Abstract
Objective
To comprehensively characterize androgens and androgen precursors in classic 21-hydroxylase deficiency (21OHD) and to gain insight to the mechanisms of their formation.
Design
Serum samples were obtained from 38 patients (19 men) with classic 21OHD, age 3-59, and 38 sex- and age-matched controls; 3 patients with 11β-hydroxylase deficiency; 4 patients with adrenal insufficiency; and 16 patients (8 men) undergoing adrenal vein sampling. Paraffin-embedded normal (n=5) and 21OHD adrenal tissue (n=3) was used for immunohistochemical studies.
Methods
We measured 11 steroids in all sera using liquid chromatography-tandem mass spectrometry. Immunofluroescence localized 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) and cytochrome b5 (CYB5A) within the normal and 21OHD adrenals.
Results
Four 11-oxygenated 19-carbon (11oxC19) steroids were significantly higher in male and female 21OHD patients than in controls: 11β-hydroxyandrostenedione, 11-ketoandrostenedione 11β-hydroxytestosterone, and 11-ketotestosterone (3-4-fold, p< 0.0001). For 21OHD patients, testosterone and 11-ketotestosterone were positively correlated in females, but inversely correlated in males. All 11oxC19 steroids were higher in adrenal vein than in inferior vena cava samples from men and women and rose with cosyntropin stimulation. Only trace amounts of 11oxC19 steroids were found in sera from patients with 11β-hydroxylase deficiency and adrenal insufficiency, confirming their adrenal origin. HSD3B2 and CYB5A immunoreactivities were sharply segregated in the normal adrenal glands, whereas areas of overlapping expression were identified in the 21OHD adrenals.
Conclusions
All four 11oxC19 steroids are elevated in both men and women with classic 21OHD. Our data suggest that 11oxC19 steroids are specific biomarkers of adrenal-derived androgen excess.
Keywords: 21-hydroxylase deficiency, congenital adrenal hyperplasia, androgens, adrenal
Introduction
Steroid 21-hydroxylase deficiency (21OHD) accounts for the majority of congenital adrenal hyperplasia cases and is one of the most common autosomal recessive diseases1. As a consequence of the steroid 21-hydroxylase (P450c21, CYP21A2) dysfunction, upstream steroids are diverted toward androgenic pathways. Severe or classic 21OHD leads to in utero virilization and ambiguous genitalia of affected girls1. Females with mild or nonclassic 21OHD may present with hirsutism, acne and irregular menses2. The excessive adrenal androgen production can lead to premature pubarche, rapid somatic growth, advanced bone age, and subfertility in both males and females3–6.
Normalization of adrenal androgen synthesis is difficult to achieve7 without supraphysiological doses of glucocorticoids. Furthermore, reliable biomarkers that accurately distinguish adrenal from gonadal androgen synthesis are lacking, and as a consequence, biochemical targets of disease control are not well defined, especially after the onset of puberty8, 9. Dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS), the most abundant 19-carbon (C19) steroids produced by the adrenal glands10, are disproportionally suppressed by glucocorticoid treatment and are not good indicators of hyperandrogenism in classic 21OHD11, 12. Similarly, there is no good correlation between the routinely measured androgens, androstenedione (AD) and, in women, testosterone (T), and clinical evidence of androgen excess in 21OHD patients13, 14, suggesting that other unrecognized androgens might be produced by the adrenal gland.
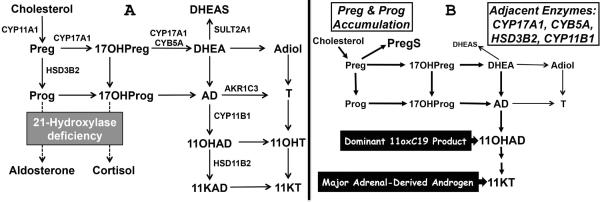
We previously found that 11β-hydroxyandrostenedione (11OHAD) is the most abundant unconjugated C19 steroid in adrenal vein blood samples and that its synthesis is adrenocorticotropin (ACTH)-dependent10. In teleost fishes, 11-ketotestosterone (11KT) is the major androgen, and its synthesis involves the 11β-hydroxylation of AD to 11OHAD with subsequent oxidation and reduction15, 16. Furthermore, 11KT is a potent agonist of the human androgen receptor (NR3C4), with an affinity comparable to T10. Given the profound accumulation of T precursors in the adrenal glands of patients with 21OHD, we reasoned that 11oxygenated C19-steroids (11oxC19) might be abundant adrenal products and a major source of active androgens. The goals of the current study were to provide a detailed characterization of the androgens and androgen precursors in classic 21OHD and to gain insight to the mechanisms and pathways of their formation (Figure 1A).
Figure 1.
Pathways of 11oxC19-steroid synthesis. (A) Anticipated flux to 11oxC19-steroids resultant from 21OHD. (B) Observed changes in steroid flux in 21OHD, with upstream precursors shunted to PregS and downstream products metabolized to 11oxC19-steroids. StAR, steroidogenic acute regulatory protein; CYP11A1, cholesterol side-chain cleavage; HSD3B2, 3β-hydroxysteroid dehydrogenase type 2; CYP17A1, 17α-hydroxylase/17,20-lyase; CYB5A, cytochrome b5 type A; CYP11B1, 11β-hydroxylase; CYP11B2, aldosterone synthase; AKR1C3, 17β-hydroxysteroid dehydrogenase type 5; HSD11B2, 11β-hydroxysteroid dehydrogenase, type 2; SULT2A1, sulfotransferase 2A1; Preg, pregnenolone; PregS, Preg sulfate; Prog, progesterone; 17OHPreg, 17α-hydroxypregnenolone; 17OHProg, 17α-hydroxyprogesterone; DHEA, dehydroepinadrosterone; DHEAS, DHEA sulfate; Adiol, androst-5-ene-3β,17β-diol; AD, androstenedione; T, testosterone; 11OHAD, 11β-hydroxyandrostenedione, 11KAD, 11-ketoandrostenedione; 11OHT, 11β-hydroxytestosterone; 11KT, 11-ketotestosterone.
Subjects and Methods
Human serum samples
We enrolled 38 patients with classic 21OHD (19 women), age 3-59 (Supplemental Data file, Supplementary Table 1). In 34 of these patients peripheral serum was obtained during routine clinical visits, while on their usual glucocorticoid replacement (Supplemental Data file, Supplementary Table 1). In addition, four samples were obtained at 8 AM, before the first morning dose of hydrocortisone, from women with a serum AD greater than 345 ng/dL (>12 nmol/L) from another study17. Patients who were potentially over-treated with glucocorticoids, as evidenced by a 17OHP <20 ng/dL, were excluded. We also enrolled 38 age- and sex-matched controls who were not receiving glucocorticoids, hormonal contraceptives or chemotherapy. In addition, we obtained peripheral serum from three patients with 11β-hydroxylase deficiency (11OHD), and four patients with adrenal insufficiency (two with classic 21OHD who underwent bilateral adrenalectomy and two with Addison's disease).
Adrenal vein (AV) samples were obtained as part of standard of care from patients undergoing evaluation for primary aldosteronism. Leftover serum from 16 patients (8 men) with aldosterone-producing adenomas, ages 32–75, was used for these studies. Only the inferior vena cava (IVC) and AV samples contralateral to the aldosterone-producing adenoma were used to minimize the influence of dysregulated tumor steroidogenesis to these profiles. Samples were obtained from the IVC and AV before and 20 minutes after 0.25 mg bolus cosyntropin administration. Successful catheterization was confirmed by a minimum AV/IVC cortisol gradient of 2 at baseline and 5 after cosyntropin stimulation. All samples were collected under Institutional Review Board (IRB) approved protocols. Written informed consent was granted by all participants who underwent AV sampling, those with 11OHD and adrenal insufficiency, and 17 patients with 21OHD. A waiver of consent was granted by the IRB for using any leftover serum collected as part of standard clinical care for the control group and 21 of the 21OHD patients.
Steroid quantitation by LC-MS/MS
Unlabeled and deuterium-labeled steroid standards were obtained from Sigma-Aldrich, Steraloids, Cerilliant, C/D/N Isotopes, and Cambridge Isotope Laboratories or synthesized (Supplementary Data file, Steroid synthesis and Supplementary Table 2). A 10–100 μL aliquot of serum was deproteinated with 225 μL acetonitrile containing 100-200 μL internal standard deuterated steroids at known concentrations, followed by 150 μL methanol. The suspension was mixed and centrifuged for 5 min at 15000 rpm. For measurement of 3-keto-Δ4–5 (Δ4, such as AD) steroids, the supernatant was mixed with 300 μL water and 1 mL of methyl-t-butyl ether (MTBE) for 4 minutes. After 10 minutes, the organic phase was separated and concentrated under nitrogen. For measurement of 3β-hydroxy-Δ5–6 (Δ5, such as DHEA) steroids, a separate aliquot was first extracted with MTBE, dried, resuspended in 50 μL 1 M ammonium hydroxide and 100 μL 1 M hydroxylamine hydrochloride, incubated at 90 °C for 30 minutes, and subsequently re-extracted with MTBE and dried as described above. Steroid sulfates were extracted with 1 mL of 1:1 chloroform:2-butanol from a serum aliquot after mixing with 200 μL 1 M ammonium sulfate. The dried extracts were reconstituted with 100-200 μL of methanol/deionized water (1:1) and transferred to a 0.25 mL vial insert. Steroids quantitation was performed as previously described18; Supplementary Table 2 gives retention times and precursor/product ion pairs for the targeted steroids. The lower limit of detection for each steroid, defined as the minimum concentration achieving an extrapolated signal-to-noise ratio of 3, ranged from 0.8 to 27 ng/dL (Supplementary Table 2). Intra-assay coefficients of variability (CV) ranged from 2–4% for steroid concentrations >100 ng/dL and from 2–11% for steroid concentrations <100 ng/dL. Inter-assay CV ranged from 2–8%. Linearity of response was assessed by measuring four separate dilutions per sample (n=3 samples), which rendered r2 values consistently >0.95.
Immunofluorescence analysis
Paraffin-embedded adrenal glands from patients with 21OHD (n=3) and deceased renal transplant donors without any adrenal pathology (n=5) were obtained under IRB approval. Immunostaining studies were performed using antibodies for human cytochrome b5 (CYB5A, mouse monoclonal, Acris) and anti-human 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2, also recognizes type 1 isoenzyme) (rabbit polyclonal, kindly provided by C. R. Parker, University of Alabama at Birmingham) antibodies. For immunofluorescence double-staining, the tissues were incubated with the primary antibody solutions overnight (1:3000 dilution for the CYB5A and 1:1000 dilution for the HSD3B2 antibodies), washed with phosphate-buffered saline, and subsequently incubated with species-specific secondary fluorescent antibodies for 1 hour (Alexa Fluor 488-conjugated anti-mouse and Alexa 594 anti-rabbit dilution 1:100). Immunofluorescence was viewed under an Olympus FV 500 Confocal microscope.
Statistical analyses
Non-parametric Mann-Whitney U test was applied to compare the 21OHD patients and controls, using GraphPad Prism6. Correlation between pairs of steroids was assessed using the nonparametric Spearman correlation test. A p<0.05 was considered statistically significant.
Results
Androgens and androgen precursors in sera of 21OHD patients
Using LC-MS/MS, we performed a targeted analysis of 11 steroids in sera from both 21OHD patients and controls, including seven unconjugated C19-steroids and 4 steroid sulfates. The four 11oxC19 steroids—11OHAD, 11-ketoandrostenedione (11KAD), 11β-hydroxytestosterone (11OHT) and 11KT— were significantly higher in 21OHD patients as compared with controls (Table 1, 3- to 4-fold, p< 0.0001 for all). Sub-analysis by sex showed that T was 3.5-fold higher in women with 21OHD (p< 0.0001) and, although not statistically significant, lower in men with 21OHD (0.53-fold, p=0.08) as compared with their corresponding sex-matched controls. AD and all four 11oxC19 steroids were significantly higher in patients with 21OHD of both sexes as compared with corresponding controls. Within the 21OHD group, T was higher in males (3.2-fold, p= 0.0003) and AD was higher in females (2.8-fold, p=0.01); however, there were no statistically significant differences between males and females for any of the 11oxC19 steroids.
Table 1.
Serum steroid concentrations (ng/dL)
| Steroid | 21OHD (n = 38) | Controls (n = 38) | Fold | p |
|---|---|---|---|---|
| Androstenedione | 155 [72–390] | 42 [22–63] | 3.7 | < 0.0001 |
| Testosterone | 80 [38–162] | 26 [12–309] | 3.0 | 0.09 |
| 11OHAD | 351 [188–792] | 118 [70–154] | 3.0 | < 0.0001 |
| 11KAD | 96 [58–143] | 31 [20–42] | 3.1 | < 0.0001 |
| 11OHT | 59 [21–104] | 15 [9–21] | 4.0 | < 0.0001 |
| 11KT | 171 [105–366] | 50 [29–78] | 3.4 | < 0.0001 |
| DHEA | 29 [16–85] | 175 [118–318] | 0.2 | < 0.0001 |
| PregS | 10600 [3400–25305] | 3738 [2853–7769] | 2.8 | 0.001 |
| 17OHPregS | 416 [290–1174] | 481 [370–683] | 0.9 | 0.6 |
| DHEAS | 18744 [7847–64308] | 139784 [58409–186697] | 0.1 | < 0.0001 |
| AdiolS | 2711 [1228–9723] | 25576 [12095–35882] | 0.1 | < 0.0001 |
Data are expressed as median [interquartile range]. Folds represent the 21OHD/controls ratio and were calculated using the medians for each steroid. To convert ng/dL to nmol/L, multiply by 0.0347 for testosterone; 0.0349 for androstenedione, 0.0328 for 11β-hydroxytestosterone (11OHT), 0.0331 for 11-ketotestosterone (11KT) and 11β-hydroxyandrostenedione (11OHAD), 0.0333 for 11-ketoandrostenedione (11KAD), 0.0347 for DHEA, 0.0252 for Pregnenolone sulfate (PregS), 0.0242 for 17α-hydroxypregnenolone sulfate (17OHPregS), 0.027 for androst-5-ene-3β,17β-diol sulfate (AdiolS) and 0.0271 for DHEAS.
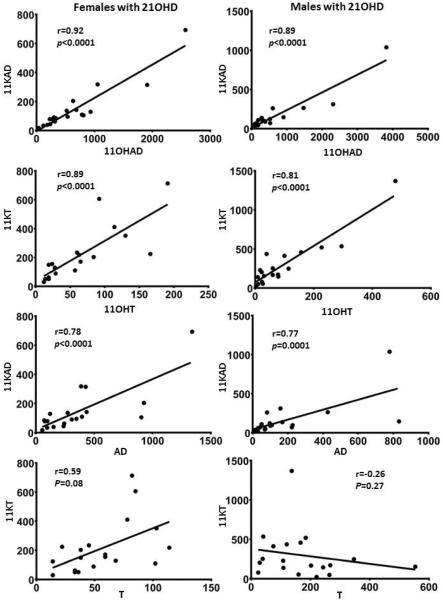
Tight correlations were observed between 11OHAD and 11KAD, as well as 11OHT and 11KT in both women (r=0.92, p<0.0001 and r=0.89, p<0.0001, respectively) and men (r=0.89, p<0.0001 and r=0.81, p<0.0001, respectively) (Figure 2, panels A–D). While 11KAD correlated positively with AD in both women (r=0.78, p<0.0001) and men (r=0.77, p=0.0001), 11KT correlated positively with T in women (r=0.59, p<0.008) but negatively -though not significantly- in men (r=−0.26, p=0.27) (Figure 2, panels E–H).
Figure 2.
Correlations between serum steroids in men and women with 21OHD. Spearman nonparametric tests were used to analyze correlations between 11β-hydroxyandrostenedione (11OHAD) and 11-ketoandrostenedione (11KAD) (A–B); between 11β-hydroxytestosterone (11OHT) and 11-ketotetsosterone (11KT) (C–D); between 11KAD and AD (E–F); and between 11KT and T (G–H), in women and men with 21-hydroxylase deficiency, respectively.
DHEA and DHEAS were significantly lower in 21OHD patients than in controls (0.2-fold and 0.1-fold respectively, p< 0.0001). Androst-5-ene-3β,17β-diol sulfate was also lower in 21OHD patients (0.1-fold, p< 0.0001), while pregnenolone sulfate was almost 3-fold higher in 21OHD patients than in controls (p=0.001). Androst-5-ene-3β,17β-diol sulfate correlated tightly with DHEAS in both 21OHD patients (r=0.96, p<0.0001) and controls (r=0.94, p<0.0001). Pregnenolone sulfate also correlated with DHEAS (r=0.44, p=0.0065 in 21OHD patients; r=0.79, p<0.0001 in controls). These data demonstrate that precursor steroids upstream of DHEA and DHEAS are diverted to 11oxC19 and to pregnenolone sulfate in patients with 21OHD.
Adrenal gland production of 11oxC19 steroids
To study the origins of 11oxC19 steroids, we measured these steroids in paired IVC and AV samples with and without cosyntropin stimulation from AVS studies. We used only AV samples contralateral to an aldosterone-producing adenoma to minimize deviations from normal adrenal steroid production. Compared with the IVC, the AV concentrations at baseline were 33-fold higher for 11OHAD, 3.3-fold higher for 11OHT, 2.5-fold higher for 11KAD and 1.8-fold higher for 11KT (Table 2). Cosyntropin stimulation further increased the AV/IVC gradient to 196-fold for 11OHAD, 17-fold for 11OHT, 6-fold for 11KAD and 3.3-fold for 11KT. Following cosyntropin stimulation, the AV concentrations of 11OHAD were augmented 12-fold, those of 11OHT 4.3-fold, while those of 11KAD and 11KT approximately 2-fold each. The IVC concentrations for all 11oxC19 steroids were similar between men and women both at baseline, as well as after cosyntropin stimulation. These data indicate that 11OHAD is a major, ACTH-stimulated product of the adrenal gland in men and women and suggest that 11OHT is also a minor adrenal product, whereas 11KAD and 11KT are primarily peripheral metabolites from their 11β-hydroxylated precursors. To confirm that adrenal 11β-hydroxylase enzymes are responsible for their synthesis, we measured 11oxC19 steroids in three patients with 11OHD and in four patients with adrenal insufficiency. Only trace amounts of 11OHAD, 11OHT, 11KAD and 11KT were found in sera from all seven patients (0–22 ng/dL).
Table 2.
Steroid concentrations and ratios in adrenal vein and inferior vena cava serum samples (ng/dL)
| 11OHT | 11KT | 11OHAD | 11KAD | T | AD | |
|---|---|---|---|---|---|---|
| AV Baseline | 72 | 47 | 3119 | 37 | 55 | 375 |
| AV post-ACTH | 357 | 112 | 27731 | 83 | 128 | 16194 |
| IVC Baseline | 15 | 31 | 100 | 15 | 40 | 29 |
| IVC post-ACTH | 17 | 31 | 146 | 17 | 32 | 35 |
| AV/IVC Baseline | 3.3 | 1.8 | 33 | 2.5 | 1.0 | 15 |
| AV/IVC post-ACTH | 17 | 3 | 196 | 6 | 5 | 451 |
| Fold stimulation post-ACTH AV | 4 | 2.0 | 12 | 2.4 | 3.4 | 48 |
| Fold stimulation post-ACTH IVC | 1.0 | 0.9 | 1.3 | 1.0 | 1.0 | 1.2 |
|
| ||||||
| p Values, Women vs Men | ||||||
| AV/IVC Baseline | 0.3 | 0.6 | 0.7 | 0.2 | 0.0002 | 0.6 |
| AV/IVC post-ACTH | 0.2 | 0.6 | 0.6 | 0.2 | < 0.0001 | 0.6 |
| Fold stimulation post-ACTH AV | 0.8 | 0.8 | 1.0 | 0.2 | 0.1 | 0.5 |
| Fold stimulation post-ACTH IVC | 0.4 | 0.7 | 0.3 | 0.8 | 0.8 | 0.1 |
Concentrations of testosterone (T), androstenedione (AD) and their 11-oxygenated derivatives in adrenal veins (AV) and inferior vena cava (IVC) of 16 sample sets (8 male), AV/IVC ratios at baseline and following cosyntropin (post-ACTH) stimulation, and fold stimulation.
Immunostaining of key enzymes in androgen synthesis in 21OHD
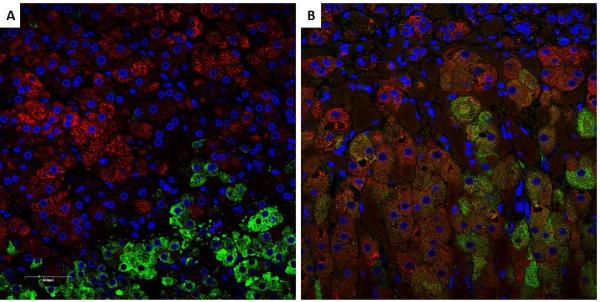
The robust synthesis of 11β-hydroxylated, 19-carbon, Δ4-steroids in 21OHD constitutes a paradox, because their synthesis requires enzymes and cofactor proteins segregated to the zona fasciculata (HSD3B2) and zona reticularis (CYB5A) in the normal adrenal. To explain this conundrum, we performed immunohistochemistry for these two key proteins in adrenal glands from patients with 21OHD (n=3) and from deceased renal transplant donors (n=5) with normal adrenal function. Representative images of HSD3B2 and CYB5A immunofluorescence in 21OHD and normal adrenal glands are shown in Figure 3. In normal adrenal glands, HSD3B2 and CYB5A immunoreactivities are precisely segregated between zona fasciculata and zona reticularis, respectively (Figure 3A). In contrast, the 21OHD adrenals exhibited areas containing a mixture of HSD3B2 and CYB5A immunoreactivities (Figure 3B).
Figure 3.
Double immunofluorescence of 3β-hydroxysteroid dehydrogenase type 2 (HSD3B2) (red) and cytochrome b5 (CYB5A) (green). Nuclei are counterstained in blue. In the normal adrenal gland (A), HSD3B2 and CYB5A are sharply segregated to the zona fasciculata and zona reticularis, respectively, while in the 21OHD adrenal (B), areas with intermingled expression of both HSD3B2 and CYB5A were identified.
Discussion
Adrenal androgen excess is a hallmark of 21OHD, but the traditional serum-steroid biomarkers, including AD, T, DHEA and DHEAS, do not serve as consistent, linear indicators of disease severity or treatment response in all patients11, 13, 14. Furthermore, DHEAS and AD are not bioactive androgens themselves but constitute a pool of precursors for potent androgens, such as T and DHT. Previous studies found elevated 11OHAD concentrations in women with nonclassic 21OHD19–21. Herein, we have shown that four 11oxC19 steroids, 11OHAD, 11KAD, 11OHT and 11KT, are significantly higher in both male and female patients with classic 21OHD than in age-matched controls. Using in vitro cell-based luciferase reporter systems, we have previously shown that both 11OHT and 11KT activate the human androgen receptor and that the maximal activity of 11KT was similar to that of T10, 22, 23. Conversely, AD and 11KAD led to only modest activation of the androgen receptor, and 11OHAD demonstrated no androgen receptor activation at concentrations up to 1000 nmol/L10, 22. These data suggest that 11KT is an important androgen in patients with 21OHD.
Unlike AD and T, which also derive from the gonads, 11oxC19 steroid derive primarily from the adrenals and thus strongly reflect the adrenal contribution to circulating androgens. The synthesis of 11OHAD occurs predominately in the adrenal gland from AD (Figure 1), through the action of steroid 11β-hydroxylase (CYP11B1), and small amounts might be produced from cortisol24. Consistent with previous reports that measured only 11OHAD25, 26, we found negligible amounts of all 11oxC19 steroids in sera from patients with 11OHD, which confirms that their synthesis relies on CYP11B1. In vitro studies with radiolabeled substrates showed that the ovarian granulosa cells cannot synthesize 11OHAD from AD27. We have previously shown that 11OHAD is the most abundant unconjugated C19 steroid produced by the adrenal glands in women and that the adrenal was also a source of 11KAD, 11OHT and 11KT10. Herein, we extend these findings to show that the adrenal contribution to the circulating 11OHT and 11KT pool is similar between men and women, supporting the fact that gonadal T is not an important precursor, if at all, for 11OHT and 11KT. Furthermore, in our 21OHD males, 11KT correlated directly and tightly with 11OHT but tended to correlate inversely with T, as 11KT will suppress gonadotropins and T production from the testes in men with poorly-controlled 21OHD. This latter result suggests that the T/11KT ratio might be an ideal parameter for titrating therapy in men with 21OHD. These findings further suggest that adrenal-derived 11OHT, rather than gonadal-derived T, is the precursor of 11KT. In addition, we found only trace amounts of 11oxC19 steroids in two 21OHD patients who had undergone adrenalectomy and in two patients with Addison's disease, further supporting the central role of the adrenal in their synthesis. Based on its high AV/IVC gradients and cosyntropin stimulation, our data suggest that 11OHAD is the major direct 11oxC19 product of the adrenal along with some 11OHT, whereas 11KAD and 11KT are primarily formed in peripheral tissues.
DHEAS, the dominant C19 steroid product of the adrenal, is often paradoxically low or low-normal in 21OHD patients even without treatment, thus limiting its clinical utility in these patients11. Even though we excluded patients with a suppressed 17OHP, we found that DHEA and DHEAS were 6-to 7-fold higher in controls rather than in 21OHD patients. The mechanisms underlying this phenomenon are poorly understood. Androst-5-ene-3β,17β-diol sulfate and DHEAS concentrations varied in parallel, in both 21OHD patients and controls. In contrast, 21OHD patients produced significantly higher amounts of pregnenolone sulfate as compared to controls (Figure 1B). Although not as robustly, pregnenolone sulfate correlated directly, rather than inversely, with DHEAS. Combined with the data for 11oxC19 steroids, these results suggest that 21-carbon steroids are diverted along several ordinarily minor pathways in the 21OHD adrenal. Several enzymes, including HSD3B2, CYP17A1, CYP11B1, and SULT2A1, compete for these accumulating common substrates. The kinetic interplay between these multiple reactions is difficult to predict and requires further study.
Another intriguing aspect of adrenal steroid biosynthesis is the mechanism by which the production of active androgens becomes sufficient to cause severe virilization in females with 21OHD. For the synthesis of AD and downstream androgens, both HSD3B2 and CYB5A are required. These two key factors in androgens synthesis are co-expressed in the testicular Leydig and ovarian theca cells28, 29. In the normal adrenal gland, HSD3B2 and CYB5A are segregated to the zonae glomerulosa and fasciculata or the zona reticularis, respectively, such that the major adrenal C19 steroids are DHEA and DHEAS. Double-immunohistochemical analysis of HSD3B2 and CYB5A in the normal adrenal glands identified a small number of cells where these two proteins overlap at the interface of the zonae fasciculata and reticularis, which might be responsible for the adrenal AD and T synthesis30. Comparison between age groups in this study showed that the co-localization of HSD3B2 and CYB5A is most prominent in the 13–20 year-old group, following adrenarche. We hypothesized that the adrenal glands of patients with 21OHD exhibit larger areas of overlapping HSD3B2 and CYB5A expression, which would confer to these cells greater androgenic production efficiency, normally present only in the gonads. Indeed, we found islands of cells with overlapping expression of HSD3B2 and CYB5A in the adrenal glands from patients with classic 21OHD, but not in normal adrenals (Figure 3).
Despite a large number of both males and females with matched controls, this initial study of androgens in classic 21OHD has several limitations. Most of our serum samples were obtained randomly, from patients on various glucocorticoid replacement regimens, and accurate clinical assessment of disease control was not possible in many participants. Prospective studies will be needed to assess the correlation of adrenal androgens in 21OHD with the clinical phenotype and response to treatment across the life span. Nevertheless, our data suggest that these 11oxC19 steroids are promising biomarkers of adrenal androgen excess in 21OHD and might be superior to AD and T. Because AD and T also derive from the gonads, these traditional biomarker steroids are problematic not only in men but also in women with 21OHD, who often secondarily develop polycystic ovarian syndrome31. An important strength of our study is the inclusion of both males and females with classic 21OHD. Although clinical stigmata of adrenal androgen excess can be subtle in males, they suffer from sexual precocity12, 32–34 and infertility4–6, similar to females. The inclusion of males in both our comparison of 21OHD with unaffected controls, as well as in our AV analysis, allowed us to conclude that the major source of 11oxC19 steroids is the adrenal gland.
In summary, we have shown that four 11oxC19 steroids are similarly elevated in patients with classic 21OHD of both sexes. Because 11KT is a potent androgen, it might be the most clinically relevant adrenal-derived androgen in 21OHD patients. In addition, our findings suggest that pregnenolone sulfate might serve as an additional biomarker for disease control in patients with 21OHD. With the expanded use of LC-MS/MS, future prospective studies will allow the characterization of steroid biomarkers that accurately reflect disease control and facilitate treatment monitoring.
Supplementary Material
Acknowledgements
We thank Michelle Vinco and the Molecular Pathology Research Laboratory (Department of Pathology, University of Michigan) for tissue procurement, David Madrigal for serum procurement, and Carole Ramm for regulatory management of clinical research protocols. Mass spectrometry used core services supported by Grant DK089503 from the National Institutes of Health to the University of Michigan under the Michigan Nutrition Obesity Center (NORC). We thank Janssen Research and Development for returning leftover serum samples from study 212082HPL1002 under written informed consent for research purposes.
Funding This work was supported by pilot grants from the University of Michigan Reproductive Sciences Program and by grants MICHR Pilot U046500 to AT, R01GM086596 to RJA, R01DK069950 to WER, and in part by the Intramural Research Program of the NIH. AFT was supported by 1F32DK103461.
Footnotes
Declaration of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Azziz R, Dewailly D, Owerbach D. Clinical review 56: Nonclassic adrenal hyperplasia: current concepts. J Clin Endocrinol Metab. 1994;78:810–815. doi: 10.1210/jcem.78.4.8157702. [DOI] [PubMed] [Google Scholar]
- 3.Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv. 2003;58:275–284. doi: 10.1097/01.OGX.0000062966.93819.5B. [DOI] [PubMed] [Google Scholar]
- 4.Cabrera MS, Vogiatzi MG, New MI. Long term outcome in adult males with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86:3070–3078. doi: 10.1210/jcem.86.7.7668. [DOI] [PubMed] [Google Scholar]
- 5.Reisch N, Flade L, Scherr M, Rottenkolber M, Pedrosa Gil F, Bidlingmaier M, Wolff H, Schwarz HP, Quinkler M, Beuschlein F, et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94:1665–1670. doi: 10.1210/jc.2008-1414. [DOI] [PubMed] [Google Scholar]
- 6.Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89:597–601. doi: 10.1016/j.fertnstert.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez LA, Moran C, Reyna R, Ochoa T, Boots LR, Azziz R. Adrenal progestogen and androgen production in 21-hydroxylase-deficient nonclassic adrenal hyperplasia is partially independent of adrenocorticotropic hormone stimulation. Fertil Steril. 2002;77:750–753. doi: 10.1016/s0015-0282(01)03236-8. [DOI] [PubMed] [Google Scholar]
- 8.Dauber A, Kellogg M, Majzoub JA. Monitoring of therapy in congenital adrenal hyperplasia. Clin Chem. 2010;56:1245–1251. doi: 10.1373/clinchem.2010.146035. [DOI] [PubMed] [Google Scholar]
- 9.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:4133–4160. doi: 10.1210/jc.2009-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rege J, Nakamura Y, Satoh F, Morimoto R, Kennedy MR, Layman LC, Honma S, Sasano H, Rainey WE. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98:1182–1188. doi: 10.1210/jc.2012-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezvani I, Garibaldi LR, Digeorge AM, Artman HG. Disproportionate suppression of dehydroepiandrosterone sulfate (DHEAS) in treated patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatr Res. 1983;17:131–134. doi: 10.1203/00006450-198302000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Volkl TM, Ohl L, Rauh M, Schofl C, Dorr HG. Adrenarche and puberty in children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2011;76:400–410. doi: 10.1159/000333696. [DOI] [PubMed] [Google Scholar]
- 13.Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, Lesser M, New MI, White PC. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90:584–595. doi: 10.1172/JCI115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85:1059–1065. doi: 10.1210/jcem.85.3.6441. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Nakanishi T. 11-ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. Gen Comp Endocrinol. 1999;115:178–187. doi: 10.1006/gcen.1999.7314. [DOI] [PubMed] [Google Scholar]
- 16.Borg B. Androgens in teleost fishes. Comp Biochem Physiol. 1994;109C:219–245. [Google Scholar]
- 17.Auchus RJ, Buschur EO, Chang AY, Hammer GD, Ramm C, Madrigal D, Wang G, Gonzalez M, Xu XS, Smit JW, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99:2763–2770. doi: 10.1210/jc.2014-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-Carbon Steroids in 21-hydroxylase Deficiency. J Clin Endocrinol Metab. 2015;100:2283–2290. doi: 10.1210/jc.2015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huerta R, Dewailly D, Decanter C, Knochenhauer ES, Boots LR, Azziz R. 11β-hydroxyandrostenedione and Δ5-androstenediol as markers of adrenal androgen production in patients with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil Steril. 1999;72:996–1000. doi: 10.1016/s0015-0282(99)00402-1. [DOI] [PubMed] [Google Scholar]
- 20.Hudson RW, Lochnan HA, Danby FW, Margesson LJ, Strang BK, Kimmett SM. 11β-hydroxyandrostenedione: a marker of adrenal function in hirsutism. Fertil Steril. 1990;54:1065–1071. [PubMed] [Google Scholar]
- 21.Carmina E, Stanczyk FZ, Chang L, Miles RA, Lobo RA. The ratio of androstenedione:11β-hydroxyandrostenedione is an important marker of adrenal androgen excess in women. Fertil Steril. 1992;58:148–152. doi: 10.1016/s0015-0282(16)55152-8. [DOI] [PubMed] [Google Scholar]
- 22.Campana C, Rege J, Turcu A, Pezzi V, Gomez-Sanchez CE, Robins DM, Rainey WE. Development of a novel cell based androgen screening model. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storbeck KH, Bloem LM, Africander D, Schloms L, Swart P, Swart AC. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol Cell Endocrinol. 2013;377:135–146. doi: 10.1016/j.mce.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Axelrod LR, Kraemer DC, Burdett J, Jr, Goldzieher JW. Biosynthesis of 11 -hydroxyandrostenedione by human and baboon adrenals. Acta Endocrinol (Copenh) 1973;72:545–550. doi: 10.1530/acta.0.0720545. [DOI] [PubMed] [Google Scholar]
- 25.Polson DW, Reed MJ, Franks S, Scanlon MJ, James VH. Serum 11β-hydroxyandrostenedione as an indicator of the source of excess androgen production in women with polycystic ovaries. J Clin Endocrinol Metab. 1988;66:946–950. doi: 10.1210/jcem-66-5-946. [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim F, Giton F, Boudou P, Villette JM, Julien R, Galons H, Fiet J. Plasma 11β-hydroxy-4-androstene-3,17-dione: comparison of a time-resolved fluoroimmunoassay using a biotinylated tracer with a radioimmunoassay using a tritiated tracer. J Steroid Biochem Mol Biol. 2003;84:563–568. doi: 10.1016/s0960-0760(03)00077-3. [DOI] [PubMed] [Google Scholar]
- 27.Holownia P, Owen EJ, Conway GS, Round J, Honour JW. Studies to confirm the source of 11 β-hydroxyandrostenedione. J Steroid Biochem Mol Biol. 1992;41:875–880. doi: 10.1016/0960-0760(92)90441-k. [DOI] [PubMed] [Google Scholar]
- 28.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5–Δ4 isomerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 29.Dharia S, Slane A, Jian M, Conner M, Conley AJ, Parker CR., Jr Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod. 2004;71:83–88. doi: 10.1095/biolreprod.103.026732. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Fujishima F, Hui XG, Felizola SJ, Shibahara Y, Akahira J, McNamara KM, Rainey WE, Sasano H. 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging. Endocr Res. 2015;40:8–13. doi: 10.3109/07435800.2014.895377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes RB, Rosenfield RL, Ehrmann DA, Cara JF, Cuttler L, Levitsky LL, Rosenthal IM. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 32.Dacou-Voutetakis C, Dracopoulou M. High incidence of molecular defects of the CYP21 gene in patients with premature adrenarche. J Clin Endocrinol Metab. 1999;84:1570–1574. doi: 10.1210/jcem.84.5.5683. [DOI] [PubMed] [Google Scholar]
- 33.Oberfield SE, Mayes DM, Levine LS. Adrenal steroidogenic function in a black and Hispanic population with precocious pubarche. J Clin Endocrinol Metab. 1990;70:76–82. doi: 10.1210/jcem-70-1-76. [DOI] [PubMed] [Google Scholar]
- 34.Balducci R, Boscherini B, Mangiantini A, Morellini M, Toscano V. Isolated precocious pubarche: an approach. J Clin Endocrinol Metab. 1994;79:582–589. doi: 10.1210/jcem.79.2.8045980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.