Abstract
Introduction
Post-kala-azar dermal leishmaniasis (PKDL) is a dermatological complication that occurs primarily among treated visceral leishmaniasis (VL) patients, and sporadically in a few without a history of VL. It mostly affects children and adolescents but is also common in adults. The conventional treatment with 120 intramuscular injections of sodium stibogluconate (SSG) is phasing out. Miltefosine (MF) is the only eventual alternative to SSG; however, its efficacy and safety profiles for treatment of children and adolescents with PKDL are lacking. In addition, risk factors for PKDL are poorly investigated. Host genetic, nutritional and environmental factors could be potential risk factors. As such, here we propose to evaluate the efficacy and safety of MF for 12 weeks at an allometric dose for children and adolescents with PKDL, and also to explore potential risk factors for PKDL.
Methods and analysis
A cross-sectional survey will look for suspected participants with PKDL among treated VL children and adolescents, a subsequent open clinical trial with MF at allometric dose, with a follow-up at 12 months. A case–control study will be carried out for PKDL risk factors. Assuming 95% cure rate, 95% CI and α=0.05, a sample size of 73 children with PKDL is needed. Considering an attrition rate of 10%, the final sample size is 80 children in each group. Descriptive and analytical analyses will be performed. Primary outcome is safety and cure rate of 12 weeks of treatment with MF.
Ethics and dissemination
International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) Ethical Review Committee (ERC) approved the protocol (PR#013045). Written informed consent will be taken from all participants and their guardians (in case of minor). A Data and Safety Monitoring Board (DSMB) of ICDDR,B ERC will monitor all study activities to ensure the safety of the participants.
Trial registration number
NCT02193022; Pre-results.
Strengths and limitations of this study.
Allometric dose of miltefosine in treating PKDL is initiated first through this study.
Development of picture-based scoring system is also initiated first through this study.
Risk factor analysis among children with PKDL is another important strength of this study.
Subjective error during scoring may happen.
There is no gold standard to validate the scoring system.
Introduction
Post-kala-azar dermal leishmaniasis (PKDL) is a skin disorder that mostly develops following treatment for visceral leishmaniasis (VL).1 Other than having skin lesions, patients with PKDL are healthy, but they are unfortunately stigmatised in the society—especially girls and women.2 The Leishmania donovani parasite, harboured in skin lesions of PKDL, can initiate an epidemic outbreak of VL in the community by transmitting the parasite through sandfly bites.2 Consequently, it is of the utmost importance to actively search for patients with PKDL and determine their safe and effective treatment, to control VL in the Indian subcontinent, including Bangladesh.2
Significance of a trial with miltefosine
Until recently, stibogluconate (SSG) was the only therapeutic option for PKDL.2 3 This involves intramuscular injections of SSG (20 mg/kg/day) for 20 consecutive days per month, for 6 months (120 injections in total). SSG injections are very painful, and serious adverse events (SAEs) including death due to sudden cardiac arrest have been observed.4–6 As such, patients with PKDL often do not seek medical care, and frequently there low treatment compliance reported when receiving SSG treatment.7 Thus, a safe, effective and affordable treatment regimen with shorter duration compared with that with conventional SSG therapy for PKDL is urgently needed.
Complementary drugs for PKDL include miltefosine (MF), amphotericin B, liposomal amphotericin B and immunotherapy in combination with SSG. One study in India compared the efficacy of amphotericin B with SSG and found that amphotericin B was superior to SSG; however, its nephrotoxicity and high price limited its use.8 A substitute for SSG therapy is the combination of SSG with immunotherapy. So far, only one study reported a high cure rate (87%) of PKDL with immunotherapy in Sudan,9 involving SSG coupled with the first-generation vaccine (alum-precipitate autoclaved Leishmania major plus BCG). Any risk of adverse events from SSG and potentially from autoclaved L. major cannot be disregarded. On the basis of a literature review, we found that 12 weeks of MF monotherapy is the best possible alternative existing to the 120 SSG injections for PKDL treatment.10–18 However, all the studies reported a very small number of PKDL cases. Unfortunately, information regarding either the safety and efficacy of 12 weeks of MF treatment of children with PKDL or the pharmacokinetics of MF in children was not available. It was established that exposure to MF in children with VL was less to oral MF at a conventional dose of 2.5 mg/kg/day. Thus, allometric dosing of MF has now been recommended by experts.19 A study is therefore urgently needed to evaluate the efficacy and safety of MF treatment at an allometric daily dose for 12 weeks in children with PKDL.
Relevance of host, environmental and nutritional risk factor analyses
PKDL is more common in Bangladesh among treated VL patients than in India and Nepal.20 The reasons for this remain to be elucidated. Perhaps genetic susceptibility, as in Sudan, nutritional status of the host and environmental factors play roles in the development of PKDL in Bangladesh.21 A study in Sudan indicated that interleukin 10 (IL-10) and interferon-γ (IFN-γ) gene polymorphisms were associated with PKDL development.1 22 23 This immune polymorphism resulted in an inefficient Th1 immune response to clear the infection with L. donovani.24 Moreover, environmental factors such as chronic exposure to arsenic may also influence the development of PKDL. Arsenic exposure has been shown to negatively influence the innate and adaptive immune responses to infections.25 Therefore, it is possible that a higher exposure to arsenic would favour PKDL development by decreasing the efficacy of drugs in maintaining an adequate Th1 response required to effectively clear the L. donovani infection. Exposure to arsenic, a global health problem, can occur from drinking water sourced from the ground.26 27 Bangladesh appears to be the most afflicted, with 59 of 64 districts affected by varying degrees of ground water contamination, including the Mymensingh district.27 Furthermore, arsenic exposure was also associated with depressed serum levels of antioxidants such as vitamins E, A and D, and of serum cholesterol.28 29 Low levels of serum cholesterol have indeed been linked with a higher risk for VL.29 In other words, vitamin E is necessary for optimum innate and adaptive immune responses to infection, and to maintain the normal immune function of the skin.30 Consequently, improving the treatment efficacy for leprosy with antioxidant(s) supplementation had been proposed.31 32 Therefore, decreased serum levels of vitamin E due to nutritional deficiency and/or arsenic exposure, and arsenic itself may independently play important roles in the pathogenesis of PKDL. That is why, here we also propose to investigate the association of serum vitamins E, A and D, zinc and arsenic exposure with PKDL.
Methods/design
The study was designed to test the following hypotheses:
Primary hypothesis:
Oral MF treatment of children with PKDL at allometric daily dose (based on body weight and height) for 12 weeks is safe, with a cure rate of ≥95%.
Secondary hypothesis:
Development of PKDL in children and adolescents is genetically predisposed and is associated with IL-10 and IFN-γ gene polymorphisms causing high and low serum level of IL-10 and IFN-γ, respectively.
Nutritional and environmental factors such as low serum vitamins E, A and D, zinc and arsenic exposure are associated with PKDL development.
Experimental design
The study includes a cross-sectional survey of children treated for VL in the past, followed by an open clinical trial of 12 weeks of oral MF treatment for parasitologically confirmed children with PKDL, and their 12 months of follow-up and a simultaneous case–control study for PKDL risk factors.
Study area and population
The study will be carried out in the district of Mymensingh, Bangladesh. VL is prevalent in 45 districts of Bangladesh, with Mymensingh being the most VL endemic. About 50–60% of total cases of VL in Bangladesh were reported from Mymensingh; 5 of the 12 upazilas (subdistricts) are endemic for VL.
Cross-sectional survey
Trained senior field research assistants (SFRAs) will collect the address details of children previously treated for VL, in the upazila hospitals of Muktagacha, Trishal, Fulbaria, Bhaluka and Gafforgaon. Following the collected information, they will visit the households of the children, and with informed written voluntary consent from parent/guardian of the child, SFRA will screen the child for suspected PKDL. Subsequently, the suspected cases will be referred to study clinics for confirmation and enrolment.
Enrolment for the trial (cases)
After confirmation of the diagnosis of PKDL, the child will be enrolled in the trial against study inclusion or exclusion criteria (table 1) and will receive treatment with MF for 12 weeks at allometric daily dose (table 2).
Table 1.
Study enrolment criteria
| Case |
Control |
||
|---|---|---|---|
| Inclusion | Exclusion | Inclusion | Exclusion |
| Age >2 years and <18 years | Do not fulfil inclusion criteria | Age >2 years and <18 years | Do not fulfil inclusion criteria |
| Treated for VL in the past | Lesions with mucosal involvement | Treated for VL in the past | Serious concomitant illness |
| Currently with skin lesions such as PKDL | Serious concomitant illness | Currently with no skin lesions such as PKDL | Cannot be followed up |
| Positive for Leishmania donovani bodies by microscopy and or DNA by qPCR in their skin | Cannot be followed up | Clinically healthy and free from other chronic illness | Controls with PKDL during follow-up |
| Clinically healthy and free from other chronic illness | Female married adolescent | Received no treatment for PKDL | |
| Received no treatment for PKDL in the last 6 months | Normal hepatic, renal and haematological functions | ||
| Normal hepatic, renal and haematological functions | Parent/guardian provided informed voluntary written consent for his/her child participation | ||
| Parent/guardian provided informed voluntary written consent for his/her child participation | |||
PKDL, post-kala-azar dermal leishmaniasis; qPCR, quantitative PCR; VL, visceral leishmaniasis.
Table 2.
Allometric dose chart19
| Weight (kg) | Total daily allometric miltefosine dose (mg) for patient of indicated height (cm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 70 | 80 | 90 | 100 | 110 | 120 | 130 | 140 | 150 | 175 | 200 | |
| Male | ||||||||||||
| 9 | 30 | 40 | 40 | 40 | 40 | |||||||
| 12 | 40 | 40 | 40 | 50 | 50 | 50 | ||||||
| 15 | 40 | 50 | 60 | 60 | 60 | |||||||
| 20 | 50 | 60 | 60 | 70 | 70 | 70 | ||||||
| 25 | 60 | 70 | 70 | 80 | 80 | 80 | ||||||
| 30 | 80 | 80 | 90 | 90 | 90 | 100 | ||||||
| 35 | 80 | 90 | 90 | 100 | 100 | 100 | 110 | |||||
| 40 | 80 | 90 | 100 | 100 | 110 | 110 | 120 | 130 | ||||
| 45 | 90 | 90 | 100 | 110 | 110 | 120 | 130 | 130 | ||||
| 50 | 90 | 100 | 100 | 110 | 120 | 120 | 130 | 140 | ||||
| 55 | 90 | 100 | 110 | 120 | 120 | 130 | 140 | 150 | ||||
| 60 | 90 | 100 | 110 | 120 | 130 | 130 | 150 | 150 | ||||
| 65 | 100 | 110 | 110 | 120 | 130 | 140 | 150 | 150 | ||||
| 75 | 100 | 110 | 120 | 130 | 140 | 140 | 150 | 150 | ||||
| 85 | 100 | 110 | 120 | 130 | 140 | 150 | 150 | 150 | ||||
| Female | ||||||||||||
| 9 | 30 | 40 | 40 | 40 | 40 | |||||||
| 12 | 40 | 40 | 40 | 50 | 50 | 50 | ||||||
| 15 | 40 | 50 | 60 | 60 | 60 | |||||||
| 20 | 50 | 60 | 60 | 70 | 70 | 70 | ||||||
| 25 | 60 | 70 | 70 | 80 | 80 | 80 | ||||||
| 30 | 80 | 80 | 90 | 90 | 90 | 100 | ||||||
| 35 | 80 | 90 | 90 | 100 | 100 | 100 | 110 | |||||
| 40 | 80 | 90 | 100 | 100 | 110 | 110 | 120 | 130 | ||||
| 45 | 90 | 90 | 100 | 110 | 110 | 120 | 130 | 130 | ||||
| 50 | 90 | 100 | 100 | 110 | 120 | 120 | 130 | 140 | ||||
| 55 | 90 | 100 | 110 | 120 | 120 | 130 | 140 | 150 | ||||
| 60 | 90 | 100 | 110 | 120 | 130 | 130 | 150 | 150 | ||||
| 65 | 100 | 110 | 110 | 120 | 130 | 140 | 150 | 150 | ||||
| 75 | 100 | 110 | 120 | 130 | 140 | 140 | 150 | 150 | ||||
| 85 | 100 | 110 | 120 | 130 | 140 | 150 | 150 | 150 | ||||
A dose of 150 mg is currently considered as maximum tolerable dose.
Study medication, adverse events and management
Children with PKDL in the trial will be treated with oral MF, obtained from the company, Paladin Labs (Canada). The daily total dose of MF will be based on allometric calculation and rounded to the nearest 10 mg (the smallest commercially available capsule of MF) (table 2). The duration of treatment will be 12 weeks. The trade name of the drug is Impavido, earlier produced by Zentaris (Germany), and now by the Paladin Labs (Canada). Impavido is available in 10 and 50 mg capsule formulation. Direct observed treatment (DOT) will be conducted to ensure 100% treatment compliance through study health workers. A treatment card will be provided through which information about pharmacovigilance will be collected. Parents or guardians of the child will be informed about the common adverse events of MF treatment, and they as well as the DOT providers will be educated in management of these common adverse events which usually includes oral rehydration in case of vomiting and diarrhoea. Each child will also be followed over cellphone on a daily basis by the study SFRA. In case of adverse events, which cannot be managed by parents, guardians or DOT providers, the child will be brought to the study clinic for examination and management by the study team and by the hospital doctor in need. In case of SAE, the child will be taken care of in the upazila hospital first and subsequently referred to the Mymensingh Medical College Hospital, if needed. Adverse events will be graded following the Common Terminology Criteria for Adverse Events (CTCAE V.4.0, 2010) of the National Institutes of Health, USA.33
Follow-up and cure assessment in cases
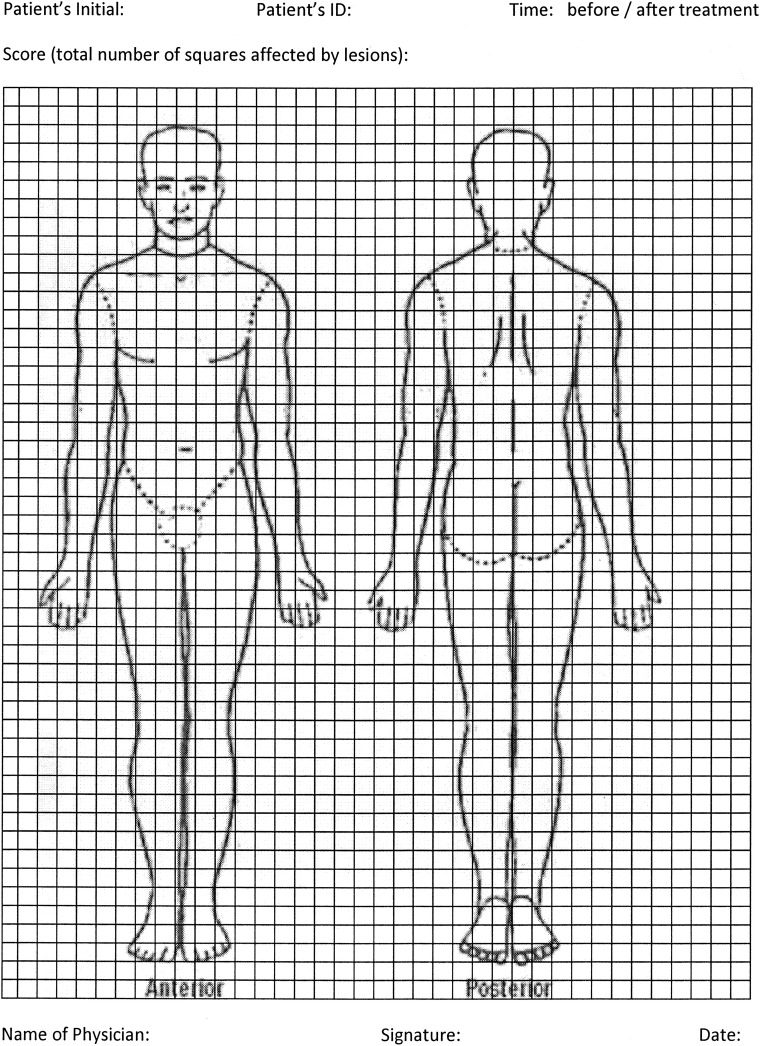
Children in the trial will be monitored for 12 months after completion of treatment, and periodic assessments every 3 months regarding response to treatment will be performed using the proposed scoring method. To date, there is no standardised scoring method for the affected area of skin by the lesions of PKDL. In the current proposal, we aim to use a new method to this end (figure 1). Using the following picture (figure 1), skin lesions will be plotted in squares of the picture. The total number of squares containing lesions will be counted before and after treatment during the 12-month follow-up. The percentage of skin lesions cured by the treatment will be calculated as follows: total number of squares free from lesions after treatment×100/total number of squares affected by the lesions at baseline.
Figure 1.
Scoring system.
The response to treatment will be evaluated clinically through assessment of scores for affected surface of skin at every 3 months following treatment. However, cure assessments will be done at 12 months only. Two end points will be used for cure assessment: (1) at >90% resolution of skin lesions plus a negative PCR assay for skin and peripheral blood buffy coat; (2) complete resolution of skin lesions.
Enrolment and follow-up of controls
Enrolment
The specific inclusion and exclusion criteria (table 1) will be used to register children as control patients in the case–control study.
Follow-up of controls
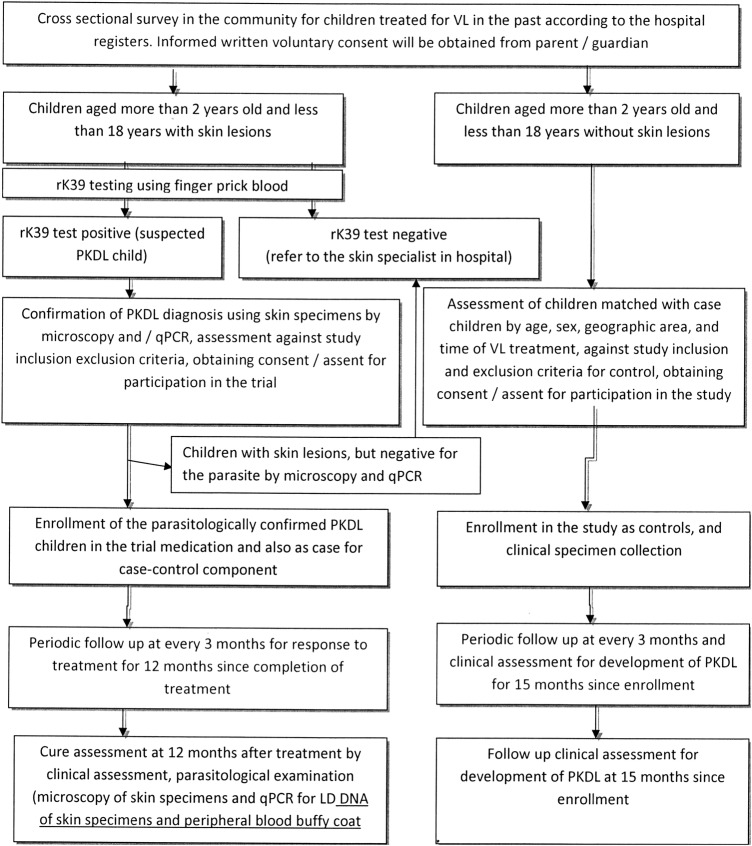
These children will be monitored for 15 months to match patients with PKDL who will be in the study for a total period of 15 months (3 months of treatment+12 months of follow-up after treatment). Each control will be assessed for signs and symptoms of PKDL every 3 months during follow-up. If any is found with suspected PKDL, then they will undergo parasitological confirmation. If a diagnosis of PKDL is confirmed, the individual will be excluded from the study as control, and instead, treated with MF, and will be referred to the hospital for further management as per national guideline. Stepwise activity is presented in figure 2.
Figure 2.
Enrolment and follow-up of study children. LD, Leishmania donovani; PKDL, post-kala-azar dermal leishmaniasis; qPCR, quantitative PCR; VL, visceral leishmaniasis.
Clinical specimen collection and laboratory methods
Clinical specimen collection
Venous blood (5 mL) from forearm, urine (10 mL) and hair (1 g) specimens will be collected from cases before and after treatment, and from controls at enrolment (baseline) only. Collected blood specimens will be analysed for haematological, hepatic and renal functions. DNA isolated from collected blood will be tested for L. donovani DNA, IFN-γ and IL-10 gene polymorphisms. The serum levels of α-tocopherol, albumin, cholesterol, serum glutamate-pyruvate transaminase (SGPT) and creatinine, and electrolytes (Na, K and Cl) will be measured before and after treatment in cases and once in control during enrolment. The concentration of arsenic will be measured in hair and urine specimens.
Laboratory methods
Haematological tests will be performed using an AutoAnalyzer and will include complete cell count, haemoglobin and erythrocyte sedimentation rate (ESR). Biochemical tests will include those for SGPT (hepatic function), serum creatinine (renal function) and serum levels of cholesterol, albumin and electrolytes (Na, K and Cl). All these tests will be performed in a certified laboratory in Mymensingh, or in ICDDR,B clinical pathology lab in Dhaka. Skin specimens will be taken by skin scraping for detection of fungal infection by potassium hydroxide (KOH) testing and by skin punch biopsy for microscopic (1000 fields will be examined for Leishman-Donovan (LD) amastigote) and quantitative PCR (qPCR) examination. The biopsy material will be prepared for Giemsa staining on glass microscope slides (air-dried, fixed with absolute methanol and finally stained by Giemsa). The remaining biopsy material will be kept in a normal saline solution until DNA isolation (Qiagen kit). Microscopic examination for L. donovani bodies and detection of L. donovani DNA by qPCR in the skin specimens will be performed in the parasitology laboratory of ICDDR,B. Serum α-tocopherol, vitamin A and Zn will be measured in the nutritional biochemistry laboratory of ICDDR,B by high-performance liquid chromatography (HPLC) (vitamins E and A) and atomic absorption (Zn). The concentrations of arsenic in hair and urine will be measured by hydride generation atomic absorption spectrometry as well. L. donovani DNA in the peripheral blood buffy coat will be detected by qPCR.34 In this study, our targeted single-nucleotide polymorphic (SNP) variant site of IFN-γ will be +874T/A, which resides in the first intron of IFN-γ gene, and for IL-10 the SNP site will be −1082G/A, one of the three SNPs in their promoter region of IL-10 gene. Both SNPs will be typed using amplification refractory mutation system PCR (ARMS PCR) method.35 36 The serum level of cytokines will be determined by Luminex assays. SNP −1082G/A and +874T/A are related to increased secretion of IL-10 (GG genotype) and lower secretion of IFN-γ, respectively.36 Specific polymorphisms and primers will be used (table 3).
Table 3.
Characteristics of IL-10 and IFN-γ polymorphism and primer sequences for amplification refractory mutation system PCR34–36
| Polymorphism location allele | Product size (bp) |
Sequence | |
|---|---|---|---|
| IL-10 (−1082 bp locus) | Generic primer (antisense) | 5′-cagcccttccattttactttc-3′ | |
| IL-10*G | 550 | Primer G (sense) | 5′-tactaaggcttctttgggag-3′ |
| IL-10*A | 550 | Primer A (sense) | 5′-ctactaaggcttctttgggaa-3′ |
| IFN-γ (intron 1) | Generic primer (antisense) | 5′-tcaacaaagctgatactcca-3′ | |
| IFN-γ *A | 261 | Primer A (sense) | 5′-ttcttacaacacaaaatcaaatca-3′ |
| IFN-γ *T | 261 | Primer T (sense) | 5′-ttcttacaacacaaaatcaaatct-3′ |
| Internal control | 426 | Primer 1 (sense) | 5′-gccttccaaccattccctta-3′ |
| Primer 2 (sense) | 5′-tcacggatttctgttgttgtgtttc-3′ | ||
Bp, base pair; IFN-γ, interferon γ; IL-10, interleukin 10.
Calculation of sample size
Sample size trial: based on an expected 95% cure rate, a sample size of 73 children with PKDL would produce a two-sided 95% CI with an estimated precision of 5%. Allowing for an attrition rate of 10%, the final sample size would be 80 children in each group, for a total sample size of 160.
Sample size for case–control study: we are interested in identifying risk factors which eventually can be candidates for a predictive marker for PKDL. Considering this, we hypothesise that children with PKDL will have a prevalence of risk factor(s) (IFN-γ gene polymorphism/IL-10 gene polymorphism/vitamin E deficiency/higher values of arsenic in hair or urine 80% and in controls 20%). Considering α of 5% and study power of 90%, a sample size of 16 in each group will be needed. Therefore, a sample size of 80 in each group will be sufficient even an attrition rate is 10%.
Statistical analysis
Information from the survey and clinical and laboratory information at baseline and during follow-up will be computerised using an MS Access database program. All information will be verified by the study research officer and research investigator prior to data entry. Descriptive and analytical statistical methods will be used. Statistically significant differences between means will be compared by parametric (t test, analysis of variance (ANOVA)) or non-parametric (non-parametric paired test or Kruskal-Wallis) tests, where applicable. Comparison between proportions will be assessed by χ2 test with Fisher's exact correction, where applicable. Intention-to-treat (ITT) as well as per-protocol (PP) analyses will be performed for assessment of initial and definitive cure with 95% CI limits, according to Clopper and Pearson. Safety analyses will include a calculation of incidence for all adverse events. Scoring will also be calculated for cure analysis. Depending on the distribution and nature of the data, bivariate as well as multivariate analysis using logistic regression, mixed-effects model or generalized estimating equation (GEE) will be used to determine risk analysis.
Quality assurance and control
The study team will be trained in good clinical practice by a WHO-certified external clinical trial monitor during the kick-off meeting of the study. The monitor will visit after the enrolment of first 30 participants and then on completion of treatment. The final study's closing visit will be expected at the end of the study. All clinical laboratory tests will be conducted in an International Organization for Standardization (ISO)-certified laboratory of ICDDR,B. The nutrient and arsenic measurements will be performed in the internationally recognised Nutritional Biochemistry Lab of ICDDR,B, where methods for determination of vitamins A and E and Zn are ISO certified. The qPCR protocol for determination of L. donovani in different specimens will be obtained from the Infectious Disease Research Institute, Seattle, USA, and will be validated in the ICDDR,B Parasitology Laboratory. The PCR methods for targeted SNP analysis will be carried out in the ICDDR,B Parasitology Laboratory, which has extensive expertise with similar tests in other infectious diseases. Experts from the University of Nagasaki, Japan, will verify these findings.
Ethical issue
The proposed study will be conducted according to the principles of the Declaration of Helsinki (2008). The study physician will obtain informed written consent from all participants, or their legal representatives (LRs), if they lack capacity before or at the time of enrolment. Patients (or their LRs) are free to withdraw from the study at any time, as this will be explicitly indicated on the patient information sheet. The data collected for the study will be identified by a code and only the study physician and researchers will be able to link those data with patients and their clinical history. Consequently, the patient's identity will not be revealed to any other person, except in cases of medical emergency or if required to do so by law. Access to patient information will be restricted to the study doctor and collaborators, the health authorities from the Government of Bangladesh, the Clinical Research Ethics Committee and personnel authorised by the sponsor when they need to check the data and procedures used in the study, but always maintaining the confidentiality of the said information in accordance with current legislation. The trial protocol, the informed consent form and the information sheet received Research Ethics Committee approval in June 2014.
Publication plan
The research team plans to publish the study results in a peer-reviewed journal within 12 months of the completion of the study. Results will be analysed and reported in accordance with data analysis plan.
Discussion
Since most of the patients with PKDL are children and complete treatment coverage is essential to terminate the root of disease transmission, a safe, effective and user-friendly treatment option should be identified for children with PKDL. It is also critical to pinpoint the contributing factors to PKDL in order to devise an effective preventive policy.
Through this study, we aim to find out the efficacy and safety profiles of 12 weeks of MF therapy at allometric dose in children with PKDL. If we find this effective and safe, we will then recommend to the national elimination programme for further application and scale up. Moreover, through the case–control component, we may identify nutritional or environmental risk factors, which will ultimately help the programme to design a preventative strategy against PKDL.
Acknowledgments
The authors thank Dr Shireen Hossain, postdoctoral research fellow, McGill University, Canada, for English editing of the manuscript.
Footnotes
Contributors: DM and MGH conceptualised the idea. DM, MGH, MSH, DG, PG, HH, RH, GM and SH wrote the proposal. DM, MGH, PG and RH defended RRC and ERC. MSH, DG, JB and RN planned and developed field activities and data collection tools. PG, HF, JB, RN, GM and SH selected methods and prepared plan for laboratory activities. DM, RH and SH are responsible for monitoring overall activities. All authors contributed to the manuscript writing. DM is the principle investigator and main grant holder of this study.
Funding: This work is supported by a grant from the Thrasher Research Fund (grant no. 11921).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Approved by Research Review Committee (RRC) and Ethical Review Committee (ERC) of the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Zijlstra EE, Musa AM, Khalil EAG et al. . Post-kala-azar dermal leishmaniasis. Lancet Infect Dis 2003;3:87–98. 10.1016/S1473-3099(03)00517-6 [DOI] [PubMed] [Google Scholar]
- 2.Desjeux P, Ramesh V. Post-kala-azar dermal leishmaniasis: facing the challenges of eliminating kala-azar from South Asia. Kala-azar in South Asia. In: Jha TK, Noiri E, eds. Kala-azar in South-Asia. New York: Springer-Verlag, 2011:111–44; Chapter 11. [Google Scholar]
- 3.Be-Nazir Ahmed, Chief Editor. National Guideline for Kala-azar Case Management, May, 2013. Kala-azar Elimination Program, Directorate General of Health Services, Ministry of Health and family Welfare, Government of the People's Republic of Bangladesh.
- 4.Rahman KM, Islam S, Rahman MW et al. . Increasing incidence of post-kala-azar dermal leishmaniasis in a population-based study in Bangladesh. Clin Infect Dis 2010;50:73–6. 10.1086/648727 [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Sivakumar R. Challenges and new discoveries in the treatment of Leishmaniasis. J Infect Chemother 2004;10:307–15. 10.1007/s10156-004-0348-9 [DOI] [PubMed] [Google Scholar]
- 6.Maheshwari A, Seth A, Kaur S et al. . Cumulative cardiac toxicity of sodium stibogluconate and amphotericin B in treatment of kala-azar. Pediatr Infect Dis J 2011;30:180–1. 10.1097/INF.0b013e3181f55843 [DOI] [PubMed] [Google Scholar]
- 7.Mondal D, Nasrin KN, Huda MM et al. . Enhanced case detection and improved diagnosis of PKDL in a kala-azar-endemic area of Bangladesh. PLoS Negl Trop Dis 2010;4:e832 10.1371/journal.pntd.0000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thakur CP, Narain S, Kumar N et al. . Amphotericin B is superior to sodium antimony gluconate in the treatment of Indian post-kala-azar dermal leishmaniasis. Ann Trop Med Parasitol 1997;91:611–16. 10.1080/00034989760707 [DOI] [PubMed] [Google Scholar]
- 9.Musa AM, Khalil EA, Mahgoub FA et al. . Leishmaniasis Research Group/Sudan. Immunochemotherapy of persistent post-kala-azar dermal leishmaniasis: a novel approach to treatment. Trans R Soc Trop Med Hyg 2008;102:58–63. 10.1016/j.trstmh.2007.08.006 [DOI] [PubMed] [Google Scholar]
- 10.Belay AD, Asafa Y, Mesure J et al. . Successful miltefosine treatment of post-kala-azar-dermal leishmaniasis occurring during antiretroviral therapy. Ann Trop Med Parasitol 2006;100:223–7. 10.1179/136485906X91440 [DOI] [PubMed] [Google Scholar]
- 11.Rihl M, Stoll M, Ulbricht K et al. . Successful treatment of post-kala-azar dermal leishmaniasis (PKDL) in a HIV infected patient with multiple relapsing leishmaniasis from Western Europe. J Infect 2006;53:e25–7. 10.1016/j.jinf.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 12.Sundar S, Kumar K, Chakravarty J et al. . Cure of antimony-unresponsive Indian post-kala-azar dermal Leishmaniasis with oral miltefosine. Trans R Soc Trop Med Hyg 2006;100:698–700. 10.1016/j.trstmh.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 13.Ramesh V, Ansari NA, Jain RK et al. . Oral miltefosine in the treatment of post-kala-azar dermal leishmaniasis. Clin Exp Dermatol 2008;33:103–4. 10.1111/j.1365-2230.2007.02547.x [DOI] [PubMed] [Google Scholar]
- 14.Khandpur S, Chaturvedi P, Kumar U et al. . Oral miltefosine in post-kala-azar dermal leishmaniasis—experience in three cases. Int J Dermatol 2010;46:565–9. 10.1111/j.1365-4632.2010.04326.x [DOI] [PubMed] [Google Scholar]
- 15.Modak D, Basu A, Bhattacharya R et al. . Miltefosine in post-kala-azar dermal leishmaniasis (PKDL). J Indian Acad Clin Med 2010;11:199–203. [Google Scholar]
- 16.Ramesh V, Katara GK, Verma S et al. . Miltefosine as an effective choice in the treatment of post-kala-azar dermal leishmaniasis. Br J Dermatol 2011;165:411–14. 10.1111/j.1365-2133.2011.10402.x [DOI] [PubMed] [Google Scholar]
- 17.Sundar S, Sinha P, Jha TK et al. . Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial. Trop Med Int Health 2013;18:96–100. 10.1111/tmi.12015 [DOI] [PubMed] [Google Scholar]
- 18.Sundar S, Singh A, Chakravarty J et al. . Efficacy and safety of miltefosine in treatment of post kala-azar dermal leishmaniasis. ScientificWorldJournal 2015;2015:414378 10.1155/2015/414378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorlo TP, Hautima ADR, Beijnen JH et al. . Optimal dosing of miltefosine in children and adults with visceral leishmaniasis. Anti Microb Agents Chemother 2012;56:3864–72. 10.1128/AAC.00292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondal D, Hamano S, Hasnain MG et al. . Challenges for management of post kala-azar dermal leishmaniasis and future directions. Res Rep Trop Med 2014;5:105–11. 10.2147/RRTM.S35707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondal D, Khan MG. Recent advances in post-kala-azar dermal leishmaniasis. Curr Opin Infect Dis 2011;24:418–22. 10.1097/QCO.0b013e32834a8ba1 [DOI] [PubMed] [Google Scholar]
- 22.Salih MA, Ibrahim ME, Blackwell JM et al. . IFNG and IFNGR1 gene polymorphisms and susceptibility to post-kala-azar dermal leishmaniasis in Sudan. Genes Immun 2007;8:75–8. 10.1038/sj.gene.6364353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farouk S, Salih MA, Musa AM et al. . Interleukin 10 gene polymorphisms and development of post kala-azar dermal leishmaniasis in a selected Sudanese population. Public Health Genomics 2010;13:362–7. 10.1159/000272457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saha S, Mondal S, Ravindran R et al. . IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol 2007;179:5592–603. 10.4049/jimmunol.179.8.5592 [DOI] [PubMed] [Google Scholar]
- 25.Vega L. Effects of arsenic on immune system and mechanism of actions. In: Gosselin JD, Fancher IM, eds. Environmental Health Risks: Lead Poisoning and Arsenic Exposure. Nova Science Publishers, 2009:155–63; Chapter 10. [Google Scholar]
- 26.Flanagan SV, Johnston RB, Zheng Y. Arsenic in tube well water in Bangladesh: health and economic impacts and implications for arsenic mitigation. Bull World Health Organ 2012;90:839–46. 10.2471/BLT.11.101253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborti D, Rahman MM, Das B et al. . Status of groundwater arsenic contamination in Bangladesh: a 14-year study report. Water Res 2010;44:5789–802. 10.1016/j.watres.2010.06.051 [DOI] [PubMed] [Google Scholar]
- 28.Mesbahuddin M, Farha N. Vitamin E levels in buccal cells of arsenicosis patients following vitamin E supplementation. Bangladesh J Pharmacol 2013;8:236–41. [Google Scholar]
- 29.Lal CS, Kumar A, Kumar S et al. . Hypocholesterolemia and increased triglycerides in pediatric visceral leishmaniasis. Clin Chim Acta 2007;382:151–3. 10.1016/j.cca.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 30.Wrenger C, Schettert I, Liebau E. Oxidative Stress in Human Infectious Diseases—Present and Current Knowledge About Its Druggability. In: El-Shemy HA, eds. Drug Discovery. INTECH, 2013:227–50.
- 31.Osadolor HB, Ihongbe JC. Effect of leprosy on non-enzymatic antioxidants (vitamin c, vitamin e and uric acid) in (edo state) Nigerian leprosy patients. Conti J Biomed Sci 2008;2:1–5. [Google Scholar]
- 32.Vijayaraghavan R, Suribabu CS, Sekar B et al. . Protective role of vitamin E on the oxidative stress in Hansen's disease (Leprosy) patients. Eur J Clin Nutr 2005;59:1121–8. 10.1038/sj.ejcn.1602221 [DOI] [PubMed] [Google Scholar]
- 33.2010. U.S. Department of health and for human services. National Institute of Health. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.03 June 14. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 34.Vallur AC, Duthie MS, Reinhart C et al. . Biomarkers for intracellular pathogens: establishing tools as vaccine and therapeutic endpoints for visceral Leishmaniasis. Clin Microbiol Infect 2014;20:O374–83. 10.1111/1469-0691.12421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lópes-Maderuelo D, Arnalich F, Serantes R et al. . Interferon-γ and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Am J Respir Crit Care Med 2003;167:970–5. 10.1164/rccm.200205-438BC [DOI] [PubMed] [Google Scholar]
- 36.Farouk S, Salih MA, Musa AM et al. . Interleukin 10 gene polymorphisms and development of post kala-azar dermal leishmaniasis in a selected Sudanese population. Public Health Genomics 2010;13:362–7. 10.1159/000272457 [DOI] [PMC free article] [PubMed] [Google Scholar]