Abstract
Objective
Within-person variability in cognitive performance is related to neurological integrity, but the association with functional abilities is less clear. The primary aim of this study was to examine the association between cognitive dispersion, or within-person variability, and everyday multitasking and the way in which these variables may influence performance on a naturalistic assessment of functional abilities.
Method
Participants were 156 community-dwelling adults, age 50 or older. Cognitive dispersion was calculated by measuring within-person variability in cognitive domains, established through principal components analysis. Path analysis was used to determine the independent contribution of cognitive dispersion to functional ability, mediated by multitasking.
Results
Results of the path analysis revealed that the number of subtasks interweaved (i.e., multitasked) mediated the association between cognitive dispersion and task sequencing and accuracy. Although increased multitasking was associated with worse task performance in the path model, secondary analyses revealed that for individuals with low cognitive dispersion, increased multitasking was associated with better task performance, whereas for those with higher levels of dispersion multitasking was negatively correlated with task performance.
Conclusion
These results suggest that cognitive dispersion between domains may be a useful indicator of multitasking and daily living skills among older adults.
Keywords: intraindividual variability, within-person variability, multitasking, cognitive disorders, ecological validity, activities of daily living
Introduction
Neuropsychological assessments are often conducted to identify patterns of cognitive dysfunction consistent with a specific neurological condition. Test scores are compared to indices of central tendency to estimate level of cognitive impairment relative to others in the same normative group. The results of these evaluations have been effectively used to estimate expected cognitive and functional trajectory, but there is still considerable heterogeneity in outcomes that is not accounted for by traditional measures of cognitive ability. A supplementary approach to comparing an individual’s neuropsychological test performance to mean scores of normative samples is to contrast levels of ability within an individual. Intra-individual variability has been used to describe within-person inconsistency in neuropsychological test performance in either a single assessment or multiple assessments over time.
In one method for assessing intra-individual variability, the standard deviation of all test scores is calculated and distilled into a single number representative of overall variability (Lindenberger & Baltes, 1997). Another method compares the discrepancy between crystallized ability, such as vocabulary, and other more fluid cognitive abilities such as speed and memory (Rabbitt, 1993). There is evidence that within-person variability at cross-sectional assessment, measured with either method, is positively correlated with increased age (Christensen et al., 1999), which suggests that this dispersion in abilities is relevant to cognitive aging. Given that intra-individual variability is a broad term with multiple definitions, we use the term cognitive dispersion to refer to the degree of between-domain cognitive variability demonstrated by an examinee at cross-sectional assessment.
Although patterns of relative strengths and weaknesses commonly comprise cognitive profiles (Schretlen, Munro, Anthony, & Pearlson, 2003), increased variability may be indicative of decreased neurological integrity. Studies have shown that compared to healthy controls, greater cognitive inconsistency is associated with mild cognitive impairment (MCI), mild dementia (Hultsch, MacDonald, Hunter, Levy-Bencheton, & Strauss, 2000), and dementia severity (Reckess, Varvaris, Gordon, & Schretlen, 2013), as well as with Alzheimer’s disease and Parkinson’s disease (Burton, Strauss, Hultsch, Moll, & Hunter, 2006). In addition, even older adults without a diagnosis of cognitive impairment demonstrate a higher degree of dispersion in neuropsychological test performance compared to younger adults (Anstey, 1999; Hultsch, MacDonald, & Dixon, 2002).
Although less clear, there is also evidence of a link between cognitive inconsistency and functional capacity. In one study of both community-dwelling and nursing home residing older adults, higher cognitive dispersion was associated with functional disability, as measured with the community affairs, home and hobbies, and personal care items on the Clinical Dementia Rating scale (Rapp, Schnaider-Beeri, Sano, Silverman, & Haroutunian, 2005). In another study with community dwelling elderly, self-reported disability in activities of daily living (ADLs) was related to high levels of cognitive dispersion (Christensen et al., 1999). Among individuals with HIV, cognitive dispersion was shown to be associated with medication adherence and functional dependence (Morgan, Woods, & Grant 2012). However, the specific dynamics of the association between dispersion and functional abilities remains unknown.
Conceptually, high levels of dispersion could manifest in an inability to efficiently integrate cognitive processes, which could potentially lead to decreased cognitive control and functional inefficiency. Multitasking, or simultaneously engaging in multiple activities, is ubiquitous to human behavior. Inefficient multitasking and increased cognitive dispersion share neuroanatomical substrates. Neuroanatomical evidence implicates an association between frontal lobe abnormalities and cognitive dispersion (Lovden et al., 2013; Murtha, Cismaru, Waechter, & Chertkow, 2002; Stuss, Murphy, Binns, & Alexander, 2003). Specifically, lesions to the dorsolateral prefrontal cortex (DLPFC) are associated with increased variability in cognitive performance (Stuss, et al., 2003). In addition, there is evidence that smaller DLPFC volume is related to higher cognitive dispersion (Lovden et al., 2013). Evidence suggests that the DLPFC is also critical to multitasking efficiency among older adults (Burgess, Veitch, de Lacy Costello, & Shallice, 2000). Specifically, right DLPFC lesions are associated with planning deficits, whereas the left DLPFC is implicated in both planning and prospective memory (Burgess et al., 2000). Given the evidence suggestive of a shared neuroanatomical etiology of cognitive dispersion and cognitive factors associated with multitasking efficiency, research examining the role of cognitive dispersion in multitasking ability is warranted.
One of the greatest challenges in developing an ecologically valid protocol is maximizing similarities between the experimental tasks and activities that would be completed in an individual’s everyday life, while maintaining experimental control and measurement reliability. The Day Out Task (DOT; Schmitter-Edgecombe, McAlister, & Weakley, 2012) is a naturalistic assessment of an individual’s ability to complete multiple activities necessary to prepare for a day out. Participants are instructed to multitask in a way that feels natural and is efficient. Multitasking involves simultaneously conducting more than one activity at a time. Research using the DOT has found that older adults with MCI perform more poorly on this task than healthy older adults (Schmitter-Edgecombe et al., 2012), and that healthy older adults perform worse than younger adults (McAlister & Schmitter-Edgecombe, 2013). However, neither of these studies found significant differences between groups in the average number of tasks conducted simultaneously (i.e., multitasked), which suggests that multitasking may not necessarily be a function of age or cognitive impairment. The majority of prior research has examined multitasking as the ability to complete multiple tasks without errors or omissions (e.g., Logie et al., 2011; McGeorge et al., 2001; Shallice & Burgess, 1991), but not simply the act of concurrently performing multiple tasks. As such, the cognitive correlates and role of multitasking in behavioral functioning in everyday activities remains unclear.
To assess multitasking behavior and functional ability we used the DOT, which requires participants to complete a variety of everyday activities (e.g., gather change for a bus ride) in a campus apartment. We hypothesized that the average number of simultaneously conducted tasks (i.e., multitasking) would mediate the association between cross-domain cognitive dispersion and performance-based functional ability.
Method
Participants
Participants were 156 community-dwelling adults between the ages of 50 and 85 years old (M = 66.9, SD = 8.57). Participant characteristics are presented in Table 1. Consistent with our aim to examine the influence of cognitive abilities on multitasking and functional skills on a continuum, both healthy older adults (n = 126) and individuals who met criteria for MCI (n = 30) were included in the sample. Examining healthy older adults and individuals with MCI together allows for better evaluation of cognitive and functional heterogeneity (Seligman et al., 2014). This is of particular importance when studying functional outcomes in the aging population because difficulties with instrumental activities of daily living have been found to be a better predictor of conversion to dementia than a diagnosis of MCI (Peres et al., 2008; Purser, Fillenbaum, Pieper, & Wallace, 2005).
Table 1.
Participant Characteristics (N = 156)
| Mean (SD) | Range | |
|---|---|---|
| Age | 66.9 (8.57) | 50 – 85 |
| Education | 16.06 (2.87) | 10 – 20 |
| Sex (% female) | 76.3% | |
| Shipley | 34.91 (3.43) | 25 – 40 |
| TICS (n = 152) | 34.75 (2.54) | 26 – 41 |
| GDS (n = 149) | 1.8 (2.05) | 0 – 10 |
| MCI (n = 30) | 19.2% |
Note. TICS = Telephone interview of cognitive status; GDS = Geriatric Depression Scale; MCI = mild cognitive impairment
Participant recruitment was primarily conducted through advertisements, health and wellness fairs, physician referrals, and from prior studies in our laboratory. This study was part of a larger study investigating the relationship between cognition and everyday functioning in the aging population (Schmitter-Edgecombe, Parsey & Cook, 2011). A subset of participants in this study also participated in one of the following studies with the DOT (McAlister & Schmitter-Edgecombe, 2013; Schmitter-Edgecombe et al., 2012). Participants were initially screened over the telephone with a medical interview to identify relevant medical conditions, the Clinical Dementia Rating scale (CDR) to screen for the presence of dementia (Hughes, Berg, Danzinger, Coben, & Martin, 1982; Morris, 1993), and the Telephone Interview of Cognitive Status (TICS; Brandt & Folstein, 2003) to provide an initial estimate of cognitive functioning. Exclusion criteria for the current study included evidence of dementia by diagnosis or cognitive assessment, a CDR greater than or equal to 1, a TICS score below 26, or the presence of a medical, neurological, or psychiatric condition with known cognitive effects (e.g., history of head injury with permanent brain lesion, brain surgery, stroke, Parkinson’s disease). Participants who endorsed greater than 10 symptoms on the short-form of the Geriatric Depression Scale (Sheikh & Yesavage, 1986), suggestive of significant depression, were also excluded. Participants were classified with MCI using established criteria (Petersen et al., 2001; Petersen & Morris, 2005). These criteria included self or informant report of subjective memory impairment for at least six months, scoring at least 1.5 standard deviations below age-matched norms or relative to prior testing on a measure in one or more cognitive domains (i.e., memory, language, executive functioning and/or speeded processing), generally preserved functional abilities as indicated by a CDR score (n = 24) of 0.5 (66.7%) or less (33.3%), and not meeting DSM-IV criteria for dementia (American Psychiatric Association, 2000). A language measure was evaluated in the classification of MCI participants but was not selected as a variable in the current study because at least three variables per domain are recommended for the proposed analyses. To determine diagnostic classification, two experienced neuropsychologists reviewed participant and informant interview data, neuropsychological results, and medical information when available. Participants with amnestic and nonamnestic and both single (n = 14) and multi-domain MCI (n = 16) were included in the sample. The Institutional Review Board at Washington State University approved this research protocol and all participants provided informed consent.
Procedures and Assessments
All participants underwent a neuropsychological assessment and a performance-based assessment of everyday living skills on separate days, approximately one-week apart, with each assessment lasting approximately three hours. The performance-based assessment was conducted in a two-story apartment on the WSU campus. The first floor has a full kitchen, living room, and dining room with task-related items placed in specific locations (e.g., cabinets, closets, refrigerator) throughout the apartment. The examiners provide instructions from the second floor of the apartment through an intercom system and monitor the participant’s progress in the task through a live video stream. Participants completed other tasks in the apartment (e.g., sweeping, dusting, preparing oatmeal) prior to the DOT. Further details of the measures used in this study are provided below.
The Shipley Institute of Living Scale—vocabulary subtest was used to characterize estimated verbal intellectual ability. The following cognitive tests were used to characterize cognitive performance: the list learning subtests from the Memory Assessment Scale (MAS; Williams, 1991); Trail Making Test, Parts A and B (Reitan, 1992); written and oral versions of the Symbol Digit Modalities Test (SDMT; Smith 1991); CLOX 1 (Royall, Cordes, & Polk, 1998); Letter-Number Sequencing (LNS; Wechsler, 1997); a temporal order sequencing task (Schmitter-Edgecombe, Woo, & Greeley, 2009); and the Zoo map subtest from the Behavioral Assessment of the Dysexecutive Syndrome (BADS; Wilson, Alderman, Burgess, Emslie, & Evans, 1996). In addition, a Letter-Number Span task was developed to assess auditory short-term memory. With the exception of Trails B, raw scores were used in all of the analyses. To minimize the processing speed component of Part B, Trails B was regressed on Trails A and the standardized residual score was used for analysis. Descriptions of each test, along with the specific scores used in the data analyses, are presented in Table 2.
Table 2.
Detailed Description of Neuropsychological Tests
| Test | Description |
|---|---|
| Memory Assessment Scale: List Learning | Participants are read a list of 12 words from four semantic categories. The list is read a maximum of six times or until the participant recalls all 12 words. Participants are then asked to recall the list after a short and long delay. The total number of words recalled is the raw score for each MAS list learning measure (i.e., list acquisition, short delay, long delay). |
| Trail Making Test | In Part A of the Trail Making Test, participants are instructed to sequentially connect 26 encircled numbers in ascending order (i.e., 1—2—3…). In Part B, the participant connects 26 circles alternating between numbers and letters (i.e., 1—A—2—B…). Time to complete each part was recorded. |
| Symbol Digit Modalities Test | Participants are instructed to match a series of symbols to the corresponding number in the key at the top of the page. Both written and oral versions were used. The number of accurate responses in 90 seconds was recorded. |
| CLOX 1 | Participants are given a piece of paper with a circle on it and instructed to draw a clock with the clock hands set to a specified time. The total correct score from Clox I was used. |
| Letter-Number Sequencing | Participants are instructed to sequentially order a set of orally presented letters and numbers, which increase in length across blocks. The task is discontinued if the participant provides the incorrect response for all three trials in a block. The number of correct trials is the total score. |
| Letter-Number Span | Participants are read a series of letters and numbers and instructed to repeat the numbers and letters in the order given. The task is discontinued after three consecutive incorrect answers in a block of trials. The total score represents the number of correct trials. |
| Temporal Order | After completing a series of eight neuropsychological tests, participant are asked to recall the tasks and then are provided with eight cards, each with a description of one of the tasks that they had completed. Participants are told to arrange the cards in the order they completed the tasks. The total number of correctly ordered cards was used as a measure of temporal order sequencing. |
| BADS Zoo Map | Participants are presented with a map of a zoo and instructed to draw a route connecting key locations without violating any of the rules provided. The profile score (range 0 – 4) represents the sequence locations, errors, and time to complete the task. |
Day Out Task: (DOT; Schmitter-Edgecombe et al., 2012)
The DOT comprises a set of activities that participants are instructed to complete to prepare for a day out. Participants are given a written list of the activities to perform in order to be prepared for a day out (e.g., planning a bus route to the museum, gathering the correct amount of change needed for the bus, taking a pill before leaving) and later traveling to a friend’s house for dinner. Participants were instructed to multitask and interweave the tasks in a way that is natural and efficient. Performance was observed through a live video feed by two examiners, blinded to the hypotheses of the current study, who recorded the time each subtask began and ended, tasks conducted simultaneously, and tasks completed. Two independent coders, who were also blinded to study hypotheses, later reviewed the video recordings and applied standardized criteria for scoring each of the eight subtasks as efficient, inefficient, incomplete or not attempted. These DOT subtask scores were averaged to create an overall DOT accuracy score. A task sequencing score, which represented the total number of a possible six activities that were correctly sequenced (e.g., determined cost for bus prior to retrieving change), was also derived using standardized criteria (see Appendix A for DOT scoring criteria). The DOT accuracy score and sequencing scores were used as the outcome measures in the path analysis.
The DOT has shown to effectively differentiate younger adults from older adults (McAlister & Schmitter-Edgecombe, 2013), as well as cognitively intact older adults from older adults with MCI (Schmitter-Edgecombe et al., 2012). In addition, the DOT scores have been shown to be related to specific cognitive abilities (e.g., memory) and knowledgeable informant reports of instrumental activities of daily living (Schmitter-Edgecombe et al., 2012).
Multitasking
The time at which each of the eight subtasks was initiated and stopped was recorded in order to quantify the number of other subtasks that overlapped within the same timeframe. For each specific subtask, the number of other subtasks that were started or ongoing during performance of the specific subtask was recorded. This number was then averaged across the eight subtasks to form the multitasking variable.
Statistical Analyses
A principal components analysis (PCA) with a varimax rotation was used to derive composite cognitive scores from the 12 neuropsychological measures. This technique was utilized to reduce the number of variables used in further analyses and to establish more stable estimates of cognitive ability. Several measures were considered for inclusion in the PCA, with the intention of creating four domains, each with at least three items, which is recommended for a stable factor. Items with component loadings greater than or equal to .4 were retained. Components with an eigenvalue greater than or equal to 1 were extracted (Kaiser, 1960). Calculating within-person standard deviation between cognitive domains created the dispersion variable. A composite neuropsychological score (NP global), representing an overall level of cognitive ability, was calculated by summing the four components extracted in the PCA. Correlation analyses were conducted to examine the association between cognitive domains and DOT performance. Path analysis was used to simultaneously test the indirect effect of dispersion on DOT variables, mediated by multitasking while accounting for the influence of NP global score. Bias-corrected bootstrapping (2,000 samples) with a 90% confidence interval was used to determine indirect effect of dispersion on DOT accuracy and sequencing, mediated by multitasking. Model fit was evaluated using multiple indices recommended by Hu and Bentler (1999) including (1) the comparative fit index (CFI), (2) the root mean square error of approximation (RMSEA), (3) the normed fit index (NFI), and (4) chi-squared test for lack of fit. The sample size for the current study (N = 156) is within the acceptable range for the recommended minimum sample size of 100 to 150, or 5 cases per parameter to be estimated, for structural models (Bentler & Chou, 1987; Ding, Velicer, & Harlow, 1995). In order to identify the most parsimonious model, coefficients were allowed to be freely estimated and then nonsignificant paths were constrained to zero.
Results
The results from the principal components analysis and mean cognitive test scores are presented in Table 3. The Kaiser-Meyer-Olkin measure of sampling adequacy was .75 (greater than .5 is recommended; Kaiser, 1974), which indicates that the sample size is adequate for PCA. Bartlett’s test of sphericity was significant, χ2 (66) = 785.04, p < .001, which indicates that between item correlations are sufficient for PCA. The Varimax rotation produced a four-factor solution, which accounted for 67.5% of the total variance. The four components extracted were considered to represent the following cognitive domains: Memory (MAS list acquisition, short recall, delayed recall), Processing Speed (TMT A, SDMT written, SDMT oral), Executive Functions (TMT B residual, Clox 1, BADS zoo map), and Working Memory (L-N Span, L-N Sequencing, Temporal Order memory).
Table 3.
Factor Loadings for Principal Components Analysis With Varimax Rotation of Cognitive Measures
| Measure | Mean | SD | Memory | Speed | Executive | Working Mem. |
|---|---|---|---|---|---|---|
| MAS long delay | 10.85 | 2.02 | .909 | .085 | .060 | .103 |
| MAS short delay | 10.46 | 2.19 | .892 | .153 | .113 | .057 |
| MAS list acquisition | 59.59 | 7.59 | .830 | .128 | .171 | .155 |
| TMTA inverse | 30.53 | 9.32 | −.035 | .819 | −.051 | .123 |
| SDMT oral | 55.94 | 10.52 | .327 | .771 | .300 | .176 |
| SDMT written | 49.03 | 9.37 | .300 | .747 | .307 | .222 |
| TMTB residual inverse | 75.34 | 31.83 | .227 | .104 | .700 | −.011 |
| CLOX 1 | 12.90 | 1.91 | −.085 | .188 | .643 | .003 |
| Zoo Map | 2.31 | 1.09 | .193 | −.006 | .618 | .176 |
| Letter-Number Span | 12.27 | 2.54 | .092 | .132 | .248 | .814 |
| Temporal Order | 2.76 | 1.34 | .069 | .115 | −.251 | .677 |
| Letter Number Sequencing | 10.53 | 2.72 | .178 | .245 | .356 | .632 |
| Eigenvalue | 4.26 | 1.59 | 1.21 | 1.03 | ||
| % of variance | 35.63 | 13.29 | 10.09 | 8.57 |
Note. MAS = Memory Assessment Scale; TMT = Trail Making Test; SDMT = Symbol Digit Modalities Test; Component loadings greater than .4 are bolded.
The correlation matrix for the dispersion variable and the cognitive and DOT variables is presented in Table 4. Dispersion (M = .887, SD = .457, range = 0.22 – 2.74) was significantly associated with the DOT multitasking (M = 3.69, SD = 1.07, range = 1.50 – 6.50), accuracy (M = 2.71, SD = .82, range = 1 – 5) and sequencing (M = 3.85, SD = 1.00, range = 1 – 6) variables. The NP Global score also correlated with all DOT measures. The dispersion variable was significantly correlated with the memory and processing speed components, but not with working memory or executive functions components extracted from the PCA. Multitasking correlated with the dispersion variable but not with any of the other cognitive domains.
Table 4.
Correlations Between Cognitive and Day Out Task Variables
| Dispersion | MEM | PS | EXE | WM | NP Global | |
|---|---|---|---|---|---|---|
| Multitasking | .271** | −.091 | −.051 | −.103 | −.102 | −.174* |
| Accuracy | .253** | −.266** | −.099 | −.141 | −.100 | −.303*** |
| Sequencing | −.184* | .078 | .050 | .135 | .176* | .220** |
| Dispersion | - | −.312*** | −.225** | −.125 | .099 | −.281*** |
Note. MEM = memory; PS = information processing speed; EXE = executive functioning; WM = working memory.
p < .05;
p < .01;
p < .001
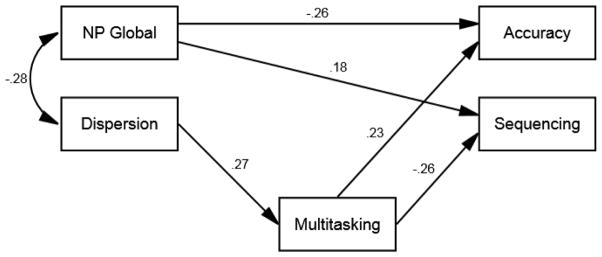
The final model was specified to estimate the direct effect of dispersion on multitasking and the indirect effect of dispersion on DOT sequencing and accuracy, mediated by multitasking. The direct effect of the NP global score on DOT accuracy and sequencing was included in the model to identify the contribution of dispersion that is independent of traditional cognitive variables. This method was utilized because the primary aim of this study was to examine the role of dispersion and multitasking on functional performance, while accounting for the potential influence of global level of cognitive impairment. The results of the path model are presented in Figure 1. The fit indices indicate an adequate fit for the data, χ2(4, N = 156) = 5.568, p = .234, RMSEA = .050, CFI = .975, NFI = .924.
Figure 1.
Path model of direct and indirect effects of cognitive variables on DOT variables. Note: To simplify model presentation, error terms are not shown. Higher sequencing and lower accuracy scores indicate better performance.
Dispersion was inversely correlated with NP global score, such that better cognitive performance was related to lower between domain variability. Dispersion between cognitive domains had a significant direct effect on multitasking, such that higher dispersion was related to more tasks being interweaved (i.e., multitasked). Dispersion had a significant indirect effect, through multitasking, on DOT accuracy, standardized coefficient = .063, SE = .032 (90% CI .020, .132), p = .002, and sequencing, standardized coefficient = −.071, SE = .029 (90% CI −.132, −.032), p = .001. This indicates that multitasking fully mediated the association between dispersion and the DOT variables. Specifically, more multitasking was associated with poorer task sequencing and lower task accuracy.
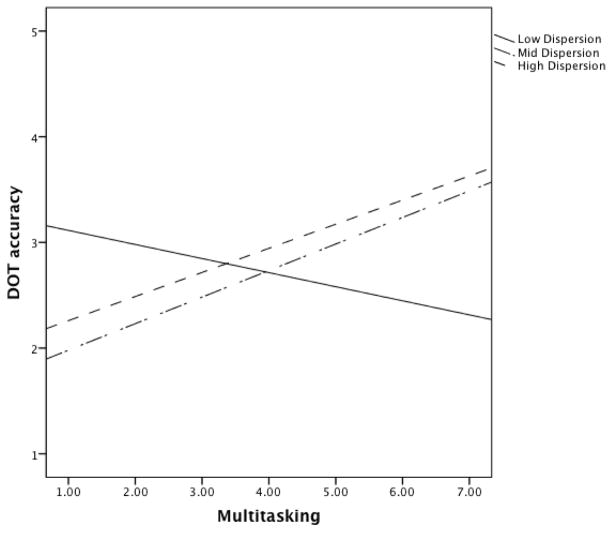
Follow-up analyses were conducted to further examine the dynamic association between dispersion, multitasking, and DOT performance. To determine whether the association between more multitasking and poorer DOT performance was a function of dispersion level, the dispersion variable was standardized (i.e., sample z-scores) and used to divide the sample into three groups based on standardized dispersion values less than one standard deviation (low dispersion; n = 15; 13 non-MCI, 2 MCI), within one standard deviation (mid dispersion; n = 115; 98 non-MCI, 17 MCI), and above one standard deviation (high dispersion; n = 26; 15 non-MCI, 11 MCI). Regression slopes are presented in Figure 2. As shown in Figure 2, for the low dispersion group, more multitasking is related to better DOT accuracy. Whereas, for the mid and high dispersion groups more multitasking is related to progressively worse overall DOT accuracy. It is important to note that a lower DOT accuracy score indicates more efficient subtask completion and a higher DOT sequencing score indicates better performance.
Figure 2.
Regression slopes of Day Out Task accuracy as a function of dispersion level and multitasking. Note: A lower DOT accuracy score indicates more efficient task completion.
Secondary analyses were conducted excluding MCI participants to determine the extent to which the MCI group influenced the results. The principal components analysis with only non-MCI participant data yielded the same four-component structure, with loadings greater than .600 on the respective domains, with no cross-loadings greater than .4. The fit indices for the path model excluding MCI participants provided a slightly lower fit for the data, χ2(4, N = 126) = 5.510, p = .239, RMSEA = .055, CFI = .950, NFI = .864. All path estimates remained significant, with the exception of the direct effect of the NP Global score on DOT accuracy (standardized estimate = −.152, p = .083). Of note, and central to the hypothesis of the current study, the path estimate from dispersion to multitasking in this analysis was nearly identical to the model that included MCI participants (standardized estimate = .282, p = .001). All of the indirect effects, mediated by multitasking, remained significant. These results suggest that the inclusion of MCI participants did not substantially bias the results from the primary analyses.
Discussion
The primary goal of this investigation was to examine the behavioral manifestation of between domain cognitive dispersion. Although performance on functional tasks has provided important information regarding real-world functional abilities of older adults, the cognitive mechanisms of functional impairment are not well understood. In this study, we examined cognitive dispersion as a potential mechanism of poorer functional abilities among community-dwelling older adults using the DOT. The results indicate that cognitive dispersion may result in increased multitasking, which mediates functional task performance. Specifically, higher cognitive dispersion was associated with more simultaneous tasks, which predicted poorer task sequencing and accuracy. The results of the secondary analyses indicated that excluding the participants with MCI did not substantially change the association between dispersion and multitasking, or the mediated indirect effects.
The ability to simultaneously complete multiple tasks requires efficient integration of cognitive processes necessary for each task individually, as well as ongoing cognitive control to follow an efficient sequence. Several studies have examined multitasking by examining accuracy of completion of each of the multiple tasks (e.g., Logie et al., 2011; McGeorge et al., 2001; Shallice & Burgess, 1991). In the current study, we extend previous research by utilizing a relatively unbiased measure of multitasking (i.e., number of subtasks being performed simultaneously) that was not part of the scoring criteria for the DOT. Overall, more multitasking was associated with poorer task sequencing and accuracy. However, further analyses revealed that individuals with low levels of dispersion performed better on the DOT if they multitasked more, whereas more multitasking among individuals with higher levels of dispersion was related to incrementally worse DOT performance.
Low dispersion may reflect more efficient integration of cognitive processes, allowing for more efficient completion of complex everyday activities. It may also be the case that individuals with lower levels of dispersion are better able to manage the cognitive load imposed by simultaneously performing multiple tasks. Conversely, for individuals with higher levels of dispersion more multitasking was associated with poorer DOT performance. It remains unclear as to whether dispersion manifests in simultaneously initiating but not completing and/or inaccurately or inefficiently completing multiple activities or whether an individual’s ability to monitor and track their activities has declined in such a way that the individual can no longer simultaneously manage multiple subtasks. These findings suggest that for individuals with low dispersion multitasking is associated with increased behavioral efficiency, whereas multitasking may be a manifestation of increased cognitive variability and/or lead to inefficient functional performance among individuals with higher levels of dispersion.
Although this study provides some insight into the mechanisms of functional inefficiency among older adults when completing an open-ended, complex, multi-component task, important limitations must be addressed. Notably, this sample was predominantly of Caucasian descent, well educated, with a relatively high level of literacy. This demographic homogeneity limits the ability to generalize to other populations. More research will be needed to determine whether these results remain consistent among more ethnically and educationally diverse populations, or whether these results will replicate with other neurologic populations. Also, although the results revealed a statistically significant indirect effect of dispersion on DOT variables, mediated by multitasking, the effect only accounted for a relatively small amount of the variance, which suggests that there are other important factors that contribute to functional ability. In addition, although the DOT was developed to provide a more ecologically valid method of assessing everyday living skills certain limitations must be addressed. In the DOT the participant is provided with a written list of the tasks to be completed, which does not necessarily replicate everyday situations, unless the individual typically makes “to do” lists. Participants were also instructed to multitask in a way that feels natural and is efficient; however, this may not be the way in which the participant typically completes multiple tasks in their everyday life. Furthermore, the tasks were completed in a testing environment to maximize standardization; it is unclear whether the findings would be similar if the task was performed in the familiar setting of the participants own home.
The neuropsychological tests evaluated in this work were limited by the battery of tests used in this study and are not without limitations. The neuropsychological tests used are validated measures of cognitive abilities, but the extent to which these tests can be used to predict performance in everyday tasks has not been well established. In addition, the executive functioning, working memory, and processing speed components comprised different measures, but the memory domain utilized three scores from a single measure (i.e., MAS list acquisition, short delay, and long delay). As such, the component loadings for the memory domain were higher than those in the other domains. However, the component loadings for the derived cognitive domains were all within an acceptable range and indicative of discriminable constructs. An examination of the cognitive component scores revealed that the memory and speed domains showed the strongest correlation with the dispersion variable, which suggests that declines in these domains may be important contributors to increased cognitive variability in advancing age and in MCI. Using dispersion between domains, rather than between individual tests, allows for an increased understanding of the dynamics of the way in which different cognitive abilities interact to influence behavior. However, more research is needed to identify whether these findings will replicate using a different set of neuropsychological tests.
In summary, the results of this study suggest that overall cognitive ability may influence functional efficiency in a naturalistic setting, whereas cognitive inefficiency, as measured by dispersion, may influence multitasking. In the path model, higher dispersion was related to more multitasking, which was associated with poorer DOT performance. However, the mediatory effect of multitasking on DOT performance was contingent on the level of dispersion. Overall, the results suggest that while multitasking may improve functional efficiency in individuals with lower levels of dispersion, older adults with higher levels of dispersion may benefit from multitasking less. As such, within-person cross-domain dispersion may be a useful index of behavioral efficiency. If the results of a neuropsychological assessment reveal a high level of dispersion, it might be useful to instruct the patient to avoid multitasking, and instead, focus on completing one task at a time. Taken together, these findings may help inform clinician directives when making recommendations for older adults based on cognitive testing results.
Acknowledgments
This study was partially supported by grants from the Life Science Discovery Fund of Washington State; NIBIB (Grant R01 EB009675); and NSF (Grant DGE-0900781). No conflicts of interest exist. We thank members of the Aging and Dementia laboratory for their help in collecting and scoring the data. We also thank Chad Sanders, Alyssa Weakley, and Jennifer Walker for their assistance in coordinating data collection.
Appendix A
Scoring Criteria for the Day Out Task
|
Subtask Completion Scores Each of the 8 subtasks is assigned one of the following scores.
|
|
Total Accuracy Score Summation of the subtask completion scores (see above0 for each of the 8 subtasks (range = 8 – 32). |
|
Task Sequencing Score Total number of the six activities below correctly sequenced (range = 0 – 6).
|
Contributor Information
Robert P. Fellows, Department of Psychology, Washington State University
Maureen Schmitter-Edgecombe, Department of Psychology, Washington State University.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text revision. [Google Scholar]
- Anstey KJ. Sensorimotor variables and forced expiratory volume as correlates of speed, accuracy, and variability in reaction time performance in late adulthood. Aging, Neuropsychology, and Cognition. 1999;6:84–95. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Chou CP. Practical issues in structural modeling. Sociological Methods & Research. 1987;16:78–117. [Google Scholar]
- Brandt J, Folstein M. Telephone Interview for Cognitive Status. Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- Burgess PW, Veitch E, DeLacy Costello A, Shallice T. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848–863. doi: 10.1016/S0028-3932(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Moll A, Hunter MA. Intraindividual variability as a marker of neurological dysfunction: A comparison of Alzheimer’s Disease and Parkinson’s Disease. Journal of Clinical and Experimental Neuropsychology. 2006;28:67–83. doi: 10.1080/13803390490918318. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P. Dispersion in Cognitive Ability as a Function of Age: A Longitudinal Study of an Elderly Community Sample. Aging, Neuropsychology, and Cognition (Neuropsychology, Development and Cognition: Section B) 1999;6(3):214–228. doi: 10.1076/anec.6.3.214.779. [DOI] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System: Examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Ding L, Velicer WF, Harlow LL. Effects of estimation methods, number of indicators per factor and improper solutions on structural equation modeling fit indices. Structural Equation Modeling. 1995;2(2):119–143. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6(1):1–55. [Google Scholar]
- Hughes CP, Berg L, Danzinger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Dixon RA. Variability in reaction time performance of younger and older adults. Journal of Gerontology, Series B: Social Sciences. 2002;57:101–115. doi: 10.1093/geronb/57.2.p101. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, Hunter MA, Levy-Bencheton J, Strauss E. Intraindividual variability in cognitive performance in the elderly: Comparison of adults with mild dementia, adults with arthritis, and healthy adults. Neuropsychology. 2000;14:588–598. doi: 10.1037//0894-4105.14.4.588. [DOI] [PubMed] [Google Scholar]
- Kaiser HF. An index of factor simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]
- Lindenberger U, Baltes PB. Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging. 1997;12(3):410–432. doi: 10.1037//0882-7974.12.3.410. [DOI] [PubMed] [Google Scholar]
- Logie RH, Law A, Trawley S, Nissan J. Multitasking, working memory and remembering intentions. Psychologica Belgica. 2010;50:309–326. [Google Scholar]
- Logie RH, Trawley S, Law A. Multitasking: multiple, domain-specific cognitive functions in a virtual environment. Memory and Cognition. 2011;39(8):1561–1574. doi: 10.3758/s13421-011-0120-1. [DOI] [PubMed] [Google Scholar]
- Lovden M, Schmiedek F, Kennedy KM, Rodrigue KM, Lindenberger U, Raz N. Does variability in cognitive performance correlate with frontal brain volume? Neuroimage. 2013;64:209–215. doi: 10.1016/j.neuroimage.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister, Schmitter-Edgecombe Naturalistic assessment of executive functions and everyday multitasking in healthy older adults. Aging, Neuropsychology, and Cognition. 2013;20(6):735–756. doi: 10.1080/13825585.2013.781990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge P, Phillips LH, Crawford JR, Garden SE, Della Sala S, Milne AB. Using virtual environments in the assessment of executive dysfunction. Presence. 2001;10:375–383. [Google Scholar]
- Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Murtha S, Cismaru R, Waechter R, Chertkow Increased variability accompanies frontal lobe damage in dementia. Journal of the International Neuropsychological Society. 2002;8(3):360–372. doi: 10.1017/s1355617702813170. [DOI] [PubMed] [Google Scholar]
- Peres K, Helmer C, Amieva H, Orgogozo JM, Rouch I, Dartigues JF, Barberger-Gateau P. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: A prospective population-based study. Journal of the American Geriatric Society. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, … Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. [Comment Research Support, N.I.H., Extramural Research Support, U.S. Gov’t, P.H.S.Review] Archives of Neurology. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- Purser JL, Fillenbaum GG, Pieper CF, Wallace RB. Mild cognitive impairment and 10-year trajectories of disability in the Iowa Established Populations for Epidemiologic Studies of the Elderly cohort. Journal of the American Geriatric Society. 2005;53:1966–1972. doi: 10.1111/j.1532-5415.2005.53566.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt P. Does it all go together when it goes? The Nineteenth Bartlett Memorial Lecture. Quarterly Journal of Experimental Psychology A, Human experimental psychology. 1993;46(3):385–434. doi: 10.1080/14640749308401055. [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, Haroutunian V. Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: Relationship to functional status. Gerontology. 2005;51:206–212. doi: 10.1159/000083995. [DOI] [PubMed] [Google Scholar]
- Reckess GZ, Varvaris M, Gordon B, Schretlen DJ. Within-person distributions of neuropsychological test scores as a function of dementia severity. Neuropsychology. 2014;28(2):254–260. doi: 10.1037/neu0000017. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Trail making test: Manual for administration and scoring. Tucson, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- Royall DR, Cordes JA, Polk M. CLOX: An executive clock drawing task. Journal of Neurology, Neurosurgery, and Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: The Freedom House Study. Journal of the American Geriatric Society. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- Sanders C, Low C, Schmitter-Edgecombe M. Assessment of planning abilities in individuals with mild cognitive impairment using and open-ended problem-solving task. Journal of Clinical and Experimental Neuropsychology. doi: 10.1080/13803395.2014.983462. in press. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, McAlister C, Weakley A. Naturalistic assessment of everyday functioning in individuals with mild cognitive impairment: The day out task. Neuropsychology. 2012;26:631–641. doi: 10.1037/a0029352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168–177. doi: 10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, Pearlson GD. Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society. 2003;9:864–870. doi: 10.1017/S1355617703960061. [DOI] [PubMed] [Google Scholar]
- Seligman SC, Giovannetti T, Sestito J, Libon DJ. A new approach to the characterization of subtle errors in everyday action: implications for mild cognitive impairment. The Clinical Neuropsychologist. 2014;28(1):97–115. doi: 10.1080/13854046.2013.852624. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986. pp. 165–173. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles, CA: 1991. [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Test. 3. New York, NY: The Psychological Corporation; 1997. [Google Scholar]
- Williams JM. Memory Assessment Scales professional manual. Odessa: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Wilson Alderman, Burgess Emslie, Evans . Behavioural assessment of the dysexecutive syndrome. Bury St Edmunds: Thames Valley Test Company; 1996. [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey MB, et al. Development and validation of a geriatric depression rating scale: A preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]