Abstract
RV144 vaccinees with low HIV-1 Envelope-specific IgA antibodies (Abs) also had Abs directed to the hypervariable region 3 (V3) that inversely correlated with infection risk. Thus, anti-V3 HIV-1 Abs may contribute to protection from HIV-1 infection. The V3 region contains two dominant clusters of epitopes; one is preferentially recognized by mAbs encoded by VH5-51 and VL lambda genes, while the second one is recognized by mAbs encoded by other VH genes. We designed a study in rhesus macaques to induce anti-V3 Abs specific to each of these two dominant clusters of V3 epitopes to test whether the usage of the VH5-51 gene results in different characteristics of antibodies. The two C4-V3 immunogens used for immunization were each comprised of a fusion of the C4 peptide containing the T cell epitope and a V3 mimotope peptide mimicking the V3 epitope. The C4-447 peptide was designed to target B cells with several VH1–VH4 genes, the C4-VH5-51 peptide was designed to specifically target B cells with the VH5-51 gene. Six animals in two groups were immunized five times with these two immunogens, and screening of 10 sequential plasma samples post immunization demonstrated that C4-447 induced higher titers of plasma anti-V3 Abs and significantly more potent neutralizing activities against tier 1 and some tier 2 pseudoviruses than C4-VH5-51. Levels of anti-V3 Abs in buccal secretions were significantly higher in sequential samples derived from C4-447-than from C4-VH5-51-immunized animals. The titers of anti-V3 Abs in plasma strongly correlated with their levels in mucosal secretions. The results show that high titers of vaccine-induced anti-V3 Abs in plasma determine the potency and breadth of neutralization, as well as the rate of transduction of Abs to mucosal tissues, where they can play a role in preventing HIV-1 infection.
Keywords: HIV-1, HIV vaccine, HIV-1 neutralizing antibodies, V3 immunogens, non-human primates immunization, rhesus macaque immunoglobulin genes
1. Introduction
Vaccine-induced antibodies (Abs) are critical for protection against infection, including HIV-1. It has been shown in the first modestly successful RV144 vaccine clinical trial that the high level of anti-V2 Abs was inversely correlated with reduction of HIV-1 infection, suggesting that these Abs can contribute to protection against virus infection (1, 2). Furthermore, in vaccine recipients with low levels of IgA Abs to envelope (Env) proteins, the level of anti-V3 Abs was also inversely correlated with the risk of the HIV-1 infection (3–5). The protective ability of anti-V3 monoclonal Abs (mAbs) against virus challenge has been shown in several animal experiments (6–9). Also, administration of anti-V3 mAbs in selected HIV-1 infected individuals reduced the viral load by 1.5 orders of magnitude (log10) in a dose-dependent manner and provided long-term viral suppression in one patient (10).
In comparative studies, anti-V3 mAbs displayed higher neutralization potency and breadth than anti-V2 mAbs (11). This suggests that the contribution of anti-V3 Abs in reducing infection may depend on their potential to neutralize HIV-1 while the role of anti-V2 Abs may depend on other functions, including the interference of virus that binds to T cells that express integrin α4β7, as some studies suggest (12, 13). Although anti-V3 mAbs neutralize mainly tier 1 pseudoviruses, most anti-V3 mAbs can neutralize one to several tier-2 and -3 viruses (11, 14). The anti-V3 Abs commonly induced by HIV-1 infection are glycan-independent; this feature limits their breadth of neutralization, although some can cross-neutralize over 30% of a panel of 41 viruses (11). The major structural obstacle to neutralization by these common anti-V3 Abs is the glycan at position 301 of V3; by contrast, anti-V3 glycan-dependent mAbs such as PGT128 can broadly neutralize viruses that incorporate glycans at position 322 (15).
Fine mapping studies of anti-V3 mAbs revealed the existence of two dominant clusters of epitopes in the crown of the V3 region that induce neutralizing Abs (16, 17). One epitope, which structurally resembles a ladle, is defined by the mAb 447-52D that is specific for the tip of the V3 loop. The second epitope, which structurally resembles a cradle, encompasses the hydrophobic face of the V3 loop and is recognized by anti-V3 mAbs encoded by the VH5-51 and VL lambda genes (16–19). Mimotopes that mimic these two dominant V3 epitopes were designed and used to produce hybrid peptides that incorporate the C4 peptide that contains a helper T cell epitope (20). These two immunogens were subsequently used to immunize rhesus macaques. The C4-VH5-51 peptide was designed to target B cells that express the receptor (BCR) encoded by VH5 family genes, and the C4-447 peptide was used to target B cells expressing the BCR encoded by VH1–VH4 family genes, but not by VH5 genes. In macaques, the peptide immunogen C4-447 induced anti-V3 Abs with significantly higher neutralizing activities than C4-VH5-51, possibly through targeting a pool of B cells that express multiple Ig genes.
2. Materials and Methods
2.1. V3 mimotopes
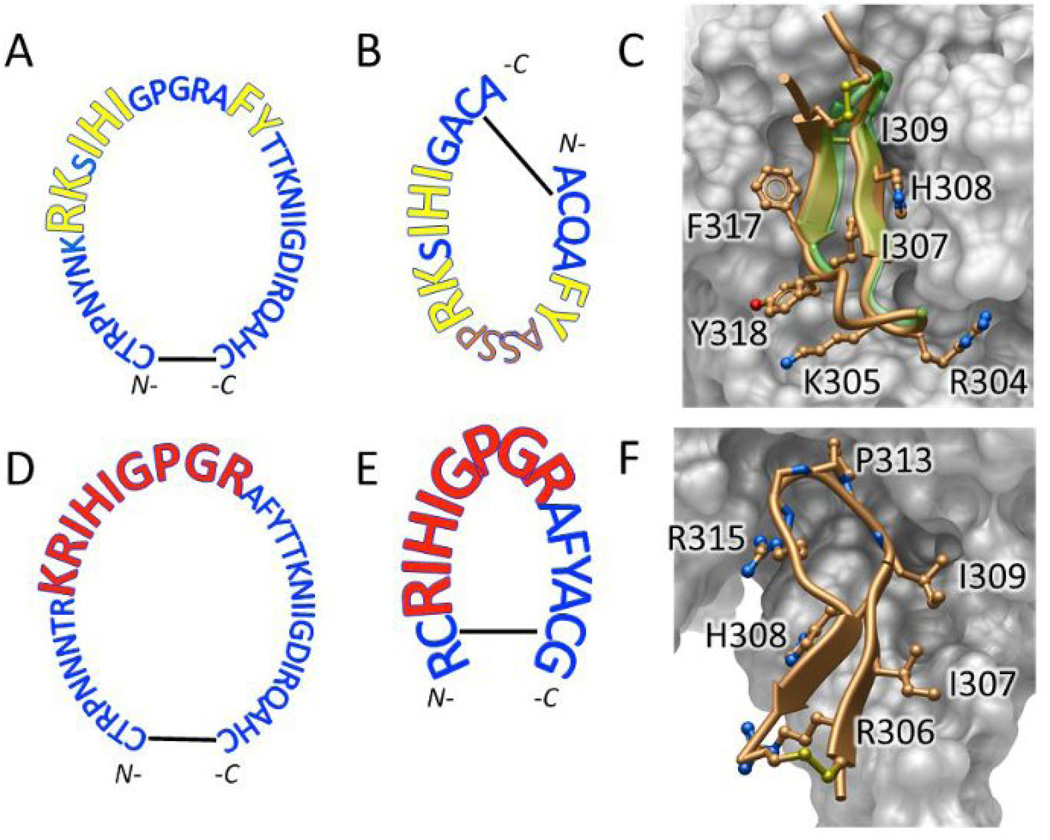
The two V3 mimotopes were designed to mimic the epitopes of the anti-V3 mAbs encoded by the VH5-51/VL lambda genes and anti-V3 mAb 447-52D (16–19). The mimotope VH5-51 was designed on the basis of X-ray structures of the Fab regions of five VH5-51 anti-V3 mAbs in complex with the V3 peptides, which revealed the epitope RK-IHI-FY (Fig. 1A, 1B) (16, 19). The 20-mer VH5-51 mimotope, ACQAFYASSPRKSIHIGACA (Fig. 1B), consists of the epitope (underlined) and a linker, ASSP, which replaces the GPGR motif to preserve the β-turn. A crystal structure of the Fab region of mAb 2557 (encoded by VH5-51) in complex with the cyclic VH5-51 mimotope (Fig. 1C) revealed the same interactions of residues identified in complexes of 2557 with different V3 peptides (19). The V3 epitope of mAb 447-52D (Fig. 1D) is included in the 16-mer 447 mimotope, RCRIHIGPGRAFYACG (Fig. 1E) which consists of the epitope (underlined) based on the X-ray structure of the 447-52D/V3 peptide complex (Fig. 1F) (17, 18).
Figure 1. The V3 epitopes and mimotopes for VH5-51-encoded mAbs and the 447-52D mAb.
(A) The V3 epitope in the sequence of HIV-1MN is shown for the VH5-51-derived mAbs (yellow, enlarged letters). (B) The VH5-51 mimotope designed to bind only the VH5-51/VL lambda genes encoded anti-V3 mAbs, which interact with V3 crown ‘hairpin’ over two separate segments of polypeptide chain that are circularly permuted in the V3 mimotope. (C) Crystal structure of the VH5-51 mimotope/Fab 2557 complex reveals that mimotope peptide (gold) segments interacting with mAb (key residues shown in ‘sticks-and-balls’ representation) closely match corresponding segments of unmodified V3 crown peptide (green transparent ‘ribbon’, from X-ray structure PDB ID 3MLT. (D) In contrast, 447-like anti-V3 mAbs interact with a continuous stretch of V3 polypeptide chain for 447-52D mAb (red, enlarged letters). (E) The 447 mimotope with disulfide-constrained V3 crown was designed to preferentially adopt the conformation recognized by mAb 447-52D. (F) X-ray structure of 447-52D/V3 peptide complex (PDB ID: 4M1D) confirms excellent fit of the modified peptide and antibody. Key peptide residue sidechains interacting with antibody are shown in ‘sticks-and-balls’ representation and labeled. Disulfide cross-link is also shown as ‘sticks-and-balls’.
2.2. Immunogens
Two immunogens were used for immunization: (i) C4-VH5-51, a 36-mer composed of the C4 peptide (underlined) and the disulfide-cyclized V3 mimotope peptide VH5-51 (KQIINMWQEVGKAMYAACAGIHISKRPSSAYFAQCA), and (ii) C4-447, a 32-mer consisting of the C4 peptide and 447 mimotope peptide (KQIINMWQEVGKAMYARCRIHIGPGRAFYACG) (Fig. 1). The C4 peptide is a 16-amino acid stretch (residues 428–443) of the C4 helper determinant of gp120MN. It contains the immunodominant helper T-cell epitope, which induced T-cell immunity to the native gp120MN in immunized mice (20). Both immunogens were synthesized by Biopeptide Co. (https://www.biopeptide.com).
2.3. Animal immunization
Six adult Indian origin rhesus macaques (Macaca mulatta), five male and one female 25328, were used in this study. Immunizations were performed under Institutional Animal Care and Use Committee (IACUC)-approved protocols at the Oregon National Primate Research Center, Beaverton, OR.
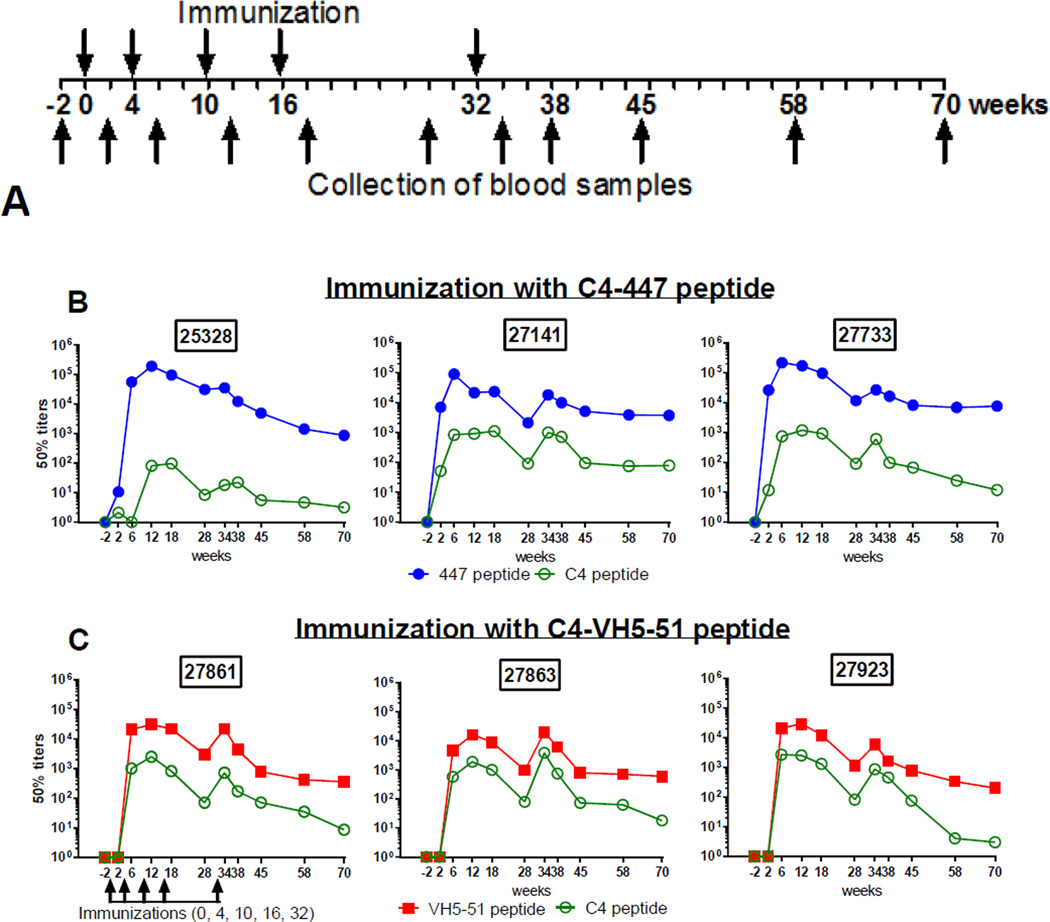
The two groups, three animals in each, were immunized five times at 0, 4, 10, 16 and 32 weeks (Fig. 2A) using 1 mg of peptide which was solubilized in 0.5 ml of phosphate-buffered saline (PBS) and then emulsified with an equal volume of incomplete Freund’s adjuvant (IFA, Sigma, St. Louis, Mo.). The peptide emulsion (1 ml) was inoculated by the intramuscular route in four sites on the back, between the shoulders, of each animal. Blood samples were collected 2 weeks prior to immunization, then 2 weeks after each immunization and an additional four times until week 70 for a total of 11 specimens per animal (Fig. 2A). For venous blood sampling, macaques were sedated with ketamine hydrochloride prior to removal from their cages. Blood was obtained from the femoral or saphenous veins using a vacuum tube apparatus. Mucosal secretions (buccal and rectal) were collected by absorption to cellulose wicks (21) at the same time as when blood samples were collected.
Figure 2. Immunization protocol and titration of plasma antibody response to homologous mimotopes and C4 peptide.
(A) Immunization protocol of rhesus macaques. Six animals, divided equally in two groups, were immunized five times intramuscularly at 0, 4, 10, 16 and 32 weeks using 1 mg of peptide with incomplete Freund’s adjuvant. Blood samples were collected 2 weeks prior to immunization, 2 weeks after each immunization, and then at weeks 38, 45, 58 and 70. (B) Binding of Abs from animals immunized by C4-447 peptide to homologous biotinylated cyclic 447 peptide. (C) Binding of Abs from animals immunized with C4-VH5-51 to homologous biotinylated cyclic VH5-51 peptide. Plasma samples were titrated in a dilution range of 1:11 to 1:106 and tested by standard ELISA against biotinylated peptides, 447 and VH5-51, immobilized onto streptavidin-coated plates; 50% titers were determined by linear regression.
2.4. Binding assay (ELISA)
Macaque plasma samples were screened against biotinylated cyclic V3 mimotope peptides (VH5-51 and 447) and the biotinylated C4 peptide using a standard ELISA. StreptaWell plates (Roche) coated with streptavidin were incubated with the biotinylated peptides at 1 µg/ml followed by washing with PBS with 0.05% Tween-20 and blocking with assay diluent (PBS containing 2.5% bovine serum albumin and 7.5% fetal bovine serum). Plasma samples were diluted by 10-fold dilutions ranging from 1:10 to 1:1,000,000, incubated for 1.5 h at 37°C, and washed again before bound Abs were detected by incubation with alkaline phosphatase-conjugated goat anti-human IgG (γ specific) (Southern Biotech) followed by washing and adding substrate to develop color. The plates were read at 405 nm. The 50% titers of plasma Abs were determined by measuring the dilutions of plasma required for 50% maximal binding by linear regression (22). Mucosal secretions, buccal and rectal, were tested by ELISA for Abs that bind to the biotinylated V3 mimotope peptides, 447 and VH5-51, and to HIV-1 SF162 gp140 trimeric protein; the second antibody was peroxidase affinipure goat anti-human IgG, Fcγ (Jackson ImmunoResearch). Binding curves were generated using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA).
2.5. Pseudovirus generation
Pseudoviruses were produced using the pSG3ΔEnv DNA plasmid that encodes the HIV backbone and a plasmid that encodes envelope variants of the tier-1 viruses Q461.e2.TAIV (clade A), DJ263.8 (clade 02_AG), SF162, SS1196.1 and 6535 (clade B), MW965.26 (clade C), and the tier-2 viruses JRCSF, REJO, and RHPA (clade B) as described (23). HIV-1 clone SF162 was provided by Leon Stamatatos (Fred Hutchinson Cancer Research Center, Seattle, WA); the Env was cloned from SHIVSF162P4 (24) and has two amino acids differences in Env from the published sequence of HIV-1 SF162. HIV-1 clone JRCSF was provided by Dennis Burton (The Scripps Research Institute, La Jolla, CA), while the other plasmids were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. For neutralization assays, the amount of 200 50% tissue culture infective doses (TCID50) was calculated according to the method of Reed and Muench (25).
2.6. Neutralization assay
The neutralizing activities of plasma Abs against six tier-1 and three tier-2 pseudotyped viruses were tested using the standard TZM.bl cell assay (23, 26). Briefly, plasma samples were titrated by 10-fold serial dilutions starting from 1:20 to 1:1,000 or 1:100,000 and were pre-incubated with the pseudoviruses at an input of 200 TCID50 for 1 h. The virus/plasma Abs mixtures were then incubated for 48 h with TZM.bl cells expressing CD4, CCR5 and CXCR4. Virus infectivity was determined by measuring luciferase activity in cell lysates. The 50% inhibitory concentration (IC50) was determined as the plasma dilution that resulted in a 50% reduction in relative light units (RLU) compared to wells with the virus only, after the subtraction of cell control RLUs (23, 27). All plasma samples were tested in duplicate. As a negative control, a pool of the pre-immune plasma was used and the lowest dilution tested (1:20) never achieved 50% neutralization. Neutralization dose-response curves were fitted by non-linear regression.
2.7. Isolation of the VH sequences from macaque B cells
Peripheral blood mononuclear cells (PBMC) isolated from blood samples collected 2 weeks prior to immunization from two macaques, 25328 and 27141, were used to study Ig gene usage. The PBMCs were stained with anti-CD20-PE, anti-IgG-FITC and anti-CD27-APC Abs for 10 min at 4°C, and positively labeled single B cells were sorted into 96-well PCR plates using a FACS Aria at the NYU School of Medicine Flow Cytometry Core. RNA was reverse–transcribed, and nested PCRs were performed using the forward and reverse primers for the variable fragment of the heavy chains (VH) of macaques’ Ig as described (28). PCR products were run in 1% agarose gel and were sequenced after purification. The amplification of VH genes from single B cells was obtained in a range of 60% to 70%. Rhesus macaques VH family genes usage was determined using the IMGT/V-QUEST/Rhesus system (http://www.imgt.org).
2.8. Statistical analysis
Plasma and mucosal secretions Abs were compared between two groups of animals by two-way ANOVA and Bonferroni’s multiple comparison tests. Neutralization activity of plasma from week to week for either group of animals was analyzed using the log-rank test. Correlation between OD values of mucosal secretions and plasma Abs titers and neutralization of pseudovirus SF162 was analyzed by use of the Spearman correlation coefficient (r) with P values (two-tailed) and by linear regression. Statistical analysis was performed using GraphPad Prism.
3. Results
3.1. Antigenicity of the V3 immunogens
Two V3 immunogens peptides, C4-VH5-51 and C4-447, were tested for reactivity with 35 human anti-V3 mAbs with known usage of VH genes (Table 1). Binding studies showed that the C4-VH5-51 peptide reacted strongly with 12 of 14 (85.7%) of VH5-51-encoded V3 mAbs, but not with the anti-V3 mAbs encoded by VH1-VH4 family genes (Table 1). The second immunogen, C4-447, reacted with the majority of VH1-VH4 encoded anti-V3 mAbs (76.2%), but not with VH5-51 mAbs; only one and two mAbs in each group cross-reacted weakly with the other peptide (Table 1). Based on these binding studies, we hypothesize that in macaques, the C4-VH5-51 immunogen may target the B cell receptor (BCR) encoded by VH5 gene family in naïve B cells, while the C4-447 may target the remaining VH1-VH4 B cells. The two macaques VH5 genes, IGHV5-A and IGHV5-B as reported by Francica et al. (29), display >94% identity with human VH5-51 germline antibody (Supplement Fig. S1) and can be targeted by C4-VH5-51 immunogen.
Table 1.
ELISA reactivity of anti-V3 mAbs with V3 immunogens1.
| mAb | VH family |
C4-VH5 immunogen |
C4-447 immunogen |
|
|---|---|---|---|---|
| 1 | 257 | 5 | 3.6 | 0.3 |
| 2 | 2558 | 5 | 3.1 | 0.5 |
| 3 | 1006-15 | 5 | 3.7 | 0.1 |
| 4 | 908 | 5 | 2.4 | 0.1 |
| 5 | 2456 | 5 | 2.6 | 0.1 |
| 6 | 3019 | 5 | 3.6 | 0.1 |
| 7 | 2557 | 5 | 3.8 | 0.1 |
| 8 | 838 | 5 | 2.7 | 0.1 |
| 9 | 2483 | 5 | 2.6 | 0.1 |
| 10 | 4022 | 5 | 2.1 | 0.1 |
| 11 | 3792 | 5 | 1.9 | 0.1 |
| 12 | 2219 | 5 | 2.1 | 0.2 |
| 13 | 3906 | 5 | 0.1 | 0.1 |
| 14 | 3694 | 5 | 0.1 | 0.1 |
| 15 | 391/95 | 1 | 0.4 | 3.6 |
| 16 | 4121 | 1 | 0.2 | 3.4 |
| 17 | 1334 | 1 | 0.1 | 1.6 |
| 18 | 1027-15 | 1 | 0.5 | 0.1 |
| 19 | 2191 | 1 | 0.1 | 0.1 |
| 20 | 694/8 | 2 | 0.1 | 3.6 |
| 21 | 2412 | 2 | 0.1 | 0.7 |
| 22 | 3527 | 3 | 0.6 | 3.8 |
| 23 | 537 | 3 | 0.1 | 3.8 |
| 24 | 418 | 3 | 0.1 | 3.9 |
| 25 | 412 | 3 | 0.1 | 3.8 |
| 26 | 447-52D | 3 | 0.1 | 3.8 |
| 27 | 504 | 3 | 0.1 | 3.1 |
| 28 | 1324E | 3 | 0.1 | 0.1 |
| 29 | 2601 | 3 | 0.1 | 0.1 |
| 30 | 2442 | 4 | 0.1 | 3.7 |
| 31 | 3074 | 4 | 0.1 | 1.8 |
| 32 | 268 | 4 | 0.1 | 3.5 |
| 33 | 2182 | 4 | 0.1 | 3.6 |
| 34 | 386 | 4 | 0.1 | 3.5 |
| 35 | 453 | 4 | 0.1 | 0.1 |
| C | 1418 | - | 0.1 | 0.1 |
The two immunogens, C4-VH5-51 and C4-447, were coated onto ELISA plastic plates at 1 µg/ml, and binding of anti-V3 mAbs at concentration of 10 µg/ml was detected by alkaline phosphate-conjugated anti-human IgG (Fc) Abs. The numbers in columns with antigens indicate the O.D. values measured at 405 nm. C – Negative control, human mAb 1418 against parvovirus B19.
3.2. Vaccine-induced antibody response to V3 mimotopes and C4 peptides
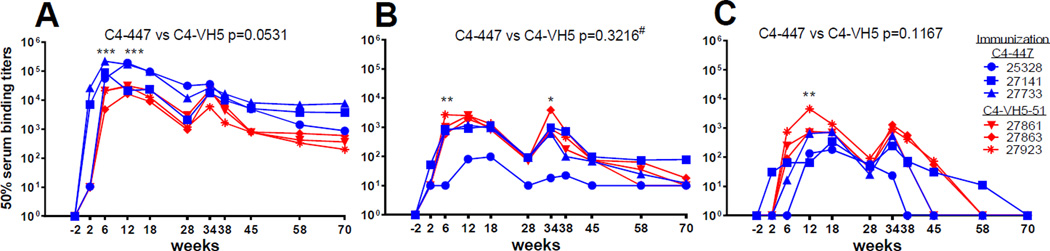
Rhesus macaques were immunized with two immunogens, C4-447 and C4-VH5-51 (Fig. 2A), and eleven sequential plasma samples from each animal were screened by ELISA against homologous and heterologous V3 mimotope peptides and against the C4 peptide (Fig. 2B, 2C and 3). In a homologous system (e.g., plasma from C4-447 immunized animals tested against a cyclic 447 peptide) the 50% titers increased at week 6 and the two peak titers were observed at weeks 12 and 34 (two weeks after the 3rd and 5th immunization). The peak titers were in a range of 1:105–1:106 dilutions for 447 peptide and 1:104–1:105 for VH5-51 peptide (Fig. 2B, 2C). The titers remain detectable (between 1:102 and 1:103 dilution) at week 70, which is 38 weeks after the last immunization (Fig. 2B, 2C). Titers of anti-V3 Abs induced by C4-447 was higher compared to C4-VH5-51; a significant difference was found at week 6 (p<0.001) and at week 12 (p=0.001). Overall variation between the groups was strongly trending toward significance, p=0.0531, by the two-way ANOVA test (Fig. 3A).
Figure 3. Summary of macaque plasma antibodies (Abs) to V3 mimotopes and C4 peptide.
(A) Binding of plasma Abs to biotinylated cyclic 447 and VH5-51 mimotope peptides (without C4 peptide) from macaques immunized with C4-447 and C4-VH5-51 immunogens, respectively (homologous system). (B) Binding of macaque Abs to biotinylated C4 peptide. (C) Binding of macaque Abs to biotinylated 447 and VH5-51 mimotope peptides in a heterologous system, e.g., C4-447 induced Abs tested against biotinylated cyclic VH5-51 peptide. Statistics: Two-Way Repeated Measures ANOVA taking into account multiple comparisons over time with Bonferroni correction. For analysis of panel 3B without 25328, an Ordinary Two-Way ANOVA was used. GraphPad Prism, v6. ***p<0.001. **p<0.01, *p<0.05; # outlier animal 25328 is excluded.
The 50% titers of anti-C4 Abs are generally 1–2 log10 lower than titers of anti-V3 peptide Abs (Fig. 3B). The titers of anti-C4 Abs are very similar in both groups of animals immunized with C4-447 and C4-VH5-51, with the exception of animal 25328 (Fig. 2B, 2C and 3B). No significant difference was found between the groups if outlier animal 25328 is excluded (p=0.3216).
The heterologous activity (plasma from C4-447 immunized animal reacted with VH5-51 peptide) was about 2 log10 lower than binding of Abs to homologous V3 peptides, and decreased to minimal or no binding activity at weeks 38 to 58 (Fig. 3C). There was no significant difference between the groups overall (p=0.1167).
3.3. Mucosal secretions
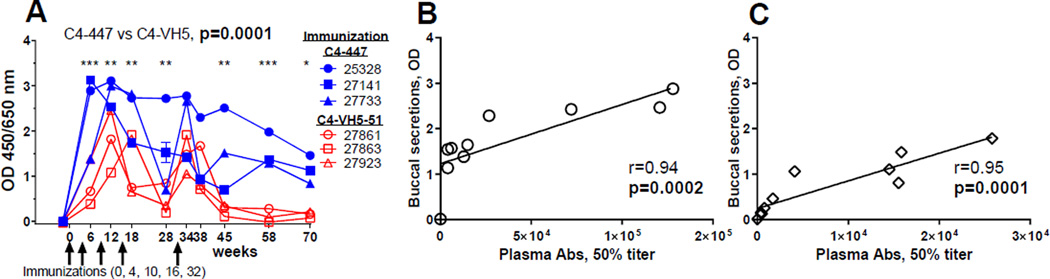
The buccal secretions were collected along with blood samples, except for one (week 2) and nine sequential specimens from each animal. ELISA was used to test undiluted buccal secretions for binding to the biotinylated cyclic V3 mimotope peptides 447 and VH5-51 (Fig. 4). The binding activities (OD) of buccal samples were significantly higher for macaques immunized with C4-447 than those immunized with C4-VH5-51 (p=0.0001) (Fig. 4A). The titers of plasma and OD values of buccal secretions of anti-V3 Abs in both groups of animals, C4-447 and C4-VH5-51, showed strong correlation (Spearman correlation coefficients of p=0.0002 and p=0.0001, respectively (Fig. 4B, 4C).
Figure 4. Vaccine-induced anti-V3 antibodies in buccal secretions.
(A) A comparison of buccal secretions collected at the different time points from each animal. ELISA was used to test undiluted samples for binding to the biotinylated cognate V3 mimotope peptides 447 and VH5-51. Secretions from four macaques collected prior to immunization (week –2) provided a negative control. Statistics: Two-Way ANOVA taking into account multiple comparisons over time with Bonferroni correction. GraphPad Prism, v6; ***p<0.0001, **p<0.01, *p=0.01. (B) Correlation between plasma and buccal secretions anti-V3 Abs in animals immunized with C4-447 and (C) C4-VH5-51, as determined using Spearman correlation coefficient (r) with p-values (two-tailed) and linear regression.
ELISA was used to test 1:3 dilutions of the same buccal secretions, and additional rectal secretions, for binding to non-biotinylated gp140 SF162. The Abs were detected in both mucosal secretions collected only from animals immunized with the C4-447, while in animals immunized with C4-VH5-51 only rectal secretions displayed minimal binding activity to gp140 SF162 (Supplement Fig. S2). There was a strong correlation between the binding OD values of Abs in the buccal and rectal secretions to gp140 SF162 and plasma neutralization of pseudovirus SF162 in animals immunized with C4-447 (Supplement Fig. S3A and S3B). There was also a significant correlation of the binding OD between buccal and rectal secretions (Supplement Fig. S3C).
3.4. Neutralization of tier 1 and -2 pseudoviruses
The plasma samples from weeks 18, 34 and 70 from each animal were tested in the TZM.bl cell assay against six tier 1 pseudoviruses (clade A, AG, B and C) (Table 2, Supplement Fig. S4–S7). Plasma from weeks 18 and 34 were also tested against three clade B tier 2 pseudoviruses (JRCSF, REJO and RHPA) (Table 2, Supplement Fig. S7). Within this virus panel, macaque plasma neutralized eight of nine viruses, with the exception being tier 1 virus DJ263.8 (CRF02_AG).
Table 2.
The 50% neutralization titers of macaque antibodies against Tier 1 and 2 pseudoviruses1.
| Animals immunized with C4-VH5-51 |
Animals immunized with C4-447 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pseudoviruses | Clade | Tier | Week | 27861 | 27863 | 27923 | 27141 | 27733 | 25328 |
| SF1622 | B | 1A | 18 | < 250 | 1837 | 991 | 3618 | 4637 | 2542 |
| Q461.e2.TAIV | A | 1 | 18 | <20 | 70 | 36 | 35 | 75 | 202 |
| MW965.26 | C | 1A | 18 | <20 | 66 | 20 | 67 | 58 | 75 |
| SS1196.1 | B | 1B | 18 | <20 | <20 | <20 | 136 | 147 | 151 |
| 6535 | B | 1B | 18 | <20 | <20 | <20 | 34 | 56 | 46 |
| DJ263.8 | 02_AG | 1B | 18 | <20 | <20 | <20 | <20 | <20 | <20 |
| SF 162 | B | 1A | 34 | <250 | 1217 | 3460 | 5250 | 1479 | 1415 |
| Q461.e2.TAIV | A | 1 | 34 | <20 | 28 | <20 | 52 | 56 | 77 |
| MW965.26 | C | 1B | 34 | <20 | 22 | 23 | 225 | 53 | 98 |
| SS1196.1 | B | 1B | 34 | <20 | <20 | <20 | 25 | 60 | 78 |
| 6535 | B | 1B | 34 | <20 | <20 | <20 | 25 | <20 | 35 |
| DJ263.8 | 02_AG | 1B | 34 | <20 | <20 | <20 | <20 | <20 | <20 |
| SF162 | B | 1A | 70 | <20 | <20 | <20 | 197 | 77 | 120 |
| Q461.e2.TAIV | A | 1 | 70 | <20 | 23 | 24 | 22 | 23 | 30 |
| MW965.26 | C | 1B | 70 | <20 | <20 | <20 | 25 | <20 | <20 |
| SS1196.1 | B | 1B | 70 | <20 | <20 | <20 | 24 | 20 | 34 |
| 6535 | B | 1B | 70 | <20 | <20 | <20 | 25 | <20 | <20 |
| DJ263.8 | 02_AG | 1B | 70 | <20 | <20 | <20 | <20 | <20 | <20 |
| JFCSF | B | 2 | 18 | <20 | <20 | <20 | 92 | 88 | 96 |
| REJO | B | 2 | 18 | <20 | <20 | <20 | <20 | 29 | 35 |
| RHPA | B | 2 | 18 | <20 | <20 | <20 | <20 | <20 | <20 |
| JFCSF | B | 2 | 34 | <20 | <20 | <20 | 78 | 62 | 70 |
| REJO | B | 2 | 34 | <20 | <20 | <20 | <20 | 27 | 32 |
| RHPA | B | 2 | 34 | <20 | <20 | <20 | <20 | 26 | 29 |
The inhibitory dilutions (ID50) values were determined by using the TZM-bl cell neutralization assay for individual macaque plasma samples at weeks 18, 34 and 70. The color-coded scale: >100 dilution, dark orange; <100, orange; <20, white, non-neutralizing. There is no significant difference from week to week (18, 34 and 70) for either group of animals as tested by Log-rank test, but for tier 1 viruses the difference between two groups, C4-VH5-51 versus C4-447 is highly significant, p=0.001.
The Env clone of the pseudovirus SF162 matches the Env sequence of the SHIV SF162P4 which is around five times more neutralization sensitive than the SHIV SF162 P3 and this explain exceptional sensitivity of SF162 tested in this experiment.
The potency of neutralization was determined by inhibitory dilution at 50% neutralization (ID50), which was calculated using linear regression analysis of percent neutralization of titrated plasma (Table 2). The plasma from three animals immunized with C4-447 neutralized eight of nine pseudoviruses in an ID50 dilution range between 1:20 and 1:5250 between weeks 18 and 70 for tier 1 viruses and in a range from 1:26 to 1:96 for tier 2 viruses between weeks 18 and 34. In contrast, the plasma from three animals immunized with C4-VH5-51 neutralized sporadically only three of nine pseudoviruses in a dilution range between 1:20 and 1:3460, and one plasma from animal 27861 did not display any neutralizing activity at a 1:20 dilution (Table 2). There is no significant difference from week to week for either group of animals as tested by log-rank, but for tier 1 viruses the difference between the two groups, C4-VH5-51 versus C4-447, is highly significant at weeks 18, 34 and 70 (p=0.001).
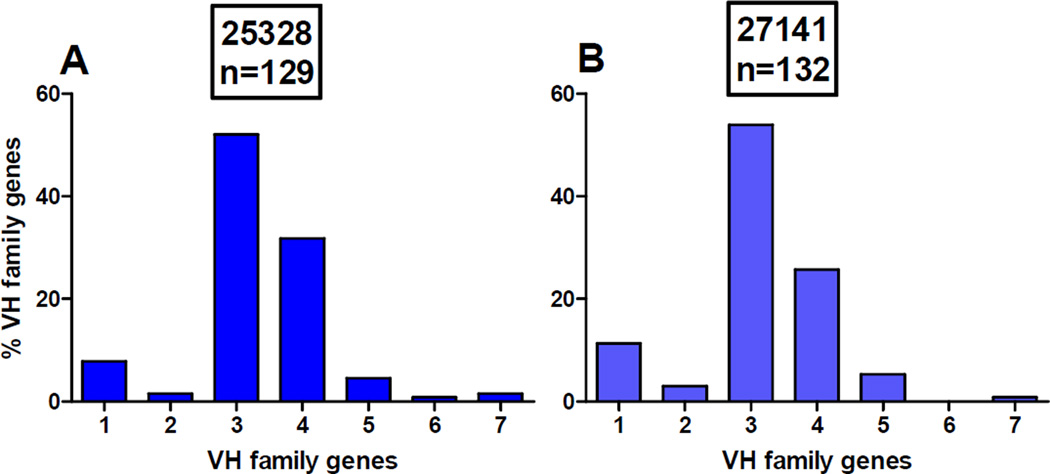
3.5. VH gene usage by IgG+ memory B cells
The frequency of the VH gene families was determined in the 261 single IgG+ memory B cells isolated from two macaques 2 weeks prior to immunization (Fig. 5, Supplement Table S1) according to methods used in our lab (30, 31). The VH3 gene family is the most frequently present in B cells from macaques 25328 and 27141 (52% and 53.9%, respectively). This is followed by the VH4 (31.8% and 25.7%, respectively) and VH1 (7.8% and 11.3%, respectively) gene families. The VH5 gene family was used by only 4.6% and 5.3% of B cells (Fig. 5, Supplement Table S1). The distribution of VH gene families in two macaques is very similar to published data showing the predominance of VH3 and VH4 families, followed by VH1, VH5 and VH2 (28).
Figure 5. Percentage of the VH gene usage by IgG+ memory B cells isolated from two rhesus macaques.
The blood samples were collected from rhesus monkey 25328 (A) and 27141 (B) two weeks prior to immunization. PBMCs were purified, stained with labeled anti-IgG, anti-CD20 and anti-CD27 Abs, and single B cells were sorted into 96-well PCR plates using a FACS Aria. RNA was reverse–transcribed, VH genes were amplified by nested PCR, and their products were sequenced. Rhesus macaques VH gene family usage was determined using the IMGT/V-QUEST/Rhesus system. n, the number of single IgG+ memory B cells analyzed for VH gene sequence.
4. Discussion
This study demonstrated differences in antibody response to the V3 region of HIV-1 gp120 in macaques immunized with two C4-V3 immunogens each containing different V3 mimotope peptides. Binding activities of anti-V3 mAbs indicate that C4-VH5-51 is recognized by anti-V3 mAbs encoded by a pair of VH5-51 and VL lambda genes, while C4-447 is recognized by anti-V3 mAbs, including 447-52D, encoded by the VH1, VH2, VH3 and VH4 gene families, but not by the VH5-51 gene (Table 1). The two V3 mimotopes were designed to target macaque naïve B cells that express Ig genes corresponding to the human VH5-51 gene and other B cells that express multiple VH genes.
Although the VH genes used by macaques for synthesis of anti-V3 Abs is unknown, serological studies of plasma Abs suggest that the antibody response to V3 mimotopes can be VH restricted. Each immunogen contained the same C4 peptide, and the titer of anti-C4 Abs was the same in the two groups of animals (with the exception of one animal); however, the titer of Abs to homologous V3 mimotope peptides was higher, trending toward significance (p=0.0531), in macaques immunized with C4-447 than in those immunized with C4-VH5-51 immunogens. The C4 peptide represents a stretch of gp120, and was not designed to target any particular B cells. In consequence, the anti-C4 Abs can use various Ig genes without restriction with similar titer in the two groups of animals. In contrast, each V3 mimotope in the immunogen was designed to elicit Abs with some VH restriction, and the C4-VH5-51 possibly targeted B cells that express the macaque VH5 genes with low frequency (5%), while the frequency of other VH genes is much higher (Fig. 5). We hypothesized that the low frequency of VH5 genes in macaque B cells resulted in the significantly lower titer of anti-V3 Abs induced by C4-VH5-51 in contrast to C4-447 immunogen, which can target B cells with a high frequency of Ig genes. There are three VH genes in the VH5 gene family in macaques (29). Of these, the VH5-A and VH5-B together have six alleles with high identity (94.79% – 95.83%) with the human VH5-51 germline sequence (Fig. S1). This suggests that the C4-VH5-51 can target macaques VH5-A and VH5-B cells and induce anti-V3 Abs.
Some support for the VH restriction of antibody response came from the three anti-V3 mAbs that we produced in preliminary experiments from macaque 27141 at week 34 (data not shown). This animal was immunized with C4-447, and the mAbs used the same pair of VH2-1/VL1-14 genes with two clonal mAbs and one unique mAb based on different sequences in the VH CDR3 domain. Although we produced only three anti-V3 mAbs, all of them used VH2-1 genes, which we expected to be used by Abs induced by C4-447 immunization.
A variety of forms and lengths of V3 peptides have been used to study their ability to elicit anti-V3 neutralizing Abs in a variety of animal species (32–38). V3 peptides – especially when they are in the cyclic form, constrained, conjugated to a carrier protein, or displayed on a scaffold protein – usually elicit Abs that cross-neutralize tier 1 viruses and sporadically elicit Abs that cross-neutralize tier 2 viruses. The anti-V3 Abs induced in macaques using the C4-V3 peptide or recombinant BCG expressing V3 antigen provided partial protection against nonpathogenic HIV-1 or SHIV, respectively (39, 40).
The experiments in this study showed how critical the titer of anti-V3 Abs is to neutralization. The higher titer of one log10 of anti-V3 Abs induced by C4-447 immunogen resulted in significantly higher neutralizing potency and breadth compared with C4-VH5-51-induced anti-V3 Abs. We hypothesize that the higher titer of anti-V3 Abs result from the broader spectrum of anti-V3 Abs induced by C4-447, which might target B cells that express multiple Ig genes and thus expand the number of clones.
The higher titer of C4-447-induced anti-V3 Abs was also critical for transducing these Abs to mucosal secretions. The buccal secretions collected from macaques immunized with C4-447 displayed significantly higher binding activities to V3 mimotope peptide than C4-VH5-51. The transduction of plasma Abs to mucosal secretions has been previously reported in animals immunized with an HIV vaccine (41) or with β-galactosidase (42). In a case of HPV16/18 vaccine, the data suggested transudation of vaccine-induced antibodies from the systemic circulation to the cervical mucosa (43). It remains to be determined whether the HIV vaccine efficacy depends on a level of transduced Abs to mucosal tissue, which is the primary route of the HIV-1 entry.
5. Conclusion
The current study was designed to induce macaque anti-V3 Abs specific to two dominant clusters of the V3 epitopes, which are recognized by mAbs coded for by different VH genes. For immunization, we used the two C4-V3 peptide immunogens containing V3 mimotopes representing the epitopes recognized by anti-V3 mAbs encoded by VH1-VH4 genes (C4-447 immunogen) and by only one VH5-51 gene (C4-VH5-51 immunogen). The serological studies revealed that C4-447 induced higher titer, trending toward significance (p=0.0531), of anti-V3 Abs than C4-VH5-51, along with significantly more potent neutralizing activities against tier 1 and some tier 2 pseudoviruses. Moreover, anti-V3 Abs were detected in buccal secretions with significantly higher binding activities in macaques immunized with C4-447 than those immunized with C4-VH5-51, which strongly correlated with plasma titer of anti-V3 Abs. This suggested that the rate of transduction of Abs to mucosal tissue is proportional to plasma concentration. The difference in the titers of anti-V3 Abs may depend on targeting a broader pool of B cells by C4-447 than C4-VH5-51, which was designed to target only VH5-51 B cells. This hypothesis will be studied by producing anti-V3 mAbs, and the first three such mAbs were already produced. The results emphasize the importance of vaccination that targets a broad range of Ig genes to elicit a high titer of Abs against a particular antigen.
Supplementary Material
Acknowledgments
The study was supported by NIH grants AI096977, AI091543 and AI112546 (MKG). We thank Dr. Arthur Nadas for statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare no conflict of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version, at
References
- 1.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zolla-Pazner S, deCamp AC, Cardozo T, Karasavvas N, Gottardo R, Williams C, Morris DE, Tomaras G, Rao M, Billings E, Berman P, Shen X, Andrews C, O'Connell RJ, Ngauy V, Nitayaphan S, de Souza M, Korber B, Koup R, Bailer RT, Mascola JR, Pinter A, Montefiori D, Haynes BF, Robb ML, Rerks-Ngarm S, Michael NL, Gilbert PB, Kim JH. Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial. PloS one. 2013;8:e53629. doi: 10.1371/journal.pone.0053629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PloS one. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, deCamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolla-Pazner S, Edlefsen PT, Rolland M, Kong XP, deCamp A, Gottardo R, Williams C, Tovanabutra S, Sharpe-Cohen S, Mullins JI, deSouza MS, Karasavvas N, Nitayaphan S, Rerks-Ngarm S, Pitisuttihum P, Kaewkungwal J, O'Connell RJ, Robb ML, Michael NL, Kim JH, Gilbert P. Vaccine-induced Human Antibodies Specific for the Third Variable Region of HIV-1 gp120 Impose Immune Pressure on Infecting Viruses. EBioMedicine. 2014;1:37–45. doi: 10.1016/j.ebiom.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrus L, Prince AM, Bernal I, McCormack P, Lee DH, Gorny MK, Zolla-Pazner S. Passive immunization with a human immunodeficiency virus type-1 neutralizing monoclonal antibody in Hu-PBL-SCID mice: Isolation of a neutralization escape variant. J. Infect. Dis. 1998;177:889–897. doi: 10.1086/515251. [DOI] [PubMed] [Google Scholar]
- 7.Emini EA, Schleif WA, Nunberg JH, Conley AJ, Eda Y, Tokiyoshi S, Putney SD, Matsushita S, Cobb KE, Jett CM, Eichberg JW, Murthy KK. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992;355:728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- 8.Watkins JD, Siddappa NB, Lakhashe SK, Humbert M, Sholukh A, Hemashettar G, Wong YL, Yoon JK, Wang W, Novembre FJ, Villinger F, Ibegbu C, Patel K, Corti D, Agatic G, Vanzetta F, Bianchi S, Heeney JL, Sallusto F, Lanzavecchia A, Ruprecht RM. An anti-HIV-1 V3 loop antibody fully protects cross-clade and elicits T-cell immunity in macaques mucosally challenged with an R5 clade C SHIV. PloS one. 2011;6:e18207. doi: 10.1371/journal.pone.0018207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eda Y, Murakami T, Ami Y, Nakasone T, Takizawa M, Someya K, Kaizu M, Izumi Y, Yoshino N, Matsushita S, Higuchi H, Matsui H, Shinohara K, Takeuchi H, Koyanagi Y, Yamamoto N, Honda M. Anti-V3 humanized antibody KD-247 effectively suppresses ex vivo generation of human immunodeficiency virus type 1 and affords sterile protection of monkeys against a heterologous simian/human immunodeficiency virus infection. J. Virol. 2006;80:5563–5570. doi: 10.1128/JVI.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita S, Yoshimura K, Ramirez KP, Pisupati J, Murakami T, Group KDS Passive transfer of neutralizing mAb KD-247 reduces plasma viral load in patients chronically infected with HIV-1. AIDS. 2015;29:453–462. doi: 10.1097/QAD.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Wang XH, Williams C, Volsky B, Steczko O, Seaman MS, Luthra K, Nyambi P, Nadas A, Giudicelli V, Lefranc MP, Zolla-Pazner S, Gorny MK. A broad range of mutations in HIV-1 neutralizing human monoclonal antibodies specific for V2, V3, and the CD4 binding site. Mol. Immunol. 2015;66:364–374. doi: 10.1016/j.molimm.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura GR, Fonseca DP, O'Rourke SM, Vollrath AL, Berman PW. Monoclonal antibodies to the V2 domain of MN-rgp120: fine mapping of epitopes and inhibition of alpha4beta7 binding. PloS one. 2012;7:e39045. doi: 10.1371/journal.pone.0039045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tassaneetrithep B, Tivon D, Swetnam J, Karasavvas N, Michael NL, Kim JH, Marovich M, Cardozo T. Cryptic determinant of alpha4beta7 binding in the V2 loop of HIV-1 gp120. PloS one. 2014;9:e108446. doi: 10.1371/journal.pone.0108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hioe CE, Wrin T, Seaman MS, Yu X, Wood B, Self S, Williams C, Gorny MK, Zolla-Pazner S. Anti-V3 monoclonal antibodies display broad neutralizing activities against multiple HIV-1 subtypes. PloS one. 2010;5:e10254. doi: 10.1371/journal.pone.0010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Miiro G, Serwanga J, Pozniak A, McPhee D, Manigart O, Mwananyanda L, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Allen S, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorny MK, Sampson J, Li H, Jiang X, Totrov M, Wang X-H, Williams C, O'Neal T, Volsky B, Li L, Cardozo T, Nyambi P, Zolla-Pazner S, Kong X-P. Human anti-V3 HIV-1 monoclonal antibodies encoded by the VH5-51/VL lambda genes define a conserved antigenic structure. PloS one. 2011;6:e27780. doi: 10.1371/journal.pone.0027780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke V, Williams C, Sukumaran M, Kim S-S, Li H, Wang X-H, Gorny MK, Zolla-Pazner S, Kong X-P. Structural Basis of the Cross-Reactivity of Genetically Related Human Anti-HIV-1 Monoclonal Antibodies: Implications for Design of V3-based Immunogens. Structure. 2009;17:1538–1546. doi: 10.1016/j.str.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanfield RL, Gorny MK, Williams C, Zolla-Pazner S, Wilson IA. Structural rationale for the broad neutralization of HIV-1 by human antibody 447-52D. Structure. 2004;12:193–204. doi: 10.1016/j.str.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Jiang X, Burke V, Totrov M, Williams C, Cardozo T, Gorny MK, Zolla-Pazner S, Kong X-P. Conserved structural elements in the V3 crown of HIV-1 gp120. Nat Struct Mol Biol. 2010;17:955–961. doi: 10.1038/nsmb.1861. [DOI] [PubMed] [Google Scholar]
- 20.Cease KB, Margalit H, Cornette JL, Putney SD, Robey WG, Ouyang C, Streicher HZ, Fischinger PJ, Gallo RC, DeLisi C, et al. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc. Natl. Acad. Sci. U. S. A. 1987;84:4249–4253. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozlowski PA, Cu-Uvin S, Neutra MR, Flanigan TP. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immunity. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorny MK, VanCott TC, Williams C, Revesz K, Zolla-Pazner S. Effects of oligomerization on the epitopes of the Human Immunodeficiency Virus Type 1 envelope glycoproteins. Virology. 2000;267:220–228. doi: 10.1006/viro.1999.0095. [DOI] [PubMed] [Google Scholar]
- 23.Blay WM, Kasprzyk T, Misher L, Richardson BA, Haigwood NL. Mutations in envelope gp120 can impact proteolytic processing of the gp160 precursor and thereby affect neutralization sensitivity of human immunodeficiency virus type 1 pseudoviruses. J. Virol. 2007;81:13037–13049. doi: 10.1128/JVI.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malherbe DC, Doria-Rose NA, Misher L, Beckett T, Puryear WB, Schuman JT, Kraft Z, O'Malley J, Mori M, Srivastava I, Barnett S, Stamatatos L, Haigwood NL. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J. Virol. 2011;85:5262–5274. doi: 10.1128/JVI.02419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reed LJM, H A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 26.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. Human Immunodeficiency Virus Type 1 env Clones from Acute and Early Subtype B Infections for Standardized Assessments of Vaccine-Elicited Neutralizing Antibodies. J. Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundling C, Phad G, Douagi I, Navis M, Karlsson Hedestam GB. Isolation of antibody V(D)J sequences from single cell sorted rhesus macaque B cells. J. Immunol. Methods. 2012;386:85–93. doi: 10.1016/j.jim.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Francica JR, Sheng Z, Zhang Z, Nishimura Y, Shingai M, Ramesh A, Keele BF, Schmidt SD, Flynn BJ, Darko S, Lynch RM, Yamamoto T, Matus-Nicodemos R, Wolinsky D, Program NCS, Nason M, Valiante NM, Malyala P, De Gregorio E, Barnett SW, Singh M, O'Hagan DT, Koup RA, Mascola JR, Martin MA, Kepler TB, Douek DC, Shapiro L, Seder RA. Analysis of immunoglobulin transcripts and hypermutation following SHIV(AD8) infection and protein-plus-adjuvant immunization. Nat. Commun. 2015;6:6565. doi: 10.1038/ncomms7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Wang X-H, Banerjee S, Volsky B, Williams C, Virland D, Nadas A, Seaman MS, Chen X, Spearman P, Zolla-Pazner S, Gorny MK. Different pattern of immunoglobulin gene usage by HIV-1 compared to non-HIV-1 antibodies derived from the same infecgted subject.e. PloS one. 2012;7:e39534. doi: 10.1371/journal.pone.0039534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Wang XH, Banerjee S, Volsky B, Williams C, Moody MA, Zolla-Pazner S, Gorny MK. Clonal analysis of human anti-V3 monoclonal antibodies selected by a V3 tetramer. Hum. Antibodies. 2012;21:65–73. doi: 10.3233/HAB-130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes BF, Torres JV, Langlois AJ, Bolognesi DP, Gardner MB, Palker TJ, Scearce RM, Jones DM, Moody MA, McDanal C, Matthews TJ. Induction of HIVMN neutralizing antibodies in primates using a prime-boost regimen of hybrid synthetic gp120 envelope peptides. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 33.Haynes BF, Ma B, Montefiori DC, Wrin T, Petropoulos CJ, Sutherland LL, Scearce RM, Denton C, Xia SM, Korber BT, Liao HX. Analysis of HIV-1 subtype B third variable region peptide motifs for induction of neutralizing antibodies against HIV-1 primary isolates. Virology. 2006;345:44–55. doi: 10.1016/j.virol.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Javaherian K, Langlois AJ, LaRosa GJ, Profy AT, Bolognesi DP, Herlihy WC, Putney SD, Matthews TJ. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990;250:1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- 35.Zolla-Pazner S, Cohen S, Pinter A, Krachmarov C, Wrin T, Wang S, Lu S. Cross-clade neutralizing antibodies against HIVI-1 induced in rabbits by focusing the immune response on a neutralizing epitope. Virology. 2009;392:82–93. doi: 10.1016/j.virol.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zolla-Pazner S, Kong X, Jiang X, Cardozo T, Nadas A, Cohen S, Totrov M, Seaman MS, Wang S, Lu S. Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA. J. Virol. 2011;85:9887–9898. doi: 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conley AJ, Conard P, Bondy S, Dolan CA, Hannah J, Leanza WJ, Marburg S, Rivetna M, Rusiecki VK, Sugg EE, Van Middlesworth F, Warne SA, Ulrich JT, Rudbach JA, Tolman RL, Emini EA. Immunogenicity of synthetic HIV-1 gp120 V3-loop peptide-conjugate immunogens. Vaccine. 1994;12:445–451. doi: 10.1016/0264-410x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 38.Moseri A, Tantry S, Sagi Y, Arshava B, Naider F, Anglister J. An optimally constrained V3 peptide is a better immunogen than its linear homolog or HIV-1 gp120. Virology. 2010;401:293–304. doi: 10.1016/j.virol.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letvin NL, Robinson S, Rohne D, Axthelm MK, Fanton JW, Bilska M, Palker TJ, Liao HX, Haynes BF, Montefiori DC. Vaccine-elicited V3 loop-specific antibodies in rhesus monkeys and control of a simian-human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate envelope. J. Virol. 2001;75:4165–4175. doi: 10.1128/JVI.75.9.4165-4175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Someya K, Cecilia D, Ami Y, Nakasone T, Matsuo K, Burda S, Yamamoto H, Yoshino N, Kaizu M, Ando S, Okuda K, Zolla-Pazner S, Yamazaki S, Yamamoto N, Honda M. Vaccination of rhesus macaques with recombinant Mycobacterium bovis bacillus Calmette-Guerin Env V3 elicits neutralizing antibody-mediated protection against simian-human immunodeficiency virus with a homologous but not a heterologous V3 motif. J. Virol. 2005;79:1452–1462. doi: 10.1128/JVI.79.3.1452-1462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Stephenson KE, Kang ZH, Lavine CL, Seaman MS, Barouch DH. Common features of mucosal and peripheral antibody responses elicited by candidate HIV-1 vaccines in rhesus monkeys. J. Virol. 2014;88:13510–13515. doi: 10.1128/JVI.02095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoji M, Katayama K, Tachibana M, Tomita K, Sakurai F, Kawabata K, Mizuguchi H. Intramuscular DNA immunization with in vivo electroporation induces antigen-specific cellular and humoral immune responses in both systemic and gut-mucosal compartments. Vaccine. 2012;30:7278–7285. doi: 10.1016/j.vaccine.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 43.Scherpenisse M, Mollers M, Schepp RM, Meijer CJ, de Melker HE, Berbers GA, van der Klis FR. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum. Vaccines & Immunotherapeutics. 2013;9:314–321. doi: 10.4161/hv.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.