Abstract
Background and Aim
Luminal nutrients stimulate enteroendocrine L cells to release gut hormones, including intestinotrophic glucagon-like peptide-2 (GLP-2). Because L cells express the bile acid receptor TGR5 and dipeptidyl peptidase-IV (DPPIV) rapidly degrades GLPs, we hypothesized that luminal TGR5 activation may attenuate intestinal injury via GLP-2 release, which is enhanced by DPPIV inhibition.
Methods
Intestinal injury was induced in mice by administration of dextran sulfate sodium (DSS) in drinking water (free access to water containing 5% DSS for 7 days). The selective TGR5 agonist betulinic acid (BTA) and the DPPIV inhibitor sitagliptin phosphate monohydrate (STG) were administered orally for 7 days. Male C57BL/6 mice (6–7 weeks old) were divided into five groups: normal control group, disease control group, BTA low group (drinking water containing 15 mg/L BTA), BTA high group (50 mg/L BTA), and BTA high + STG (3 mg/kg, i.g.) group.
Results
The selective TGR5 agonist BTA dose-dependently suppressed disease activity index and mRNA expression of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α in the colon. Nevertheless, STG administration had little additive effect on BTA-induced protection. Fibroblast activation protein mRNA expression, but not expression of other DPP family members, was increased in the colon of DSS-treated mice with increased mucosal DPPIV. Co-administration of the selective GLP-2 antagonist GLP-2 (3–33) reversed the effect of BTA.
Conclusion
The selective TGR5 agonist BTA ameliorated DSS-induced colitis in mice via the GLP-2 pathway with no effect of DPPIV inhibition, suggesting that other DPP enzymatic activity is involved in GLP-2 degradation.
Keywords: dextran sulfate sodium, DPPIV, GLP-2, sitagliptin, TGR5
Introduction
Luminal nutrients stimulate cognate intestinal epithelial nutrient receptors in order to exert rapid physiological responses.1 Enteroendocrine cells detect luminal nutrients, such as long- or short-chain fatty acids, bile acids, and amino acids, and secrete peptide hormone via a number of nutrient-sensing G protein-coupled receptors (GPCRs) expressed in the apical membranes of enteroendocrine cells.2 Enteroendocrine L cells produce glucagon-like peptide-1 (GLP-1) and GLP-2 simultaneously, processed from the prohormone proglucagon.3 L cells, principally located on the distal small intestine and colon, also release the intestinotrophic hormone GLP-2.4 Repeated administration of GLP-2 stimulates crypt cell growth and reduces enterocyte apoptosis.5 So far, it has been elucidated that exogenous GLP-2 administration reduces parenteral nutrition support in patients with short bowel syndrome.6
Recently, the bile acid GPCR TGR5 was reported to be expressed in the enteroendocrine GLP-1 secreting cell line STC-1 and in primary L cells isolated from mice.7 In L cell model GLUTag cells, bile acids increase the release of GLP-1 and GLP-2.8 In rat duodenum, luminal bile acids modulate bicarbonate secretion via TGR5 activation and GLP-2 release.9
Dipeptidyl peptidase-IV (DPPIV) is a member of the serine protease family, which also includes three structurally homologous enzymes, DPP-8, DPP-9, and fibroblast activation protein (FAP).10 DPPIV, DPP-8, DPP-9, and FAP possess similar enzyme activity and cleave N-terminal dipeptides containing proline or alanine in the penultimate position from regulatory factors, thereby rapidly deactivating hormones and cytokines.11 Because GLP-2 is also a DPPIV substrate, we hypothesized that luminal TGR5 activation may attenuate intestinal injury via GLP-2 release, which is enhanced by DPPIV inhibition.
Methods
Chemicals
The DPPIV inhibitor, sitagliptin phosphate monohydrate (STG), was obtained from Carbosynth Limited (Berkshire, UK). K-579 and betulinic acid (BTA; (+)-(3β)-3-hydroxylup-20(29)-en-28-oic acid) were obtained from Tocris Bioscience (Ellisville, MO, USA). Dextran sulfate sodium (DSS) with molecular weight of 4000–5000 was from Nacalai Tesque, Inc. (Kyoto, Japan). Mouse GLP-2 (3–33) (sequence; NH2- DGS FSDEM STILD NLATR DFINW LIQTK ITD- COOH) was custom synthesized by Eurofins NWG Operon, Inc (Tokyo, Japan).
Animals
Six- to seven-week-old male C57BL/6 mice (Oriental Bio Service, Inc, Kyoto, Japan), weighing 20–25 g, were maintained in an animal colony with controlled temperature (23°C) and light (12 h light–dark cycle) at the Osaka Medical College, Osaka, Japan, and were permitted free access to standard mice chow pellets (MM-3, Funabashi Farms, Chiba, Japan) and water. Ethical permission for the experiments presented in this study was given by the Animal Ethical Committee of the Osaka Medical College and all procedures were conducted according to the guidelines of the Institute for Laboratory Animal Research at Osaka Medical College.
Experimental procedures
For induction of experimental colitis, mice were given water containing 5% DSS for 7 days instead of tap water. Mice were divided into five groups: normal control group (n = 5), disease control group (n = 6), BTA low group (n = 6) (free access to water containing 15 mg/L BTA for 7 days), BTA high group (n = 5) (free access to water containing 50 mg/L BTA for 7 days), and BTA high + STG (3 mg/kg, i.g. for 7 days) co-administration group (n = 5). To examine the effect of endogenous GLP-2, a GLP-2 receptor antagonist GLP-2 (3–33) was administered to DSS-treated mice for 7 days (2.5 or 25 μg/body/day, i.p.).
Evaluation of severity of clinical colitis
Disease activity index (DAI) was determined in all animals during the administration of DSS by scoring body weight, Hemoccult reactivity, or the presence of gross blood and stool consistency in accordance with the method described by Murthy et al.12 This method of scoring is a comprehensive functional measure that correlates well with the degree of inflammation. The individuals who examined mice and determined the DAI scores were blinded as to the experimental group to which the animal belonged. For histology, the rectum was fixed in 10% neutral buffered formalin, paraffin-embedded, sectioned, and stained with hematoxylin and eosin (HE). Histological score of HE-stained specimens of the rectum was determined in a blinded fashion according to the method reported by Murthy et al. as follows: Grade 0, normal colonic mucosa; Grade 1, loss of one-third of the crypts; Grade 2, loss of two-third of the crypts; Grade 3, the lamina propria is covered with a single layer of epithelium and mild inflammatory cell infiltration is present; and Grade 4, erosions and marked inflammatory cell infiltration are present.12,13 In this study, sites with the most severe inflammation were evaluated. The mean score in each section was calculated.
Real-time quantitative polymerase chain reaction
To measure expression of DPPIV, DPP-8, DPP-9, FAP, and pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α mRNA, a small amount of proximal colonic mucosa was scraped with a glass slide, frozen in liquid nitrogen, and stored at −80°C until RNA isolation. Total RNA was extracted using a total RNeasy mini-Kit (Qiagen, Tokyo, Japan). For cDNA synthesis, RNA was reverse-transcribed with a Prime Script RT reagent Kit (Takara Bio Inc., Shiga, Japan) with a SYBR Premix Ex Taq Kit (Takara Bio Inc.) on the Thermal Cycler Dice Real Time System TP870 (Takara Bio Inc.). The sequences of sense and antisense primers for mouse DPPIV, DPP-8, DPP-9, FAP, TNF-α, IL-1β, IL-6, and β-actin are depicted in Table 1. Cycling conditions were one cycle of 95°C for 30 s for denaturing followed by 40 cycles of 95°C for 5 s and 60°C for 30 s and one cycle of 95°C for 15 s and 60°C for 30 s. The expression of target genes was normalized to the expression of β-actin.
Table 1.
Primers used to detect gene expression at mRNA level
| Genes | Sequence of primers | |
|---|---|---|
| DPPIV | Sense | 5′-AAATGGGATTTGTGGACAGCAAG-3′ |
| Anti-sense | 5′-CCGATCCCAGGACCATTGAG-3′ | |
| DPP-8 | Sense | 5′-GCGGTAAGCTGATCCCTGACA-3′ |
| Anti-sense | 5′-CCAGCCAGCACTTGAAAGCTC-3′ | |
| DPP-9 | Sense | 5′-TCATCCACAAGCCACAAGTGTTC-3′ |
| Anti-sense | 5′-GGGCTACAGACCCTGCCTCATA-3′ | |
| FAP | Sense | 5′-TGGCATAGCAGTGGCTCCAG-3′ |
| Anti-sense | 5′-TTCTGCTCTTGCCATCACAGTTG-3′ | |
| TNF-α | Sense | 5′-TATGGCCCAGACCCTCACA-3′ |
| Anti-sense | 5′-GGAGTAGACAAGGTACAACCCATC-3′ | |
| IL-1β | Sense | 5′-TCCAGGATGAGGACATGAGCAC-3′ |
| Anti-sense | 5′-GAACGTCACACACCAGCAGGTTA-3′ | |
| IL-6 | Sense | 5′-CCACTTCACAAGTCGGAGGCTTA-3′ |
| Anti-sense | 5′-CCAGTTTGGTAGCATCCATCATTTC-3′ | |
| β-actin | Sense | 5′-CATCCGTAAAGACCTCTATGCCAAC-3′ |
| Anti-sense | 5′-ATGGAGCCACCGATCCACA-3′ |
Mucosal DPPIV activity
Mucosal scraped tissues were collected in lysis buffer (TRIS-buffered saline containing protease inhibitors) (Complete Mini, Roche Diagnostics, Indianapolis, IN, USA) with or without DPPIV inhibitor (100 μM K-579). Blood samples were also collected into chilled Eppendorf tubes (Eppendorf Japan, Tokyo, Japan) containing 1 μL each of 0.5 M EDTA and 100 μM K-579. The samples were immediately centrifuged at 3000 × g for 5 min and their supernatants and plasma were stored at −80°C until measurements. Mucosal DPPIV activity was determined by the cleavage rate of 7-amino-4-methylcoumarin (AMC; SensoLyte AMC DPPIV Assay Kit, AnaSpec, Inc., Fremont, CA, USA) from the synthetic substrate according to the manufacturer’s instruction. In brief, each sample of scraped colon was homogenized. Lysates containing 10 μg/50 μL of protein was mixed with 50 μL of the DPPIV substrate AMC (AnaSpec, Inc.). After 30 min of incubation at room temperature, AMC release was determined using a fluorescence plate reader (excitation at 354 nm and emission at 442 nm) (Fluoroskan Ascent, Thermo Fisher Scientific K.K., Kanagawa, Japan). DPP activity in mucosa was expressed as the relative fluorescence units.
Measurement of plasma and mucosal GLPs
Plasma and mucosal active GLP-1 (7–36) and total GLP-2 were measured using a GLP-1 (Active 7–36) ELISA kit and GLP-2 (Mouse) ELISA kit (ALPCO Diagnostics, Salem, NH, USA) according to the manufacturer’s instruction.
Statistics
All data are expressed as mean ± SD. Data were derived from five or six mice in each group. Comparisons were performed using one-way ANOVA or Kruskal–Wallis followed by Fisher’s PLSD test. Statistical significance was defined as P < 0.05.
Results
Effects of BTA and STG on clinical index and histological scores
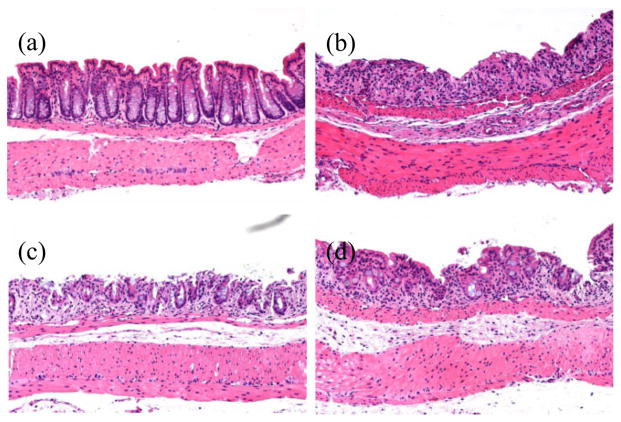
Treatment of C57BL/6 mice with 5% DSS in drinking water for 7 days evoked clinical and histological signs of colitis. Clinical symptoms of colitis included bloody stool, diarrhea, and loss of body weight, which progressed over the 7-day experimental duration. BTA administration significantly and dose-dependently suppressed the DAI and histological scores on Day 7. Nevertheless, co-administration of STG had no additive effect on the BTA-induced protection (Table 2). Histologically, specimens obtained from disease control mice were observed to have sporadic erosions with marked inflammatory cell infiltration in the lamina propria (Fig. 1b). In contrast, fewer erosions and a less-marked inflammatory rectal cell infiltration were observed in the BTA high group (Fig. 1c). STG administration did not potentiate the protective effect of high-dose BTA (Fig. 1d). BTA dose-dependently reduced histological scores, whereas STG had no additive effect (Table 2).
Table 2.
The effect of BTA and STG on clinical index and histological scores
| Group | n | DAI | Histological scores |
|---|---|---|---|
| Disease control | 6 | 3.56 ± 0.42 | 2.83 ± 1.17 |
| BTA low | 5 | 3.44 ± 0.60 | 1.80 ± 0.84 |
| BTA high | 5 | 2.33 ± 0.37* | 1.40 ± 0.55* |
| BTA high + STG | 5 | 2.13 ± 0.16* | 1.40 ± 0.55* |
P < 0.05 versus disease control group.
Results are expressed as the mean ± SD.
BTA, betulinic acid; STG, sitagliptin phosphate monohydrate.
Figure 1.
DSS-induced colitis in mice. (a) Normal control, (b) disease control, (c) BTA high, and (d) BTA high + STG. Treatment with high-dose BTA produced fewer erosions and decreased the amount of rectal inflammatory cell infiltration. STG only partially potentiated the effect of BTA. BTA, betulinic acid; DSS, dextran sulfate sodium; STG, sitagliptin phosphate monohydrate.
Effect of BTA and STG on pro-inflammatory cytokine expression
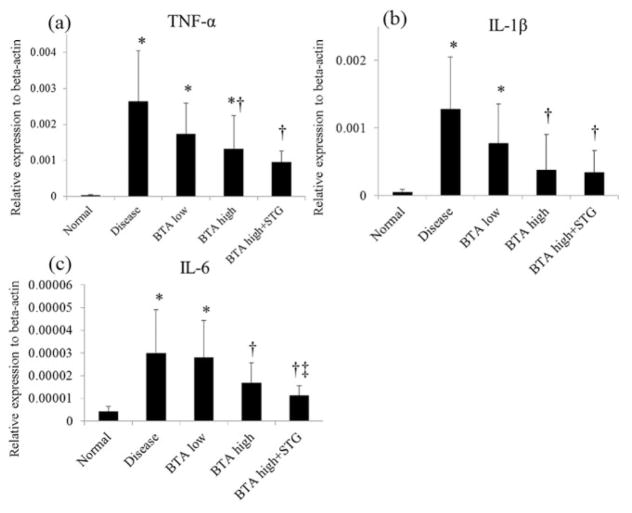
As shown in Figure 2, a significant increase of mRNA expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, as measured by real-time quantitative polymerase chain reaction, was observed in mice with DSS-induced colitis compared with normal control mice. The administration of BTA significantly suppressed pro-inflammatory cytokine mRNA expression. Co-administration of STG significantly potentiated the suppressive effects of BTA on the mRNA expression of IL-6, but not of TNF-α or of IL-1β.
Figure 2.
Inflammatory cytokine mRNA expression in the colon. The administration of BTA significantly suppressed the mRNA expressions of pro-inflammatory cytokines. *P < 0.05 versus normal control, †P < 0.05 versus disease control, ‡P < 0.05 versus BTA high group. BTA, betulinic acid; IL, interleukin; STG, sitagliptin phosphate monohydrate; TNF, tumor necrosis factor.
Mucosal DPPIV activity, DPP family genes expressions, and GLP concentrations in colonic tissues
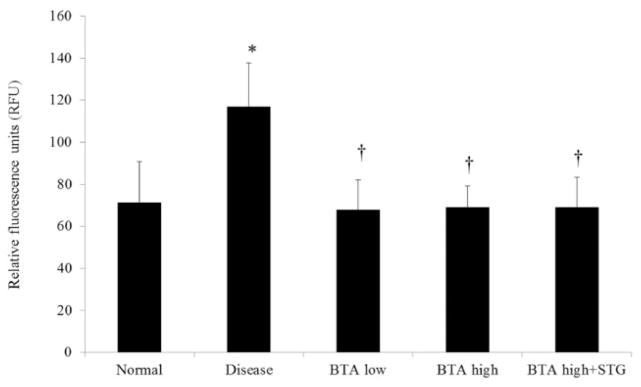
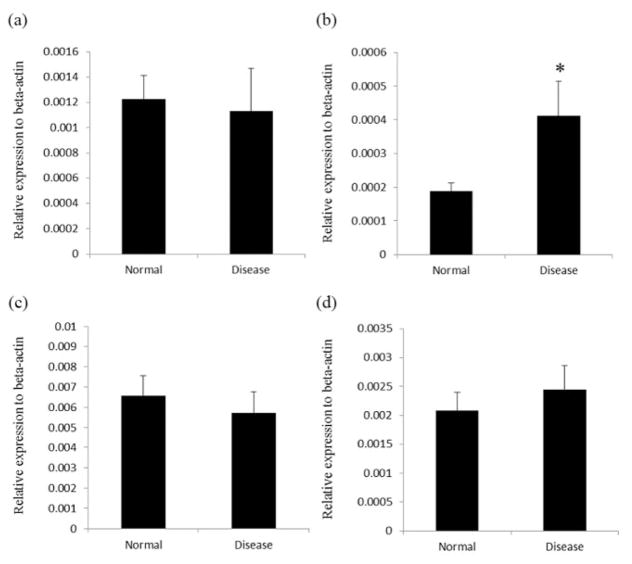
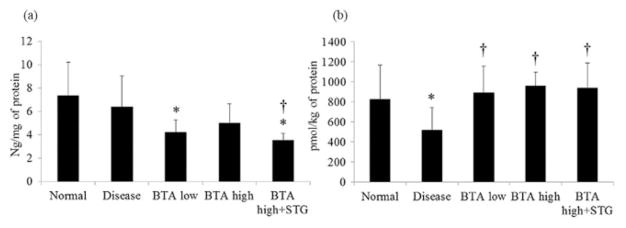
Mucosal DPPIV activity was significantly increased in mice with DSS-induced colitis (Fig. 3). The administration of BTA significantly suppressed DPPIV activity compared with disease control mice. There was no significant difference in DPPIV activity, however, between the BTA high group and the BTA high + STG group. Mucosal FAP mRNA expression was significantly increased in the disease control mice, whereas the expression of other DPP family members (DPPIV, 8, and 9) was unchanged (Fig. 4). While mucosal concentration of total GLP-2 was significantly decreased in the BTA high + STG group compared with disease control (Fig. 5a), mucosal concentration of GLP-1 (7–36) was significantly increased in the BTA-treated mice (Fig. 5b).
Figure 3.
Dipeptidyl peptidase-IV (DPPIV) activity in intestinal mucosa expressed by RFU. Mucosal DPPIV activity was significantly increased in the disease control mice. *P < 0.05 versus normal, †P < 0.05 versus disease control. BTA, betulinic acid; STG, sitagliptin phosphate monohydrate.
Figure 4.
mRNA expression of DPP family members in the colon. The expression of FAP mRNA was upregulated in the disease control mice colon. *P < 0.05 versus normal. (a) DPPIV; (b) FAP; (c) DPP8; and (d) DPP9. DPP, dipeptidyl peptidase; FAP, fibroblast activation protein.
Figure 5.
Mucosal concentration of total glucagon-like peptide (GLP)-2 (ng/mg of protein) (a) and active GLP-1 (pmol/kg of protein) (b). Although mucosal total concentration of GLP-2 was not changed, the concentration of active GLP-1 was significantly decreased in the disease control colonic tissues. *P < 0.05 versus normal, †P < 0.05 versus disease control. BTA, betulinic acid; STG, sitagliptin phosphate monohydrate.
Effect of co-administration of GLP-2 (3–33)
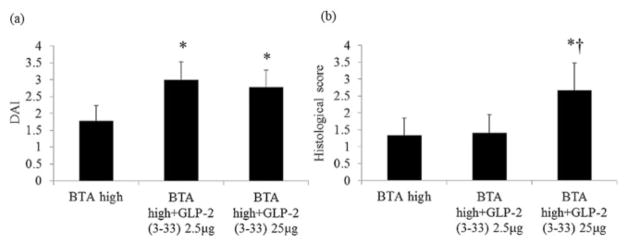
GLP-2 (3–33) was co-administered with a high dose of BTA for 7 days (2.5 or 25 μg/body/day, i.p.) to identify the effect of endogenously active GLP-2. Co-administration of GLP-2 (3–33) significantly increased DAI and histological scores compared with each of the BTA high dose-treated mice (Fig. 6), suggesting that the protective effect of BTA high dose is mediated by endogenously active GLP-2.
Figure 6.
The effect of the glucagon-like peptide (GLP)-2 receptor antagonist on betulinic acid (BTA)-induced protection. GLP-2 (3–33) increased disease activity index (DAI) (a) and histological scores (b) compared with BTA high group. *P < 0.05 versus BTA high group, †P < 0.05 versus BTA high group + GLP-2 (3–33) 2.5 μg.
Discussion
We demonstrated that the selective TGR5 agonist BTA significantly ameliorated DSS-induced colitis in mice. Co-administration of GLP-2 (3–33) reversed the protective effect of BTA, suggesting that the effect of BTA is mediated by the released GLP-2. Co-administration of a DPPIV inhibitor, STG, however did not enhance the effect of BTA.
In the small and large intestines, TGR5 is localized to the enteric ganglia of the myenteric and submucosal plexuses, and is also present in villous and crypt enterocytes and in unidentified cells such as mucosal enteroendocrine L cells.14,15 TGR5 is a class A GPCR expressed in enteroendocrine L cells localized principally in the distal small and proximal large intestines, transducing the chemical signal through the Gs subunit, which mediates cAMP synthesis and evokes GLP-1 release.16 Because TGR5 activation stimulates GLP-1 release from L cells, TGR5 is an attractive target for the potential treatment of metabolic disorders.17 With regard to the association of TGR5 and GLP-2, we have demonstrated that TGR5 agonist induced bicarbonate secretion via GLP-2 release and that DPPIV inhibition potentiated the effect of a TGR5 agonist on bicarbonate secretion.9 These results suggest that the combination of TGR5 agonists and DPPIV inhibition may be a novel approach to GLP-2-related therapeutics for intestinal mucosal injury. Because TGR5 expressing, GLP-2 releasing L cells are primarily located in the distal small intestine and colon,4,18 we focused on the effect of the TGR5 agonist on DSS-induced colitis.
Whether DPPIV inhibitors are potential therapeutic agents for inflammatory bowel disease remains conjectural because of conflicting data.19–21 Yazbeck et al. evaluated the effect of DPPIV inhibitor, Ile-Pyrr-(2-CN)TFA or Ile-Thia, in DPPIV−/− and wild type mice with DSS colitis demonstrating that disease activity was reduced in wild type mice treated with the DPPIV inhibitor but not in DPPIV−/− mice.22 DPPIV−/− mice still possessed a significant level of plasma DPPIV-like activity. Although DPPIV shares sequence homology with the enzymes FAP, DPP8, and DPP9, their expression and activities are differentially regulated.11,23 Therefore, they concluded that loss of DPPIV activity does not increase resistance to experimental colitis and hypothesized that other DPP family members may also be involved in the cleavage of GLP-2.22,24 In this study, the DPPIV inhibitor sitagliptin did not potentiate the protective effect of TGR5 in the colitic mice. Regarding GLP-2, it was interesting to observe that the mucosal concentration of GLP-2 was significantly decreased in the BTA and BTA + STG groups. BTA dose-dependently suppressed mucosal expression of pro-inflammatory cytokines, especially IL-6, which enhances GLP secretion from enteroendocrine cells. Therefore, we consider that mucosal total GLP-2 could be suppressed via a suppressed IL-6 pathway.25 In the colitic mice, mucosal DPPIV activity and FAP mRNA expression were significantly increased, and so the concentration of mucosal active GLP-1 was significantly decreased in the disease control mice and reversed in the BTA groups. Unfortunately, as no ELISA kit for active GLP-2 is available yet, we measured active GLP-1 instead of measuring active GLP-2. Because GLP-1 and GLP-2 are equally cleaved by DPPIV, we considered that a significantly decreased active GLP-1 level indicates a decreased active GLP-2 level even though the mucosal concentration of total GLP-2 was not different. Ban et al. evaluated the effect of DPPIV inhibitor ER-319711 on DSS-induced colitis in mice and reported that DPPIV inhibitor showed a minimal effect in the acute phase of colitis, and Mimura et al. reported that DPPIV inhibitor facilitates restoration of colitis in the recovery phase.26 We consider that these results are based on increased and decreased mucosal FAP-induced DPP activity. Indeed, in this study, co-administration of STG did not affect mucosal DPP activity in the colitic mice, consistent with FAP influencing DPP activity in induced colitis.
Because BTA is a bile acid-type TGR5 agonist and a naturally occurring pentacyclic triterpene,27 BTA may exhibit a variety of biological activities including anti-inflammatory and anti-tumor properties independent of the GLP-2 pathway. Actually, in this study, we administered GLP-2 (3–33), as a GLP-2 receptor antagonist, and attempted to reverse the anti-inflammatory effect of BTA. However, low dose GLP-2 (3–33) treatment did not improve the histological score.
We conclude that the TGR5 agonist protected mice from DSS-induced colitis, which was partially mediated by active GLP-2 release. Lack of potentiation by pharmacological DPPIV inhibition suggests that DPP family enzymes other than DPPIV degrade GLP-2 released by TGR5 activation.
Footnotes
Conflict of interest: No conflicts of interest exist for either of the authors of this manuscript.
References
- 1.Akiba Y, Kaunitz JD. Duodenal chemosensing and mucosal defenses. Digestion. 2011;83:25–31. doi: 10.1159/000323401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–9. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Yazbeck R, Howarth GS, Abbott CA. Growth factor based therapies and intestinal disease: is glucagon-like peptide-2 the new way forward? Cytokine Growth Factor Rev. 2009;20:175–84. doi: 10.1016/j.cytogfr.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Drozdowski L, Thomson AB. Intestinal hormones and growth factors: effects on the small intestine. World J Gastroenterol. 2009;15:385–406. doi: 10.3748/wjg.15.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci US A. 1996;93:7911–6. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–14. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katsuma S, Hirasawa A, Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–90. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 8.Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532–9. doi: 10.1016/j.cmet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoue T, Wang JH, Higashiyama M, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman MR, Sulda ML, Kuss B, Abbott CA. Dipeptidyl peptidase 8 and 9-guilty by association? Front Biosci. 2009;14:3619–33. doi: 10.2741/3476. [DOI] [PubMed] [Google Scholar]
- 11.Abbott CA, Yu DM, Woollatt E, Sutherland GR, McCaughan GW, Gorrell MD. Cloning, expression and chromosomal localization of a novel human dipeptidyl peptidase (DPP) IV homolog, DPP8. Eur J Biochem. 2000;267:6140–50. doi: 10.1046/j.1432-1327.2000.01617.x. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 13.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through a NF-kB half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knop FK. Bile-induced secretion of glucagon-like peptide-1: pathophysiological implications in type 2 diabetes? Am J Physiol Endocrinol Metab. 2010;299:E10–13. doi: 10.1152/ajpendo.00137.2010. [DOI] [PubMed] [Google Scholar]
- 15.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–25. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foord SM, Bonner TI, Neubig RR, et al. International Union of Pharmacology. XLVI. G protein coupled receptor list. Pharmacol Rev. 2005;57:279–88. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 17.Tiwari A, Miti P. TGR5: an emerging bile acid G-protein-coupled receptor target for the potential treatment of metabolic disorders. Drug Discov Today. 2009;14:523–30. doi: 10.1016/j.drudis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Eissele R, Göke R, Willemer S, et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22:283–91. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 19.Yazbeck R, Howarth GS, Geier MS, Demuth HU, Abbott CA. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci. 2008;13:6850–8. doi: 10.2741/3193. [DOI] [PubMed] [Google Scholar]
- 20.Abbott CA, Yazbeck R, Geier MS, Demuth HU, Howarth GS. Dipeptidyl peptidases and inflammatory bowel disease. Adv Exp Med Biol. 2006;575:155–62. doi: 10.1007/0-387-32824-6_16. [DOI] [PubMed] [Google Scholar]
- 21.Ban H, Bamba S, Imaeda H, et al. The DPP-IV inhibitor ER-319711 has a proliferative effect on the colonic epithelium and a minimal effect in the amelioration of colitis. Oncol Rep. 2011;25:1699–703. doi: 10.3892/or.2011.1223. [DOI] [PubMed] [Google Scholar]
- 22.Yazbeck R, Sulda ML, Howarth GS, et al. Dipeptidyl peptidase expression during experimental colitis in mice. Inflamm Bowel Dis. 2010;16:1340–51. doi: 10.1002/ibd.21241. [DOI] [PubMed] [Google Scholar]
- 23.Ajami K, Abbott CA, McCaughan GW, Gorrell MD. Dipeptidyl peptidase 9 has two forms, a broad tissue distribution, cytoplasmic localization and DPIV-like peptidase activity. Biochim Biophys Acta. 2004;1679:18–28. doi: 10.1016/j.bbaexp.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Geier MS, Tenikoff D, Yazbeck R, McCaughan GW, Abbott CA, Howarth GS. Development and resolution of experimental colitis in mice with targeted deletion of dipeptidyl peptidase IV. J Cell Physiol. 2005;204:687–92. doi: 10.1002/jcp.20333. [DOI] [PubMed] [Google Scholar]
- 25.Ellingsgaard H, Hauselmann I, Schuler B, et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17:1481–9. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mimura S, Ando T, Ishiguro K, et al. Dipeptidyl peptidase-4 inhibitor anagliptin facilitates restoration of dextran sulfate sodium-induced colitis. Scand J Gastroenterol. 2013;48:1152–9. doi: 10.3109/00365521.2013.832366. [DOI] [PubMed] [Google Scholar]
- 27.Jonnalagadda SC, Corsello MA, Sleet CE. Betulin-betulinic acid natural product based analogs as anti-cancer agents. Anticancer Agents Med Chem. 2013;13:1477–99. doi: 10.2174/18715230113129990094. [DOI] [PubMed] [Google Scholar]