Abstract
Between March 2007 and July 2009, 325 Highly Pathogenic Avian Influenza (HPAI, subtype H5N1) outbreaks in poultry were reported in 154 out of a total of 486 sub-districts in Bangladesh. This study analyzed the temporal and spatial patterns of HPAI H5N1 outbreaks and quantified the relationship between several spatial risk factors and HPAI outbreaks in sub-districts in Bangladesh. We assessed spatial autocorrelation and spatial dependence, and identified clustering sub-districts with disease statistically similar to or dissimilar from their neighbors. Three significant risk factors associated to HPAI H5N1 virus outbreaks were identified; the quadratic log-transformation of human population density [humans per square kilometer, P = 0.01, OR 1.15 (95% CI: 1.03–1.28)], the log-transformation of the total commercial poultry population [number of commercial poultry per sub-district, P < 0.002, OR 1.40 (95% CI: 1.12–1.74)], and the number of roads per sub-district [P = 0.02, OR 1.07 (95% CI: 1.01–1.14)]. The distinct clusters of HPAI outbreaks and risk factors identified could assist the Government of Bangladesh to target surveillance and to concentrate response efforts in areas where disease is likely to occur. Concentrating response efforts may help to combat HPAI more effectively, reducing the environmental viral load and so reducing the number of disease incidents.
Keywords: Highly Pathogenic Avian Influenza, Bangladesh, HPAI, H5N1, Risk factor, Spatial analysis
1. Introduction
Since 2003, Highly Pathogenic Avian Influenza (HPAI, H5N1 subtype) virus has spread from China (Guan et al., 2004) to Southeast Asia (MMWR, 2004), Europe and Africa (Science, 2006), and HPAI H5N1 outbreaks occurred in over 60 countries (FAO, 2007). HPAI H5N1 events had significant impacts on poultry production, farmers’ livelihoods and human health. It has resulted in over 400 human infections with more than 260 deaths (WHO, 2009).
Animal disease reporting and surveillance in Bangladesh is conducted through the governmental public veterinary network that covers all 64 districts and 486 sub-districts of the country. The administrative structure consists of one Chief Veterinary Officer (CVO), six Divisional Veterinary Officers, 64 District Veterinary Officers and 486 Sub-district Veterinary Officers. The Sub-district Veterinary Officers report animal disease and collect animal census data. This data is collated through the chain of command and send to the CVO. Emerging diseases and emergencies (as HPAI) are reported by phone. Since May 2008 there was an active HPAI surveillance network in place in 150 sub-districts, consisting out of 50 full time employed and specifically trained veterinarians and 150 community animal health workers. The surveillance teams report to the Sub-district Veterinary Officers directly and through an Short Message Service gateway to the central command (FAO, 2009; Loth et al., 2009). HPAI H5N1 was first reported in poultry in Bangladesh in March 2007 (OIE, 2009). Since then the Department of Livestock Services responded to 325 outbreaks in 47 districts in Bangladesh (EMPRES-i, 2009). According to the Ministry of Fishery and Livestock (MoFL), out of a total poultry population of 220 million, over 200,000 poultry died and 1.6 million poultry have been culled as part of the disease response and control measures (MoFL, 2009). One human H5N1 infection has been reported from Bangladesh in 2008, the patient survived (WHO, 2008).
Previous studies in Hong Kong (Kung et al., 2007), Thailand (Tiensin et al., 2005) and Vietnam (Pfeiffer et al., 2007) have shown that disease introduction, spread and persistence are associated with poultry trading patterns, poultry densities, poultry production systems, live bird markets (Sims, 2007) and domestic ducks (Gilbert et al., 2006a; Hulse-Post et al., 2005). In Bangladesh, agricultural and ecological conditions of HPAI H5N1 spread and persistence may somewhat differ from those reported in Thailand and Vietnam as agriculture in Bangladesh is primarily extensive and subsistence, with much lower levels of long-distance trading (Dolberg, 2009). Although two epidemiological studies (Biswas et al., 2008, 2009) identified farm-level risk factors, spatial risk factors associated with the HPAI H5N1 spread at the national level is unknown. Additionally, epidemiological research needs to include the cluster analysis of disease occurrence and risk factors in specific locations and during specific time periods, which leads to define location-sensitive “hot spots” and time-sensitive “hot times” risk maps (Gilbert et al., 2008). To date, HPAI H5N1 clusters in poultry have been investigated in China (Oyana et al., 2006), Thailand (Tiensin et al., 2009) and Vietnam (Henning et al., 2009; Minh et al., 2009), but not in Bangladesh.
Bangladesh has very limited resources and is one the most densely populated countries in the world with over 140 million citizens. These conditions pose great challenges to develop a HPAI H5N1 control strategy, including early detection, stamping-out and prevention. In those conditions, better understanding of the disease epidemiology allowing a better targeting of the available surveillance and control resources is particularly critical. In this study we investigated temporal and spatial patterns and the relationship between several spatial risk factors and HPAI outbreaks in Bangladesh at sub-district level. Our objective is to understand the relative significance of the different components of this complex epidemiological system. This will provide useful information to the HPAI response programme for policy and intervention measures that aim to interrupt the disease transmission cycle.
2. Materials and methods
2.1. Data
HPAI H5N1 outbreak data were provided by the Department of Livestock Services, Dhaka, Bangladesh. The outbreak data were based on both the national clinical disease reporting system at sub-district level for the entire period and the active disease surveillance program in the 150 high-risk sub-districts (Loth et al., 2009) from May 2008 to July 2009; these two reporting systems were complementary as all suspect HPAI cases were reported to the Sub-district Veterinary Officer. The diagnosis of a HPAI outbreak was based upon a three stage diagnosis: (A); a case definition of “unusually high mortality or morbidity rates” in chickens and ducks, commercial and backyard; (B); combined with a positive rapid antigen test (The Flu Detect™ Antigen Capture test, Synbiotics Corp., San Diego, CA) for Influenza A on the farm or at a regional laboratory; and (C); a positive reverse-transcriptase polymerase chain reaction (PCR) test for Hemagglutinin-5 (H5) at the National Reference Laboratory for HPAI near the capital Dhaka.
HPAI H5N1 outbreak data were grouped at sub-district level over the period of March 2007 to July 2009. Sub-districts with one or more HPAI event were classified “positive”, sub-districts without HPAI events as “negative”. Bangladesh is administratively divided into 6 divisions, 64 districts and 486 sub-districts. The area of sub-district ranges from 4.4 km2 to 1730 km2, with a mean area of 281 km2; Sub-district data were used as the epidemiological unit in this study. Geographical information system (Arc-GIS, version 9.2, ESRI), was used for mapping and visualization of the data.
2.2. Spatial and cluster analysis
HPAI events in Bangladesh over the reporting period took place in distinct phases. These phases were separated by periods without any reported events. Three types of analyses were undertaken to examine the spatial clustering of H5N1 outbreaks at a sub-district level (Tiensin et al., 2009).
First, global (non-specific) Moran's I spatial autocorrelation statistic with a “Queen Contiguity” weight matrix was used to quantify spatial dependence, for each phase in time and for the entire period (Moran, 1950). Second, we used two methods of cluster detection. Two, because different analytical methods may identify different underlying spatial patterns (Jacquez and Greiling, 2003). The same cluster sub-districts should be identified with two methods:
-
(a)
local spatial autocorrelation analysis was conducted using the GeoDa cluster detection programme to generate Anselin's Local Moran test statistics (GeoDa-0.9.5-i). Local Moran's statistics assess spatial autocorrelation and identify clustering sub-districts with disease statistically similar to or dissimilar from their neighbors on the basis of aggregated data (Anselin, 1995, 2005).
-
(b)
a spatial scan test by SaTScan, version 7.0.3 (SatScan, 2009), was used to examine spatial clustering over sub-districts (Kulldorff et al., 1997; Kulldorff and Nagarwalla, 1995). The (purely spatial, Bernoulli) scan statistic test assessed disease distribution with the use of sub-district centroids and a circular scan for cases (HPAI positive sub-district) and controls (HPAI negative sub-districts) (Kulldorff et al., 1998). The maximum cluster size was set to 50% of the sub-districts. Third, we estimate the extent of the autocorrelation of the outcome variable, HPAI H5N1 positive or negative sub-districts, using the range of the spatial correlogram ρ(h) (Pebesma, 2004).
2.3. Risk factor analysis
Risk factors considered in this analysis include agricultural production systems relevant to poultry farming and factors identified in earlier studies (Gilbert et al., 2006a; Morris et al., 2005; Pfeiffer et al., 2007; Rushton et al., 2004; VSF, 2004).
- - Human population related transmission:
- The Bangladesh Population Census data of 2001, which recorded the number of people per village, was used (BBS, 2009). Village-level human population data were aggregated to sub-district level, using the sub-district-level administration boundary map (Source: (CEGIS, 2009)). Human population densities at sub-district were calculated based on land areas at sub-district. Human population density is log-normally distributed, and therefore, a log-transformation of human population density was carried out, and used in the models. In addition, a quadratic term for population density was also added to account for possible curvilinear relationship between human population and HPAI risk in poultry. It was assumed that risk of HPAI poultry could be high in peri-urban areas where human population density was intermediate but poultry density was high. Conversely, a lower risk could be assumed in areas with very high human densities (e.g., big cities), and in areas with very low human population density, due to lower poultry densities.
- Poultry markets have been identified as a major risk factor contributing to the spread of HPAI in poultry (Dharmayanti, 2008; Wang et al., 2006), but no data on market locations in Bangladesh were available. As an alternative, the centers of cities with populations over 150,000 and villages (population size < 150,000) were assumed to be the indicators of market locations. The number of cities and villages in the sub-districts were used for the risk analysis (Source: (CEGIS, 2009)).
- Poultry movement was regarded as a risk factor (Rappole and Hubalek, 2006). To estimate between sub-districts movements, the number of major (=sealed, two lanes or more) roads and the total length of these major roads in a sub-district were calculated (Source: (CEGIS, 2009)). Minor (sealed and unsealed, single lane) roads were assumed only to be used for within sub-district movements and not further considered.
- - Risk factors related to poultry and the amplification of H5N1 virus:
- The Year 2005 poultry dataset was composed of the total number of chickens (backyard, broilers and layers) and ducks (combined backyard and commercial), as well as the numbers of layer and broiler farms per sub-district (BBS, 2009). The densities of backyard, broiler, layer chickens and ducks in a sub-district were calculated based upon the land surface area of that sub-district. Total commercial poultry density was calculated by combining the layer and broiler densities. Duck populations are reported as a major risk factor for HPAI transmission to chickens (Gilbert et al., 2006a) and therefore included in the analysis. Poultry density data has a log-normal distribution, and therefore, a log-transformation of poultry data was carried out and used in the models.
- - Agricultural and environmental risk factors:
- Free ranging ducks are associated with rice production as they feed on post-harvested rice fields (Gilbert et al., 2007). Geospatial datasets of paddy rice and cropping intensity (single-, double- and triple-cropping in a year) at 500-m spatial resolution, derived from analysis of time-series satellite images from Moderate Resolution Imaging Spectroradiometer (MODIS, 2010) onboard the NASA Terra satellite in 2005, were used. (Gilbert et al., 2008; Xiao et al., 2005, 2006).
In this study we used a similar approach as reported in previous studies in Thailand (Gilbert et al., 2006a), Vietnam (Gilbert et al., 2008) and Indonesia (Pfeiffer, 2006). The analysis was conducted with HPAI H5N1 positive or negative sub-districts as binary outcome. Odds ratios were estimated using multivariable logistic regression analyses, where variables with P ≤ 0.1 from the univariate analyses were included (18 variable and 8 transformations, Table 1) in the initial logistic model. A backward stepwise variable–selection strategy was used to construct a final model with a significance level of P < 0.05. Once all variables in the model were significant, the negativity or positivity of their association was checked and verified that the sign was identical to the sign in a bivariate relationship. This is done to ensure that significant relationships were not resulting from co-linearity between variables (Gupta, 2003). Spatial autocorrelation in the model was accounted for by applying an autologistic approach (Augustin et al., 1996), The extent of the autocorrelation [the range of the spatial correlogram ρ(h), as discussed under “Spatial and Cluster Analysis (Pebesma, 2004)”] was used to derive an autoregressive term that was added as predictor in the logistic model. The model fit was assessed with the Hosmer–Lemeshow test (Hosmer and Lemeshow, 2000). The performance of the model was assessed by determining the area under the curve (AUC) of the receiver operating characteristics (ROC) plots. AUC is a quantitative measure of the overall fit of the model that varies from 0.5 (chance event) to 1.0 (perfect fit) (Greiner et al., 2000).
Table 1.
Variables used in the initial univariate analyses.
| Variable | Mean | Standard deviation | Univariate analysis P-value |
|---|---|---|---|
| Number of people per sub-district | 2.49E+05 | 1.32E+05 | <0.001 |
| Densitya of people per sub-district | 1.98E+03 | 5.81E+03 | 0.734 |
| Log[number of people per sub-district] | 5.33 | 0.25 | <0.001 |
| [Number of people per sub-district]2 | 7.97E+10 | 9.67E+10 | <0.001 |
| Log[number of people per sub-district]2 | 28.51 | 2.65 | <0.001 |
| [Density of people per sub-district]2 | 3.76E+07 | 2.78E+08 | 0.830 |
| Log[Density of people per sub-district]2 | 9.07 | 2.56 | 0.001 |
| Number of roads per sub-district | 3.01 | 3.85 | <0.001 |
| Total length of roads per sub-district (m) | 1.49E+04 | 1.70E+04 | <0.001 |
| Number of villages per sub-district | 1.87E+02 | 1.01E+02 | 0.429 |
| Number of major towns per sub-district | 0.29 | 0.49 | 0.078 |
| Number of chickens per sub-district | 3.69E+05 | 6.00E+05 | <0.001 |
| Number of backyard chickens per sub-district | 2.36E+05 | 1.98E+05 | 0.001 |
| Number of layer farms per sub-district | 43.78 | 2.56E+02 | 0.012 |
| Number of layer birds per sub-district | 5.88E+04 | 3.36E+05 | <0.001 |
| Number of broiler farms per sub-district | 79.27 | 1.49E+02 | <0.001 |
| Number of broiler birds per sub-district | 7.44E+04 | 2.21E+05 | <0.001 |
| Number of commercial chickens per sub-district | 1.33E+05 | 5.36E+05 | <0.001 |
| Log[Number of commercial chickens per sub-district] | 4.37 | 1.31 | <0.001 |
| Density of chickens per sub-district | 1.97E+03 | 4.76E+03 | 0.009 |
| Density of backyard chickens per sub-district | 1.15E+03 | 2.07E+03 | 0.444 |
| Density of layer farms per sub-district | 0.23 | 1.01 | 0.049 |
| Density of layer birds per sub-district | 4.69E+02 | 1.90E+03 | 0.025 |
| Density of broiler farms per sub-district | 0.47 | 1.49 | 0.009 |
| Density of broiler birds per sub-district | 5.45E+02 | 2.41E+03 | 0.007 |
| Density of commercial chickens per sub-district | 8.19E+02 | 3.35E+03 | 0.001 |
| Log[Density of chickens per sub-district] | 2.93 | 0.73 | <0.001 |
| Log[Density of backyard chickens per sub-district] | 2.70 | 0.80 | 0.049 |
| Log[Density of commercial chickens per sub-district] | 2.16 | 0.88 | <0.001 |
| Number of ducks per sub-district | 8.04E+04 | 9.71E+04 | 0.682 |
| Density of ducks per sub-district | 3.42E+02 | 3.98E+02 | 0.858 |
| Log[Density of ducks per sub-district] | 2.19 | 0.74 | 0.731 |
| Mean number of crops per year per sub-district | 1.17 | 0.27 | 0.139 |
| Mean number of rice harvests per year per sub-district | 0.47 | 0.30 | 0.166 |
| Number of crops per year per sub-district | 1.38E+03 | 8.88E+02 | 0.566 |
| Number of rice harvests per year per sub-district | 5.42E+02 | 4.48E+02 | 0.670 |
| Autoregressive term | 0.12 | 0.06 | <0.001 |
Density: number/km2.
3. Results
3.1. Disease mapping and cluster analysis
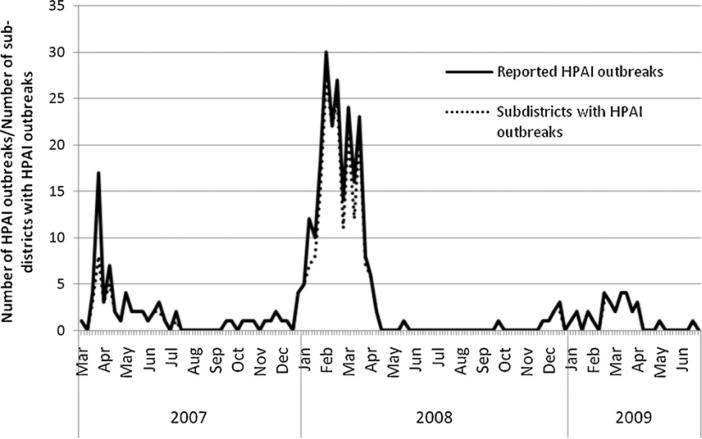
Over the period from March 2007 to July 2009, 325 HPAI outbreaks were reported in 154 out of a total of 486 sub-districts in Bangladesh. The weekly epidemic curve of H5N1 outbreaks from March 2007 through July 2009 and a curve of the total number of sub-districts with HPAI outbreaks by week show the changes in spatial extent of disease outbreaks (Fig. 1). The number of HPAI outbreaks and the number of sub-districts with outbreaks per week is similar; almost every new HPAI outbreak occurred in a different sub-district. Fig. 1 also illustrated clearly the epidemic occurring in three distinct phases. The first phase is from March 2007 to July 2007 (n = 54), the second phase from August 2007 to July 2008 (n = 233) and the third phase from August 2008 to July 2009 (n = 38).
Fig. 1.
Epidemic curve of the number of HPAI outbreaks and the number of sub-districts with HPAI outbreaks by week. Phase I, March–July 2007; Phase II, August 2007–July 2008, Phase III, August 2008–July 2009.
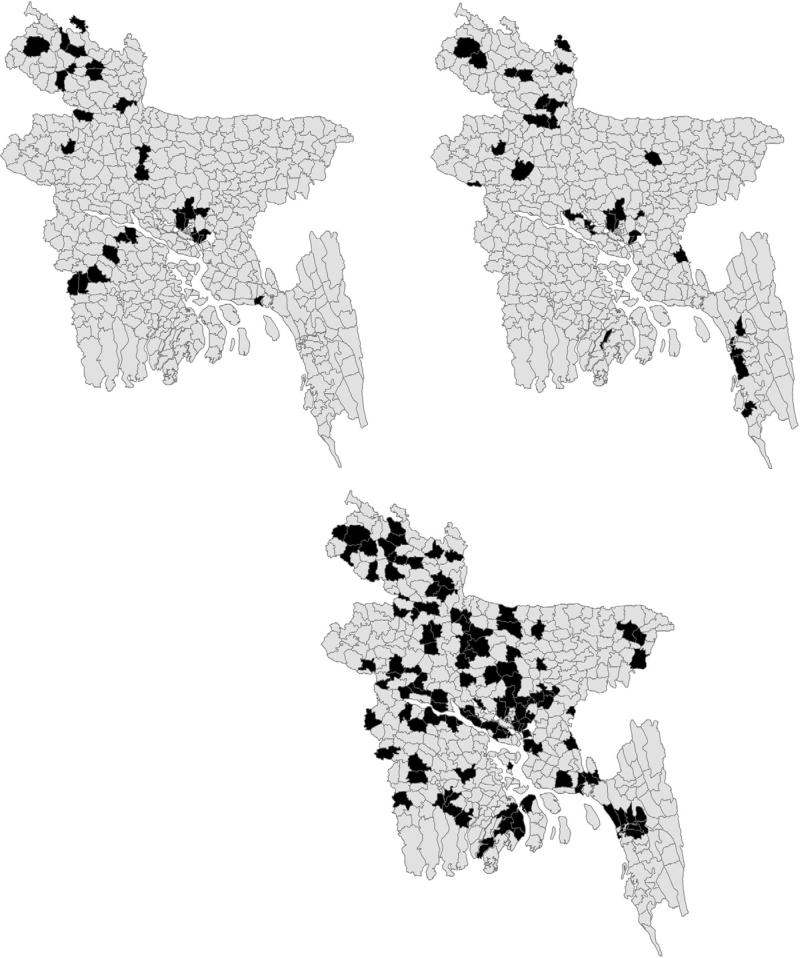
The spatial extent of HPAI events over the abovementioned three phases is shown in Fig. 3. HPAI H5N1 outbreaks occurred in 29 sub-districts in Phase I, 129 sub-districts in Phase II and 30 sub-districts in Phase III. Eight sub-districts had H5N1 outbreaks throughout the three phases, 18 sub-districts had H5N1 outbreaks in both Phase I and II and 17 sub-districts had outbreaks in both Phase II and III.
Fig. 3.
Sub-districts with HPAI outbreaks reported in black: Left; Phase I, March–July 2007; Middle; Phase II, August 2007–July 2008, Right; Phase III, August 2008–July 2009.
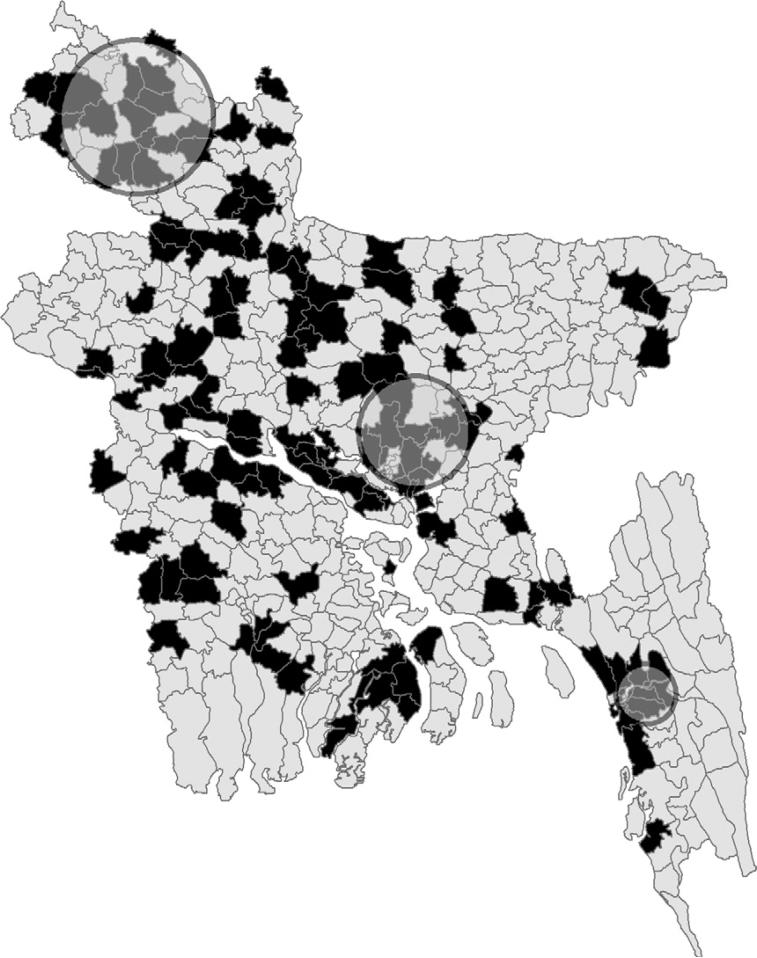
Moran's I statistics investigated non-specific spatial autocorrelation and found spatial aggregation of the negative and positive sub-districts for the entire period and for every time phases (P < 0.01) (Table 2). Both non-focused cluster analysis techniques revealed overlapping clusters of high risk HPAI areas in Phase I, Phase II and the entire period. No significant clusters were found in Phase III by either Anselin's Local Moran test statistics or SatScan. The spatial location of three significant clusters, calculated with SatScan, for the entire period, is shown in Fig. 4. All three clusters identified areas with a high HPAI relative risk (RR) (Central: RR 2.4, 95% CI 2.3–2.5; North-West: RR 1.9, 95% CI 1.7–2.2; South-East RR 2.0, 95% CI 1.1–3.0, Table 3).
Table 2.
Results of the global spatial autocorrelation by Moran's I statistics, based on HPAI outbreaks by sub-districts, for the 3 study periods and the combined period March 2007-July 2009.
| Study period | Moran's I statistic | Z score | P value | Pattern | Sub-districts |
|
|---|---|---|---|---|---|---|
| No. of case | No. of control | |||||
| March-July 2007 | 0.666 | 6.45 | 0.001 | Clustered | 28 | 458 |
| August 2007-July 2008 | 0.776 | 4.73 | <0.001 | Clustered | 112 | 374 |
| August 2008-July 2009 | 0.663 | 3.85 | <0.01 | Clustered | 30 | 456 |
| March 2007-July 2009 | 0.183 | 6.03 | 0.001 | Clustered | 138 | 348 |
Fig. 4.
Sub-districts with all HPAI outbreaks reported in black and three significant circular clusters of HPAI hot spots.
Table 3.
Cluster analyses’ results.
| Cluster | Location | Radius (km) | Log likelihood ratio | P value | Observed | Expected | Relative risk | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| 1 | Central | 35 | 49.4 | 0.001 | 90 | 46 | 2.4 | 2.3 | 2.5 |
| 2 | North-West | 47 | 13.2 | 0.003 | 39 | 22 | 1.9 | 1.7 | 2.2 |
| 3 | South-East | 19 | 9.2 | 0.045 | 17 | 9 | 2.0 | 1.1 | 3.0 |
3.2. Risk factors
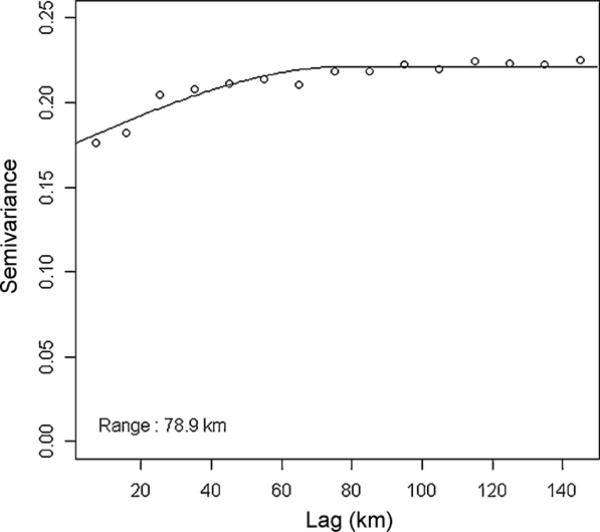
The semivariogram of HPAI presence/absence data showed autocorrelation at distances <78.9 km, which was set as the maximum distance of the autoregressive term (Fig. 2). The significant variables (risk factors) identified by the autologistic regression models were the quadratic log-transformation of human population density [humans per square kilometer, P = 0.01, OR 1.15 (95% CI: 1.03–1.28)], the log-transformation of the total commercial poultry population [number of commercial poultry per sub-district, P < 0.002, OR 1.40 (95% CI: 1.12–1.74)], and the number of roads per sub-district [P = 0.02, OR 1.07 (95% CI: 1.01–1.14)] (Table 4). The final logistic regression model was:
Fig. 2.
The semivariogram of HPAI presence or absence data showed autocorrelation at distances <78.9 km.
Table 4.
Results of the autologistic regression model with the outcome variable positive or negative HPAI sub-districts.
| Variable | Estimate | Standard error | Z score | P value | Odd ratio | 95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (Intercept) | –7.96 | 1.60 | –4.99 | <0.001 | - | - | - |
| Log[number of people per square kilometer]2 | 0.14 | 0.06 | 2.44 | 0.01 | 1.15 | 1.03 | 1.28 |
| Log[Number of commercial chickens per sub-district] | 0.35 | 0.11 | 3.14 | <0.002 | 1.40 | 1.12 | 1.74 |
| Number of roads per sub-district | 0.07 | 0.03 | 2.27 | 0.02 | 1.07 | 1.01 | 1.14 |
| Autoregressive term | 11.1 | 1.98 | 5.58 | <0.001 | 2.93E+04 | 587 | 1.46E+06 |
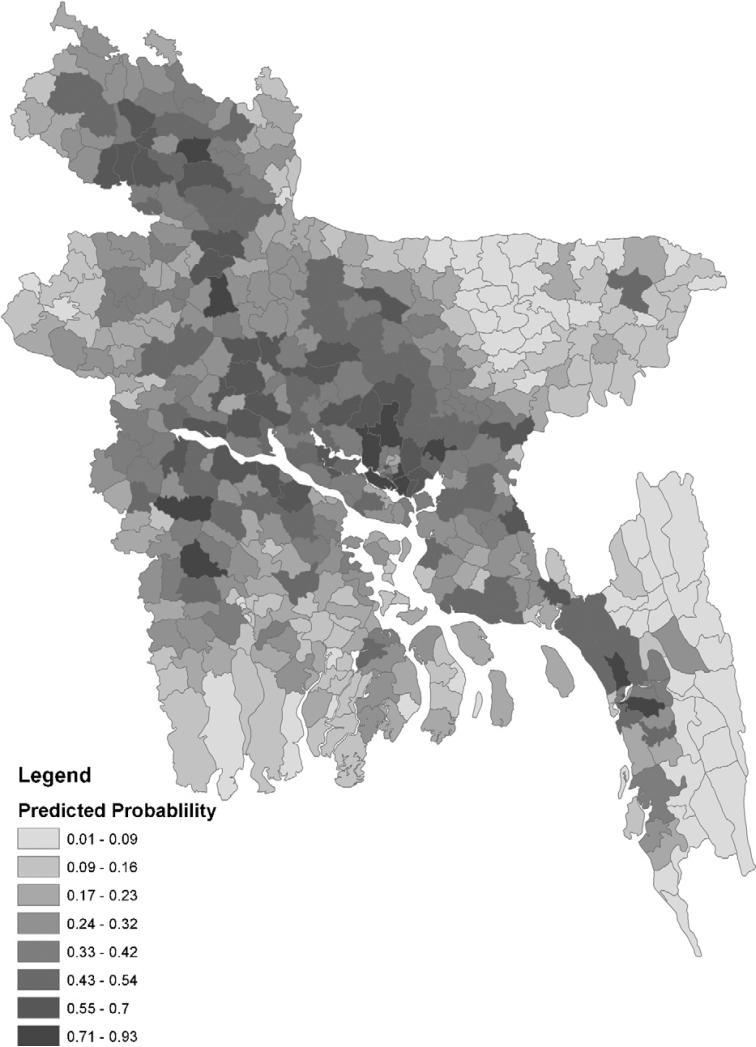
The autoregressive term was highly significant (P < 0.000001), which again confirmed the spatial autocorrelation in the data. The Hosmer and Lemeshow goodness-of-fit Chi-squared test of the model was 5.87 (P = 0.66). The ROC curve assessed the predictive power of the model; and the AUC was 0.75 (95% Confidence Interval 0.70–0.78). The autologistic regression model's prediction of the probability of HPAI positive sub-districts is shown in Fig. 5. The model predicts that HPAI is more likely to occur on a broad diagonal line from the Northwest to the Southeast.
Fig. 5.
Predicted probability of HPAI in Bangladesh.
4. Discussion
The distinct three phases of HPAI outbreaks in Bangladesh was difficult to explain (Fig. 1). More HPAI was reported in the cool (average daily temperature of 15–20 °C) and dry winter months (December–March), while no disease was reported during the hot (average daily temperature of 30 °C) and wet summer season [June–August (Islam, 2009)]. It has been suggested that the H5N1 virus was introduced in Bangladesh by migratory birds (Biswas et al., 2009). Analyses of molecular DNA sequence data from 27 virus isolates collected in early 2007 (n = 10), mid 2008 (n = 10) and early 2009 (n = 7) showed that all virus isolates belonged to sub-Clade 2.2 with little difference (<5%) in genetic makeup (Genbank, 2002). This suggests that of the same HPAI H5N1 virus strains of Phase I re-emerged in Bangladesh at the start of Phase II and III. The virus possibly goes undetected between the phases, circulating in backyard chickens, ducks, wildlife or migratory birds.
The quality of the data used in this study may have some limitations. First of all; if no HPAI outbreaks were detected during certain periods, this may indicate that the surveillance programme is not sensitive and fails to detect HPAI in chickens based on clinical symptoms or the disease goes undetected in ducks as no diagnostic surveillance is conducted. Further, the detection of HPAI in certain areas may be caused by surveillance bias. Secondly; the animal census data used in this analysis (BBS, 2009) is the data collected in 2005 and was not accurate anymore in 2009. Thirdly; the centers of major cities and villages were assumed to be the indicators of market locations. This may have been far from accurate and could have resulted in erroneous results for the risk factor “Presence of Markets”.
The national-wide spatial distribution of detected HPAI outbreaks was evident. Fig. 1 showed that almost all weekly cumulated HPAI outbreaks occurred in different sub-districts. Spatial distribution of outbreaks during the three phases showed large extent of outbreaks for each phase. During the Phase I, HPAI events occurred west of a Northwest–Southeast diagonal line. During the Phase II, HPAI events occurred almost everywhere, except in the Deep South. The 38 HPAI events during the Phase III included areas where previously no disease was detected. Cox's Bazar, the nation's most southerly town incurred HPAI in May 2009. All this suggests that HPAI is endemic in Bangladesh, with the virus circulating in unknown animal populations.
The clusters identified by both cluster detection methods indicated that the disease was more likely to be detected in geographical areas surrounding or bordering large cities; in the middle Dhaka and in the Southeast Chittagong. This is largely attributed to the fact that most commercial poultry is reared close to large human populations. After numerous incidents of HPAI in the first half of 2008 (Phase II, number of HPAI events = 233) the Government of Bangladesh revised the disease control strategy. Surveillance was improved by conducting active surveillance in 150 sub-districts with 450 Community Animal Health workers and 50 veterinary surgeons (Loth et al., 2009) assigned fulltime to door-to-door surveillance. Stamping-out was conducted immediately after an Avian Influenza A positive test (on the farm or within 24 h at the local laboratory), instead of awaiting confirmation of a H5 PCR test in the only HPAI reference laboratory, which could take several days. Far less outbreaks were reported in Phase III (n = 38) and no more significant local clusters were found during Phase III, which possibly indicates that control measures (early detection and timely stamping-out of disease) were effective, resulting in less local spread of disease. Although far less numbers of HPAI outbreaks were reported in Phase III, the extensive spatial spread of HPAI events was worrisome.
In Bangladesh, HPAI outbreaks have been mainly reported in commercial layer flocks (242 out of 325, unpublished data; Department of Livestock Services). Layer flock are particular susceptible to HPAI infections due to frequent movements on and off the farms. Possible contaminated transport vehicles return dirty egg-trays back to the farms, increasing the chance of disease spread (Biswas et al., 2008; Thomas et al., 2005). Increased movement over longer distances may increase the disease spread even further (Rivas et al., 2007). This may explain why “roads” was a significant risk factor.
In previous studies in Thailand and Vietnam (Gilbert et al., 2006a, 2008), domestic free-grazing ducks had been identified as a major risk factor, but not in this study. Two possible explanations: Firstly; the duck numbers used in the model may not have been accurate. Or, secondly; the difference in duck trading intensity between Bangladesh and other Southeast Asia countries (Songserm et al., 2006). In Bangladesh ducks are mainly kept for egg production in small numbers in backyards, and very few commercial duck (meat or layer) farms exist (Das et al., 2008) compared to Thailand and Vietnam where they were identified as a key risk factor. Wildlife and migratory birds have been identified as risk factors for the spread of HPAI (Gilbert et al., 2006b; Jourdain et al., 2007; Zhang et al., 2007). Neither migratory bird nor wildlife surveillance has been conducted in Bangladesh to date. Data on wildlife and migratory birds was not available and was not included in the analysis. Additional research on the roles of wildlife, migratory birds and ducks in the epidemiology of HPAI H5N1 in Bangladesh is urgently needed.
5. Conclusions
Since 2007, HPAI H5N1 outbreaks in Bangladesh occurred in three distinct temporal phases with most outbreaks reported in the first trimester of the year. Spatial analysis showed that the disease was widespread with three significant circular clusters of HPAI H5N1 hotspots located near large cities. The three risk factors identified and modelled could predict with 75% accuracy the presence of HPAI outbreaks per sub-district. These findings, together with the cluster analysis, can be used to identify high risk areas and assist targeted risk-based surveillance in those areas. Further improved data collection and analysis would likely lead to improving our understanding of ecology and epidemiology of HPAI H5N1 in Bangladesh.
Acknowledgements
The authors would like to thank the Department of Livestock Services, Bangladesh for providing the HPAI H5N1 data and technical support. This study was supported by the FAO Avian Influenza Control Program – Bangladesh, the United States Agency for International Development, the Asian Development Bank, the US National Institutes of Health Fogarty International Center (NIH, 5R01TW007869-03) and the NASA Land Use and Land Cover Change Program (NNX09AC39G).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Anselin L. Local indicators of spatial association–LISA. Geogr. Anal. 1995;27:93–115. [Google Scholar]
- Anselin L. Exploring Spatial Data with GeoDa: A Workbook. 2nd edition University of Illinois; Urbana: 2005. [Google Scholar]
- Augustin NH, Mugglestone MA, Buckland ST. An autologistic model for the spatial distribution of wildlife. J. Appl. Ecol. 1996;33:339–347. [Google Scholar]
- BBS [12 November 2009];Bangladesh Bureau of Statistics. 2009 from http://www.bbs.gov.bd/
- Biswas PK, Christensen JP, Ahmed SS, Barua H, Das A, Rahman MH, Giasuddin M, Hannan AS, Habib AM, Debnath NC. Risk factors for infection with highly pathogenic influenza A virus (H5N1) in commercial chickens in Bangladesh. Vet. Rec. 2009;164:743–746. doi: 10.1136/vr.164.24.743. [DOI] [PubMed] [Google Scholar]
- Biswas PK, Christensen JP, Ahmed SS, Barua H, Das A, Rahman MH, Giasuddin M, Hannan AS, Habib MA, Ahad A, Rahman AS, Faruque R, Debnath NC. Avian influenza outbreaks in chickens, Bangladesh. Emerg. Infect. Dis. 2008;14:1909–1912. doi: 10.3201/eid1412.071567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEGIS [12 March 2009];Center for Environmental and Geographic Information Services. 2009 from http://www.cegisbd.com/
- Das SC, Chowdhury SD, Khatun MA, Nishibori M, Isobe N, Yoshimura Y. Poultry production profile and expected future projection in Bangladesh. World's Poult. Sci. J. 2008:99–118. [Google Scholar]
- Dharmayanti I. Environmental Sampling of AI Virus in Live Bird Markets in Indonesia. Regional Task Force Meeting on Avian Influenza; Bali, Indonesia. 2008. [6 November 2008]. from http://www.searo.who.int/LinkFiles/Publication CD-177.pdf. [Google Scholar]
- Dolberg F. [12 August 2009];Poultry sector country review—Bangladesh. 2009 from ftp://ftp.fao.org/docrep/fao/011/ai319e/ai319e00.pdf.
- EMPRES-i . Information System for Early Warning and Response of Transboundary Animal Diseases. Food and Agriculture Organization of the United Nations; 2009. [12 May 2009]. from http://empres-i.fao.org/empres-i/home. [Google Scholar]
- FAO . Emergency Prevention System (EMPRES) for Transboundary Animal and Plant Pest and Diseases. EMPRESMAP; 2007. [6 December 2007]. from http://www.fao.org/ag/AGAinfo/programmes/en/empres/maps.html. [Google Scholar]
- FAO [11 March 2010];SMS Gateway data collection in Bangladesh. 2009 from ftp://ftp.fao.org/docrep/fao/ 012/ak132e/ak132e00.pdf.
- Genbank [July 2009];Genetic sequence database, an annotated collection of all publicly available DNA sequences. 2002 from http://www.ncbi.nlm.nih.gov/Genbank/
- [12 July 2009];GeoDa-0.9.5-i, Arizona State University. GeoDa Center for Geospatial Analysis and Computation. from http://geodacenter.asu.edu.
- Gilbert M, Chaitaweesub P, Parakamawongsa T, Premashthira S, Tiensin T, Kalpravidh W, Wagner H, Slingenbergh J. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerg. Infect. Dis. 2006a;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao X, Chaitaweesub P, Kalpravidh W, Premashthira S, Boles S, Slingenbergh J. Avian influenza, domestic ducks and rice agriculture in Thailand. Agric. Ecosyst. Environ. 2007;119:409–415. doi: 10.1016/j.agee.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao X, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, Chaitaweesub P, Kalpravidh W, Minh PQ, Otte MJ, Martin V, Slingenbergh J. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proc. Natl. Acad. Sci. U.S.A. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao XM, Domenech J, Lubroth J, Martin V, Slingenbergh J. Anatidae migration in the Western Palearctic and spread of highly pathogenic avian influenza H5N1 virus. Emerg. Infect. Dis. 2006b;12:1650–1656. doi: 10.3201/eid1211.060223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Guan Y, Poon LL, Cheung CY, Ellis TM, Lim W, Lipatov AS, Chan KH, Sturm-Ramirez KM, Cheung CL, Leung YH, Yuen KY, Webster RG, Peiris JS. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RC. On some association measures in bivariate distributions and their relationships. J. Stat. Plann. Inference. 2003;117:83–98. [Google Scholar]
- Henning J, Pfeiffer DU, Vu le T. Risk factors and characteristics of H5N1 Highly Pathogenic Avian Influenza (HPAI) post-vaccination outbreaks. Vet. Res. 2009;40:15. doi: 10.1051/vetres:2008053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd edition. John Wiley; New York: 2000. p. 307. [Google Scholar]
- Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, Krauss S, Scholtissek C, Puthavathana P, Buranathai C, Nguyen TD, Long HT, Naipospos TS, Chen H, Ellis TM, Guan Y, Peiris JS, Webster RG. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam AKMS. [12 July 2009];Regional Climate Change modeling for Bangladesh using PRECIS 1.7.1. 2009 2009, from http://teacher.buet.ac.bd/akmsaifulislam/climate/
- Jacquez GM, Greiling DA. Local clustering in breast, lung and colorectal cancer in Long Island. Int. J. Health Geogr. 2003;2:3. doi: 10.1186/1476-072X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain E, Gauthier-Clerc M, Bicout DJ, Sabatier P. Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg. Infect. Dis. 2007;13:365–372. doi: 10.3201/eid1303.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulldorff M, Athas WF, Feurer EJ, Miller BA, Key CR. Evaluating cluster alarms: a space-time scan statistic and brain cancer in Los Alamos, New Mexico. Am. J. Public Health. 1998;88:1377–1380. doi: 10.2105/ajph.88.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulldorff M, Feuer EJ, Miller BA, Freedman LS. Breast cancer clusters in the northeast United States: a geographic analysis. Am. J. Epidemiol. 1997;146:161–170. doi: 10.1093/oxfordjournals.aje.a009247. [DOI] [PubMed] [Google Scholar]
- Kulldorff M, Nagarwalla N. Spatial disease clusters: detection and inference. Stat. Med. 1995;14:799–810. doi: 10.1002/sim.4780140809. [DOI] [PubMed] [Google Scholar]
- Kung NY, Morris RS, Perkins NR, Sims LD, Ellis TM, Bissett L, Chow M, Shortridge KF, Guan Y, Peiris MJS. Risk for infection with highly pathogenic influenza A virus (H5N1) in chickens, Hong Kong, 2002. Emerg. Infect. Dis. 2007;13:412–418. doi: 10.3201/eid1303.060365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loth L, Mahabub Ahmed M, Hannan A, Kalam MA, Stocchi A. Short Message Service Gateway as Tracking and Reporting Communication Tool during Active Highly Pathogenic Avian Influenza Surveillance in Bangladesh. International Meeting on Emerging Diseases and Surveillance. 20092009 from http://ww2.isid.org/Downloads/IMED2009_AbstrAuth.pdf. [Google Scholar]
- Minh PQ, Morris RS, Schauer B, Stevenson M, Benschop J, Nam HV, Jackson R. Spatio-temporal epidemiology of highly pathogenic avian influenza outbreaks in the two deltas of Vietnam during 2003–2007. Prev. Vet. Med. 2009;89:16–24. doi: 10.1016/j.prevetmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- MMWR Cases of influenza A (H5N1)—Thailand, 2004. Morb. Mortal. Wkly. Rep. 2004;53:100–103. [PubMed] [Google Scholar]
- MODIS . Moderate Resolution Imaging Spectroradiometer. National Aeronautics and Space Administration; 2010. [12 August 2009]. from http://modis.gsfc.nasa.gov/ [Google Scholar]
- MoFL [21 July 2009];Ministry of Fishery and Livestock, Government of the People's Republic of Bangladesh. 2009 2009, from http://www.mofl.gov.bd/
- Moran PA. Notes on continuous stochastic phenomena. Biometrika. 1950;37:17–23. [PubMed] [Google Scholar]
- Morris R, Jackson R, Stevenson R, Benard H, Cogger N. [12 July 2009];Epidemiology of H5N1 Avian Influenza in Asia and Implications for Regional Control. 2005 from http://www.fao.org/docs/eims/upload//246974/aj122e00.pdf.
- OIE [12 May 2009];WAHID Interface Animal Health Information. 2009 2007, from http://www.oie.int/wahid-prod/public.php?page=home.
- Oyana TJ, Dai D, Scott KE. Spatiotemporal distributions of reported cases of the avian influenza H5N1 (bird flu) in Southern China in early 2004. Avian Dis. 2006;50:508–515. doi: 10.1637/7597-040506.1. [DOI] [PubMed] [Google Scholar]
- Pebesma EJ. Multivariable geostatistics in S: the gstat package. Comput. Geosci. 2004;30:683–691. [Google Scholar]
- Pfeiffer D. [12 December 2007];Assistance in the Geospatial Analysis of HPAI Outbreaks in Indonesia. 2006 2007, from http://www.fao.org/docs/eims/upload/199669/Pfeiffer_Report_Indonesia_2005.pdf.
- Pfeiffer DU, Minh PQ, Martin V, Epprecht M, Otte MJ. An analysis of the spatial and temporal patterns of highly pathogenic avian influenza occurrence in Vietnam using national surveillance data. Vet. J. 2007;174(2):302–309. doi: 10.1016/j.tvjl.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Rappole JH, Hubalek Z. Birds and influenza H5NI virus movement to and within North America. Emerg. Infect. Dis. 2006;12:1486–1492. doi: 10.3201/eid1210.051577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas AL, Chowell G, Hoogesteijn AL, Chaffer M, Smith SD, Schwager SJ. Diffusion patterns of the 2006 Nigerian Avian Influenza epidemic. GISVet; Copenhagen, Denmark: 2007. [Google Scholar]
- Rushton J, Viscarra R, Guerne-Bleich E, Mcleod A. Impact of Influenza Outbreaks in the Poultry Sectors of Five South-east Asian Countries (Cambodia, Indonesia, Lao PDR, Thailand, Viet Nam) Outbreak Costs, Responses and Potential Long-term Control. Food and Agriculture Organization of the United Nations; 2004. [18 July 2009]. from www.fao.org/docs/eims/upload/214194/poultrysector_seasia_en.pdf. [Google Scholar]
- [5 May 2009];SatScan. 2009 from http://www.satscan.org.
- Science Influenza. Bird flu moves west, spreading alarm. Science. 2006;311:1084. doi: 10.1126/science.311.5764.1084b. [DOI] [PubMed] [Google Scholar]
- Sims LD. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 2007;51:174–181. doi: 10.1637/7637-042806R.1. [DOI] [PubMed] [Google Scholar]
- Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, Webster RG. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerg. Infect. Dis. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ME, Bouma A, Ekker HM, Fonken AJ, Stegeman JA, Nielen M. Risk factors for the introduction of high pathogenicity Avian Influenza virus into poultry farms during the epidemic in the Netherlands in 2003. Prev. Vet. Med. 2005;69:1–11. doi: 10.1016/j.prevetmed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tiensin T, Ahmed SS, Rojanasthien S, Songserm T, Ratanakorn P, Chaichoun K, Kalpravidh W, Wongkasemjit S, Patchimasiri T, Chanachai K, Thanapongtham W, Chotinan S, Stegeman A, Nielen M. Ecologic risk factor investigation of clusters of avian influenza A (H5N1) virus infection in Thailand. J. Infect. Dis. 2009;199:1735–1743. doi: 10.1086/599207. [DOI] [PubMed] [Google Scholar]
- Tiensin T, Chaitaweesub P, Songserm T, Chaisingh A, Hoonsuwan W, Buranathai C, Parakamawongsa T, Premashthira S, Amonsin A, Gilbert M, Nielen M, Stegeman A. Highly pathogenic avian influenza H5N1, Thailand, 2004. Emerg. Infect. Dis. 2005;11:1664–1672. doi: 10.3201/eid1111.050608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VSF . Organization of Avian Production and Description of HPAI Epidemiological Patterns in Vietnam—Intermediate Report. Veterinaires Sans Frontieres/World Bank; 2004. [12 August 2009]. from www.fao.org/docs/eims/upload//246973/aj121e00.pdf. [Google Scholar]
- Wang M, Di B, Zhou D, Zheng B, Jing H, Lin Y, Liu Y, Wu X, Qin P, Wang Y, Jian L, Li X, Xu J, Lu E, Li T, Xu J. Food markets with live birds as source of avian influenza. Emerg. Infect. Dis. 2006;12:1773–1775. doi: 10.3201/eid1211.060675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Avian influenza—situation in Bangladesh. World Health Organization; 2008. [12 September 2009]. 15 May 2009 from http://www.who.int/csr/don/2008_05_28/en/print.html. [Google Scholar]
- WHO . Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) World Health Organization; 2009. [12 July 2009]. Reported to WHO. from http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_07_01/en/index.html. [Google Scholar]
- Xiao X, Boles S, Frolking S, Li C, Babu JY, Salas W, Moore B. Mapping paddy rice agriculture in South and Southeast Asia using multi-temporal MODIS images. Remote Sens. Environ. 2006;100:95–113. [Google Scholar]
- Xiao X, Boles S, Liub J, Zhuangb D, Frolkinga S, Lia C, Salasc W, Moore B. Mapping paddy rice agriculture in southern China using multi-temporal MODIS images. Remote Sens. Environ. 2005;95:480–492. [Google Scholar]
- Zhang J, Zhao D, Yin Z, Lei F. The threat of Highly Pathogenic Avian Influenza H5N1 and its ecological basis. Chin. J. Zool. 2007;42:152–156. [Google Scholar]