Abstract
Background
Protein losing enteropathy (PLE) is a challenging complication after Fontan operation. Subclinical enteric protein loss may precede development of overt PLE. We evaluated the acute effects of Fontan circulation on enteric protein loss and mesenteric vascular resistance.
Methods
A prospective cohort study was performed evaluating enteric protein loss in children undergoing Fontan operation. Stool alpha-1-antitrypsin (A1AT) concentration was measured in the pre-operative, early post-operative, and intermediate post-operative (3–9 months) periods. The intestinal circulation was characterized by Doppler-derived resistance indices of the superior mesenteric artery, and serum albumin and protein levels were obtained.
Results
We enrolled 33 subjects at a median age at operation of 3.0 (2.5–3.3) years. No clinical PLE was observed. Six of the 93 stool A1AT samples obtained were elevated (>54 mg/dl), with two abnormal samples at each of the three time points. Two of the five subjects with elevated stool A1AT values had significant hemodynamic disturbances requiring intervention (junctional bradycardia or tricuspid stenosis). There was no difference in superior mesenteric artery resistance in the pre-operative versus early post-operative period (p=0.9). Serum albumin levels were lower in the early post-operative period compared to the pre-operative period (3.2 mg/dl [IQR 2.9–3.5] vs. 4.1 mg/dl [IQR 3.4–4.5], p=0.01) but did not correlate with abnormal stool A1AT concentration or superior mesenteric artery resistance indices.
Conclusions
The Fontan operation does not commonly result in acute development of increased enteric protein loss. However, increased enteric protein loss may occur in children before or after Fontan operation, particularly when hemodynamic disturbances are present.
Keywords: CHD, Fontan, Glenn, Pediatric
Approximately 4 to 13 percent of patients with single ventricle circulation develop protein losing enteropathy (PLE) weeks to years after the Fontan operation (1, 2). This abnormal loss of serum proteins into the gastrointestinal tract can result in a clinical state characterized by peripheral edema, ascites, and effusions as well as disturbances in coagulation, the immune system, and growth (3). Despite its significant morbidity and mortality, the pathophysiology of and time course for development of PLE is incompletely understood. While a subclinical state of PLE has been described in adults with Fontan circulation (4), and children with Fontan circulations have elevated resistance of their mesenteric vasculature (5), these have not been well studied in children before and early after the Fontan operation. We sought to determine the acute effects of Fontan circulation on enteric protein loss and mesenteric vascular resistance. We hypothesize that a preclinical state of PLE may exist in which children have abnormal enteric protein loss without significant depletion of serum protein levels and the clinical manifestations of PLE.
PATIENTS AND METHODS
A single center, prospective, longitudinal cohort study was performed at the Children’s Hospital of Philadelphia (Philadelphia, PA). Subjects were enrolled from 8/30/13 – 11/5/14. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board (IRB # 12-009574), and consent was obtained in all subjects.
Patient Selection
The institutional surgical database was screened for subjects who had completed superior cavopulmonary anastomosis surgery who had not yet undergone Fontan operation. The surgical schedule was then reviewed during the enrollment period to identify subjects presenting for Fontan operation. The families of subjects meeting inclusion and exclusion criteria were contacted for consent to participate.
Subjects were considered for inclusion if they were undergoing a Fontan procedure, had a prior superior cavopulmonary anastomosis, and were age 1–5 years. The age limitation was imposed as children far outlying the typical age of this palliation were thought not to represent the typical population of children scheduled for Fontan. Subjects were excluded if they already had a clinical diagnosis of PLE prior to the Fontan surgery, had a known condition causing abnormal serum protein levels prior to Fontan surgery (including synthetic liver dysfunction, inflammatory bowel disease, or renal protein loss such as nephrotic syndrome), or who no longer had Fontan physiology due to surgical or catheterization take-down of the Fontan circulation or cardiac transplantation. Additionally, a small subset of subjects who failed to provide adequate stool samples was excluded from the analysis.
Study Design
The medical records of consented and enrolled subjects were reviewed for demographic, treatment, echocardiographic, catheterization, magnetic resonance imaging (MRI) and surgical data. Families were also requested to complete a questionnaire to self-report family history of gastrointestinal disease. To longitudinally investigate the impact of the Fontan operation on enteric protein loss, subjects were evaluated at the following time points:
Pre-operative: Between superior cavopulmonary surgery to the Fontan operation.
Early post-operative: Five to 30 days after Fontan operation. All subjects met the following criteria for stability and adequate enteric intake: spontaneous respiration, not receiving inotropic support, taking enteric feeds, and stooling, with at least one formed bowel movement produced prior to collection of the study sample. Subjects had to be at least five days post-operative as there are reports that gut permeability and absorption return to normal after bypass by this time frame. (6)
Intermediate post-operative: Three to nine months after Fontan operation.
Enteric protein loss was evaluated through stool alpha-1-antitrypsin (A1AT) concentration at all three time points. Doppler-derived resistance indices of the superior mesenteric artery (SMA) and serum albumin and total protein levels were obtained at the pre-operative and early post-operative time points.
Stool Alpha-1-Antitrypsin (A1AT)
Stool samples were analyzed for A1AT concentration by the Mayo Medical Laboratories (Rochester, MN). Increased stool A1AT concentration has been shown to be diagnostic for abnormal enteric protein loss (7–11). A cut off of > 54 mg/dL was used for abnormal, as derived by the Mayo clinical laboratory. Two prior studies revealed stool A1AT concentrations of 17.2 mg/dL or 26.8mg/dL to be two standard deviations from the mean of healthy controls (8, 12).
Superior Mesenteric Artery Doppler Analysis
The technique for SMA Doppler assessment has been previously described (5). Subjects were requested to receive nothing by mouth for at least four hours prior to study except for clear liquids up to two hours prior. Using a S5-1 or S8-3 transducer on the Philips iE33 or Philips CX-50 ultrasound machine (Philips corporation, Andover, MA), the abdominal aorta was identified and the superior mesenteric artery (SMA) was located as the second vessel arising from the aorta caudal to the diaphragm. A pulse wave Doppler sample was placed at the origin of the SMA from the descending aorta, and tracings with a simultaneous electrocardiogram were recorded. Peak systolic and diastolic velocities were measured and the average of two beats recorded. The resistance index (RI) of Pourcelot (RI = [S − D]/S) was calculated, where S is peak systolic velocity and D is end diastolic velocity.
Statistical Analysis
Data were evaluated for normality with Shapiro-Wilk. Descriptive statistics were expressed as count (percentage) or median (interquartile range). Serum albumin and total protein as well as SMA Doppler indices between the pre-operative and early post-operative time points were compared with the Wilcoxon signed rank test. Linear regression was used to test the association between serum albumin and total protein levels with SMA resistance indices. Chi-square test and Kruskal-Wallis equality-of-populations rank test were employed to evaluate categorical and continuous variables, respectively, between subjects with any abnormal stool A1AT sample and subjects with all normal A1AT. Significance was reached for P values < 0.05. All statistical analyses were performed with STATA software (version 12.1, StataCorp, College Station, Texas).
RESULTS
Patient Population
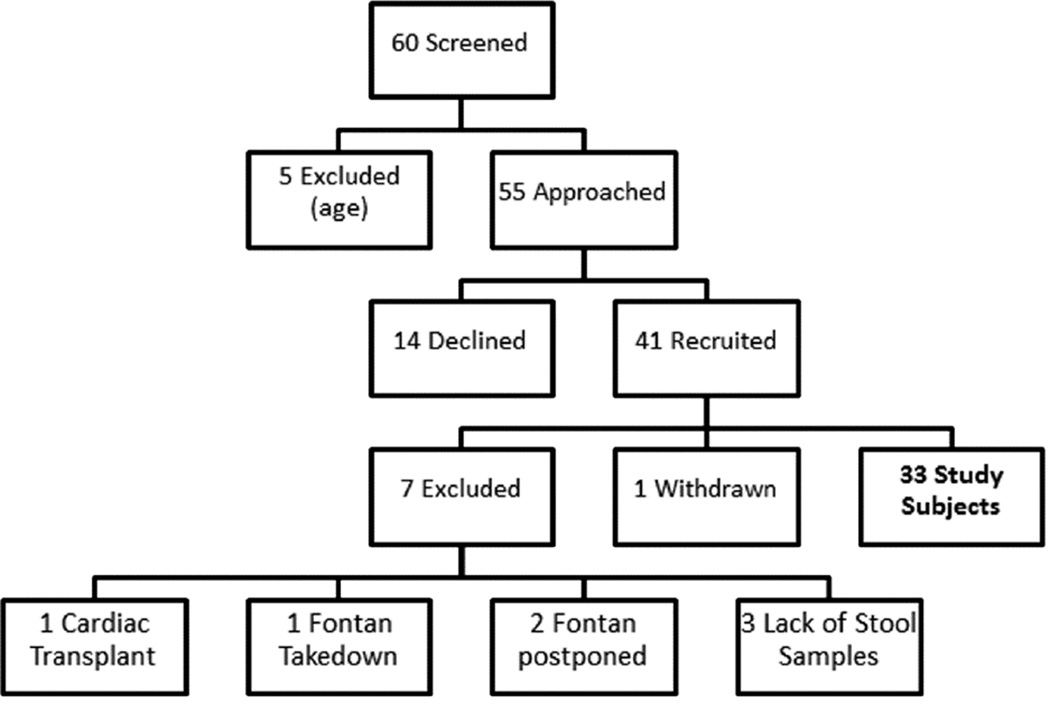
A total of 60 children were identified to undergo Fontan operation during the study period (Figure 1). Five children were excluded due to age, and the remaining 55 were approached for consent, which was obtained in 41 subjects. Of these, one subject withdrew due to family preference. Four subsequently were excluded as two had Fontan operation postponed beyond the study time period, one had a Fontan takedown, and one had cardiac transplantation. Three subjects were also excluded from analysis due to failure to provide any stool samples. The remaining 33 subjects were included in analysis.
Figure 1.
Scheme of patient enrollment.
Patient Characteristics
As summarized in Table 1, the population was predominantly male (n=28, 85%) with a median age at surgery of 3.0 years (2.5–3.3). Twenty-four patients (73%) had dominant right ventricle. Most patients (n=31, 93%) underwent extracardiac conduit type Fontan procedures, and all patients received a 4mm fenestration. Nine subjects (27%) had prior gastrointestinal surgery. Three had mothers and one had a father with irritable bowel syndrome; the father was being evaluated for inflammatory bowel disease. There were no first-degree relatives reported to have inflammatory bowel disease, celiac disease, or other gastrointestinal condition.
Table 1.
Patient Characteristics
| Number of Subjects | 33 |
| Patient demographic data | |
| Gender (female) | 5 (15%) |
| Age at Fontan operation (years) | 3.0 (2.5–3.3) |
| Age at Stage 2 operation (years) | 0.38 (0.34–0.47) |
| Cardiac Diagnosis | |
| Heterotaxy syndrome | 2 (7%) |
| Common atrioventricular canal | 3 (9%) |
| Ventricular morphology | |
| Right | 4 (12%) |
| Left | 7 (21%) |
| Both | 2 (6%) |
| Hypoplastic Left Heart Syndrome (HLHS) | 20 (61%) |
| Dominant right (HLHS + Right) | 24 (73%) |
| Fontan type | |
| Extracardiac conduit | 31 (93%) |
| Lateral tunnel | 1 (3%) |
| Hepatic vein inclusion | 1 (3%) |
| Gastrointestinal History | |
| Feeding type | |
| All Oral | 26 (79%) |
| Oral and enteric | 5 (15%) |
| All enteric | 2 (6%) |
| Prior Gastrointestinal surgery (any) | 9 (27%) |
| Gastric or jejunal tube | 9 (27%) |
| Fundoplication | 4 (12%) |
| Ladds procedure | 1 (3%) |
| Tracheoesophageal fistula repair | 1 (3%) |
| Surgical necrotizing enterocolitis | 1 (3%) |
| Any gastrointestinal diagnosis in first degree relative |
4 (12%) |
| Time points Relative to Fontan Operation | |
| Pre-Fontan: Days pre-operative | 9 (2–46) |
| Early Post-Fontan: Days post-operative | 6 (5–8) |
| Intermediate Post-Fontan: Months post- operative |
4.4 (3.9–6.4) |
Alpha-1-Antitrypsin Level Findings and Clinical Conditions
There were 32 stool A1AT samples obtained at each the first and second time points, and 29 stool A1AT samples obtained at the third time point. Of the total 93 stool samples analyzed, there were six abnormal stool A1AT samples (>54mg/dL), with two abnormal values identified at each time point. There were five subjects with an abnormal stool A1AT value (designated A–E, Table 2).
Table 2.
Clinical description of subjects with abnormal stool alpha-1-antitrypsin (A1AT) values.
| Subject | Pre-Fontan A1AT |
Early Post-Fontan A1AT |
Intermediate Post-Fontan A1AT |
Clinical History |
|---|---|---|---|---|
| A | 61 mg/dL | Normal | Normal | Junctional bradycardia |
| B | 81 mg/dL | Normal | Normal | Well |
| C | Normal | 123 mg/dL | 71mg/dL | Tricuspid valve stenosis and regurgitation |
| D | Normal | 55 mg/dL | Normal | Well |
| E | Not performed |
Normal | 64 mg/dL | Well. History of repaired tracheoesophageal fistula and necrotizing enterocolitis with bowel resection |
Of the 33 subjects in the cohort, only subjects A and C required additional procedures at the time of Fontan operation (pacemaker placement for junctional bradycardia in subject A and tricuspid valvuloplasty in subject C). Additionally, subject C was one of two subjects requiring reoperation after the Fontan operation (the other subject, who underwent a mediastinal exploration and further suturing of conduit anastomosis due to bleeding had normal stool A1AT samples at all time points). Subject C, despite tricuspid valvuloplasty during Fontan operation, underwent tricuspid valve replacement between early and intermediate post-operative time points for continued tricuspid valve regurgitation and stenosis (16mmHg a wave to end diastolic pressure gradient and 11mmHg mean gradient during catheterization). This re-operation was followed by four days of extracorporeal membrane oxygenation, and subsequent catheterization in which balloon dilation of Fontan fenestration was performed demonstrated continued low cardiac output (Qs 2.3L/min/m2) with right ventricular dysfunction and elevated Fontan pressures (24mmHg) after intervention. Finally, while subject E had no obvious hemodynamic derangements at the time of Fontan operation, he had the most complicated gastrointestinal surgical history of the cohort consisting of repaired tracheoesophageal fistula and necrotizing enterocolitis requiring bowel resection.
Comparisons between Subjects with Abnormal versus Normal Stool A1AT Samples
There was no difference in the gender distribution, age at Fontan, age at second stage operation, or feeding type between subjects with an abnormal A1AT sample at any time point and subjects with all normal samples. Additional examination of pre-Fontan catheterization data demonstrated that subjects with abnormal stool A1AT samples had a trend to higher median pulmonary artery pressures (12 mmHg [10.5–16] vs. 9.5 mmHg [9.0–11.0], p=0.08) and lower median superior vena cava saturation (58% [56–67] vs. 67% [64–72], p=0.09). However, there was no difference in pre-Fontan catheterization systemic saturation, systemic ventricle end diastolic pressure, or pulmonary vascular resistance. Finally, there was no difference in pre-Fontan MRI pulmonary blood flow, collateral burden, or volume of inferior vena caval blood return.
Superior Mesenteric Artery Doppler Indices
Superior mesenteric artery Doppler-derived resistance indices were not significantly different in the pre-operative (0.84 [0.83–0.88]) and early post-operative (0.83 [0.78–0.86]) time points for all subjects. There was no significant difference between SMA resistance indices at the pre-operative (p=0.9) or early post-operative (p=0.2) point between subjects with all normal and any abnormal stool A1AT samples.
Serum Albumin and Protein Levels
The serum albumin levels were lower in the early post-operative time point compared to the pre-operative time point (3.2 mg/dl [2.9–3.5] vs. 4.1 mg/dl [3.4–4.5], p=0.009). There also was a trend towards lower serum protein levels (5.8 mg/dl [5.4–6.3] vs. 6.6 mg/dl [5.7–7.0], p=0.07). There was no significant difference in serum albumin or protein levels between subjects with all normal and any abnormal stool A1AT samples at the pre-operative (p=0.8 for albumin and p=0.7 for protein) or early post-operative (p=0.4 for both albumin and protein) time point. There was also no correlation between serum albumin and protein level and SMA resistance indices at the pre-operative (p=0.9) or early post-operative (p=0.4) time points.
COMMENT
The time course for development of PLE in association with Fontan operation and the prevalence of abnormal enteric protein loss as a possible precursor to clinical PLE in children with single ventricle type congenital heart disease are unknown. In this longitudinal, prospective investigation, we found that increased enteric protein loss is not common after Fontan operation, but can occur in patients both before and after Fontan operation. Indeed, five of 33 subjects had abnormal stool A1AT samples either before, early after, or at an intermediate time point (3–9 months) after Fontan operation. Additionally, our findings suggest that hemodynamic derangement may predispose subjects toward having abnormal stool A1AT samples; both of the two children in this study requiring additional procedures with their Fontan operation and one of the two children requiring repeat operation post-Fontan had abnormal stool A1AT samples. Having significant gastrointestinal pathology may also, when compounded with single ventricle physiology, lead to abnormal stool A1AT samples, as the one subject with surgical repair of trachoesophageal fistula and necrotizing enterocolitis had an abnormal stool A1AT sample. Our findings support the hypothesis that children with single ventricle type of congenital heart disease may have inherently altered intestinal physiology, perhaps due to decreased cardiac output and splanchnic perfusion or due to abnormal intestinal lymphatics either as part of the congenital malformation itself or acquired during early palliative intervention (13). We hypothesize that children with single ventricle type of congenital heart disease remain in a state of acute compensation, even before Fontan operation. Hemodynamic or gastrointestinal insult may alter this delicate balance and lead to abnormal enteric protein loss.
Previous work performed over two decades ago was suggestive of early Fontan postoperative enteric protein loss in a subset of patients. Davis et al found that 26 patients had normal stool A1AT concentration (range 4–29 mg/dL, mean 12.9mg/dL) 2 to 8 weeks after the operation, but one patient had transient PLE symptoms and abnormal A1AT stool concentration (247mg/dL) when re-evaluated 2 to 4 months post-operatively (14). Caution must be taken in applying these results to the modern single ventricle cohort as children in the study underwent strict candidacy selection for surgery, had classic atriopulmonary Fontan operation with no prior superior cavopulmonary anastomosis, were predominately morphological single left ventricles, and underwent operation at a median age of 8.7 years.
Another study by Fujii et al in 2003 evaluating early enteric protein loss was also suggestive of occasional enteric protein loss after Fontan operation (12). Compared to 12 control patients, 12 Fontan patients had significantly higher stool A1AT concentration (17.3 ± 7.8 mg/dL vs. 10.0 ± 3.6 mg/dL, p<0.01) 12 to 43 months after the Fontan operation. The stool A1AT concentration increased significantly when re-evaluated 14 to 20 months thereafter (37.2 ± 26.2 mg/dL, p<0.01). The non-uniform sampling of time points in this study complicates the interpretation of these results. Neither of these studies evaluated pre-Fontan enteric protein loss with longitudinal follow up into the postoperative period
Enteric protein loss has been studied in adults many years after Fontan operation. Thorne et al. found that nine of 20 (45%) of Fontan subjects had abnormal stool A1AT samples, but only one had clinical evidence of PLE (4). Findings from our study suggest that this subclinical state may also exist to a degree before or early after the Fontan operation, but appears less common in young children than adults. Enteric protein loss may be due to a combination of intrinsic factors compounded by the imposition of elevated venous pressures following inferior cavo-pulmonary anastomosis with duration of Fontan circulation as an additional variable. Further investigations with serial longitudinal evaluation would be of great value in determining the time course of enteric protein loss development in the ensuing years after Fontan operation.
We were curious to evaluate for a possible association between altered mesenteric blood flow and enteric protein loss in our population. Fontan operation, which results in a relative decrease in cardiac output and increase in central venous pressure, is associated with changes in mesenteric circulation. Patients with abnormal enteric protein loss have been found to have lower mesenteric-to-celiac artery flow ratio on abdominal Doppler ultrasonography (15). Additionally, mesenteric vascular resistance is elevated after the Fontan operation compared to control patients as determined by the Doppler derived resistance indices of the SMA, and this difference may be even more significant in patients with Fontan and clinical PLE than in those with Fontan physiology but no PLE (5). Reduction in mesenteric resistance through sildenafil therapy has led to elimination of PLE, further supporting the notion that in part, enteric protein loss is related to mesenteric hemodynamics after Fontan operation (16).
We hypothesized that the inferior cavopulmonary anastomosis with acute imposition of Fontan circulation would increase mesenteric resistance. The finding of no significant change in mesenteric resistance before and in the days after the Fontan operation is intriguing. It is possible that the alteration in mesenteric resistance occurs in the weeks to months after the operation, and an increased resistance may be seen later after the Fontan operation as an accommodative process with the progression of time. As the resistance indices of the SMA in our subjects are comparable to those seen in older children after the Fontan operation (5), it is also possible that the single ventricle defect and Glenn physiology are inherently related to increased vascular resistance even before the Fontan operation. Higher preoperative SMA resistance values could explain the lack of significant change postoperatively.
Our examination of serum albumin and protein before and after the Fontan operation revealed that serum albumin and protein levels decreased after the Fontan operation. Low albumin levels may be due to the inflammation and volume resuscitation in the post-operative period. They also may be due to increased loss in pleural effusions or perhaps mildly decreased synthesis. Given that most subjects had normal stool A1AT samples, the protein and albumin loss does not appear to be mediated by gastrointestinal loss.
Indeed, the notion that end-organ dysfunction may commence acutely after imposition of the Fontan circulation has been proposed in other organ systems. In a longitudinal study, investigators found an acute change in specific coagulation factors early after Fontan operation (17). This may occur either through the mechanism of an acute impact on hepatic synthetic function, or perhaps through selective loss.
Limitations
There are several limitations to this study. We opted to use a single, random stool A1AT value rather than a 24 hour stool collection in order to optimize compliance and subject convenience; however, studies have shown good correlation between the random single stool value and A1AT clearance levels (7, 8). Additionally, the commercial laboratory analyzing our stool A1AT samples was unable to provide accurate quantitative values for normal samples and prevented analysis of subtle enteric protein loss. We also did not have a separate control group of children analyzed, partially due to the difficulty of finding a similarly aged sample of children undergoing bypass surgery. However, the subjects were compared by matched pairs at various time points, and laboratory and published data was referenced for the normal range of A1AT stool concentration. Finally, we were unable to obtain SMA resistance indices for the intermediate post-operative time point, which may have elucidated any changes that occur after recovery from the Fontan operation.
Conclusion
Imposition of the Fontan circulation does not regularly result in increased early enteric protein loss. Instead, increased enteric protein loss may occur before or after Fontan operation, particularly when hemodynamic disturbance is present. This is suggestive that abnormal enteric protein loss may be inherently associated with single ventricle type congenital heart disease, and not solely a consequence of the physiology following Fontan operation. Prospective, longitudinal assessment of the stool of patients at various stages of palliation and follow-up after Fontan operation as part of a comprehensive program of evaluation or multi-center trial may be elucidative. Such an endeavor may provide insight as to who may develop full clinical PLE, perhaps allowing for initiation of early therapeutic strategies that may prove to be more effective in managing this challenging condition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein-losing enteropathy after the fontan operation: An international multicenter study. Ple study group. J Thorac Cardiovasc Surg. 1998;115(5):1063–1073. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 2.Feldt RH, Driscoll DJ, Offord KP, et al. Protein-losing enteropathy after the fontan operation. J Thorac Cardiovasc Surg. 1996;112(3):672–680. doi: 10.1016/S0022-5223(96)70051-X. [DOI] [PubMed] [Google Scholar]
- 3.Rychik J. Protein-losing enteropathy after fontan operation. Congenit Heart Dis. 2007;2(5):288–300. doi: 10.1111/j.1747-0803.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SA, Hooper J, Kemp M, Somerville J. Gastro-intestinal protein loss in late survivors of fontan surgery and other congenital heart disease. Eur Heart J. 1998;19(3):514–520. doi: 10.1053/euhj.1997.0777. [DOI] [PubMed] [Google Scholar]
- 5.Rychik J, Gui-Yang S. Relation of mesenteric vascular resistance after fontan operation and protein-losing enteropathy. Am J Cardiol. 2002;90(6):672–674. doi: 10.1016/s0002-9149(02)02584-5. [DOI] [PubMed] [Google Scholar]
- 6.Ascione R, Talpahewa S, Rajakaruna C, et al. Splanchnic organ injury during coronary surgery with or without cardiopulmonary bypass: A randomized, controlled trial. Ann Thorac Surg. 2006;81(1):97–103. doi: 10.1016/j.athoracsur.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Crossley JR, Elliott RB. Simple method for diagnosing protein-losing enteropathies. Br Med J. 1977;1(6058):428–429. doi: 10.1136/bmj.1.6058.428-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier JJ, Florent C, Desmazures C, Aymes C, L'Hirondel C. Diagnosis of protein-losing enteropathy by gastrointestinal clearance of alpha1-antitrypsin. Lancet. 1978;2(8093):763–764. doi: 10.1016/s0140-6736(78)92650-8. [DOI] [PubMed] [Google Scholar]
- 9.Florent C, L'Hirondel C, Desmazures C, Aymes C, Bernier JJ. Intestinal clearance of alpha 1-antitrypsin. A sensitive method for the detection of protein-losing enteropathy. Gastroenterology. 1981;81(4):777–780. [PubMed] [Google Scholar]
- 10.Hill RE, Hercz A, Corey ML, Gilday DL, Hamilton JR. Fecal clearance of alpha 1-antitrypsin: A reliable measure of enteric protein loss in children. J Pediatr. 1981;99(3):416–418. doi: 10.1016/s0022-3476(81)80332-0. [DOI] [PubMed] [Google Scholar]
- 11.Thomas DW, Sinatra FR, Merritt RJ. Random fecal alpha-1-antitrypsin concentration in children with gastrointestinal disease. Gastroenterology. 1981;80(4):776–782. [PubMed] [Google Scholar]
- 12.Fujii T, Shimizu T, Takahashi K, et al. Fecal alpha1-antitrypsin concentrations as a measure of enteric protein loss after modified fontan operations. J Pediatr Gastroenterol Nutr. 2003;37(5):577–580. doi: 10.1097/00005176-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Dori Y, Keller MS, Fogel MA, et al. Mri of lymphatic abnormalities after functional single-ventricle palliation surgery. American Journal of Roentgenology. 2014;203(2):426–431. doi: 10.2214/AJR.13.11797. [DOI] [PubMed] [Google Scholar]
- 14.Davis CA, Driscoll DJ, Perrault J, et al. Enteric protein loss after the fontan operation. Mayo Clin Proc. 1994;69(2):112–114. doi: 10.1016/s0025-6196(12)61035-0. [DOI] [PubMed] [Google Scholar]
- 15.Ostrow AM, Freeze H, Rychik J. Protein-losing enteropathy after fontan operation: Investigations into possible pathophysiologic mechanisms. The Annals of Thoracic Surgery. 2006;82(2):695–700. doi: 10.1016/j.athoracsur.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 16.Uzun O, Wong JK, Bhole V, Stumper O. Resolution of protein-losing enteropathy and normalization of mesenteric doppler flow with sildenafil after fontan. The Annals of Thoracic Surgery. 2006;82(6):e39–e40. doi: 10.1016/j.athoracsur.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Odegard KC, Zurakowski D, DiNardo JA, et al. Prospective longitudinal study of coagulation profiles in children with hypoplastic left heart syndrome from stage i through fontan completion. The Journal of Thoracic and Cardiovascular Surgery. 2009;137(4):934–941. doi: 10.1016/j.jtcvs.2008.09.031. [DOI] [PubMed] [Google Scholar]