Abstract
Objectives
KRAS mutations are the most commonly found mutations in patients with non-small cell lung cancer (NSCLC) adenocarcinoma histology. The clinical implications of KRAS mutations in patients with advanced NSCLC are not well defined. We sought to determine if there is a correlation between KRAS mutation status, response to cytotoxic chemotherapy, and survival in patients with metastatic or recurrent NSCLC.
Materials and Methods
Patients with metastatic or recurrent NSCLC and tumor mutation analyses were analyzed for response to conventional chemotherapy. The presence or absence of tumor mutations was assessed with the SNaPshot assay, which detects >40 somatic mutations in eight genes, including KRAS. ALK fluorescence in-situ hybridization analysis was done separately. Associations between KRAS mutation status and response to chemotherapy and survival were assessed.
Results
We identified 80 patients with metastatic or recurrent NSCLC and a KRAS activating mutation, and we compared these patients to 70 patients who were pan negative (no detectable mutation by the SNaPshot assay and ALK negative). Patients with KRAS-mutant advanced NSCLC demonstrated a significantly shorter progression-free survival in response to first line chemotherapy (4.5 months versus 5.7 months, p = 0.008) compared to pan-mutation negative patients. Overall survival was also significantly shorter in patients with KRAS-mutant advanced NSCLC compared to patients without KRAS activating mutations (8.8 months versus 13.5 months, p = 0.038).
Conclusions
Within this single institution retrospective analysis, patients with advanced NSCLC and a KRAS activating mutation exhibited inferior responses to cytotoxic chemotherapy and decreased survival compared to patients with advanced NSCLC and no KRAS mutation.
Keywords: Non-small cell lung cancer, KRAS, metastatic, chemotherapy
1.1 Introduction
In 2015, it is estimated that there will be 221,200 Americans diagnosed with lung cancer, and 158,040 people will die of the disease, making this disease the leading cause of cancer-related mortality in the United States [1]. Non-small cell lung cancer (NSCLC) comprises 80-85% of patients with lung cancer, and small cell lung cancer (SCLC) comprises 10-15% of all lung cancer cases [1]. Within NSCLC there are three main histologic subtypes: adenocarcinoma (∼40%), squamous cell carcinoma (∼25%), and large cell carcinoma (∼10%) [1-3].
Approximately 50% of NSCLC tumors have an identified single mutated oncogene which exerts a primary role in its pathogenesis. To date, the two most commonly mutated oncogenes in patients with NSCLC are epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma viral oncogene (KRAS) [4]. EGFR mutations occur with increased frequency in patients that have never smoked; KRAS mutations occur more commonly in patients who have had significant tobacco exposure [5,6]. Both mutations are found predominantly in patients with lung adenocarcinoma [7,8,9]. Patients with EGFR mutations have demonstrated better overall survival (OS) than patients with KRAS mutations [8-12]. Patients with NSCLC with EGFR-activating mutations have the potential to be treated with FDA-approved targeted therapies, including erlotinib and afatinib [5,6,13,14].
Recently there has been a movement within oncology to personalize anti-cancer therapy on the basis of tumor genotypes in order to provide enhanced prognostic and treatment planning. Despite the frequency of KRAS mutations in patients with NSCLC, data are conflicting regarding the impact these mutations have on treatment response and patient outcomes. In patients receiving adjuvant chemotherapy and radiotherapy for primary resected stage II and IIIA NSCLC, a subset analysis showed there was a trend toward improved OS in patients without KRAS mutations compared to patients with a KRAS mutation [15]. Smaller studies have failed to demonstrate a consistent prognostic implication of KRAS mutation status in patients with advanced NSCLC. Rodenhuis and colleagues reviewed KRAS mutation status in patients receiving mesna, ifosfamide, carboplatin, and etoposide (MICE) chemotherapy. This trial showed no difference in progression free survival (PFS) or OS in patients with KRAS mutations compared to patients with no KRAS mutation [16]. In a more recent study by Camps, et al., patients with advanced NSCLC and codon 12 KRAS mutations were compared to patients with KRAS wild type tumors. This trial also showed that median OS was similar across all KRAS genotype groups [17]. Similarly, multiple studies have concluded the presence of a KRAS tumor mutation is not predictive of worse progression-free or overall survivalin advanced NSCLC patients treated with platinum-based chemotherapy in the first or second line settings [18-21]. However, the data from these analyses contrast to the study by Metro and colleagues in which patients with KRAS mutations were shown to have a decreased PFS and OS in response to first line platinum-based chemotherapy [22].
With the current conflicting evidence regarding treatment prognosis in patients with KRAS-mutant NSCLC, we sought to determine if there is a correlation between KRAS mutation status and response to first-line cytotoxic chemotherapy in patients with metastatic or recurrent NSCLC within our single institution cohort.
1.2 Materials and Methods
1.2.1 Study Subjects/Design
This was a retrospective study using an IRB approved protocol (IRB# 121671) which reviewed the medical records of adult patients with metastatic or recurrent NSCLC who were seen at Vanderbilt-Ingram Cancer Center (VICC) and had molecular tumor profiling available (SNaPShot assay, sizing assay) between the dates of July 1, 2010 and December 31, 2013. The practice at VICC is to assess for >40 different mutations in 9 oncogenes/tumor suppressor genes implicated in lung cancer pathogenesis using multiplex polymerase chain reaction (PCR) and sizing assay on tumor samples. In addition, chromosomal translocations involving the ALK gene are assessed by fluorescence in-situ hybridization (FISH). We retrospectively reviewed medical records to correlate tumor molecular profiling data with clinical and pathologic features. Inclusion criteria for this study were any adult (age 18 years or older) patient with recurrent or metastatic NSCLC seen at VICC who had received mutational testing (SNaPShot assay, sizing assay, ALK FISH). Patients were excluded from this analysis if they did not have histologically proven NSCLC, if they did not receive mutational testing at VICC, or if they had any non-KRAS mutation on the SNaPShot assay, sizing assay, or ALK FISH analysis.
A comparative analysis was used to evaluate differences between patients with tumors with KRAS mutations and patients without any mutation by SNaPshot analysis (deemed pan-mutation negative for the purposes of this analysis). We chose to use patients with tumors that were pan-mutation negative by SNaPshot as the control group since these patients do not have any targetable mutations with FDA-approved drug therapies, thus the primary treatment option for this group remains cytotoxic chemotherapy in the metastatic setting, as is currently used to treat patients with KRAS mutations. Patients with KRAS mutations formed a consecutive cohort, however, patients in the pan-mutation negative cohort who met the inclusion/exclusion criteria were selected in a randomized fashion to yield an approximate 1:1 distribution between groups. Co-primary endpoints were OS and PFS.
1.2.2. Genetic Analysis
Molecular profiling was performed with the SNaPshot analysis on formalin-fixed paraffin-embedded tissue (FFPE). The SNaPshot process utilizes multiplex PCR, multiplex primer extension, and capillary electrophoresis, and it has been extensively validated. The SNaPshot analysis for NSCLC detects >40 somatic mutations in eight genes (AKT1, BRAF, EGFR, KRAS, MEK1, NRAS, PIK3CA, and PTEN), including 16 mutation types within the KRAS gene [23,24].
1.2.3. Statistical Analysis
PFS was defined as the date from initiation of first-line cytotoxic chemotherapy in the metastatic setting until the start date of second-line therapy or death, whichever occurred first. OS was defined as the date from initiation of first-line cytotoxic chemotherapy in the metastatic setting until date of death. Both PFS and OS curves were calculated from Kaplan-Meier method for KRAS mutation status and compared using log rank test. We used Cox Proportional Hazard (PH) regression to estimate the hazard ratio and 95% confidence interval for KRAS with the adjustment of sex and smoking status. Descriptive statistics, including the median and the ranges for continuous parameters, as well as percentages and frequencies for categorical parameters, were presented. All analyses are conducted using R software version 3.2.
1.3 Results
A total of 150 patients who received treatment for advanced NSCLC were analyzed, 70 patients with pan-mutation negative tumors, and 80 patients with KRAS-mutant advanced NSCLC. The median age was 62 years (range, 55-70). The majority of patients (82%) had stage IV disease at diagnosis, with 71% of patients having adenocarcinoma histology. The baseline characteristics of the patient population were well matched between groups with the exception of gender, smoking status, and tissue histology (Table 1).
Table 1.
Baseline characteristics of the study population.
| KRAS (N = 80) |
Pan-Mutation Negative (N = 70) |
p-value | ||
|---|---|---|---|---|
|
| ||||
| Age at diagnosis (years) | 62 (55.8 – 70) | 61 (55 – 70) | 0.976 | |
|
| ||||
| Male | 52% | 73% | 0.01 | |
| Female | 48% | 27% | ||
|
| ||||
| Race | ||||
| Caucasian | 90% | 84% | 0.303 | |
| African American | 10% | 12% | ||
| Asian | 0% | 1% | ||
| Hispanic | 0% | 3% | ||
|
| ||||
| Smoker (current or former) | 96% | 84% | 0.012 | |
|
| ||||
| Stage IV at diagnosis | 83% | 81% | 0.798 | |
| Recurrent/Relapsed Disease | 17% | 19% | ||
|
| ||||
| Histology | ||||
| Adenocarcinoma | 80% | 60% | <0.001 | |
| Squamous | 2% | 27% | ||
| Poorly differentiated carcinoma | 8% | 10% | ||
| Large Cell | 4% | 1% | ||
| Adenosquamous | 4% | 1% | ||
| Other | 2% | 1% | ||
Eighty nine percent of the patients received first line chemotherapy with carboplatin-based doublet chemotherapy with or without bevacizumab (Table 2). The proportion of patients who received a carboplatin-based doublet as first line treatment within the KRAS and the pan-negative cohort was similar (64% versus 65%, respectively). These numbers remained similar when reviewing patients who received bevacizumab in addition to a carboplatin-based doublet (22% in the KRAS-mutant cohort and 26% in the pan-negative cohort). Of note, numerically more KRAS-mutant patients received a cisplatin-based doublet when compared to pan-negative patients (14% versus 7%, respectively). Within the cohort of patients treated with carboplatin-based doublets, more patients in the KRAS-mutant group received carboplatin-pemetrexed (28% in KRAS-mutant group versus 13% in the pan-negative group), whereas more patients within the pan-negative group received carboplatin-paclitaxel (30% in the KRAS-mutant group versus 39% in the pan-negative group). (Table 2)
Table 2.
Treatment(s) received within the KRAS mutant and pan-mutation negative cohorts.
| KRAS (N=80) |
Pan-Mutation Negative (N=70) |
|
|---|---|---|
|
| ||
| Platinum doublets | ||
| Carboplatin Containing | 64% | 65% |
| Paclitaxel | 30% | 39% |
| Pemetrexed | 28% | 13% |
| Gemcitabine | 4% | 10% |
| Docetaxel | 1% | 3% |
| Vinorelbine | 1% | -- |
| Cisplatin Containing | 14% | 7% |
| Carboplatin doublet + Bevacizumab | 22% | 26% |
| Other | -- | 2% |
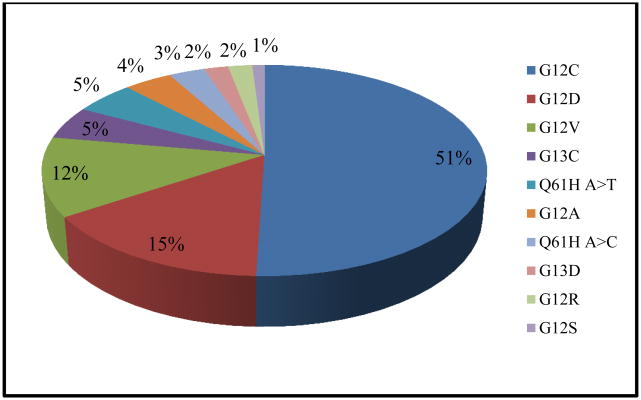
The different types of KRAS mutations found within our population are presented in Figure 1. Amongst different exon locations of KRAS mutations, mutations in exon G12 occurred most frequently, followed by exon Q61 and exon G13 (89%, 6%, and 5%, respectively). The percentage of each KRAS mutation in our study population was similar to the historical population in the COSMIC database [25]. Of the 16 mutation types that were analyzed within our study population, G12C, G12D, and G12V mutations occurred with the highest frequency, with a combined frequency of 80%. There was a statistically significant difference in OS when comparing within these KRAS mutation types and the pan-negative cohort, with G12C and G12D having decreased OS when compared to G12V and the pan-negative cohorts (8.8 months G12C, 6.7 months G12D, 14.2 months G12V, and 13.5 months pan-negative cohort, p=0.045).
Figure 1.
Distribution of KRAS mutations in the study population.
In regards to the primary endpoints, PFS was 1.2 months longer in the pan-negative group compared with the KRAS-mutant group (5.7 months versus 4.5 months, p = 0.008). Also, OS was 4.7 months longer in the pan-negative group compared with the KRAS-mutant group (13.5 months versus 8.8 months, p = 0.038). (Figure 2, Table 3) Subgroup analyses were completed in patients with adenocarcinoma histology and metastatic disease at diagnosis. In patients with adenocarcinoma histology, PFS was shorter in the KRAS-mutant group versus the pan-negative group (4.8 months versus 5.6 months, p=0.004). There was also a shorter, although not statistically significant, OS in patients with KRAS-mutant adenocarcinoma histology (8.8 months versus 14.2 months, p=0.067). This same trend was observed in the patients with metastatic disease at diagnosis with significantly decreased PFS (4.4 months versus 5.7 months, p=0.004) and OS (7.8 months versus 13.5 months, p=0.019) in patients with KRAS mutations versus the pan-negative group.(Figure 2)
Figure 2.
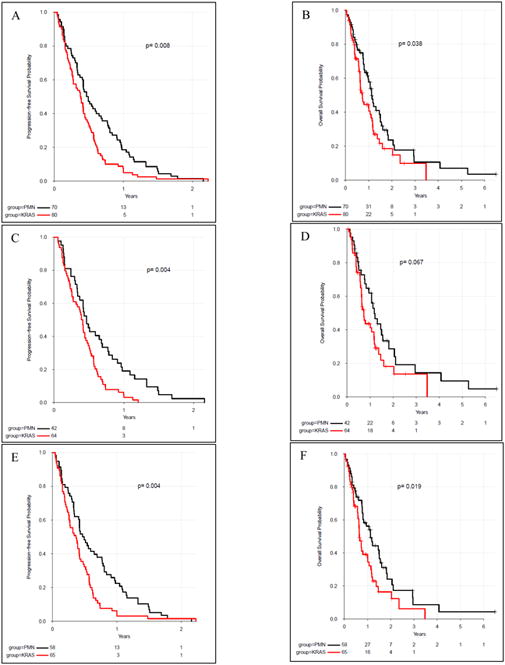
Progression-free survival (A) and overall survival (B) in KRAS mutant versus pan-mutation negative (PMN) patients within the total patient population. Subgroup analysis of PFS (C) and OS (D) in KRAS mutant versus PMN patients with adenocarcinoma histology. Subgroup analysis of PFS (E) and OS (F) in KRAS mutant versus PMN patients with metastatic NSCLC at diagnosis.
Table 3.
Median progression-free survival and overall survival in KRAS mutant versus pan-mutation negative patients in the total cohort and within the subgroup analyses, adenocarcinoma histology and metastatic at diagnosis.
| Median Progression-Free Survival (Range, months) |
Median Overall Survival (Range, months) |
|
|---|---|---|
|
| ||
| All patients | ||
| Pan-Mutation Negative | 5.7 months (4.4 – 7.8) |
13.5 months (11.4 – 18) |
| KRAS | 4.5 months (3.2 – 5.3) |
8.8 months (7.5 – 13.2) |
|
| ||
| Adenocarcinoma histology | ||
| Pan-Mutation Negative | 5.6 months (4.1 – 8.1) |
14.2 months (10 – 18.6) |
| KRAS | 4.8 months (3.3 – 5.5) |
8.8 months (7.5 – 14) |
|
| ||
| Stage IV at Diagnosis | ||
| Pan-Mutation Negative | 5.7 months (4.1 – 9.2) |
13.5 months (9.3 – 18.6) |
| KRAS | 4.4 months (3.1 – 5.1) |
7.8 months (6.9 – 12) |
Among patients who received first line treatment with carboplatin and pemetrexed, there was no difference in PFS or OS. Although there was no statistical difference in OS in patients that received carboplatin and paclitaxel, there was a 4.2 month survival advantage in pan-mutation negative patients (11.7 months pan-negative versus 7.5 months KRAS-mutant, p=0.118). There was a two month PFS advantage for pan-mutation negative patients who received carboplatin and paclitaxel (4.9 months pan-mutation negative versus 2.9 months KRAS-mutant, p=0.028). This same trend was also seen when comparing pan-mutation negative and KRAS mutated patients who received carboplatin, paclitaxel, and bevacizumab as first line therapy, with patients having no statistically significant difference in OS (14.5 months versus 9.5 months, respectively, p=0.071), but a difference in PFS (8.3 months versus 5.2 months, respectively, p=0.023).
Within the multivariate analysis, we evaluated sex, smoking history and KRAS status (KRAS mutation versus pan-mutation negative) to determine impact on survival (Table 4). Smoking history did not affect outcome for either PFS (HR: 1.64, 95% CI: 0.92-2.93; p = 0.091) nor OS (HR: 1.34, 95% CI: 0.68-2.63; p=0.399). However, male sex did increase the risk of death versus female sex (HR: 1.67, 95% CI: 1.07-2.61; p=0.025). KRAS mutations were associated with both decreased PFS and OS in the multivariate analysis when controlling for both sex and smoking history. KRAS mutations were associated with shorter overall survival compared with the pan-negative mutation (HR: 1.73, 95% CI: 1.12-2.67; p=0.014).
Table 4.
Multivariate analysis of progression free survival (A) and overall survival (B) for KRAS versus pan-mutation negative patients.
| A. Progression-free survival | ||
| Variable | HR | p-value |
| KRAS | 1.56 (95% CI: 1.09-2.23) | 0.015 |
| Sex (W vs M) | 1.30 (95% CI: 0.91-1.85) | 0.152 |
| Smoking (Y vs N) | 1.64 (95% CI: 0.92-2.93) | 0.091 |
| B. Overall survival | ||
| Variable | HR | p-value |
| KRAS | 1.73 (95% CI: 1.12-2.67) | 0.014 |
| Sex (W vs M) | 1.67 (95% CI: 1.07-2.61) | 0.025 |
| Smoking (Y vs N) | 1.34 (95% CI: 0.68-2.63) | 0.399 |
1.4 Discussion
In patients with NSCLC, multiple prognostic factors have been identified, such as stage at diagnosis, age and EGFR mutation status. However, the prognostic significance of certain mutations, such as KRAS mutations, remains controversial. In this retrospective analysis, we found that patients with KRAS mutations had a worse prognosis in terms of PFS and OS when compared to patients with no detectable NSCLC tumor mutations.
Within our patient population, we found several characteristics that differed between patients with KRAS-mutant tumors and patients with no detectable tumor mutation. In agreement with prior findings, patients with KRAS mutations in our cohort were more likely to have been smokers and to demonstrate adenocarcinoma histology compared to patients with no detectable tumor mutation [26,27]. We also noted that within our KRAS-mutant patient population, the most common mutation type was a G12C mutation (50%), and this transversion mutation is more likely to be smoking related; it was also noted that the next two most frequently encountered mutations, G12D and G12V (15% and 12%, respectively) are transition mutations that have been shown to occur more frequently in non-smokers [27]. However, KRAS mutations were not exclusively seen in patients with adenocarcinoma histology, which provides rationale for testing all patients with NSCLC for KRAS tumor mutations. There were more women in the KRAS-mutant group compared to the group of patients with no detectable tumor mutation; the significance of this finding is unknown. Although male sex was associated with worse outcomes in our multivariate analysis, survival was still shorter in the KRAS cohort despite having a higher proportion of females.
The majority of the patients in this analysis received treatment with platinum doublet chemotherapy, most commonly carboplatin plus paclitaxel, which is consistent with first line recommendations in the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of metastatic or recurrent NSCLC [28,29]. Approximately the same proportion of patients in each group received treatment with carboplatin-paclitaxel.
There have been several larger analyses reporting conflicting data. In a retrospective analysis by Metro et al., patients with KRAS mutations experience significantly lower response rates and disease control rates as well as decreased PFS compared to EGFR wild type/KRAS wild type patients [22]. Conversely, a retrospective analysis by Mellema and colleagues which included 161 patients demonstrated no difference in OS or response to chemotherapy when comparing patients with KRAS mutations to patients without KRAS mutations [18]. This analysis, as well as others conducted in patients with advanced NSCLC, sharply contrast with studies in patients with operable NSCLC, where it is known that KRAS mutations portend a highly unfavorable prognosis [15-18]. In the operative setting it has been speculated that KRAS mutations encourage early metastasis which is undetectable at the time of resection, thereby leading to an overall worse prognosis when micrometastases grow to detectable sizes.
This study had several limitations, primarily its retrospective design at a single institution. Also, statistical analysis comparing treatments was not completed, and a statistical analysis controlling for potential confounding variables was not completed. Another potential limitation was the higher incidence of patients with adenocarcinoma within the KRAS cohort since non-squamous histology is a known adverse prognostic factor. However, subgroup analysis among patients with only adenocarcinoma still demonstrated significantly shorter PFS among patients with KRAS mutant tumors in our study.
1.4.1. Conclusion
In conclusion, the results of this analysis showed that the presence of KRAS activating mutations in patients with metastatic or recurrent NSCLC portends a worse prognosis compared to the absence of detectable tumor mutations when patients are treated with first line cytotoxic chemotherapy. With the currently available literature conflicting on the prognostic significance of KRAS mutations in advanced NSCLC, future studies should prospectively compare responses to cytotoxic chemotherapy in patients with advanced NSCLC based on KRAS mutation status.
Highlights.
G12C, G12D, and G12V were the most frequent KRAS mutations identified.
G12C and G12D mutations were associated with shorter OS compared to G12V mutations
KRAS mutations were associated with decreased PFS and OS in advanced NSCLC.
Acknowledgments
We would like to thank the Vanderbilt-Ingram Cancer Center Biomedical Informatics team including: Mia Levy, Joseph Burden, Pam Carney, Lucy Wang and Jeremy Warner for their contributions to this analysis.
Footnotes
Footnotes: Study data were collected and managed using REDCap electronic data capture tools hosted at Vanderbilt University Medical Center.1 REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. 1Paul A. Harris, Robert Taylor, Robert Thielke, Jonathon Payne, Nathaniel Gonzalez, Jose G. Conde, Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377-81.
Conflict of Interest: Megan Hames: MedAssets compensated regional speaker
Heidi Chen: N/A
Wade Iams: N/A
Jonathan Aston: N/A
Christine Lovly: Supported in part by the National Institutes of Health (NIH) and National Cancer Institute (NCI) K12CA9060625, R01CA121210, and P01CA129243. CML was additionally supported by a Damon Runyon Clinical Investigator Award and a LUNGevity Career Development Award.
Leora Horn: Advisory board member compensated: Merck, Genentech; Advisory boards uncompensated: Bristol Myers Squibb, Biodesix; Consulting uncompensated: Xcovery and Bayer.
References
- 1.Lung and Bronchus Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969-2012) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [Accessed May 15, 2015]. www.seer.cancer.gov/popdata) released April 2015. Available at: http://seer.cancer.gov/statfacts/html/lungb.html. [Google Scholar]
- 2.National Cancer Institute. PDQ® Non-Small Cell Lung Cancer Treatment [database online] Bethesda, MD: National Cancer Institute; 2015. Updated: August 6, 2014. [Google Scholar]
- 3.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, editors. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press; Lyon: 2004. World Health Organization Classification of Tumours. [Google Scholar]
- 4.Riley GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pao W, Wang TY, Riely GJ, et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2005;2:e17. doi: 10.1371/journal.pmed.0020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002 1 Dec;62(23):6997–7000. [PubMed] [Google Scholar]
- 8.Marks JL, Broderick S, Zhou Q, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3:111–116. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 9.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 10.Mok TS, Wu Y, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 11.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 12.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405):an open label, randomized phase 3 trial. Lancet Oncol. 2010;11(2):121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 13.Gilotrif (afatinib) [package insert] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals Inc; Apr, 2014. [Google Scholar]
- 14.Tarceva (erlotinib) [package insert] San Francisco, CA: Genentech USA, Inc; Apr, 2012. [Google Scholar]
- 15.Graziano SL, Gamble GP, Newman NB, et al. Prognostic significance of K-ras codon 12 mutations in patients with resected stage I and II non-small-cell lung cancer. J Clin Oncol. 1999;17:668–675. doi: 10.1200/JCO.1999.17.2.668. [DOI] [PubMed] [Google Scholar]
- 16.Rodenhuis S, Boerrigter L, Top B, et al. Mutational activation of the K-ras oncogene and the effect of chemotherapy in advanced adenocarcinoma of the lung: a prospective study. J Clin Oncol. 1997;15:285–291. doi: 10.1200/JCO.1997.15.1.285. [DOI] [PubMed] [Google Scholar]
- 17.Camps C, Sirera R, Bremnes R, et al. Is there a prognostic role of K-ras point mutations in the serum of patients with advanced non-small cell lung cancer? Lung Cancer. 2005;50:339–346. doi: 10.1016/j.lungcan.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Mellema WW, Dingemans AM, Thunnissen E, et al. KRAS mutations in advanced nonsquamous non-small-cell lung cancer patients treated with first-line platinum-based chemotherapy have no predictive value. J Thorac Oncol. 2013 Sep;8(9):1190–5. doi: 10.1097/JTO.0b013e318298764e. [DOI] [PubMed] [Google Scholar]
- 19.Rulli E, Marabese M, Torri V, et al. Value of KRAS as prognostic or predictive marker in NSCLC: results from the TAILOR trial. Annals of Oncology. 2015;26:2079–2084. doi: 10.1093/annonc/mdv318. [DOI] [PubMed] [Google Scholar]
- 20.Cserepes M, Ostoros G, Lohinai Z, et al. Subtype-specific KRAS mutations in advanced lung adenocarcinoma: a retrospective study of patients treated with platinum-based chemotherapy. European Journal of Cancer. 2014;50:1819–1828. doi: 10.1016/j.ejca.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Marcerelli M, Caramella C, Faivre L, et al. Does KRAS mutational status predict chemoresistance in advanced non-small cell lung cancer (NSCLC)? Lung Cancer. 2014;83:383–388. doi: 10.1016/j.lungcan.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Metro G, Chiari R, Bennati C, et al. Clinical outcome with platinum-based chemotherapy in patients with advanced nonsquamous EGFR wild-type non-small cell lung cancer segregated according to KRAS status. Clinical Lung Cancer. 2014;15(1):86–92. doi: 10.1016/j.cllc.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Orita M, Shiraishi M, Hayashi K, Sekiya T. Detection of RAS gene mutations in human lung cancers by single-strand conformation polymorphism analysis of polymerase chain reaction products. Oncogene. 1990;5:1037–1043. [PubMed] [Google Scholar]
- 24.Lovly CM, Dahlman KB, Fohn LE, Su Z, Dias-Santagata D, Hicks DJ, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7:e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forbes S, Clements J, Dawson E, et al. Cosmic 2005. Br J Cancer. 2006;94:318–322. doi: 10.1038/sj.bjc.6602928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Riely GJ, Kris MG, Marks JL, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. J Clin Oncol. 2008;425s(suppl; abstr 8006):26. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly K, Crowley J, Bunn PA, Jr, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non-small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001 Jul 1;19(13):3210–8. doi: 10.1200/JCO.2001.19.13.3210. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger S, Wood DE, Loo BW, Martins W, et al. NCCN Clinical practice guidelines in oncology (NCCN guidelines): Non-Small Cell Lung Cancer Version 7. 2015 [Google Scholar]