similar to this study, Bajwa et al. and El-Hennawy et al. also found the incidence side-effects to be statistically nonsignificant on comparison. Clonidine and dexmedetomidine acts on central thermoregulatory system to reduce the vasoconstriction threshold and the shivering threshold and prevents postoperative shivering.[35,36]
Most of the previous studies have used a higher dexmedetomidine dose and found superior results to clonidine.[5,26,27] This study clearly shows the superiority of lower dose of dexmedetomidine (1 μg/kg) when compared to clonidine (2 μg/kg).
Although this study adds to the existing knowledge on dexmedetomidine and clonidine, the results should be considered taking into consideration the obvious limitations: It was conducted on patients of lower limb surgeries to avoid much differences in the perception of pain, because the perception of postoperative pain will certainly differ depending on the level of surgery. We also could not assess pain; quality and effectiveness of analgesia postoperatively on limb movement. We were unable to assess the total dose of local anesthetic consumption postoperatively, as a different mode of analgesia was chosen.
Further scope for study
Clinical studies can be done to find the equivalent epidural doses of dexmedetomidine and clonidine.
Conclusion
Dexmedetomidine appears to be a better alternative to clonidine as an epidural adjuvant as it provides comparable stable hemodynamics, early onset and establishment of sensory and motor anesthesia, prolonged postoperative analgesia and superior sedation levels. Overall the clinical experience with dexmedetomidine was satisfactory for surgery under regional anesthesia, for the patient, the anesthetist, and the surgeon as compared to clonidine because of its superior sedative and block characteristics during the surgical procedure.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Schultz AM, Werba A, Ulbing S, Gollmann G, Lehofer F. Peri-operative thoracic epidural analgesia for thoracotomy. Eur J Anaesthesiol. 1997;14:600–3. doi: 10.1046/j.1365-2346.1994.00183.x. [DOI] [PubMed] [Google Scholar]
- 2.Höhener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth. 2008;100:8–16. doi: 10.1093/bja/aem342. [DOI] [PubMed] [Google Scholar]
- 3.Helgeson LE. Sedation during regional anesthesia: Inhalation versus intravenous. Curr Opin Anaesthesiol. 2005;18:534–9. doi: 10.1097/01.aco.0000183106.81554.a9. [DOI] [PubMed] [Google Scholar]
- 4.Némethy M, Paroli L, Williams-Russo PG, Blanck TJ. Assessing sedation with regional anesthesia: Inter-rater agreement on a modified Wilson sedation scale. Anesth Analg. 2002;94:723–8. doi: 10.1097/00000539-200203000-00045. [DOI] [PubMed] [Google Scholar]
- 5.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–65. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 7.Bucklin B, Eisenach JC, Tucker B. Pharmacokinetics and dynamic studies of intrathecal, epidural and intravenous dexmedetomidine. Anesthesiology. 1991;75:662. [Google Scholar]
- 8.Bischoff P, Kochs E. Alpha 2-agonists in anesthesia and intensive medicine. Anasthesiol Intensivmed Notfallmed Schmerzther. 1993;28:2–12. doi: 10.1055/s-2007-998867. [DOI] [PubMed] [Google Scholar]
- 9.Villela NR, Nascimento Júnior Pd. Dexmedetomidine in anesthesiology. Rev Bras Anestesiol. 2003;53:97–113. doi: 10.1590/s0034-70942003000100013. [DOI] [PubMed] [Google Scholar]
- 10.Linde He MO. The clinical use of dexmedetomidine. Rev Bras Anestesiol. 2004;54:1–4. [Google Scholar]
- 11.Schnaider TB, Vieira AM, Brandão AC, Lobo MV. Intraoperative analgesic effect of cetamine, clonidine and dexmedetomidine, administered through epidural route in surgery of the upper abdomen. Rev Bras Anestesiol. 2005;55:525–31. doi: 10.1590/s0034-70942005000500007. [DOI] [PubMed] [Google Scholar]
- 12.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 14.Gajraj NM, Sharma SK, Souter AJ, Pole Y, Sidawi JE. A survey of obstetric patients who refuse regional anaesthesia. Anaesthesia. 1995;50:740–1. doi: 10.1111/j.1365-2044.1995.tb06110.x. [DOI] [PubMed] [Google Scholar]
- 15.Lonsdale M, Hutchison GL. Patients' desire for information about anaesthesia. Scottish and Canadian attitudes. Anaesthesia. 1991;46:410–2. doi: 10.1111/j.1365-2044.1991.tb09560.x. [DOI] [PubMed] [Google Scholar]
- 16.Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid. The perspective of patients? Anesth Analg. 1999;89:652–8. doi: 10.1097/00000539-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Wresch KP. Analgesia and sedation to supplement incomplete regional anesthesia. Anaesthesist. 1995;44(Suppl 3):S580–7. [PubMed] [Google Scholar]
- 18.Mauro VA, Brandão ST. Clonidine and dexmedetomidine through epidural route for post-operative analgesia and sedation in a colecistectomy. Rev Bras Anestesiol. 2004;4:1–10. [Google Scholar]
- 19.Gabriel JS, Gordin V. Alpha 2 agonists in regional anesthesia and analgesia. Curr Opin Anaesthesiol. 2001;14:751–3. doi: 10.1097/00001503-200112000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paris A, Tonner PH. Dexmedetomidine in anaesthesia. Curr Opin Anaesthesiol. 2005;18:412–8. doi: 10.1097/01.aco.0000174958.05383.d5. [DOI] [PubMed] [Google Scholar]
- 22.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 23.Al Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind study. American journal of applied sciences. 2009;1:882–887. [Google Scholar]
- 24.Lawhead RG, Blaxall HS, Bylund DB. Alpha-2A is the predominant alpha-2 adrenergic receptor subtype in human spinal cord. Anesthesiology. 1992;77:983–91. doi: 10.1097/00000542-199211000-00022. [DOI] [PubMed] [Google Scholar]
- 25.Murthy TV, Singh R. Alpha 2 adrenoceptor agonist-dexmedetomidine role in anaesthesia and intensive care: A clinical review. J Anaesth Clin Pharmacol. 2009;25:267–72. [Google Scholar]
- 26.Saravana Babu M, Verma AK, Agarwal A, Tyagi CM, Upadhyay M, Tripathi S. A comparative study in the post-operative spine surgeries: Epidural ropivacaine with dexmedetomidine and ropivacaine with clonidine for post-operative analgesia. Indian J Anaesth. 2013;57:371–6. doi: 10.4103/0019-5049.118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Módolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 28.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–61. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 29.Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci. 2008;27:3182–90. doi: 10.1111/j.1460-9568.2008.06260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 31.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 32.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 34.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Gupta V, Sehgal C, Kumar R. Bupivacaine-clonidine mixture for epidural anaesthesia. J Evol Med and Dent Sci. 2013;2:38–45. [Google Scholar]
- 36.Jabbary Moghaddam M, Ommi D, Mirkheshti A, Dabbagh A, Memary E, Sadeghi A, et al. Effects of clonidine premedication upon postoperative shivering and recovery time in patients with and without opium addiction after elective leg fracture surgeries. Anesth Pain Med. 2013;2:107–10. doi: 10.5812/aapm.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]


