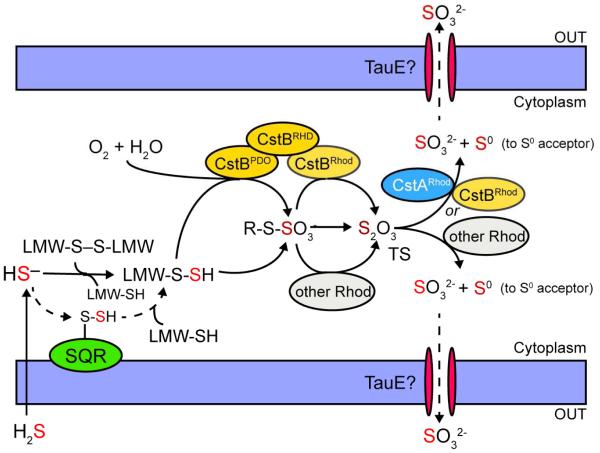
Figure 9.
Current model for cellular H2S detoxification in Staphylococcus aureus highlighting the known or proposed enzymatic activities encoded by the cst operon. (this work). Extracellular H2S freely penetrates the cell membrane and exists as HS− once in the cytoplasm. SQR then carries out the initial two-electron oxidation of HS− to form SQR-bound persulfide and the SQR-bound persulfide is transferred to reduced cellular LMW thiols (LMW-SH) to form LMW persulfides (LMW-SSH), which can also be generated from HS− directly via reaction with oxidized LMW thiols (LMW-S-S-LMW). In the presence of molecular oxygen (O2), CstBPDO further oxidizes LMW persulfides to possibly form a LMW thiol-S-sulfonate or CstB-S-sulfonate (denoted R-S-SO3−) intermediate, which requires further investigation. Free thiosulfate (TS) is then immediately generated either through the persulfide transferase activity of CstBRhod or other cellular rhodanese proteins (other Rhod), or directly generated via reaction of a LMW persulfide with LMW thiol-S-sulfonate or CstB-S-sulfonate. Finally, accumulating thiosulfate is processed by the thiosulfate sulfurtransferase activities of CstBRhod, CstARhod or other cellular rhodanese proteins. Sulfite (SO32−) is effluxed from the cell (perhaps by TauE) and sulfane sulfur (S0) proposed to be transferred to as yet unknown downstream cellular sulfur acceptor(s) for assimilation.