Abstract
Purpose
To compare the receipt of clofarabine plus cytarabine (Clo+Ara-C arm) with cytarabine (Ara-C arm) in patients ≥ 55 years old with refractory or relapsed acute myelogenous leukemia (AML).
Patients and Methods
Patients were randomly assigned to receive either clofarabine (Clo) 40 mg/m2 or a placebo followed by Ara-C 1 g/m2 for five consecutive days. The primary end point was overall survival (OS). Secondary end points included event-free survival (EFS), 4-month EFS, overall remission rate (ORR; complete remission [CR] plus CR with incomplete peripheral blood count recovery), disease-free survival (DFS), duration of remission (DOR), and safety.
Results
Among 320 patients with confirmed AML (median age, 67 years), the median OS was 6.6 months in the Clo+Ara-C arm and 6.3 months in the Ara-C arm (hazard ratio [HR], 1.00; 95% CI, 0.78 to 1.28; P = 1.00). The ORR was 46.9% in the Clo+Ara-C arm (35.2% CR) versus 22.9% in the Ara-C arm (17.8% CR; P < .01). EFS (HR: 0.63; 95% CI, 0.49 to 0.80; P < .01) and 4-month EFS (37.7% v 16.6%; P < .01) favored the Clo+Ara-C arm compared with Ara-C arm, respectively. DFS and DOR were similar in both arms. Overall 30-day mortality was 16% and 5% for CLO+Ara-C and Ara-C arms, respectively. In the Clo+Ara-C and Ara-C arms, the most common grade 3 to 4 toxicities were febrile neutropenia (47% v 35%, respectively), hypokalemia (18% v 11%, respectively), thrombocytopenia (16% v 17%, respectively), pneumonia (14% v 10%, respectively), anemia (13% v 0%, respectively), neutropenia (11% v 9%, respectively), increased AST (11% v 2%, respectively), and increased ALT (10% v 3%, respectively).
Conclusion
Although the primary end point of OS did not differ between arms, Clo+Ara-C significantly improved response rates and EFS. Study follow-up continues, and the role of clofarabine in the treatment of adult patients with AML continues to be investigated.
INTRODUCTION
Although the 5-year survival for younger patients with acute myelogenous leukemia (AML) increased significantly from 11.9% in 1975 to 52.1% in 2002, survival for patients ≥ 50 years of age only increased from 4.3% to 11.3% during the same period.1 More recent data, which showed a 5-year survival of 5.1% for patients ≥ 65 years of age did not change between 1999 and 2006, confirmed the continued clinical challenge in these patients.2 In addition to a generally poorer performance status, older patients with AML tend to have disease marked by unfavorable cytogenetics, which lowers the chance of achieving the durable complete remissions (CRs) needed for long-term survival.3 Older patients with AML also have the added burden of comorbidities that increase their risk of death during induction.
No currently available treatment option for older patients with relapsed and refractory AML has demonstrated a survival advantage over any other. Most randomized clinical trials in patients with relapsed or refractory AML have included younger patients (< 55 years old), and in these studies, no overall survival (OS) improvement has been demonstrated.4–11 Hematopoietic stem-cell transplantation (HSCT) after remission induction in this setting may lead to long-term remissions or cure. Fortunately, HSCT has increasingly become an option for older patients through the availability of reduced-intensity conditioning regimens.12,13 Currently, there is no standard of care for these patients, and enrollment onto clinical trials is recommended.14,15
Clofarabine (2-chloro-9-[2-deoxy-2-fluoro-b-D-arabinofuranosyl] adenine) is a second-generation deoxyadenosine analog rationally synthesized to take advantage of the cytotoxic characteristics of fludarabine and cladribine while reducing the potential for dose-limiting toxicity.16 After experiments in myeloid-derived cells, which demonstrated the synergistic cytotoxic activity of clofarabine and cytarabine (Ara-C),17 a phase I/II study showed that this combination was safe and active in adult patients with relapsed and refractory acute leukemias, primarily AML.18 Other phase I/II studies have shown the feasibility of the clofarabine and Ara-C combination in various populations including first-line AML,19 R/R AML,20–22 and myelodysplastic syndrome.23
To follow-up on the phase I/II study,18 we conducted the CLASSIC I (Clofarabine and Ara-C Studying Survival via Induction and Consolidation) study. This phase III, randomized, double-blind, placebo-controlled trial compared clofarabine plus Ara-C (Clo+Ara-C arm) with a placebo plus Ara-C (Ara-C arm) in patients ≥ 55 years old with refractory or relapsed AML. The primary end point of this study was OS.
PATIENTS AND METHODS
Patient Criteria
Patients ≥ 55 years of age with refractory or relapsed (≥5% leukemic blasts in the bone marrow) AML, after no more than two previous induction regimens, were eligible to participate in the study. A blinded confirmation of AML diagnosis was made (with prerandomization specimens) by a central laboratory. Additional major inclusion criteria included Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 1, or 2, adequate renal (estimated glomerular filtration rate ≥ 60 mL/min/1.73 m2 per modification of diet in renal disease [MDRD] equation) and hepatic (serum bilirubin ≤ 1.5× the upper limit of normal; AST, ALT, and alkaline phosphatase ≤ 2.5× the upper limit of normal) functions. Major exclusion criteria included previous clofarabine or intermediate- or high-dose Ara-C treatment (unless the total duration of remission [DOR] after the most recent Ara-C–containing regimen exceeded 6 months, and the DOR after the last day of exposure to intermediate or high-dose Ara-C exceeded 3 months), a previous HSCT within 3 months of study entry, the presence of grade 2 or higher acute graft versus host disease (GvHD) or moderate to severe chronic graft versus host disease.
The institutional review boards of participating institutions approved the study. All patients gave written informed consent according to institutional guidelines.
Treatment Plan and Evaluations
After screening and eligibility assessment, patients were randomly assigned in an 1:1 ratio by using an interactive voice-response system to receive either Clo+Ara-C or a placebo plus Ara-C. Randomization stratified by the DOR after the first prestudy induction regimen was categorized as follows: primary refractory disease or first pretrial induction remission (CR1) duration less than 6 months (REF) or relapse after CR1 duration ≥ 6 months (REL).
Clofarabine 40 mg/m2 or placebo was administered as a 1-hour intravenous infusion, which was followed 3 hours from the end of infusion by Ara-C 1 g/m2 administered as a 2-hour intravenous infusion. Patients who achieved remission after their induction cycle could receive a single (optional) consolidation cycle; patients who did not achieve remission after induction but who demonstrated hematologic improvement could receive a reinduction cycle followed by a single (optional) consolidation cycle. The use of prophylactic antibacterial, antifungal, and antiviral agents was recommended according to institutional guidelines.
Response Criteria
International Working Group criteria24 were used to determine response. CR was defined as recovery to normal hematopoiesis with and absolute neutrophil count ≥ 1.0 × 109/L and a platelet count ≥ 100 × 109/L and normalization of marrow blasts (< 5%). CR with incomplete peripheral blood count recovery (CRi) was defined as meeting all criteria for CR but failing to meet either platelet or absolute neutrophil count recovery. Patients who achieved overall remission (CR or CRi) had follow-up bone marrow assessments or peripheral blood smears until documentation of disease recurrence or death; patients who experienced disease recurrence or who never achieved remission were followed for survival. Patients who did not achieve CR or CRi during induction or reinduction were considered treatment failures. Response was assessed by an Independent-response review panel (IRRP); these results were used in formal analyses of the overall remission rate (ORR = CR + CRi), DOR, disease-free survival (DFS), and event-free survival (EFS), but not in patient treatment decisions.
Statistical Analysis
The primary end point was OS, which was defined as the time from random assignment to the date of death as a result of any cause. The total follow-up in the study was end-point driven (until 260 deaths occurred) and was designed to achieve 90% power to detect a statistically significant improvement in OS (assumed median survival, 8.25 and 5.5 months in the Clo+Ara-C and Ara-C arms, respectively). The time-to-event analysis compared treatment arms by using a stratified log-rank test with durations of first remission as strata. The primary analysis was assessed for statistical significance with a two-sided significance level of 0.05. Secondary end points included EFS, 4-month EFS, ORR, DFS, DOR, and safety.
An independent data monitoring committee (DMC) assessed serious adverse events (SAEs) every 3 months and full safety results (SAEs and nonserious adverse events [AEs] and laboratory findings) every 6 months. The DMC also performed a single efficacy interim analysis when approximately one half of the planned number of events (deaths) had been reported. At this efficacy interim analysis, the DMC could have recommended early termination of the trial as a result of oustanding efficacy (P < .0031) or futility (P > .8461); the DMC recommended continuing the trial without modification. Trial modifications or termination by the DMC at any time as a result of safety concerns was permitted; no such recommendations were made.
End Point Definitions
OS (ie, months from random assignment to the time of death or date last known to be alive) and EFS (ie, months from randomization to the time of treatment failure, relapse, death, or date last known to be alive and in remission) analyses were performed for all randomly assigned patients with centrally confirmed AML. OS and EFS data were not censored for the initiation of alternative antileukemic therapy, including HSCT. DFS (ie, the time from IRRP documented remission to the time of death or relapse), and DOR (ie, the time from documented remission to the time of death, relapse, or initiation of alternative antileukemic therapy while in remission) were evaluated for all patients with an overall remission (OR). The administration of subsequent therapy, including off-protocol reinduction, consolidation, and HSCT were censored for DOR but not the primary analysis of OS.
RESULTS
Patient Characteristics
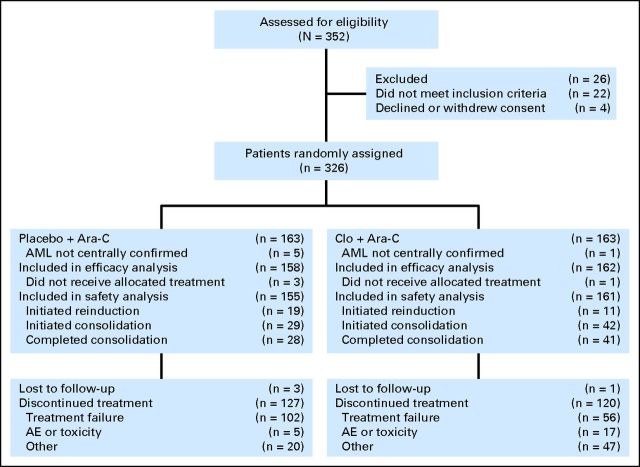
Of 326 patients enrolled at 57 sites (44 patients in North America and 13 patients in Europe), 163 patients were randomly assigned to each treatment arm (Fig 1) between September 2006 and November 2009. Six patients (one patient in the Clo+Ara-C arm; five patients in the Ara-C arm) were excluded from the efficacy and safety analyses because their baseline AML diagnosis was not centrally confirmed. In addition, four patients (one patient in the Clo+Ara-C arm; three patients in the Ara-C arm) did not receive any study treatment and were also excluded from the safety analysis (consent withdrawal [n = 2], one patient in each arm; ineligible [n = 1], and herpes zoster infection [n = 1]).
Fig 1.
CONSORT diagram of patient distribution in the CLASSIC I (Clofarabine and Ara-C Studying Survival via Induction and Consolidation) trial. AE, adverse event; AML, acute myelogenous leukemia; Ara-C, cytarabine; Clo+Ara-C, clofarabine plus cytarabine.
Patient demographics and baseline disease characteristics were balanced between treatment arms, with the exception of the karyotype category of unfavorable/other abnormalities and ECOG PS 2 (Table 1). A median of one cycle was completed in each arm. Among patients who initiated a second cycle (n = 94), 11 Clo+Ara-C patients (6.8%) versus 19 Ara-C patients (12.3%) initiated the second cycle as reinduction, whereas 39 Clo+Ara-C patients (24.2%) versus 25 Ara-C patients (16.1%) initiated the second cycle as consolidation. Less than 3% of patients in either arm initiated a third cycle as consolidation after reinduction (1.9% of patients in the Clo+Ara-C arm; 2.6% of patients in the Ara-C arm).
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Demographic or Characteristic | Clo+Ara-C (n = 162) |
Ara-C (n = 158) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age at randomization, years | ||||
| Median | 67 | 67 | ||
| Range | 55-82 | 55-86 | ||
| Sex | ||||
| M | 114 | 70.4 | 101 | 63.9 |
| F* | 48 | 29.6 | 57 | 36.1 |
| Race | ||||
| White | 150 | 92.6 | 142 | 89.9 |
| Asian | 3 | 1.9 | 2 | 1.3 |
| Black or African American | 7 | 4.3 | 11 | 7.0 |
| Other | 2 | 1.2 | 3 | 1.9 |
| ECOG performance status | ||||
| 0 | 57 | 35.2 | 48 | 30.4 |
| 1 | 79 | 48.8 | 92 | 58.2 |
| 2 | 26 | 16.0 | 18 | 11.4 |
| Karyotype | ||||
| CBF† | 7 | 4.3 | 9 | 5.7 |
| Normal | 65 | 40.1 | 84 | 53.2 |
| Unfavorable/other | 80 | 49.4 | 61 | 38.6 |
| Not assessed | 8 | 4.9 | 3 | 1.9 |
| Unknown | 2 | 1.2 | 1 | 0.6 |
| Disease | ||||
| Primary refractory | 74 | 46 | 69 | 44 |
| Relapsed | 88 | 54 | 88 | 56 |
| Clinical strata | ||||
| REF | 88 | 54.3 | 83 | 52.5 |
| REL | 74 | 45.7 | 74 | 46.8 |
| IVRS strata | ||||
| REF | 86 | 53 | 84 | 53.2 |
| REL | 76 | 46.9 | 74 | 46.8 |
Abbreviations: Ara-C, cytarabine; CBF, core binding factor; Clo+Ara-C, clofarabine plus cytarabine; ECOG, Eastern Cooperative Oncology Group; IVRS, interactive voice-response system; REF, primary refractory disease or first pretrial induction remission duration < 6 months; REL, first pretrial induction remission duration ≥ 6 months.
All women were postmenopausal or surgically sterile.
Contains abnormalities of chromosome 16 such as [inv16(p13;q22) or t(16;16)(p13;q22)] as well as t(8;21)(q22;q22). CBF also contains one patient with a t(15;17)(q22;q12) in the Clo + Ara-C arm.
OS
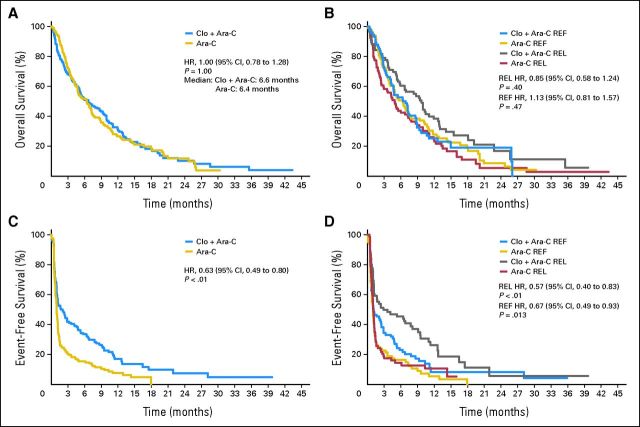
Among the 320 patients with centrally confirmed AML (162 Clo+Ara-C patients; 158 Ara-C patients), the median OS was 6.6 months in the Clo+Ara-C arm and 6.3 months in the Ara-C arm (P = 1.00; hazard ratio [HR], 1.00; 95% CI, 0.78 to 1.28; Fig 2A) with a median follow-up of 5.9 months (range, 0.03 to 43.32 months) and 6.4 months (range, 0.07 to 30.26 months), respectively. The effect of clofarabine on OS did not achieve statistical significance in either randomization stratum (Fig 2B). In the REF stratum, the median OS was 5.1 months for the Clo+Ara-C arm and 5.5 months for the Ara-C arm (P = .47, HR, 1.13; 95% CI, 0.81 to 1.57). In the REL stratum, the median OS was 8.7 months in the Clo+Ara-C arm and 7.2 months in the Ara-C arm (P = .40; HR, 0.85; 95% CI, 0.58 to 1.24). OS subarm comparisons yielded similar results to the overall population (data not shown).
Fig 2.
Kaplan-Meier curves for (A) overall survival, (B) overall survival by random assignment strata, (C) event-free survival, and (D) event-free survival by random assignment strata. Ara-C, cytarabine; Clo+Ara-C, clofarabine plus cytarabine; HR, hazard ratio; REF, primary refractory disease or first pretrial induction remission duration < 6 months; REL, first pretrial induction remission duration ≥ 6 months.
Response Assessed by IRRP
Overall response (46.9% v 22.9%; P < .01) and CR rates (35.2% v 17.8%; P < .01) were significantly higher for Clo+Ara-C compared with Ara-C for the overall population and the two randomization strata (Table 2). An evaluation of ORR by a duration of pretrial CR1 ≥ 12 months, presence of FLT3 mutation, and post hoc calculation of CRF data for previous CR1 stratum had similar results to those for the overall population (data not shown). The concordance between IRRP and investigator assessments of ORR was high (94.1%).
Table 2.
Best Responses by Using IRRP Assessment, Overall and by Strata
| Response | All Patients* |
REF Stratum |
REL Stratum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clo+Ara-C (n = 162) |
Ara-C (n = 157) |
Clo+Ara-C (n = 88) |
Ara-C (n = 83) |
Clo+Ara-C (n = 74) |
Ara-C (n = 74) |
|||||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Overall | 76 | 46.9 | 36 | 22.9 | 40 | 45.5 | 19 | 22.9 | 36 | 48.6 | 17 | 23.0 |
| P† | < .01 | < .01 | < .01 | |||||||||
| CR | 57 | 35.2 | 28 | 17.8 | 29 | 33.0 | 15 | 18.1 | 28 | 37.8 | 13 | 17.6 |
| P† | < .01 | .04 | < .01 | |||||||||
| CRi | 19 | 11.7 | 8 | 5.0 | 11 | 12.5 | 4 | 4.8 | 8 | 10.8 | 4 | 5.4 |
| Treatment failure | 86 | 53.1 | 122 | 77.1 | 48 | 54.5 | 64 | 77.1 | 38 | 51.4 | 57 | 77.0 |
| PR‡ | 3 | 1.9 | 2 | 1.3 | 2 | 2.3 | 0 | 1 | 1.4 | 2 | 2.7 | |
| Not PR | 83 | 51.2 | 119 | 75.8 | 46 | 52.3 | 64 | 77.1 | 37 | 50.0 | 55 | 74.3 |
Abbreviations: Ara-C, cytarabine; Clo+Ara-C, clofarabine plus cytarabine; CR, complete remission; CRi, complete remission with incomplete peripheral blood count recovery; IRRP, independent-response review panel; PR, partial remission; REF, primary refractory disease or first pretrial induction remission duration < 6 months; REL, first pretrial induction duration ≥ 6 months.
Evaluable patients (n = 319); one patient was removed because the duration of the first pretrial induction was unknown.
Comparison P value was calculated by using the Cochran-Mantel-Haenszel test and stratified by randomization strata (REF v REL). Within strata, the comparison P value was calculated by using Fisher's exact test.
PR was defined as follows: recovery of peripheral counts (platelets ≥ 100 × 109/L and absolute neutrophil count ≥ 1.0 × 109/L) and either a decrease ≥ 50% in the percentage of leukemic blasts to 5% to 25% in the bone marrow aspirate or biopsy or a bone marrow aspirate or biopsy of ≤ 5% leukemic blasts with Auer rods present.
Other Secondary End Points
Secondary end points were significantly in favor of the Clo+ Ara-C–treatment arm for EFS (HR, 0.63; 95% CI, 0.49 to 0.80; P < .01) and for 4-month EFS (37.7% v 16.6%; P < .01) in the overall patient population as well as in both randomization strata (Table 3; Figs 2C and 2D). The median DOR, censoring for alternative treatment, was 7.6 months in the Clo+Ara-C arm and 3.8 months in the Ara-C arm. By strata, the difference between treatment arms in the median DOR appeared greatest in the REL stratum (11.5 v 3.8 months). No formal comparison or statistical analyses were performed for DOR because an interpretation of arm comparison was complicated by different proportions of patients who achieved remission in each treatment arm.
Table 3.
Efficacy End Points, Overall and by Strata
| End Point | All Patients* |
REF Stratum |
REL Stratum |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clo+Ara-C |
Ara-C |
Clo+Ara-C |
Ara-C |
Clo+Ara-C |
Ara-C |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| OS | ||||||||||||
| Patients | 162 | 158 | 88 | 84 | 74 | 74 | ||||||
| Events | 129 | 79.6 | 129 | 81.6 | 73 | 84.9 | 72 | 85.7 | 56 | 73.7 | 57 | 77.0 |
| HR | 1.00 | 1.13 | 0.85 | |||||||||
| 95% CI | 0.78 to 1.28 | 0.81 to 1.57 | 0.58 to 1.24 | |||||||||
| P† | 1.00 | .47 | .40 | |||||||||
| EFS | ||||||||||||
| Patients | 162 | 157 | 88 | 83 | 74 | 74 | ||||||
| Events | 130 | 80.2 | 144 | 91.7 | 73 | 83.0 | 78 | 94.0 | 57 | 77.0 | 66 | 89.2 |
| HR | 0.63 | 0.67 | 0.57 | |||||||||
| 95% CI | 0.49 to 0.80 | 0.49 to 0.93 | 0.40 to 0.83 | |||||||||
| P† | < .01 | .013 | < .01 | |||||||||
| 4-month EFS | ||||||||||||
| Patients | 162 | 158 | 86 | 84 | 76 | 74 | ||||||
| Events | 61 | 37.7 | 26 | 16.6 | 31 | 35.2 | 14 | 16.9 | 30 | 40.5 | 12 | 16.2 |
| P‡ | < .01 | < .01 | < .01 | |||||||||
| DOR, months§∥ | ||||||||||||
| Patients | 76 | 36 | 40 | 19 | 36 | 17 | ||||||
| Events | 25 | 32.9 | 13 | 36.1 | 16 | 40.0 | 8 | 42.1 | 9 | 25.0 | 5 | 29.4 |
| Median | 7.6 | 3.8 | 5.7 | 6.3 | 11.5 | 3.8 | ||||||
| 95% CI | 7.7 to 20.6 | 3.8 to 12.1 | 6.7 to 27.0 | 2.6 to 12.1 | 10.2 to 20.6 | 3.8 to N/E | ||||||
| DFS, months∥¶ | ||||||||||||
| Patients | 76 | 36 | 40 | 19 | 36 | 17 | ||||||
| Events | 44 | 57.9 | 23 | 63.9 | 25 | 62.5 | 14 | 73.7 | 19 | 52.8 | 9 | 52.9 |
| Median | 8.1 | 7.0 | 5.7 | 6.7 | 10.3 | 9.1 | ||||||
| 95% CI | 10.3 to 27.0 | 7.2 to 16.7 | 7.7 to N/E | 6.7 to 16.7 | 11.5 to N/E | 9.1 to N/E | ||||||
Abbreviations: Ara-C, cytarabine; Clo+Ara-C, clofarabine plus cytarabine; DFS, disease-free survival; DOR, duration of remission; EFS, event-free survival; HR, hazard ratio; N/E, not estimable; OS, overall survival; REF, primary refractory disease or first pretrial induction remission duration < 6 months; REL, first pretrial induction duration ≥ 6 months.
Evaluable patients (n = 319); one patient was removed because the duration of the first pretrial induction remission (CR1) was unknown.
Comparison P value is from a log-rank test, stratified by randomization strata (REF v REL). Within strata, comparison P value is from a log-rank test with no strata.
Comparison P value is from Cochran-Mantel-Haenszel test, stratified by randomization strata (REF v REL). Within strata, comparison P value is from a Fisher's exact test.
Measured from the time point of remission to relapse or death, censored if alternative therapy is initiated.
Statistical comparisons were not made for DOR/DFS because they only assessed patients in remission and, thus, would have been invalid.
Measured from the time point of remission to relapse or death.
Exploratory End Points
In the Clo+Ara-C arm, 34 of 162 patients(21%) underwent HSCT after study treatment; comparatively, 30 of 158 patients (19%) in the Ara-C arm underwent HSCT. Among transplant patients, 16% versus 9% of patients were in remission after study treatments at the time of HSCT, respectively. Fewer patients in the Clo+Ara-C arm (n = 68 [43%]) received alternative antileukemic therapy before HSCT compared with in the Ara-C arm (n = 97 [61%]). Among the 64 patients who underwent an HSCT, all but two patient received an allogeneic transplant. After treatment failure, 22% of patients in the Clo+Ara-C arm and 46% of patients in the Ara-C arm received subsequent therapy.
Safety
The two arms had similar frequencies of grade 3 to 4 AEs regardless of causality (77% in the Clo+Ara-C arm v 74% in the Ara-C arm), although the frequency of all grade treatment-related AEs was slightly higher in the Clo+Ara-C arm than in the Ara-C arm (98% v 86%, respectively). The most common grade 3 to 4 nonhematologic AEs regardless of causality were febrile neutropenia, hypokalemia, and pneumonia (Table 4). Grade 3 to 4 acute renal failure occurred in eight patients (5%) in the Clo+Ara-C arm and in no patients in the Ara-C arm.
Table 4.
Maximum Common Terminology Criteria Grade 3 to 4 Treatment-Emergent Adverse Events Reported in ≥ 5% of Patients
| Adverse Event | Clo+Ara-C (n = 161) |
Ara-C (n = 155) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 3 Toxicity |
Grade 4 Toxicity |
Total |
Grade 3 Toxicity |
Grade 4 Toxicity |
Total |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Any | 77 | 48 | 47 | 29 | 124 | 77 | 79 | 51 | 36 | 23 | 115 | 74 |
| Febrile neutropenia | 72 | 45 | 4 | 2 | 78 | 47 | 49 | 32 | 4 | 3 | 53 | 35 |
| Hypokalemia | 24 | 15 | 5 | 3 | 29 | 18 | 15 | 10 | 1 | 1 | 16 | 11 |
| Thrombocytopenia | 2 | 1 | 24 | 15 | 26 | 16 | 3 | 2 | 23 | 15 | 26 | 17 |
| Pneumonia | 19 | 12 | 3 | 2 | 22 | 14 | 16 | 10 | 0 | 16 | 10 | |
| Anemia | 18 | 11 | 3 | 2 | 21 | 13 | 12 | 8 | 0 | 12 | 8 | |
| Neutropenia | 1 | 1 | 16 | 10 | 17 | 11 | 2 | 1 | 12 | 8 | 14 | 9 |
| Increased AST | 16 | 10 | 1 | 1 | 17 | 11 | 3 | 2 | 0 | 3 | 2 | |
| Increased ALT | 16 | 10 | 0 | 16 | 10 | 4 | 3 | 0 | 4 | 3 | ||
| Bacteremia | 12 | 7 | 3 | 2 | 15 | 9 | 6 | 4 | 0 | 6 | 4 | |
| Hypertension | 13 | 8 | 1 | 1 | 14 | 9 | 11 | 7 | 2 | 1 | 13 | 8 |
| Diarrhea | 13 | 8 | 0 | 13 | 8 | 4 | 3 | 0 | 4 | 3 | ||
| Enterococcal bacteremia | 12 | 7 | 1 | 1 | 13 | 8 | 2 | 1 | 0 | 2 | 1 | |
| Leukopenia | 0 | 9 | 6 | 9 | 6 | 1 | 1 | 7 | 5 | 8 | 5 | |
| Fatigue | 9 | 6 | 0 | 9 | 6 | 8 | 5 | 0 | 8 | 5 | ||
| Sepsis | 4 | 2 | 4 | 2 | 8 | 5 | 3 | 2 | 1 | 1 | 4 | 3 |
| Staphylococcal bacteremia | 10 | 6 | 0 | 10 | 6 | 15 | 10 | 0 | 15 | 0 | ||
| Hyperglycemia | 9 | 6 | 0 | 9 | 6 | 6 | 4 | 0 | 6 | 4 | ||
| Renal failure, acute | 6 | 4 | 2 | 1 | 8 | 5 | 0 | 0 | 0 | |||
| Hyponatremia | 9 | 6 | 0 | 9 | 6 | 1 | 1 | 0 | 0 | |||
| Hypotension | 7 | 4 | 3 | 2 | 10 | 6 | 1 | 1 | 2 | 1 | 3 | 2 |
| Increased bilirubin | 7 | 4 | 1 | 1 | 8 | 5 | 1 | 1 | 0 | 1 | 1 | |
| Increased lipase | 6 | 4 | 2 | 1 | 8 | 5 | 2 | 1 | 1 | 1 | 3 | 2 |
| Hypocalcemia | 7 | 4 | 1 | 1 | 8 | 5 | 1 | 1 | 0 | 1 | 1 | |
NOTE. Only laboratory abnormalities identified as clinically significant by the investigator were reported as adverse events. Clinically significant is defined as any variation in laboratory parameters, which has medical relevance resulting in an alteration in medical care.
Abbreviations: Ara-C, cytarabine; Clo+Ara-C, clofarabine plus cytarabine.
A greater proportion of patients in the Clo+Ara-C arm had SAEs compared with in the Ara-C arm (60% v 49%, respectively). The total incidence of serious infections was higher in the Clo+Ara-C arm than in the Ara-C arm (38% v 22%, respectively). In the Clo+Ara-C arm, the most frequent infection SAEs were bacteremia (seven patients), sepsis (eight patients), pneumonia (13 patients), and septic shock (six patients). In the Ara-C arm, the most common infection SAEs were cellulitis (three patients), bacteremia (three patients), and pneumonia (12 patients).
Most skin and subcutaneous tissue disorders were grades 1 to 2; however, 15% of patients in the Clo+Ara-C arm and 6% of patients (all grade 3) in the Ara-C arm experienced grade 3 to 4 skin and subcutaneous tissue–disorder AEs. Treatment-related palmar-plantar erythrodysesthesia occurred in 33 patients (20%) in the Clo+Ara-C arm (grades 1 to 3; grade 3, 6%) and two patients (1%) in the Ara-C arm (grades 1 to 2).
Deaths as a result of AEs occurred in 14.3% of patients in the Clo+Ara-C arm compared with 5.2% of patients in the Ara-C arm, although deaths as a result of treatment related AEs occurred in 6.2% versus 1.9% of patients, respectively.
The all-cause 30-day induction mortality rate was 16% (25 of 161 patients) in the Clo+Ara-C arm compared with 5% (eight of 155 patients) in the Ara-C arm (P < .01). Of the 25 Clo+Ara-C patients who died within 30 days, five patients died as a result of disease progression, 19 patients died as a result of AEs, and the cause of death of one patient was unknown.
Among the 19 Clo+Ara-C patients with fatal AEs, six patients died of sepsis-related AEs, three patients had acute respiratory distress/failure, two patients had a cerebral hemorrhage, two patients had pneumonia, and one patient each had a pulmonary hemorrhage, subdural hematoma, renal failure, veno-occlusive liver disease, acute myocardial infarction, and toxic epidermal necrolysis. Among the eight Ara-C–arm patients who died within 30 days, three patients died because of disease progression, and five patients had a fatal AE (one patient each with a cerebral infarction, cerebral hemorrhage, multiorgan failure, Klebsiella sepsis, and cardiorespiratory arrest).
In the comparison of induction mortality, rates were higher in Clo+Ara-C arms within each strata (REF, 16.1%; REL, 14.9%) compared with Ara-C alone (REF: 3.7% and REL: 5.5%).
DISCUSSION
To our knowledge, this placebo-controlled, double-blind, randomized phase III CLASSIC I study was the largest completed phase III trial to date that involved older patients with R/R AML. In addition to the enrollment of patients older (median age, 67 years) than those included in most other AML trials, a large proportion of patients (Clo+Ara-C arm, 45%; Ara-C arm, 43%) had primary refractory disease. These patients represent a population that is difficult to treat (as evidenced by CR rates that ranged from 4% to 63% and a median OS of 2.4 to 8 months in published studies), has limited treatment options, and for whom a survival benefit has not been previously demonstrated in randomized comparative clinical trials.4–11
With a median OS of 6.6 months in the Clo+Ara-C arm and 6.3 months in the Ara-C arm, this study did not meet its primary end point of improved OS. However, our results fell within the higher end of the median OS (eg, 5.2 months for lintuzumab,4 4.6 months for valspodar,5 3.7 months for ozagamicin,7 2.4 months for liposomal daunorubicin,7 and 3.8 months for topotecan7) reported in other studies of novel agents combined with Ara-C–based chemotherapy in this patient population. Moreover, CR and ORR rates were significantly higher with Clo+Ara-C compared with Ara-C for the overall population and the randomization strata, unlike the rates reported in other large randomized studies in which statistically significant differences have not been observed.4–11,25 This difference may be clinically significant because patients who are not in CR at the time of transplant have poorer outcomes compared with those in CR.26,27
In addition, other secondary end points, including overall and 4-month EFS, significantly favored the Clo+Ara-C combination compared with Ara-C. Analyses of EFS eliminated the contribution of subsequent therapy after relapse, which may have obfuscated the OS analyses. A statistically significant difference in 4-month EFS was observed in the Clo+Ara-C arm. Because HSCT is the only curative therapy for patients with R/R AML, the ability to achieve a sustained remission is a critical measure of therapeutic success for treatment regimens in this population. The 4-month EFS serves as a measure of sustained remission. Consistent with the higher EFS in the Clo+Ara-C arm, more patients in the Clo+Ara-C arm proceeded to HSCT directly after remission from the study treatment. This difference was particularly evident when the REL stratum wasexamined.
There were no unexpected toxicities associated with Clo+Ara-C compared with the known safety profile of clofarabine monotherapy or the Clo+Ara-C combination previously reported in the literature.18,20–23 However, there were more toxicities in the Clo+Ara-C arm than in the Ara-C arm. The 16% 30-day induction mortality rate in the Clo+Ara-C arm was similar to that seen in earlier studies of this combination in various populations of patients with AML.18,20–23 This early mortality rate for Clo+Ara-C is in line with 8% to 32% rates reported for other combination regimens in this patient population.25 The higher early mortality rate in the Clo+Ara-C arm, a slight baseline imbalance in unfavorable karyotype and ECOG PS2, and the impact of subsequent therapy may explain why the doubling of the remission rate and the improvement in EFS did not translate into improved OS. The lower rate of early mortality in the Ara-C arm in this study raises the question of whether the combination regimen of Clo+Ara-C may be too toxic for some patients or whether the most appropriate doses were selected for this relapsed/refractory elderly population. Future analyses of prognostic factors and risk determination for patient subgroups should provide important information for drug development and clinical practice.
The observation from this trial provides several lessons. Older patients with primary refractory disease or CR duration less than 6 months represent a disease biology that is difficult to impact with conventional chemotherapy combinations. Moreover, alternative therapy confounds the contributions of new induction regimens. Last, because the principle objective in AML is HSCT, EFS or 4-month EFS, rather than OS, should be considered a measure of clinical benefit in the evaluation of novel therapies for AML.
Few other new agents are used in phase III clinical studies in patients with R/R AML. Recruitment is ongoing for the placebo-controlled VALOR (vosaroxin and Ara-C combinations evaluating OS in R/R AML) study. Unfortunately, the ACCEDE (amonafide plus Ara-C compared to daunorubicin plus Ara-C in patients with secondary AML) phase III study of the novel agent amonafide failed to meet its primary end point of ORR.
In conclusion, the CLASSIC I study did not demonstrate an improvement in OS; however, compared with Ara-C alone, the addition of clofarabine to Ara-C significantly improved ORR and EFS and allowed more patients to proceed to transplant in remission from study treatment. Study follow-up continues, and the role of clofarabine in the treatment of adult patients with AML is being investigated in other randomized trials.
Supplementary Material
Acknowledgment
We thank Monica Nicosia, PhD (Science Wordsmith), Caryn Cramer, PhD (Genzyme), and Angela Partisano, PharmD, MS (Genzyme), for their editorial assistance. In addition, we are grateful to the patients and their families and the investigators and institutions that participated in this study.
Footnotes
Supported by Genzyme, Cambridge, MA.
Presented in part at the 53rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2011, and at the 16th Congress of the European Hematology Association, London, United Kingdom, June 9-12, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00317642.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Michael Vasconcelles, Genzyme (C); Dirk Huebner, Genzyme (C) Consultant or Advisory Role: Stefan Faderl, Genzyme (C); Meir Wetzler, Genzyme (C); Gary Schiller, Genzyme (C); Robert Stuart, Genzyme (C); Siddhartha Ganguly, Genzyme (C); David Avigan, Genzyme (C); Norbert Vey, Genzyme (C); Christian Recher, Genzyme (C), Celgene (C); Farhad Ravandi, Genzyme (C); Hagop M. Kantarjian, Genzyme (C) Stock Ownership: None Honoraria: Stefan Faderl, Genzyme; Meir Wetzler, Genzyme; David Rizzieri, Genzyme; Gary Schiller, Genzyme; Siddhartha Ganguly, Genzyme; Tibor Kovacsovics, Genzyme; Karen Seiter, Genzyme; Parameswaran Hari, Genzyme; Norbert Vey, Genzyme; Christian Recher, Genzyme, Celgene Research Funding: Stefan Faderl, Genzyme; Meir Wetzler, Genzyme; Gary Schiller, Genzyme; Robert Stuart, Genzyme; Siddhartha Ganguly, Genzyme; David Avigan, Genzyme; Michael Craig, Genzyme; Robert Collins, Genzyme; Stuart Goldberg, Genzyme; Karen Seiter, Genzyme; Parameswaran Hari, Genzyme; Christian Recher, Celgene, Jansen-Cilag; Farhad Ravandi, sanofi-aventis; Hagop M. Kantarjian, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Stefan Faderl, David Rizzieri, David Avigan, Michael Vasconcelles, Dirk Huebner, Hagop M. Kantarjian
Provision of study materials or patients: Stefan Faderl, Meir Wetzler, David Rizzieri, Gary Schiller, Siddhartha Ganguly, Michael Craig, Stuart Goldberg, Norbert Vey, Eunice S. Wang
Collection and assembly of data: Stefan Faderl, Meir Wetzler, David Rizzieri, Gary Schiller, Madan Jagasia, Robert Stuart, Siddhartha Ganguly, David Avigan, Michael Craig, Robert Collins, Michael Maris, Tibor Kovacsovics, Stuart Goldberg, Karen Seiter, Parameswaran Hari, Jochen Greiner, Norbert Vey, Christian Recher, Farhad Ravandi, Eunice S. Wang, Hagop M. Kantarjian
Data analysis and interpretation: Stefan Faderl, Meir Wetzler, David Rizzieri, Madan Jagasia, Siddhartha Ganguly, David Avigan, Michael Maris, Stuart Goldberg, Karen Seiter, Parameswaran Hari, Jochen Greiner, Michael Vasconcelles, Dirk Huebner, Hagop M. Kantarjian
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Surveillance Epidemiology and End Results. Fast Stats. Acute myeloid leukemia 5-year relative survival by year diagnosis and age at diagnosis/death 1975-2002. 2011. http://www.seer.cancer.gov/faststats/selections.php?series=age.
- 2.Altekruse SF, et al. SEER Cancer Statistics Review 1975-2007. National Cancer Institute. 2011. http://seer.cancer.gov/csr/1975_2007/
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman EJ, Brandwein J, Stone R, et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J Clin Oncol. 2005;23:4110–4116. doi: 10.1200/JCO.2005.09.133. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg PL, Lee SJ, Advani R, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: A phase III trial (E2995) J Clin Oncol. 2004;22:1078–1086. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karanes C, Kopecky KJ, Head DR, et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res. 1999;23:787–794. doi: 10.1016/s0145-2126(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 7.Litzow MR, Othus M, Cripe LD, et al. Failure of three novel regimens to improve outcome for patients with relapsed or refractory acute myeloid leukaemia: A report from the Eastern Cooperative Oncology Group. Br J Haematol. 2010;148:217–225. doi: 10.1111/j.1365-2141.2009.07917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milligan DW, Wheatley K, Littlewood T, et al. Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: Results of the MRC AML-HR randomized trial. Blood. 2006;107:4614–4622. doi: 10.1182/blood-2005-10-4202. [DOI] [PubMed] [Google Scholar]
- 9.Ohno R, Naoe T, Kanamaru A, et al. A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Kohseisho Leukemia Study Group. Blood. 1994;83:2086–2092. [PubMed] [Google Scholar]
- 10.Thomas X, Fenaux P, Dombret H, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) to increase efficacy of intensive sequential chemotherapy with etoposide, mitoxantrone and cytarabine (EMA) in previously treated acute myeloid leukemia: A multicenter randomized placebo-controlled trial (EMA91 Trial) Leukemia. 1999;13:1214–1220. doi: 10.1038/sj.leu.2401474. [DOI] [PubMed] [Google Scholar]
- 11.Vogler WR, McCarley DL, Stagg M, et al. A phase III trial of high-dose cytosine arabinoside with or without etoposide in relapsed and refractory acute myelogenous leukemia. A Southeastern Cancer Study Group trial. Leukemia. 1994;8:1847–1853. [PubMed] [Google Scholar]
- 12.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 13.Forman SJ. What is the role of reduced-intensity transplantation in the treatment of older patients with AML? Hematology Am Soc Hematol Educ Program. 2009;2009:406–413. doi: 10.1182/asheducation-2009.1.406. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia V 2.2011. National Comprehensive Cancer Network. 2011. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 15.Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89:998–1008. [PubMed] [Google Scholar]
- 16.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, et al. Synthesis and biologic activity of 2′-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 17.Cooper T, Ayres M, Nowak B, et al. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother Pharmacol. 2005;55:361–368. doi: 10.1007/s00280-004-0906-y. [DOI] [PubMed] [Google Scholar]
- 18.Faderl S, Gandhi V, O'Brien S, et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood. 2005;105:940–947. doi: 10.1182/blood-2004-05-1933. [DOI] [PubMed] [Google Scholar]
- 19.Faderl S, Verstovsek S, Cortes J, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 20.Faderl S, Ravandi F, Ferrajoli A, et al. Clofarabine and clofarabine plus low-dose cytarabine (ara-C) as induction therapy for patients (pts) ≥ 60 years with newly diagnosed acute myeloid leukemia (AML) Blood. 2005;(suppl):106. abstr 2804. [Google Scholar]
- 21.Becker PS, Kantarjian H, Appelbaum FR, et al. Multivariate analysis of response and survival after treatment with clofarabine, cytarabine and G-CSF priming (GCLAC) in relapsed/refractory acute myeloid leukemia (AML): Comparison with prior experience using fludarabine and cytarabine combination regimens. Blood. 2010;(suppl):116. abstr 1065. [Google Scholar]
- 22.Agura E, Cooper B, Holmes H, et al. Report of a phase II study of clofarabine and cytarabine in de novo and relapsed and refractory AML patients and in selected elderly patients at high risk for anthracycline toxicity. Oncologist. 2011;16:197–206. doi: 10.1634/theoncologist.2010-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 25.Kell J. Treatment of relapsed acute myeloid leukaemia. Rev Recent Clin Trials. 2006;1:103–111. doi: 10.2174/157488706776876445. [DOI] [PubMed] [Google Scholar]
- 26.Hamadani M, Awan FT, Copelan EA. Hematopoietic stem cell transplantation in adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2008;14:556–567. doi: 10.1016/j.bbmt.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Société Française de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.