Abstract
Purpose
This multicenter, randomized, open-label, phase III trial compared the efficacy and safety of decitabine with treatment choice (TC) in older patients with newly diagnosed acute myeloid leukemia (AML) and poor- or intermediate-risk cytogenetics.
Patients and Methods
Patients (N = 485) age ≥ 65 years were randomly assigned 1:1 to receive decitabine 20 mg/m2 per day as a 1-hour intravenous infusion for five consecutive days every 4 weeks or TC (supportive care or cytarabine 20 mg/m2 per day as a subcutaneous injection for 10 consecutive days every 4 weeks). The primary end point was overall survival (OS); the secondary end point was the complete remission (CR) rate plus the CR rate without platelet recovery (CRp). Adverse events (AEs) were recorded.
Results
The primary analysis with 396 deaths (81.6%) showed a nonsignificant increase in median OS with decitabine (7.7 months; 95% CI, 6.2 to 9.2) versus TC (5.0 months; 95% CI, 4.3 to 6.3; P = .108; hazard ratio [HR], 0.85; 95% CI, 0.69 to 1.04). An unplanned analysis with 446 deaths (92%) indicated the same median OS (HR, 0.82; 95% CI, 0.68 to 0.99; nominal P = .037). The CR rate plus CRp was 17.8% with decitabine versus 7.8% with TC (odds ratio, 2.5; 95% CI, 1.4 to 4.8; P = .001). AEs were similar for decitabine and cytarabine, although patients received a median of four cycles of decitabine versus two cycles of TC. The most common drug-related AEs with decitabine were thrombocytopenia (27%) and neutropenia (24%).
Conclusion
In older patients with AML, decitabine improved response rates compared with standard therapies without major differences in safety. An unplanned survival analysis showed a benefit for decitabine, which was not observed at the time of the primary analysis.
INTRODUCTION
Acute myeloid leukemia (AML) is a common adult leukemia with approximately 12,330 new cases annually in the United States1,2 and approximately 18,000 new cases in the European Union.3 AML is more common in the elderly,4 and treatments for older patients are limited, particularly in those with poor performance status (PS) and comorbidities. The National Comprehensive Cancer Network,4 European LeukemiaNet,5 and European Society for Medical Oncology6 recently updated their AML treatment recommendations to include low-intensity cytarabine, 5-azacytidine, and decitabine.
Decitabine, which is a hypomethylating agent, inhibits DNA methyltransferase, which appears to have direct cytotoxic effects and/or affect cellular differentiation and apoptosis. Decitabine is indicated for treatment of previously treated and untreated de novo and secondary myelodysplastic syndrome (MDS) of all French-American-British subtypes and intermediate-1, intermediate-2, and high-risk International Prognostic Scoring System groups.7 In phase I and II studies, decitabine demonstrated activity alone8 and combined with amsacrine or idarubicin9 in patients with relapsed AML.
Methylation is involved in silencing the tumor suppressor CCAAT/enhancer binding protein δ in AML pathogenesis.10 In a randomized study of three decitabine regimens in patients with MDS or chronic myelomonocytic leukemia, intravenous (IV) decitabine 20 mg/m2 per day infused over 1 hour for 5 days optimally induced hypomethylation and provided the best complete remission (CR) rates.11 This regimen demonstrated activity in patients older than age 60 years with AML and poor- or intermediate-risk cytogenetics, with CR in 13 (24%) of 55 patients and acceptable tolerability.12 In patients ≥ age 60 years with untreated AML, IV decitabine 20 mg/m2 per day infused over 1 hour for 10 days elicited CR in 25 (47%) of 53 patients.13
This study compared the efficacy and safety of decitabine with patient choice, with physician advice, of supportive care (SC) or cytarabine in older patients with AML.
PATIENTS AND METHODS
Patients
Eligible patients were ≥ 65 years old with newly diagnosed, histologically confirmed de novo or secondary AML (≥ 20% blasts) and poor- or intermediate-risk cytogenetics (Southwest Oncology Group categorization)14, Eastern Cooperative Oncology Group (ECOG) PS of 0 to 2, WBC count ≤ 40,000/mm, bilirubin ≤ 1.5× the upper limit of normal, AST or ALT ≤ 2.5× the upper limit of normal, creatinine clearance ≥ 40 mL/min, and life expectancy ≥ 12 weeks.
Exclusion criteria included acute promyelocytic leukemia, t(8;21) or inv(16) karyotype abnormalities, CNS leukemia, active systemic malignancies, unstable angina or New York Heart Association class 3/4 congestive heart failure, inaspirable bone marrow, comorbidities or organ dysfunction, uncontrolled active infection, or HIV. Patients must not have had previous chemotherapy (except hydroxyurea) for any myeloid disorder or used experimental drugs for 4 weeks prerandomization, been candidates for bone marrow or stem-cell transplantation for 12 weeks prerandomization, or received radiotherapy for extramedullary disease for 2 weeks prerandomization.
Study Design and Treatment
This randomized, open-label, phase III study conducted in 15 countries was approved by institutional review boards or independent ethics committees and was conducted according to the Declaration of Helsinki. Patients provided written informed consent.
Patients indicated, with physician advice, their preferred treatment choice (TC), either SC (to maximize the quality of life) or cytarabine 20 mg/m2 one time per day subcutaneously for 10 consecutive days every 4 weeks. Dosing one time per day was selected because patient compliance was previously poor with dosing two times per day. Patients were randomly assigned 1:1 to receive decitabine or TC by using a stratified permuted block method. Random assignment was stratified by age, cytogenetic risk, and ECOG PS. Patients assigned to receive decitabine received 1-hour IV infusions of decitabine 20 mg/m2 one time per day for five consecutive days every 4 weeks. Treatment continued until relapse or progressive disease (PD), death, unacceptable toxicity, lack of clinical benefit, intercurrent illness preventing treatment, or patient/physician request.
Patients were allowed hydroxyurea until cycle 1 on day 15. The study drug was not administered unless the absolute blast count was less than 30,000/μL. Treatment was delayed at the discretion of the investigator for febrile neutropenia (≥ 38.5°C; absolute neutrophil count [ANC], < 1,000/μL), clinical and/or microbiologic infection with grade 3 to 4 neutropenia (ANC < 1,000/μL), or hemorrhage with grade 4 thrombocytopenia (< 25,000 platelets/μL). If renal or hepatic dysfunction occurred, treatment was stopped until resolution or withheld if dysfunction persisted more than 4 days.
Progressive disease during cycle 1 was based on peripheral blood counts, new extramedullary disease, and investigator judgment. If peripheral blast counts increased more than 50% over baseline or blast counts increased more than 25% over baseline during cycle 1, patients could be discontinued for PD. During subsequent cycles, PD was defined as a greater than 50% increase in the peripheral blast count from baseline, a greater than 25% increase in blast counts from baseline on bone marrow aspirate collected ≥ 7 days before every second cycle beginning at cycle 3 or as clinically indicated, new extramedullary disease, or investigator judgment.
Objectives and End Points
The primary objective was to compare overall survival (OS) in patients who received decitabine or TC. Secondary objectives were to compare CR rates and adverse events (AEs).
Assessments
OS was measured from randomization to death (any cause). For secondary end points, bone marrow biopsies and aspirates were obtained from patients at screening. Aspirates were obtained ≤ 7 days before cycles 3 and 5 and at the end-of-study visit. With decitabine or cytarabine, aspirates were performed every second cycle thereafter. For SC patients, aspirates were performed every third month thereafter. Morphologic CR (< 5% blasts in bone marrow aspirates, marrow spicules, ≥ 200 nucleated cells, ANC > 1,000/μL, platelets ≥ 100,000/μL, and absence of transfusions for ≥ 1 week before each assessment) and morphologic CR with incomplete platelet recovery (CRp; ie, no requirement to reach a platelet count ≥ 100,000/μL) were determined. Patients with CR or CRp had additional bone marrow assessments with cytogenetics after cycles 1 and 3 after initial documentation of CR. Remission was evaluated by using modified 2003 International Working Group criteria.15 Morphologic CR with incomplete blood count recovery was also recorded. Response assessments were centrally made by independent, blinded, expert reviewers.
Safety was assessed via AEs, medical histories, physical examinations, concomitant medications, and central laboratory assessments. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events (version 3.0) with investigators determining the relationship to the study drug.
Patients were followed monthly for 2 years postrandomization and then every 2 months for 3 years for OS and PD until death or loss to follow-up.
Statistical Analysis
The planned sample size was 480 patients (approximately 240 patients per arm), required to observe ≥ 385 events at the time of analysis. The study was designed to detect a 25% reduction in mortality risk with ≥ 80% power and a significance level of 0.05 (two-sided). The median survival was assumed to be 8 months for decitabine and 6 months for TC. Two planned interim analyses were performed after approximately one third and two thirds of the targeted number of deaths occurred. Additional unplanned analyses of mature survival and updated safety data were performed 1 year after the planned survival analysis at the suggestion of European regulatory authorities.
The primary efficacy population comprised all randomly assigned patients. The safety population comprised all patients who received at least one dose of decitabine or cytarabine and all patients who received SC. OS analysis used a log-rank test stratified by baseline age, cytogenetic risk, and ECOG PS. The Kaplan-Meier method was used to describe OS. Hazard ratios (HRs) and 95% CIs were calculated by using a Cox proportional hazards model stratified by age, cytogenetic risk, and ECOG PS. The following two planned sensitivity analyses were performed: an unstratified log-rank test and HR for OS; and an analysis of OS in which patients who received subsequent disease-modifying therapy were censored on the first day of the first subsequent therapy. Exploratory subgroup analyses assessed primary and secondary end points, including age, baseline bone marrow blasts, type of AML, cytogenetics, and ECOG PS. Effects of these characteristics on OS and PFS were investigated by using a multivariate Cox proportional hazards model; effects on the probability of achieving CR or CRp were investigated by using a logistic regression model. The incidence of CR plus CRp and corresponding CIs were compared between decitabine and TC arms by using Fisher's exact test. Significance was set at P = .05.
RESULTS
Patient Disposition and Treatments
Between January 2006 and April 2009, 485 patients were randomized at 65 sites. The primary OS analysis was based on a clinical cutoff date of October 28, 2009, by which time 385 deaths were projected, and 396 deaths occurred. To provide additional clinical data, mature survival and updated safety data (clinical cutoff date, October 29, 2010) were used for an ad hoc mature survival analysis (446 deaths).
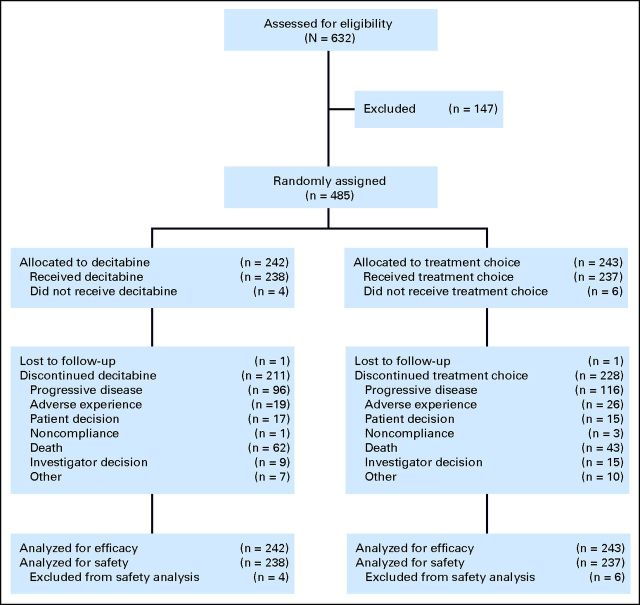
The efficacy populations comprised 242 patients randomly assigned to the decitabine group and 243 patients randomly assigned to the TC group (cytarabine, n = 215; SC, n = 28). The safety population comprised 475 patients (decitabine, n = 238; cytarabine, n = 209; SC, n = 28). At the 2009 cutoff, 211 patients (87.2%) who received decitabine and 228 patients (93.8%) who received TC had discontinued, primarily because of PD (96 and 116 patients, respectively; Fig 1). Patient demographics and baseline clinical characteristics were balanced (Table 1). This was a high-risk population: more than two thirds of patients were ≥ 70 years of age, 35.3% of patients had secondary AML, 36.0% of patients had poor-risk AML, 24.3% of patients had ECOG PS of 2, and median baseline blasts in bone marrow were 46.0%.
Fig 1.
CONSORT diagram showing patient disposition from random assignment to time of ad hoc mature analysis.
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Characteristic | TC |
Decitabine (n = 242) |
All Patients (N = 485) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Supportive Care (n = 28) |
Cytarabine (n = 215) |
Total TC (n = 243) |
||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||||||
| Median | 75.0 | 73.0 | 73.0 | 73.0 | 73.0 | |||||
| Range | 66.0-86.0 | 64.0-91.0 | 64.0-91.0 | 64.0-89.0 | 64.0-91.0 | |||||
| 65-69 | 5 | 17.9 | 64 | 29.8 | 69 | 28.4* | 68 | 28.1† | 137 | 28.2 |
| ≥ 70 | 23 | 82.1 | 150 | 69.8 | 173 | 71.2 | 171 | 70.7 | 344 | 70.9 |
| Sex | ||||||||||
| F | 8 | 28.6 | 84 | 39.1 | 92 | 37.9 | 105 | 43.4 | 197 | 40.6 |
| M | 20 | 71.4 | 131 | 60.9 | 151 | 62.1 | 137 | 56.6 | 288 | 59.4 |
| BSA, m2 | ||||||||||
| Median | 1.75 | 1.80 | 1.80 | 1.82 | 1.81 | |||||
| Range | 1.3-2.4 | 1.4-2.7 | 1.3-2.7 | 1.4-2.6 | 1.3-2.7 | |||||
| Time since AML diagnosis, days | ||||||||||
| Median | 27.0 | 15.0 | 15.0 | 14.0 | 15.0 | |||||
| Range | 0.0-363.0 | 0.0-398.0 | 0-398.0 | 3.0-346.0 | 0-398.0 | |||||
| Type of AML | ||||||||||
| De novo | 17 | 60.7 | 140 | 65.1 | 157 | 64.6 | 155 | 64.0 | 312 | 64.3 |
| Secondary | 11 | 39.3 | 73 | 34.0 | 84 | 34.6 | 87 | 36.0 | 171 | 35.3 |
| Bone marrow blasts‡ | ||||||||||
| 20-30% | 5 | 17.9 | 53 | 24.9 | 58 | 24.1 | 65 | 27.0 | 123 | 25.5 |
| > 30-50% | 10 | 35.7 | 64 | 30.0 | 74 | 30.7 | 67 | 27.8 | 141 | 29.3 |
| > 50% | 11 | 39.3 | 90 | 42.3 | 101 | 41.9 | 105 | 43.69 | 206 | 42.7 |
| ECOG PS | ||||||||||
| 0 or 1 | 19 | 67.9 | 164 | 76.3 | 183 | 75.3 | 184 | 76.0 | 367 | 75.7 |
| 2 | 9 | 32.1 | 51 | 23.7 | 60 | 24.7 | 58 | 24.0 | 118 | 24.3 |
| Cytogenetics | ||||||||||
| Intermediate risk | 20 | 71.4 | 134 | 62.6 | 154 | 63.6 | 152 | 63.1 | 306 | 63.4 |
| Poor risk | 8 | 28.6 | 79 | 36.9 | 87 | 36.0 | 87 | 36.1 | 174 | 36.0 |
| Hemoglobin, g/dL | ||||||||||
| Median | 9.3 | 9.4 | 9.4 | 9.3 | 9.3 | |||||
| Range | 6.6-10.7 | 5.0-12.6 | 5.0-12.6 | 5.2-15.0 | 5.0-15.0 | |||||
| White blood cells, 109/L | ||||||||||
| Median | 2.73 | 3.71 | 3.69 | 3.10 | 3.43 | |||||
| Range | 0.7-26.5 | 0.5-80.9 | 0.5-80.9 | 0.3-127.0 | 0.3-127.0 | |||||
Abbreviations: AML, acute myeloid leukemia; BSA, body surface area; ECOG PS, Eastern Cooperative Oncology Group performance status; TC, treatment choice.
One patient (not included) was age < 65 years.
Three patients (not included) were age < 65 years.
Twelve patients with < 20% blasts in the safety population included one patient with M6 AML (defined by marrow erythroblasts), three patients with a misdiagnosis of AML, five patients with unknown blast counts at screening, and three protocol deviations.
By the 2009 cutoff, patients received a median of four cycles (range, 1 to 29 cycles) of decitabine, two cycles (range, 1 to 30 cycles) of cytarabine, and two cycles (range, 1 to 28 cycles) of SC. Of 238 decitabine recipients, 63 patients (26.5%) remained on study for at least nine cycles versus 32 (15.4%) of 208 cytarabine recipients. At the mature analysis (2010 cutoff), the median numbers of cycles were unchanged, and again, more decitabine than cytarabine patients remained on study for at least nine cycles.
Efficacy
OS.
At the final analysis (2009 cutoff), 396 deaths (81.6%) occurred (decitabine, n = 197; TC, n = 199; Fig 2A). There was a nonsignificant but favorable trend toward an increased median OS with decitabine (7.7 months; 95% CI, 6.2 to 9.2 months) versus TC (5.0 months; 95% CI, 4.3 to 6.3 months), with a 54% improvement for decitabine over TC. The estimated HR for death (decitabine:TC) was 0.85 (95% CI, 0.69 to 1.04). A sensitivity analysis (2009 cutoff), wherein patients who received subsequent disease-modifying therapy were censored, showed a median OS for decitabine of 8.5 months (95% CI, 6.5 to 9.5 months) versus 5.3 months for TC (95% CI, 4.3 to 6.7 months; P = .044). Of patients in the decitabine and TC groups, 37.6% and 44.4%, respectively, received subsequent therapy; 10.3% in each group received induction therapy, 8.7% and 4.5%, respectively, received low-dose cytarabine, 1.7% and 5.8%, respectively, received azacitidine, and 0.8% and 2.1%, respectively, received decitabine.
Fig 2.
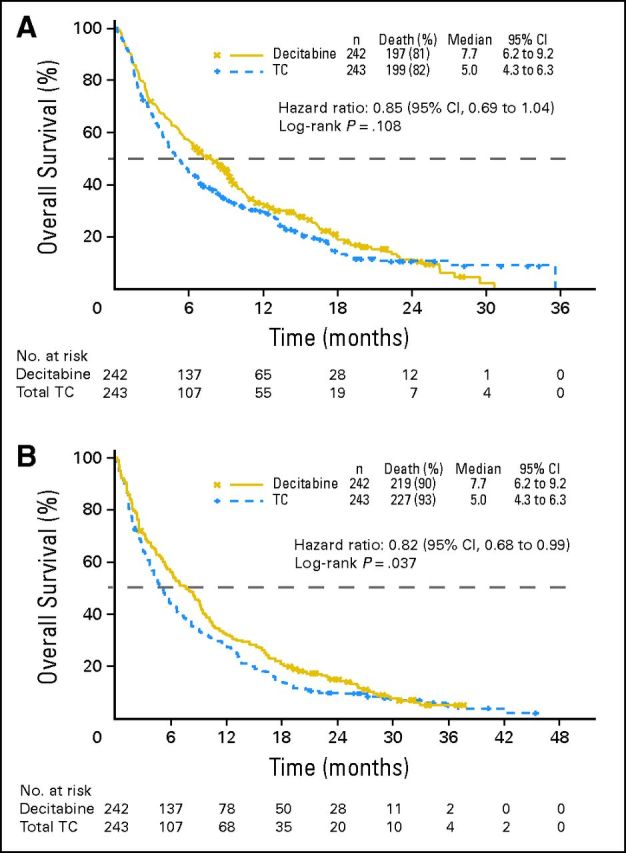
(A) Overall survival (Kaplan-Meier method) in a protocol-specified 2009 clinical cutoff analysis of decitabine and treatment choice (TC) in the intent-to-treat population. (B) Overall survival (Kaplan-Meier method) in an ad hoc mature (2010) analysis of decitabine and TC in the intent-to-treat population.
A 20% reduction in risk of death occurred with decitabine (HR, 0.80; 95% CI, 0.64 to 0.99). At the mature analysis (2010 cutoff), 446 deaths (92.0%) were reported (decitabine, n = 219; TC, n = 227). Median OS values were the same as in the 2009 analysis (Fig 2B) but with an improved HR (0.82; 95% CI, 0.68 to 0.99) and nominal P = .037.
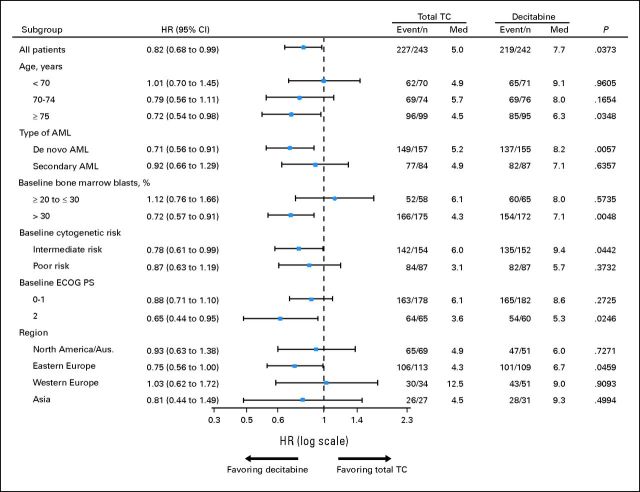
In an exploratory subgroup analysis of mature data (2010 cutoff) using a multivariate Cox proportional hazards model, the trend toward a decitabine treatment benefit was more clearly observed in patients ≥ 70 years old versus in patients less than 70 years old, with de novo versus secondary AML, baseline bone marrow blasts more than 30% versus ≤ 30%, intermediate- versus poor-risk cytogenetics, and ECOG PS of 2 versus 0 to 1 (Fig 3). Patients with baseline bone marrow blasts ≤ 30% did not show any survival advantage with decitabine, but this may have been due to low patient numbers and poorer prognostic factors in the decitabine versus TC groups (OS by geographic region is provided in the Appendix, online only).
Fig 3.
Subanalyses of mature overall survival data (2010 clinical cutoff) for decitabine and patient treatment choice with physician advice (TC) in the intent-to-treat population. P values were based on two-sided log-rank test and stratified by age, cytogenetic risk, and Eastern Cooperative Oncology Group (ECOG) performance status (PS). AML, acute myeloid leukemia; Aus., Australia; HR, hazard ratio; Med, median (months).
Remission rate.
At the 2009 cutoff, decitabine was associated with a significantly higher CR rate plus CRp compared with TC (17.8% v 7.8%, respectively; P = .001; Table 2 ; odds ratio of decitabine to TC, 2.5; 95% CI, 1.4 to 4.8). In patients with CR or CRp, the median time to best response was 4.3 months (95% CI, 3.8 to 5.1 months) with decitabine and 3.7 months (95% CI, 2.8 to 4.6) with TC. In the decitabine and TC groups, 23.6% and 30.9% of patients, respectively, were not evaluable because they were lost to follow-up or did not have repeat bone marrow samples because of discontinuation or death.
Table 2.
Treatment Response (2009 clinical cutoff)
| Response | TC |
Decitabine (n = 242) |
||||||
|---|---|---|---|---|---|---|---|---|
| Supportive Care (n = 28) |
Cytarabine (n = 215) |
Total TC (n = 243) |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| CR | 1 | 3.6 | 17 | 7.9 | 18 | 7.4 | 38 | 15.7 |
| CRi | 1 | 3.6 | 6 | 2.8 | 7 | 2.9 | 24 | 9.9 |
| CRp | 0 | 0 | 1 | 0.5 | 1 | 0.4 | 5 | 2.1 |
| CR + CRp | 1 | 3.6 | 18 | 8.4 | 19 | 7.8* | 43 | 17.8* |
| Partial remission | 1 | 3.6 | 8 | 3.7 | 9 | 3.7 | 6 | 2.5 |
| Stable disease | 3 | 10.7 | 52 | 24.2 | 55 | 22.6 | 67 | 27.7 |
| Progressive disease | 10 | 35.7 | 69 | 32.1 | 79 | 32.5 | 50 | 20.7 |
| Not evaluable | 12 | 42.9 | 63 | 29.3 | 75 | 30.9 | 57 | 23.6 |
Abbreviations: CR, complete remission; CRi, complete remission with incomplete blood count recovery; CRp, complete remission with incomplete platelet recovery; TC, treatment choice.
P = .001 (Fisher's exact test); odds ratio, 2.5; 95% CI, 1.40 to 4.78.
Safety
At the 2009 and 2010 cutoffs, exposure to study medication was greater with decitabine (median, 4.4 months) than with TC (2.4 months with cytarabine). Reasons for greater decitabine exposure may have included patient motivation and perceived efficacy by the investigator. The 83% longer exposure to decitabine versus TC meant that the AE reporting period for decitabine was also longer, which is important when interpreting AE data. The incidence of AEs overall, grades 3 and 4, and AEs that led to treatment discontinuation or death were similar between decitabine and TC and between decitabine and cytarabine. At the 2009 cutoff, most patients (≥ 97%) had at least one on-study AE. The most common grade 3 and 4 treatment-emergent AEs with decitabine and TC were thrombocytopenia (decitabine, 40%; cytarabine, 35%; SC, 14%) and anemia (decitabine, 34%; cytarabine, 27%; SC, 14%; Table 3). The mature analysis (2010 cutoff) showed similar results. Most patients reported at least one serious AE (decitabine, 80%; cytarabine, 72%; SC, 41%; Table 4). At the 2009 cutoff, the most common serious AEs were febrile neutropenia (decitabine, 24%; cytarabine, 16%; SC, 0%), pneumonia (decitabine, 20%; cytarabine, 16%; SC, 10%), and PD (decitabine, 11%; cytarabine, 14%; SC, 7%). A similar pattern was seen at the 2010 cutoff.
Table 3.
Treatment-Emergent Adverse Events of Grades 3 or 4 in ≥ 10% of Patients in Any Group (2009 clinical cutoff)
| Adverse Event | TC |
Decitabine (n = 238) |
||||||
|---|---|---|---|---|---|---|---|---|
| Supportive Care (n = 29) |
Cytarabine (n = 208) |
Total TC (n = 237) |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Any grade 3 or 4 adverse event | 16 | 55 | 188 | 90 | 204 | 86 | 221 | 93 |
| Thrombocytopenia | 4 | 14 | 73 | 35 | 77 | 32 | 95 | 40 |
| Anemia | 4 | 14 | 56 | 27 | 60 | 25 | 80 | 34 |
| Febrile neutropenia | 0 | 0 | 51 | 25 | 51 | 22 | 76 | 32 |
| Neutropenia | 1 | 3 | 41 | 20 | 42 | 18 | 76 | 32 |
| Leukopenia | 0 | 0 | 20 | 10 | 20 | 8 | 47 | 20 |
| Pneumonia | 4 | 14 | 39 | 19 | 43 | 18 | 51 | 21 |
| Bronchopneumonia | 3 | 10 | 9 | 4 | 12 | 5 | 10 | 4 |
| Disease progression | 2 | 7 | 46 | 22 | 48 | 20 | 43 | 18 |
| General physical health deterioration | 5 | 17 | 33 | 16 | 38 | 16 | 30 | 13 |
| Pyrexia | 3 | 10 | 17 | 8 | 20 | 8 | 24 | 10 |
| Hypokalemia | 5 | 17 | 19 | 9 | 24 | 10 | 27 | 11 |
| Dyspnea | 3 | 10 | 11 | 5 | 14 | 6 | 16 | 7 |
Abbreviation: TC, treatment choice.
Table 4.
Treatment-Emergent Serious Adverse Events in ≥ 5% of Patients in Any Group (2009 clinical cutoff)
| Adverse Event | TC |
Decitabine (n = 238) |
||||||
|---|---|---|---|---|---|---|---|---|
| Supportive Care (n = 29) |
Cytarabine (n = 208) |
Total TC (n = 237) |
||||||
| No. | % | No. | % | No. | % | No. | % | |
| Any serious adverse event | 12 | 41 | 150 | 72 | 162 | 68 | 190 | 80 |
| Febrile neutropenia | 0 | 0 | 33 | 16 | 33 | 14 | 57 | 24 |
| Thrombocytopenia | 0 | 0 | 11 | 5 | 11 | 5 | 21 | 9 |
| Anemia | 0 | 0 | 12 | 6 | 12 | 5 | 15 | 6 |
| Neutropenia | 0 | 0 | 7 | 3 | 7 | 3 | 15 | 6 |
| Pneumonia | 3 | 10 | 33 | 16 | 36 | 15 | 48 | 20 |
| Sepsis | 1 | 3 | 9 | 4 | 10 | 4 | 15 | 6 |
| Septic shock | 0 | 0 | 8 | 4 | 8 | 3 | 15 | 6 |
| Bronchopneumonia | 3 | 10 | 9 | 4 | 12 | 5 | 9 | 4 |
| Disease progression | 2 | 7 | 29 | 14 | 31 | 13 | 27 | 11 |
| Pyrexia | 2 | 7 | 18 | 9 | 20 | 8 | 23 | 10 |
| General physical health deterioration | 1 | 3 | 13 | 6 | 14 | 6 | 15 | 6 |
Abbreviation: TC, treatment choice.
Drug-related AEs (2009 cutoff) of any grade occurred in 175 decitabine recipients (74%) and 152 cytarabine recipients (73%). Grade 3 and 4 drug-related AEs occurred in 141 decitabine recipients (59%) and 114 cytarabine recipients (55%). The most common drug-related AEs with decitabine and cytarabine were thrombocytopenia (27% v 26%, respectively), anemia (21% v 20%), neutropenia (24% v 15%), and febrile neutropenia (21% v 15%). Drug-related AEs led to discontinuation in 14 decitabine recipients (6%) and 17 cytarabine recipients (8%); drug-related AEs reported in more than one patient were febrile neutropenia (n = 3 in each arm), pneumonia (decitabine, n = 3; cytarabine, n = 2), anemia (decitabine, n = 2; cytarabine, n = 1), and septic shock (decitabine, n = 1; cytarabine, n = 2). A similar pattern was seen at the 2010 cutoff.
Within 30 days after the first treatment, 21 decitabine recipients (9%) and 17 cytarabine recipients (8%) died. Sixty-day mortality was 19.7% with decitabine and 24.9% with TC (cytarabine, 23%; SC, 34.5%).
During treatment or ≤ 30 days after the last study drug dose, 77 decitabine-treated patients (32%) and 59 cytarabine-treated patients (28%) died. Of these patients, 58 deaths (24%) with decitabine and 39 deaths (19%) with cytarabine were due to AEs and 16 deaths (7%) and 17 deaths (8%), respectively, were due to PD. After adjustment for study drug exposure, which was 40% lower for cytarabine versus decitabine (969 v 1610 patient-months), the overall death rate (per patient-year) was lower for decitabine (0.57; 95% CI, 0.45 to 0.72) than for cytarabine (0.73, 95% CI, 0.56 to 0.94). The rate of deaths as a result of AEs was similar for decitabine (0.43; 95% CI, 0.33 to 0.56) and cytarabine (0.48; 95% CI, 0.34 to 0.66).
DISCUSSION
This study, which was one of the largest randomized, controlled, phase III trials to date in older patients with newly diagnosed AML compared the efficacy and safety of decitabine with the two most common treatments (SC or low-dose cytarabine). In these patients with poor prognostic factors, the median OS was 7.7 months in the decitabine group compared with 5.0 months in the control group (P = not significant) at the time of the 2009 cutoff. In addition, significantly improved remission rates (CR rate or CRp, 17.8% v 7.8%; P = .001) were observed with decitabine versus TC, respectively. Mature survival data collected up to the 2010 cutoff showed that the difference in OS in favor of decitabine became statistically significant (nominal P = .037), although this analysis was unplanned.
Decitabine was well tolerated, and patients received a median of four treatment cycles (v 2 cytarabine cycles); 26.5% of patients received at least nine cycles. Treatment-related AEs for decitabine were similar to those for cytarabine and consistent with the known decitabine safety profile7 and clinical presentation (predominantly myelosuppression) of AML. Despite a longer decitabine treatment duration and AE reporting period, the incidence and severity of AEs that led to discontinuation or death were similar between arms. After adjustment for study drug exposure, the overall death rate per patient-year was lower for decitabine (0.57) than for cytarabine (0.73), and between-group death rates as a result of AEs were similar (0.43 v 0.48). These findings are important because older patients with AML have limited treatment options, and the toxicity of standard therapies limits treatment.4
This trial was limited by its open-label design, which was necessary to compare the optimal regimen of IV decitabine with the standard subcutaneous cytarabine regimen.
Our results are consistent with those from a multicenter, open-label, phase II trial that used the same 5-day decitabine regimen in 55 patients (median age, 74 years) with intermediate- or poor-risk AML (CR, 21% and 24%, respectively; median OS, 7.7 months).12 Higher response rates were found in a randomized, adaptive-design trial in 64 patients (median age, 65 years) with higher-risk MDS and chronic myelomonocytic leukemia who received the same 5-day decitabine regimen (CR, 39%).11 In a single-center, open-label, phase II trial in 53 patients (median age, 74 years) with untreated AML, in which approximately one half of patients had poor-risk disease, the longer decitabine regimen of 20 mg/m2 IV per day infused over 1 hour for 10 days elicited a CR of 47%.13 However, these trials differed from the current trial in the number of centers, study design, patient numbers (lower), indication, and/or decitabine regimen.
A retrospective subanalysis of data from a phase III trial in patients with MDS evaluated survival with azacitidine (n = 55) versus conventional care (best SC, low-dose cytarabine, or intensive chemotherapy; n = 58).16,17 The study population, which was originally classified as having refractory anemia with excess blasts in transformation (≥ 20% blasts), was retrospectively recoded as having AML.17 The study suggested a survival advantage for azacitidine over conventional care (median OS, 25 v 16 months; P = .005), although no survival advantage was found versus low-dose cytarabine (median OS, 25 v 17 months; P = .08). However, the median OS with conventional care was atypically high, which suggested bias in the patient selection, particularly the exclusion of patients with proliferative AML (WBCs > 10,000/μL), who are known to have worse prognoses. Patients in the azacitidine trial had primary MDS and low baseline blast percentages (median, 23%), which are both prognostic factors for improved survival. Our study included high-risk patients with primary or secondary AML, median baseline blasts of 46%, and poor cytogenetics. In comparison, in a study of 446 older patients with AML who received cytarabine-based intensive chemotherapy,18 the median survival was 4.6 months, and in a recent compassionate usage program19 in 138 mostly older patients (median age, 73 years) with AML who received azacitidine, the median survival was 10.2 months.
In conclusion, results of this large, international, multicenter, phase III trial indicated that decitabine achieved a higher response rate, with a possible survival advantage, compared with low-dose cytarabine or SC in this difficult-to-treat, older population with AML.
Supplementary Material
Acknowledgment
Supported by Eisai; partially supported by the Leukemia Specialized Program of Research Excellence Grant No. CA 100632. Medical writing, editing, and graphics assistance was provided by Peloton Advantage and funded by Eisai.
Presented in part at the 47th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011.
We thank Yvonne E. Yarker, PhD, CMPP, for her editorial assistance during the development of this article.
Appendix
Enrollment and Overall Survival by Geographic Region and Country
A breakdown of patient enrollment by geographic region for the intent-to-treat population is presented in Appendix Table A1 (online only). There was no stratification for geographic region at randomization, and there was some imbalance in the number of patients in each treatment arm in some of the regions. This was particularly seen in the Western Europe subgroup, which had more patients in the decitabine arm (51 patients [v 34 patients for treatment choice (TC) with physician advice]), and in the North American/Australian subgroup, which had more patients in the TC arm (69 patients [v 51 patients in the decitabine arm]).
Table A1.
Patient Enrollment by Geographic Region for the Intent-to-Treat Population
| Region and Country | TC |
Decitabine (n = 242) |
Total (N = 485) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SC (n = 28) |
Cytarabine (n = 215) |
Total TC (n = 243) |
||||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Eastern Europe | 12 | 42.9 | 101 | 47.0 | 113 | 46.5 | 109 | 45.0 | 222 | 45.8 |
| Poland | 8 | 28.6 | 39 | 18.1 | 47 | 19.3 | 41 | 16.9 | 88 | 18.1 |
| Russian Federation | 0 | 29 | 13.5 | 29 | 11.9 | 28 | 11.6 | 57 | 11.8 | |
| Czech Republic | 0 | 23 | 10.7 | 23 | 9.5 | 24 | 9.9 | 47 | 9.7 | |
| Serbia | 4 | 14.3 | 6 | 2.8 | 10 | 4.1 | 12 | 5.0 | 22 | 4.5 |
| Hungary | 0 | 3 | 1.4 | 3 | 1.2 | 4 | 1.7 | 7 | 1.4 | |
| Romania | 0 | 1 | 0.5 | 1 | 0.4 | 0 | 1 | 0.2 | ||
| North America/Australia | 9 | 32.1 | 60 | 27.9 | 69 | 28.4 | 51 | 21.1 | 120 | 24.7 |
| United States | 3 | 10.7 | 24 | 11.2 | 27 | 11.1 | 21 | 8.7 | 48 | 9.9 |
| Australia | 5 | 17.9 | 17 | 7.9 | 22 | 9.1 | 16 | 6.6 | 38 | 7.8 |
| Canada | 1 | 3.6 | 19 | 8.8 | 20 | 8.2 | 14 | 5.8 | 34 | 7.0 |
| Western Europe | 3 | 10.7 | 31 | 14.4 | 34 | 14.0 | 51 | 21.1 | 85 | 17.5 |
| France | 0 | 24 | 11.2 | 24 | 9.9 | 31 | 12.8 | 55 | 11.3 | |
| Spain | 3 | 10.7 | 7 | 3.3 | 10 | 4.1 | 20 | 8.3 | 30 | 6.2 |
| Asia | 4 | 14.3 | 23 | 10.7 | 27 | 11.1 | 31 | 12.8 | 58 | 12.0 |
| Taiwan | 4 | 14.3 | 23 | 10.7 | 27 | 11.1 | 31 | 12.8 | 58 | 12.0 |
NOTE. Percentages were calculated with the number of patients in each group as the denominator.
Abbreviations: SC, supportive care; TC, treatment choice.
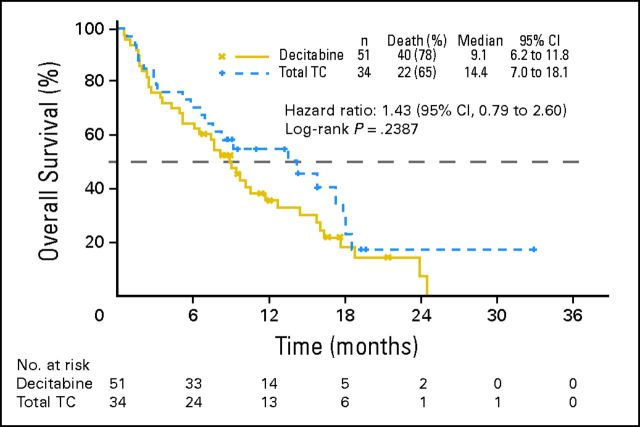
Appendix Table A2 summarizes the results of the multivariate proportional hazards analysis of overall survival (OS) for the intent-to-treat population. The Western European subgroup seemed to differ markedly in OS (2009 analysis) from the general study trend. In this small subgroup (n = 85; 17.5% of total), the observed median survival in the TC arm (14.4 months [v 9.1 months in the decitabine arm]) for the primary analysis of OS was much longer than in any other region (Fig 3) and was influenced by censored observations before the median, which resulted in a hazard ratio (HR) of 1.43 (95% CI, 0.79 to 2.60; P = .2387; Appendix Fig A1, online only).
Table A2.
Overall Survival by Geographic Region for the Intent-to-Treat Population: Multivariate Proportional Hazards Analysis
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Treatment, decitabine v total TC | 0.846 | 0.683 to 1.049 | 0.1270 |
| Geographic region | |||
| Eastern Europe v North America/Australia | 1.038 | 0.779 to 1.384 | 0.7968 |
| Western Europe v North America/Australia | 0.691 | 0.487 to 0.981 | 0.0385 |
| Asia v North America/Australia | 1.054 | 0.720 to 1.541 | 0.7878 |
| Western Europe v Eastern Europe | 0.665 | 0.480 to 0.922 | 0.0143 |
| Western Europe v Asia | 0.656 | 0.434 to 0.991 | 0.0454 |
Abbreviation: TC, patient treatment choice with physician advice.
Fig A1.
Overall survival in Western Europe for the intent-to-treat population. TC, treatment choice.
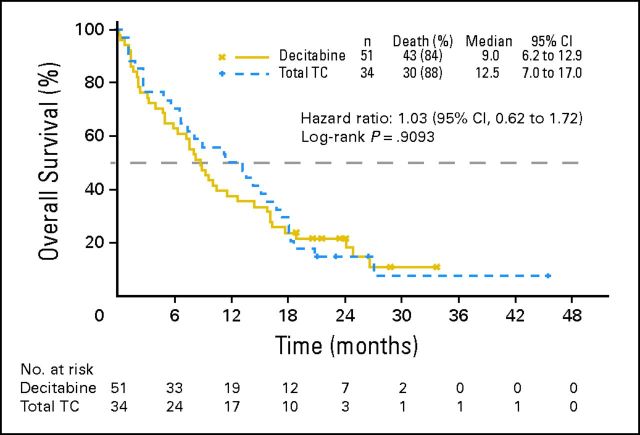
This apparent difference became less prominent in the mature (2010) OS analysis in which the number of patients with censored data was lower (median, 12.5 months in the TC arm, with an HR close to unity [1.03; 95% CI, 0.62 to 1.72, P = .9093; Appendix Fig A2, online only]).
In this region, fewer patients were randomly assigned to the TC arm (n = 34) than to the decitabine arm (n = 51), which contributed to the greater variability observed in OS curves in the TC arm and to the wide CIs for HRs. Patients in both arms received more cycles of therapy compared with in other regions. TC patients in the subgroup had fewer patients with an Eastern Cooperative Oncology Group performance status of 2, poor-risk cytogenetics, or a baseline bone marrow count greater than 50%. When both treatment arms in this subgroup were compared, there were more patients older than age 75, with poor-risk cytogenetics, with a baseline bone marrow blast count greater than 50%, or with secondary acute myeloid leukemia (in particular, proven leukemogenic exposure) in the decitabine arm, which conferred a worse prognosis. An additional examination of this Western European subgroup has also revealed that, unlike other regions, there was a marked subsequent therapy crossover to hypomethylating agents (ie, azacitidine or decitabine). More than one third of patients (12 patients [35%]) in the TC arm crossed over, although in the decitabine arm, only two patients (4%) received subsequent azacitidine and another two patients (4%) were retreated with decitabine. The seemingly reversed OS trend in this subgroup might be explained by the combination of the imbalance in baseline characteristics, the high crossover rate, and the greater variability of data because of the small sample size. Of note, secondary end points that were not influenced by subsequent therapy (ie, the response rate and progression-free survival) were more in line with the other regional and overall study results.
Fig A2.
Mature overall survival in Western Europe for the intent-to-treat population. TC, treatment choice.
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00260832.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Anna Dmoszynska, Mundipharma (C), Janssen-Cilag Polska (C); Agnieszka Wierzbowska, Genzyme (C); Jiri Mayer, Eisai (C); Rena Buckstein, Celgene (C), Novartis (C); Jacques Delaunay, Genzyme (C), Novartis (C); Christopher Arthur, Amgen (C), Bristol-Myers Squibb (C), Celgene (C), Jassen-Cilag (C), Novartis (C) Stock Ownership: None Honoraria: Anna Dmoszynska, Janssen-Cilag Polska; Rena Buckstein, Celgene, Novartis; Farhad Ravandi, Eisai, Johnson & Johnson; Christopher Arthur, Bristol-Myers Squibb, Janssen-Cilag, Novartis Research Funding: Hagop M. Kantarjian, Celgene, Eisai; Jiri Mayer, Eisai; Rena Buckstein, Celgene, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Provision of study materials or patients: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: Hagop M. Kantarjian, Xavier G. Thomas, Anna Dmoszynska, Agnieszka Wierzbowska, Jacques Delaunay
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.American Cancer Society. Cancer facts and figures. 2010. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol. 2007;18(suppl 1):i3–i8. doi: 10.1093/annonc/mdl443. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology. Acute myeloid leukemia v 2. 2011. http://guidelines.nccn.org/epc-guideline/guideline/id/F4F85BCF-EB5A-32AC-3926-8FDB31B89419#.
- 5.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Fey MF, Dreyling M, ESMO Guidelines Working Group Acute myeloblastic leukaemias and myelodysplastic syndromes in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;5(21 suppl):v158–v161. doi: 10.1093/annonc/mdq179. [DOI] [PubMed] [Google Scholar]
- 7.Dacogen [package insert] Woodcliff Lake, NJ: Eisai; 2010. [Google Scholar]
- 8.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 9.Willemze R, Suciu S, Archimbaud E, et al. A randomized phase II study on the effects of 5-Aza-2′-deoxycytidine combined with either amsacrine or idarubicin in patients with relapsed acute leukemia: An EORTC Leukemia Cooperative Group phase II study (06893) Leukemia. 1997;11(suppl 1):S24–S27. [PubMed] [Google Scholar]
- 10.Agrawal S, Hofmann WK, Tidow N, et al. The C/EBPδ tumor suppressor is silenced by hypermethylation in acute myeloid leukemia. Blood. 2007;109:3895–3905. doi: 10.1182/blood-2006-08-040147. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 12.Cashen AF, Schiller GJ, O'Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010;28:556–561. doi: 10.1200/JCO.2009.23.9178. [DOI] [PubMed] [Google Scholar]
- 13.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–569. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 18.Kantarjian H, Ravandi F, O'Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thepot S, Itzykson R, Seegers V, et al. Azacytidine (AZA) as first line therapy in AML: Results of the French ATU program. Blood. 2009:114. abstr 843. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.