Abstract
Purpose
Antiangiogenic therapies targeting the vascular endothelial growth factor (VEGF) pathway have yielded more modest clinical benefit to patients with non–small-cell lung cancer (NSCLC) than initially expected. Clinical data suggest a distinct biologic role of the VEGF pathway in the different histologic subtypes of lung cancer. To clarify the influence of histologic differentiation in the prognostic relevance of VEGF-mediated signaling in NSCLC, we performed a concomitant analysis of the expression of three key elements of the VEGF pathway in the earliest stages of the following two principal histologic subtypes: squamous cell carcinoma (SCC) and adenocarcinoma (ADC).
Patients and Methods
We evaluated tumor cell expression of VEGF, VEGF receptor (VEGFR) 1, and VEGFR2 using automatic immunostaining in a series of 298 patients with early-stage NSCLC recruited as part of the multicenter European Early Lung Cancer Detection Group project. A score measuring the VEGF signaling pathway was calculated by adding the tumor cell expression value of VEGF and its two receptors. The results were validated in two additional independent cohorts of patients with NSCLC.
Results
The combination of high VEGF, VEGFR1, and VEGFR2 protein expression was associated with lower risk of disease progression in early SCC (univariate analysis, P = .008; multivariate analysis, hazard ratio, 0.62; 95% CI, 0.42 to 0.92; P = .02). The results were validated in two independent patient cohorts, confirming the favorable prognostic value of high VEGF signaling score in early lung SCC.
Conclusion
Our results clearly indicate that the combination of high expression of the three key elements in the VEGF pathway is associated with a good prognosis in patients with early SCC but not in patients with ADC.
INTRODUCTION
Antiangiogenic therapies targeting the vascular endothelial growth factor (VEGF) A pathway have proven to be useful in slowing metastatic disease progression and in improving progression-free survival in several types of cancer. VEGF acts as an endothelial mitogen and survival factor, can increase vascular permeability, and is a crucial regulator of angiogenesis in healthy and neoplastic tissues.1–3 The principal effects of VEGF are mediated by VEGF receptor (VEGFR) 1 and VEGFR2. VEGFR1 is a regulator of monocyte and macrophage migration, whereas VEGFR2 is the main regulator of angiogenesis.3 There are four US Food and Drug Administration–approved drugs that disrupt the VEGF pathway.4 Hundreds of clinical trials using these drugs are currently under way in a variety of tumor types and drug combinations. However, despite an intensive effort over the last two decades, the clinical yield remains poor. New questions have emerged about the mechanisms of resistance to antiangiogenic therapy. Moreover, recent reports have demonstrated unforeseen prometastatic effects of targeting the VEGF pathway in cancer animal models.5–7
In lung cancer, both VEGFR tyrosine kinase inhibitors and anti-VEGF antibodies have demonstrated clinical benefit.8–10 Bevacizumab (Avastin; Genentech/Roche, South San Francisco, CA), a monoclonal antibody against VEGF, is widely used in combination with platinum-based chemotherapy for first-line treatment of advanced non–small-cell lung cancer (NSCLC).11–13 Nevertheless, the indication for anti-VEGF therapy is restricted to nonsquamous histology. Patients with squamous cell carcinoma (SCC) of the lung are excluded from anti-VEGF treatment as a result of the risk of severe pulmonary hemorrhages.14 More recently, the addition of sorafenib (Nexavar; Bayer, Leverkusen, Germany), a VEGFR inhibitor, to first-line carboplatin and paclitaxel treatment has been reported to be associated with an increased risk of death in patients with lung squamous histology.15 The mechanisms that concern the association of these adverse effects with the squamous histologic subtype have not been elucidated. Antiangiogenic therapies are primarily designed to hit the endothelial compartment via inactivation of VEGF or its receptors. Nevertheless, these inhibitors may also impact other cell types expressing VEGF receptors, specifically tumor cells.2 In fact, NSCLC cells express the three principal elements of the VEGF pathway, namely VEGF, VEGFR1, and VEGFR2.16,17 The expression of VEGF in NSCLC cells has been extensively analyzed, primarily to assess its prognostic value.18–20 However, the conclusions remain contentious. A meta-analysis suggests that VEGF overexpression by tumor cells is an indicator of poor prognosis only for patients with adenocarcinoma (ADC), but not for patients with SCC.21
A more precise picture of the implications of VEGF and its receptors in the progression of the two main types of lung cancer is clearly needed. Thus, we aimed to study the expression of the key elements of the VEGF pathway in the early stages of ADC and SCC. Moreover, we examined, for the first time, the prognostic value of VEGF in combination with its receptors in SCC and ADC separately. For that purpose, we simultaneously analyzed VEGF and its two receptors, VEGFR1 and VEGFR2, in a large series of early (stage I to III) SCC and ADC, and the results were validated in two independent cohorts. Our results clearly demonstrated that high expression of the VEGF pathway elements was associated with a good prognosis in patients with early SCC, in contrast to what was observed in patients with early ADC. These data stress the relevance of histology in understanding VEGF-mediated biologic responses in early stages of lung cancer. Our results also suggest that the levels of VEGF autocrine loop activity have a different impact in SCC and ADC cell phenotypes and, thus, potentially lead to diverse clinical outcomes in patients treated with anti-VEGF therapies.
PATIENTS AND METHODS
Patients
Patients were recruited as part of the European Early Lung Cancer Detection Group (EUELC) project.22 The inclusion criteria used to enroll the patients were histology (NSCLC), stage at diagnosis (stage I to III), complete resection of the primary lung tumor, absence of cancer within the 5 years before surgery, and no treatment with chemotherapy or radiotherapy before surgery. Tumors were classified according to the WHO 2004 classification.23 Follow-up for the whole multi-institutional series continued through June 2009. A total of 913 patients with primary lung cancer were recruited for the project, and we selected 298 patients based on availability of paraffin-embedded material and clinical information. Patients who experienced recurrence within 6 months after surgery were excluded. Patients were separated into the following two groups: patients with no sign of the disease at the last follow-up were defined as disease free, and patients with recurrence were included in the progressive disease group. Deaths related to lung cancer were included in the progressive disease group, and those related to other causes were included in the disease-free group. Relapse time was calculated from the date of surgery to the date of recurrence or death. The median follow-up period for these patients was 25 months (range, 6 to 109 months). The study protocol was approved by local ethics committees. Written informed consent was obtained from each patient. Reported recommendations for tumor marker prognostic studies (REMARK) criteria were followed throughout the study.24
For validation, we analyzed two independent series of patients with NSCLC. The first series was a tissue microarray set of 341 patients with NSCLC diagnosed from 1994 to 2004 at the University of Texas MD Anderson Cancer Center (Houston, TX). From this cohort, 173 patients were selected based on the inclusion criteria described earlier. The median follow-up period was 66 months (range, 7 to 161 months). The second validation series was composed of 36 patients diagnosed with stage I SCC from 1988 to 2006 at the Centre Hospitalier Universitaire (CHU) Albert Michallon (Grenoble, France). The median follow-up period for these patients was 68 months (range, 6 to 226 months). Clinicopathologic features of the patients are listed in Table 1.
Table 1.
Demographics and Clinical Characteristics ofthe Patients
| Demographic or Clinical Characteristic | EUELC Series (n = 298) |
MDA Series (n = 173) |
CHU Series (n = 36) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Age, years | ||||||
| Median | 64 | 67 | 63 | |||
| SD | 9 | 11 | 9 | |||
| Sex | ||||||
| Male | 260 | 87.2 | 84 | 48.6 | 32 | 89 |
| Female | 38 | 12.8 | 89 | 51.4 | 4 | 11 |
| Stage | ||||||
| I | 191 | 64 | 141 | 67 | 36 | 100 |
| II | 86 | 29 | 42 | 20 | — | |
| III | 11 | 4 | 24 | 11 | — | |
| IV* | 9 | 3 | 5 | 2 | — | |
| pT | ||||||
| T1 | 93 | 31 | 74 | 43 | 36 | 100 |
| T2 | 176 | 59 | 89 | 51 | — | |
| T3 | 19 | 7 | 3 | 2 | — | |
| T4 | 9 | 3 | 7 | 4 | — | |
| pN | ||||||
| N0 | 202 | 68 | 135 | 79 | 36 | 100 |
| N1 | 79 | 27 | 33 | 19 | — | |
| N2 | 3 | 1 | 5 | 2 | — | |
| Nx | 13 | 4 | — | — | ||
| Histology | ||||||
| ADC | 151 | 51 | 103 | 60 | — | |
| SCC | 138 | 46 | 68 | 39 | 36 | 100 |
| Other | 9 | 3 | 2 | 1 | — | |
| Smoking status | ||||||
| Current smoker | 175 | 59 | 71 | 41 | NA | |
| Former smoker | 111 | 37 | 76 | 44 | NA | |
| Nonsmoker | 12 | 4 | 26 | 15 | NA | |
Abbreviations: ADC, adenocarcinoma; CHU, Grenoble Centre Hospitalier Universitaire Albert Michallon; EUELC, European Early Lung Cancer project; MDA, MD Anderson Cancer Center; NA, not available; SCC, squamous cell carcinoma; SD, standard deviation.
All tumors were classified as stage I to III at presentation, fulfilling the inclusion criteria. However, the final staging of the disease was determined based on a combination of surgical, pathologic, and radiologic findings, and some patients were reclassified as stage IV.
Immunohistochemistry
Immunohistochemical staining was performed on formalin-fixed paraffin-embedded tissue sections with anti-VEGF (A20, rabbit polyclonal immunoglobulin G [IgG]; Santa Cruz Biotechnology, Santa Cruz, CA), anti-VEGFR1 (C-17, rabbit polyclonal IgG; Santa Cruz), or anti-VEGFR2 (A-3, mouse monoclonal IgG; Santa Cruz) diluted at 1:100, 1:25, and 1:50, respectively. To perform technical validation, other commercially available antibodies against the same proteins were used (Data Supplement). To analyze microvascular density, anti-CD34 (QBend10; Immunotech, Marseille, France) at 1:25 was used. The immunohistochemical procedures were performed on an automated immunostainer (Dako, Glostrup, Denmark). Briefly, paraffin was removed from the tissues, and the sections were hydrated through a graded series of ethanol. Endogenous peroxidase activity was quenched with 3% H2O2 for 10 minutes. For VEGF, VEGFR1, and VEGFR2 detection, microwave antigen retrieval was conducted with citrate buffer (10 mmol/L, pH6) for 2 × 15 minutes. Nonspecific binding sites were blocked with 5% goat serum in triethanolamine (TRIS)-buffered saline–Tween for 30 minutes. Sections were incubated with primary antibody for 30 minutes at room temperature. After a rinse with TRIS-buffered saline–Tween, sections were incubated with Envision complex (Dako). Finally, sections were developed with diaminobenzidine, lightly counterstained with hematoxylin, and mounted in distyrene plasticizer xylene. Tissues expressing different levels of antigen were included in each immunohistochemical run to control for variation between experiments. The specificity of each antibody was demonstrated using an extensive variety of controls, including Western blot analysis, adsorption controls with antigenic peptides, inhibition with two short interfering RNA sequences, and comparison of staining patterns between different antibodies (Data Supplement).
Evaluation of Immunostaining
Specimens were evaluated by two observers (M.J.P. and J.A.) who were unaware of the clinical features and outcomes of patients. For VEGF, VEGFR1, and VEGFR2, the extension was scored as the percentage of positive cells (0% to 100%), and the intensity of staining was assessed by comparison with a known external positive control (0, below the level of detection; 1, weak; 2, moderate; and 3, strong). Scores were calculated by multiplying the staining intensity and extension at each intensity level, as previously described.25 For VEGFR1 and VEGFR2, the combined expression of cytoplasmic and membrane staining was assessed. The sum of the VEGF, VEGFR1, and VEGFR2 scores rendered the combined variable termed VEGF signaling score (VSS). The microvessel density (MVD) scores were evaluated using a 25-point Chalkley eyepiece graticule, as previously described.26 Median values were used as the cutoff. For VSS, the cutoff point was 400 in the EUELC and CHU series and 460 in the MD Anderson series. Automatic immunohistochemical and semiquantification procedures were cross validated in 10% of the patients by an independent investigator (E.B.; Data Supplement).
Statistics
The expression of each marker was dichotomized into low and high according to the median value of the semiquantitative expression score. The Fisher's exact test was used to assess the association between the expression of each marker and clinical parameters. Cumulative incidence curves for relapse or lung cancer–related death were constructed reflecting time to progression (TTP) and time to death from other cause as competing risk. The Fine and Gray model was used to identify the risk factors associated with disease progression in both univariate and multivariate analyses.27 The Fine and Gray model is an adaptation of the Cox model that is used to cope for the informative censoring assumption violation. In this study, the risk of disease progression was the main event, and the risk of death from a cause other than lung cancer was the competing event. Sensitivity analyses on histologic subtypes and tumor stages were also performed. P < .05 was considered significant. All statistical analyses were performed using SAS version 9.1 (SAS, Cary, NC) and R version 2.4 (R Project, Vienna, Austria).
RESULTS
VEGF, VEGFR1, and VEGFR2 Are Expressed in NSCLC Cells
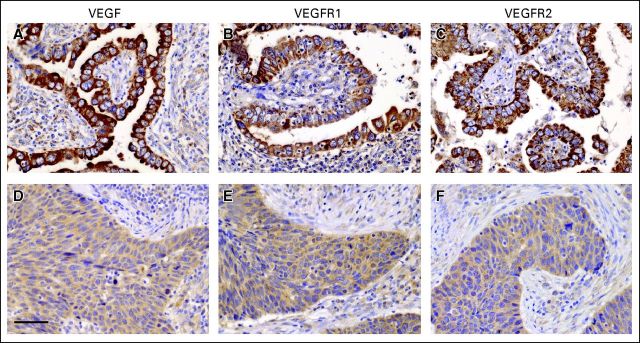
Cytoplasmic staining of VEGF and its receptors (VEGFR1 and VEGFR2) was found in NSCLC cells (Fig 1). We found a significant association between the expression of VEGF and its receptors in tumor cells (VEGF v VEGFR1, P < .001; VEGF v VEGFR2, P < .001; and VEGFR1 v VEGFR2, P < .001). VEGF, VEGFR1, and VEGFR2 expression was significantly higher in ADC than in SCC (VEGF, P = .009; VEGFR1, P = .007; VEGFR2, P = .0186; Fig 1). However, there was no difference in the expression of VEGF or its receptors according to different stages of the disease (VEGF, P = .42; VEGFR1, P = .56; VEGFR2, P = .48) or smoking status (VEGF, P = .45; VEGFR1, P = .94; VEGFR2, P = .84). Endothelial and stromal cells also showed immunoreactivity for VEGF and its receptors (Data Supplement). No evident differences were observed in endothelial staining between ADC and SCC. The accuracy of both the immunohistochemical and the quantification methods was demonstrated by a variety of controls (Data Supplement).
Fig 1.
The expression of vascular endothelial growth factor (VEGF), VEGF receptor (VEGFR) 1, and VEGFR2 is higher in adenocarcinoma (ADC) compared with squamous cell carcinoma (SCC). Representative immunostaining for (A, D) VEGF, (B, E) VEGFR1, and (C, F) VEGFR2 in (A-C) ADC and (D-F) SCC tumors. VEGF, VEGFR1, and VEGFR2 are predominantly found in the cytoplasm of tumor cells. Scale bar: 25 μm.
High Expression of VEGF and Its Receptors Is Associated With Favorable Prognosis in SCC
To determine which factors were important predictors of lung cancer–related relapse, a multivariate analysis was performed. As expected, there was a significant association between pT and pN status and high risk of disease progression (P < .001 and P < .001, respectively; Table 2). Other clinicopathologic variables (sex, age, and smoking status) were determined to not be prognostic factors in the EUELC cohort of patients with NSCLC. We subsequently evaluated the prognostic role of VEGF, VEGFR1, VEGFR2, and MVD and found no statistically significant association between any of these markers, considered separately, and the progression of the disease (Data Supplement). Additionally, we found no correlation between MVD and tumor cell expression of VEGF (P = .192).
Table 2.
Clinical Risk Factors of Disease Progression in the EUELC Cohort
| Factor | DF (n = 172) |
PD (n = 126) |
Hazard Ratio* | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||||
| Sex | |||||||
| Male | 155 | 59.6 | 105 | 40.4 | 1 | ||
| Female | 17 | 44.7 | 21 | 55.3 | 1.62 | 0.96 to 2.75 | .07 |
| Age, years | |||||||
| ≤ 70 | 129 | 58.9 | 90 | 41.1 | 1 | ||
| > 70 | 43 | 54.4 | 36 | 45.6 | 1.18 | 0.79 to 1.78 | .42 |
| pN | |||||||
| N0 | 134 | 66.3 | 68 | 33.7 | 1 | ||
| N1, N2, Nx | 38 | 40.0 | 57 | 60.0 | 2.11 | 1.45 to 3.07 | < .001 |
| pT | |||||||
| T1, T2 | 69 | 74.2 | 24 | 25.8 | 1 | ||
| T3, T4 | 103 | 50.5 | 101 | 49.5 | 2.68 | 1.59 to 4.50 | < .001 |
| Smoking status | |||||||
| Former/never smoker | 72 | 58.5 | 51 | 41.5 | 1 | ||
| Current smoker | 100 | 57.1 | 75 | 42.9 | 1.04 | 0.72 to 1.50 | .84 |
Abbreviations: DF, disease free; EUELC, European Early Lung Cancer project; PD, progressive disease.
Fine and Gray hazard ratio and P value for disease progression risk.
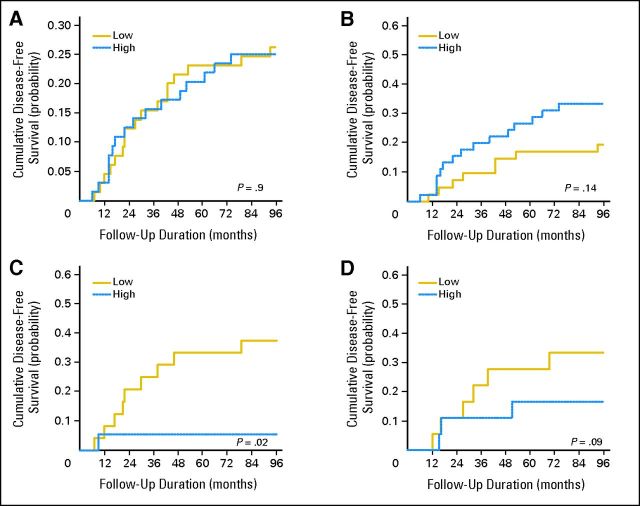
To analyze the relevance of the VEGF pathway in lung tumor cells, we created a score entitled VSS (VEGF + VEGFR1 + VEGFR2). This score enabled us to use a surrogate to measure the VEGF pathway activity in tumor cells. The cumulative incidence plots clearly indicated that a high VSS was associated with longer TTP (Fig 2A). The independent prognostic impact of VSS was demonstrated by multivariate analysis (hazard ratio [HR], 0.62; 95% CI, 0.42 to 0.92; P = .02; Table 3). To investigate whether these striking results were dependent on tumor histology, ADC and SCC subgroups were analyzed separately (Figs 2B and 2C). The favorable prognostic value of VSS was restricted to patients with SCC (HR, 0.41; 95% CI, 0.22 to 0.76; P = .004). In contrast, this association was not observed in patients with ADC (HR, 0.90; 95% CI, 0.52 to 1.56; P = .7). We also performed a subset analysis by disease stage. VSS was only associated with good prognosis in stage I SCC (HR, 0.31; 95% CI, 0.12 to 0.80; P = .015; Table 3; Figs 2D to 2F; Data Supplement).
Fig 2.
High vascular endothelial growth factor signaling score (VSS) is a good prognostic factor in patients with squamous cell carcinoma (SCC) in the European Early Lung Cancer project cohort. Cumulative incidence plots of disease progression were grouped by VSS for (A) all patients, (B) patients with adenocarcinoma (ADC), (C) patients with SCC, (D) patients with stage I disease, (E) patients with stage I ADC, and (F) patients with stage I SCC. A competing risk analysis of disease progression was performed to analyze the cumulative competing risks of progression.
Table 3.
Multivariate Proportional Hazard Model for Competing Risk of VSS in Patients With SCC
| Median VSS Cutoff | DF |
PD |
Adjusted HR* | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||||
| EUELC series | |||||||
| ≤ 400 | 75 | 50.3 | 74 | 49.7 | 1 | ||
| > 400 | 97 | 65.1 | 52 | 44.9 | 0.62 | 0.42 to 0.92 | .02 |
| EUELC series (stage I) | |||||||
| ≤ 400 | 25 | 52.1 | 23 | 47.9 | 1 | ||
| > 400 | 26 | 81.3 | 6 | 18.8 | 0.31 | 0.12 to 0.80 | .015 |
| MDA series (stage I) | |||||||
| ≤ 460 | 15 | 62.5 | 9 | 37.5 | 1 | ||
| > 460 | 17 | 94.4 | 1 | 5.6 | 0.05 | 0.004 to 0.62 | .02 |
Abbreviations: DF, disease free; EUELC, European Early Lung Cancer project; HR, hazard ratio; MDA, MD Anderson Cancer Center; PD, progressive disease; SCC, squamous cell carcinoma; VSS, vascular endothelial growth factor signaling score.
Fine and Gray adaptation of Cox proportional hazard model and P value for disease progression risk adjusted by pT, pN, and smoking duration (EUELC series); smoking duration (EUELC series, stage I); and pN, pT, sex, age, and smoking status (MDA series, stage I).
We next sought to validate the association between VSS and the risk of disease progression in early SCC in two independent series of patients with NSCLC (MD Anderson and CHU cohorts). In the first validation cohort (MD Anderson series), we found a strong association between high VSS and lower risk of disease progression among patients with stage I SCC (HR, 0.05; 95% CI, 0.004 to 0.62; P = .02; Figs 3A to 3C and Data Supplement). Interestingly, the cumulative incidence plots of disease progression exhibited an opposite effect according to the histologic subtypes; thus, in patients with ADC, high VSS tended to be associated with shorter TTP (Fig 3B; P = .14), whereas it was clearly associated with longer TTP in SCC (Fig 3C; P = .02). In the second validation series (Grenoble CHU Hospital) composed of 36 patients with stage I SCC, we found an association close to the statistical significance between high VSS and longer TTP (Fig 3D; P = .09), although the differences did not reach significance using multivariate analysis. Overall, these results clearly indicate that VEGF signaling exhibits a completely different prognostic significance in early SCC compared with early ADC.
Fig 3.
The MD Anderson (MDA) and Grenoble Centre Hospitalier Universitaire (CHU) Albert Michallon validation series confirm that high vascular endothelial growth factor signaling score is associated with longer survival in patients with stage I squamous cell carcinoma (SCC). Cumulative incidence plots are shown for (A) all patients with stage I disease, (B) patients with stage I adenocarcinoma, and (C) patients with stage I SCC in the MDA series. (D) Cumulative incidence plot is shown for patients with stage I SCC in the CHU series.
DISCUSSION
In this study, we demonstrate that the combined high expression of proteins of the VEGF pathway in lung cancer cells is associated with good prognosis in patients with stage I SCC. This was a striking result, given that anti-VEGF therapy is considered a major anticancer treatment strategy. To robustly validate our results, we used a wide array of controls. More importantly, we validated our results in two independent series.
Immunocytochemical analysis revealed a significant association between the expression of VEGF and the expression of its receptors, suggesting an autocrine/paracrine VEGF signaling in these lung tumors. Koukourakis et al28 demonstrated the presence of the VEGF/VEGFR2 complex in tumor cells from patients with NSCLC. Moreover, a functional VEGF/VEGFR autocrine loop has been previously reported for gastric,29 breast,30,31 and lung tumor cell lines.32,33 Gratzinger et al34 analyzed the combined expression of VEGF and VEGFR1 in lymphoma cells and identified a subgroup of patients with high VEGF/VEGFR1 who exhibited improved survival. Similarly, we conducted a simultaneous analysis of the three principal proteins of the VEGF pathway (VEGF + VEGFR1 + VEGFR2 = VSS) and found high VSS to be an independent factor of good prognosis in a subset of patients with NSCLC, namely those with squamous differentiation at the earliest stages.
Several studies have been conducted to assess the prognostic role of VEGF, VEGFR1, and/or VEGFR2 in tumor cells of patients with NSCLC, with contentious results. Many investigations documented a correlation of these factors with poor prognosis,19,20,35–39 whereas many others failed to demonstrate any correlation.16,40–44 One possible explanation for these discrepancies is the fact that the cohorts of patients were notably diverse in terms of stage and histology, median follow-up, and size. Remarkably, in the majority of these studies, SCC and ADC were considered as a single entity. However, in recent years, many reports clearly indicate that these histologic subtypes are biologically different in terms of gene expression, drug toxicity, tumor response to therapy, and clinical outcome.45–47 In our study, the prognostic value of the expression of the VEGF pathway differs significantly between SCC and ADC. Interestingly, in the MD Anderson series, despite the low event frequency in breakdown analyses, a good prognostic value for VSS in patients with stage I disease with squamous histology was found, whereas a trend toward poor prognosis was observed in patients with early ADC.
Data demonstrating an association of histology with toxicity or drug efficacy have stressed the relevance of the histopathologic diagnosis when evaluating NSCLC clinical trials. In the Evaluation of Sorafenib, Carboplatin, and Paclitaxel Efficacy in NSCLC (ESCAPE) phase III trial, the addition of sorafenib (a VEGFR inhibitor) to first-line chemotherapy was associated with increased risk of death in patients with lung squamous histology.15 Interestingly, there were no differences between treatment arms in the incidence of fatal bleeding in patients with squamous histology. These results suggest a potentially distinct response to inhibition of the VEGF pathway in SCC and ADC. Unfortunately, apart from histology, there are currently no well-established biomarkers to predict the subset of patients with NSCLC who would benefit from these molecular targeted drugs. High VSS could potentially be considered in clinical studies as a biomarker for selecting patients with SCC who should not receive anti-VEGF/VEGFR–related therapies, which may even carry negative effects. These negative effects may be related to the fact that the antiangiogenic therapies hit not only endothelial cells, but also other cells that express the receptors, in particular lung tumor cells and several types of stromal inflammatory cells.2,47 Recent data in animal models suggest that inhibition of angiogenic factors increases the invasion capabilities of some types of tumor cells5,6 and that VEGFR2 signaling protects from metastatic spread in carcinoid cell lines.7 Although VEGFR1 has been described as a negative regulator of VEGFR2 signaling in endothelial cells,3 the function of VEGFR1 and VEGFR2 in tumor cells is poorly understood.
The differences found between ADC and SCC may be explained by the fact that both subtypes are different biologic entities in terms of their more prevalent genetic alterations.47–49 Moreover, the expression of the main VEGF signaling molecules is different between ADC and SCC. In the present study, we have demonstrated that the expression of VEGF, VEGFR1, and VEGFR2 is higher in ADC than in SCC, and it has been previously described that the expression of the VEGFR coreceptor neuropilin-1 is differentially regulated in ADC and SCC.50 In addition, differences in VEGFR downstream signaling proteins, such as PI3K or AKT, have also been found. In this sense, it has been demonstrated that PIK3CA copy number gains are more frequent in SCC (33.1%) than in ADC (6.2%),51 and AKT1 mutations are only found in SCC.49 More extensive mechanistic studies on the specific molecular traits of the VEGF pathway in the different histologic lung cancer subtypes, as well as in the specific microenvironment, are warranted.
In summary, we demonstrated that high expression of a VSS correlates with good prognosis in patients with stage I SCC. Notably, this provocative observation raises the question of a differential role of VEGF-related proteins according to the histology of lung tumors. For early lung SCC, these results challenge the established paradigm that high VEGF expression is associated with poor prognosis. These data also suggest that the strategy of targeting the VEGF pathway in patients with stage I NSCLC with squamous histology requires a clearer understanding of the molecular mechanisms specifically involved in the biology of these tumors.
Supplementary Material
Acknowledgment
We thank Patricia Martín, Ana Remírez, and Usua Montes for technical support and all researchers and patients of the centers included in the European Early Lung Cancer Consortium.
Appendix
European Early Lung Cancer (EUELC) Study Group: Christian Brambilla and Elisabeth Brambilla (INSERM U823, Albert Bonniot Institute, Grenoble, France); Yves Martinet (Centeral Hospitalier Universitaire de Nancy, Nancy, France); Frederik B. Thunnissen (Canisius Wilhelmina Ziekenhuis, Nijmegen, the Netherlands); Peter J. Snijders (University Hospital Vrije Universiteit, Amsterdam, the Netherlands); Gabriella Sozzi (Department of Experimental Oncology, Milan, Italy); Angela Risch (DKFZ-German Cancer Research Centre, Heidelberg, Germany); Heinrich D. Becker (Thoraxklinik am Universitätsklinikum, Heidelberg, Germany); J. Stuart Elborn (Belfast City Hospital, Belfast, United Kingdom); Luis M. Montuenga (University of Navarra, Pamplona, Spain); Ken J. O'Byrne (St James Hospital, Dublin, Ireland); David J. Harrison (University of Edinburgh, Edinburgh, United Kingdom); Jacek Niklinski (Medical Academy of Bialystok, Bialystok, Poland); and John K. Field (Institute of Translational Medicine, University of Liverpool, Liverpool, United Kingdom).
Footnotes
Author affiliations appear at the end of this article.
See accompanying article on page 1137
Written on behalf of the European Early Lung Cancer Study Group (Appendix).
Supported by European Union (Grants No. QLG1-CT-2002-01735 and HEALTH-F2-2010-258677), UTE project CIMA, Spanish Government (Grants No. ISCIII-RTICC, RD06/0020/0066, and PI10/00166), Spanish Ministry of Science and Innovation, European Regional Development Fund una manera de hacer Europa, and Government of Navarra, Department of Health (39/2007). M.J.P. was funded by a Fellowship from the International Association for the Study of Lung Cancer, and J.A. and M.L. were funded by Instituto de Salud Carlos III, Ministry of Science and Innovation, Spain. J.K.F. was funded by the Roy Castle Lung Cancer Foundation.
Presented at the 102nd Annual Meeting of the American Association of Cancer Research, April 2-6, 2011, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: María J. Pajares, Jackeline Agorreta, Wenceslao Torre, Ruben Pio, John K. Field, Luis M. Montuenga
Provision of study materials or patients: Wenceslao Torre, María D. Lozano, Elisabeth Brambilla, Ignacio I. Wistuba, John K. Field
Collection and assembly of data: María J. Pajares, Jackeline Agorreta, Marta Larrayoz, Teresa Ezponda, Isabel Zudaire, María D. Lozano, Elisabeth Brambilla, Ignacio I. Wistuba, Carmen Behrens, Ruben Pio, John K. Field, Luis M. Montuenga
Data analysis and interpretation: María J. Pajares, Jackeline Agorreta, Marta Larrayoz, Aurélien Vesin, Teresa Ezponda, Isabel Zudaire, Elisabeth Brambilla, Christian Brambilla, Jean-Francois Timsit, Ruben Pio, John K. Field, Luis M. Montuenga
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: Insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM, Hicklin DJ. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 3.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 4.Ebos JML, Kerbel RS. Antiangiogenic therapy: Impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva SR, Bowen KA, Rychahou PG, et al. VEGFR-2 expression in carcinoid cancer cells and its role in tumor growth and metastasis. Int J Cancer. 2011;128:1045–1056. doi: 10.1002/ijc.25441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagri A, Kouros-Mehr H, Leong KG, et al. Use of anti-VEGF adjuvant therapy in cancer: Challenges and rationale. Trends Mol Med. 2010;16:122–132. doi: 10.1016/j.molmed.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Socinski MA, Novello S, Brahmer JR, et al. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenschein GR, Jr, Gatzemeier U, Fossella F, et al. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 12.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 13.Cohen MH, Gootenberg J, Keegan P, et al. FDA drug approval summary: Bevacizumab (Avastin) plus carboplatin and paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12:713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti G, Novello S, von Pawel J, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–1842. doi: 10.1200/JCO.2009.26.1321. [DOI] [PubMed] [Google Scholar]
- 16.Decaussin M, Sartelet H, Robert C, et al. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): Correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 18.Kadota K, Huang CL, Liu D, et al. The clinical significance of lymphangiogenesis and angiogenesis in non-small cell lung cancer patients. Eur J Cancer. 2008;44:1057–1067. doi: 10.1016/j.ejca.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Donnem T, Al-Saad S, Al-Shibli K, et al. Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res. 2007;13:6649–6657. doi: 10.1158/1078-0432.CCR-07-0414. [DOI] [PubMed] [Google Scholar]
- 20.Fontanini G, Vignati S, Boldrini L, et al. Vascular endothelial growth factor is associated with neovascularization and influences progression of non-small cell lung carcinoma. Clin Cancer Res. 1997;3:861–865. [PubMed] [Google Scholar]
- 21.Zhan P, Wang J, Lv XJ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: A systematic review with meta-analysis. J Thorac Oncol. 2009;4:1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 22.Field JK, Liloglou T, Niaz A, et al. EUELC project: A multi-centre, multipurpose study to investigate early stage NSCLC, and to establish a biobank for ongoing collaboration. Eur Respir J. 2009;34:1477–1486. doi: 10.1183/09031936.00077809. [DOI] [PubMed] [Google Scholar]
- 23.Travis WD, Brambilla E, Müller-Hermelink HK, et al. ed 3. Lyon, France: IARC Press; 2004. Tumours of the Lung, Pleura, Thymus and Heart. [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 25.Ezponda T, Pajares MJ, Agorreta J, et al. The oncoprotein SF2/ASF promotes non-small cell lung cancer survival by enhancing survivin expression. Clin Cancer Res. 2010;16:4113–4125. doi: 10.1158/1078-0432.CCR-10-0076. [DOI] [PubMed] [Google Scholar]
- 26.Vermeulen PB, Gasparini G, Fox SB, et al. Quantification of angiogenesis in solid human tumours: An international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/s0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 27.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 28.Koukourakis MI, Giatromanolaki A, Thorpe PE, et al. Vascular endothelial growth factor/KDR activated microvessel density versus CD31 standard microvessel density in non-small cell lung cancer. Cancer Res. 2000;60:3088–3095. [PubMed] [Google Scholar]
- 29.Tian X, Song S, Wu J, et al. Vascular endothelial growth factor: Acting as an autocrine growth factor for human gastric adenocarcinoma cell MGC803. Biochem Biophys Res Commun. 2001;286:505–512. doi: 10.1006/bbrc.2001.5409. [DOI] [PubMed] [Google Scholar]
- 30.Bachelder RE, Crago A, Chung J, et al. Vascular endothelial growth factor is an autocrine survival factor for neuropilin-expressing breast carcinoma cells. Cancer Res. 2001;61:5736–5740. [PubMed] [Google Scholar]
- 31.Lee TH, Seng S, Sekine M, et al. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanno S, Ohsaki Y, Nakanishi K, et al. Human small cell lung cancer cells express functional VEGF receptors, VEGFR-2 and VEGFR-3. Lung Cancer. 2004;46:11–19. doi: 10.1016/j.lungcan.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Wu W, Onn A, Isobe T, et al. Targeted therapy of orthotopic human lung cancer by combined vascular endothelial growth factor and epidermal growth factor receptor signaling blockade. Mol Cancer Ther. 2007;6:471–483. doi: 10.1158/1535-7163.MCT-06-0416. [DOI] [PubMed] [Google Scholar]
- 34.Gratzinger D, Zhao S, Tibshirani RJ, et al. Prognostic significance of VEGF, VEGF receptors, and microvessel density in diffuse large B cell lymphoma treated with anthracycline-based chemotherapy. Lab Invest. 2008;88:38–47. doi: 10.1038/labinvest.3700697. [DOI] [PubMed] [Google Scholar]
- 35.Giatromanolaki A, Koukourakis MI, Kakolyris S, et al. Vascular endothelial growth factor, wild-type p53, and angiogenesis in early operable non-small cell lung cancer. Clin Cancer Res. 1998;4:3017–3024. [PubMed] [Google Scholar]
- 36.Mineo TC, Ambrogi V, Baldi A, et al. Prognostic impact of VEGF, CD31, CD34, and CD105 expression and tumour vessel invasion after radical surgery for IB-IIA non-small cell lung cancer. J Clin Pathol. 2004;57:591–597. doi: 10.1136/jcp.2003.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Held-Feindt J, Buhl R, et al. Expression of VEGF and its receptors in different brain tumors. Neurol Res. 2005;27:371–377. doi: 10.1179/016164105X39833. [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz A, Ernam D, Unsal E, et al. Vascular endothelial growth factor immunostaining correlates with postoperative relapse and survival in non-small cell lung cancer. Arch Med Res. 2007;38:764–768. doi: 10.1016/j.arcmed.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 39.Carrillo de Santa Pau E, Arias FC, Caso Pelaez E, et al. Prognostic significance of the expression of vascular endothelial growth factors A, B, C, and D and their receptors R1, R2, and R3 in patients with nonsmall cell lung cancer. Cancer. 2009;115:1701–1712. doi: 10.1002/cncr.24193. [DOI] [PubMed] [Google Scholar]
- 40.Baillie R, Carlile J, Pendleton N, et al. Prognostic value of vascularity and vascular endothelial growth factor expression in non-small cell lung cancer. J Clin Pathol. 2001;54:116–120. doi: 10.1136/jcp.54.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Offersen BV, Pfeiffer P, Hamilton-Dutoit S, et al. Patterns of angiogenesis in nonsmall-cell lung carcinoma. Cancer. 2001;91:1500–1509. [PubMed] [Google Scholar]
- 42.Tanaka F, Ishikawa S, Yanagihara K, et al. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62:7124–7129. [PubMed] [Google Scholar]
- 43.Tomita M, Matsuzaki Y, Shimizu T, et al. Vascular endothelial growth factor expression in pN2 non-small cell lung cancer: Lack of prognostic value. Respirology. 2005;10:31–35. doi: 10.1111/j.1440-1843.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim SJ, Rabbani ZN, Dewhirst MW, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer. 2005;49:325–335. doi: 10.1016/j.lungcan.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Neal JW. Histology matters: Individualizing treatment in non-small cell lung cancer. Oncologist. 2010;15:3–5. doi: 10.1634/theoncologist.2009-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheatley-Price P, Blackhall F, Lee SM, et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: A pooled analysis of five randomized trials. Ann Oncol. 2010;21:2023–2028. doi: 10.1093/annonc/mdq067. [DOI] [PubMed] [Google Scholar]
- 47.Langer CJ, Besse B, Gualberto A, et al. The evolving role of histology in the management of advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:5311–5320. doi: 10.1200/JCO.2010.28.8126. [DOI] [PubMed] [Google Scholar]
- 48.Dutt A, Ramos AH, Hammerman PS, et al. Inhibitor-sensitive FGFR1 amplification in human non-small cell lung cancer. PloS One. 2011;6:e20351. doi: 10.1371/journal.pone.0020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 50.Pidgeon GP, Barr MP, Cathcart MC, et al. Neuropilin-1 expression in adenocarcinoma and squamous cell carcinoma of the lung is differentially regulated by hypoxia. J Clin Oncol. 2006;24(suppl 18s):679s. abstr 17152. [Google Scholar]
- 51.Brambilla E, Gazdar A. Pathogenesis of lung cancer signalling pathways: Roadmap for therapies. Eur Respir J. 2009;33:1485–1497. doi: 10.1183/09031936.00014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.