Abstract
Acute myelogenous leukemia is propagated by a subpopulation of leukemia stem cells (LSCs). In this article, we review both the intrinsic and extrinsic components that are known to influence the survival of human LSCs. The intrinsic factors encompass regulators of cell cycle and prosurvival pathways (such as nuclear factor kappa B [NF-κB], AKT), pathways regulating oxidative stress, and specific molecular components promoting self-renewal. The extrinsic components are generated by the bone marrow microenvironment and include chemokine receptors (CXCR4), adhesion molecules (VLA-4 and CD44), and hypoxia-related proteins. New strategies that exploit potentially unique properties of the LSCs and their microenvironment are discussed.
INTRODUCTION
The past 10 to 15 years have witnessed substantial progress in the biologic characterization of acute myelogenous leukemia (AML). In particular, the long-established view that myeloid leukemia is developmentally similar to normal hematopoiesis and is driven by a relatively small subset of stem or progenitor cells has been established via extensive studies using both primary human tissues and various mouse models.1,2 Studies have clearly demonstrated that AML populations are highly heterogeneous and that the disease is propagated by a subpopulation of leukemia stem cells (LSCs). Initial reports described a specific cell surface phenotype for LSCs that allowed primitive leukemia cells to be distinguished from normal stem and progenitor cells (CD34+, CD38−, CD123+, chronic lymphatic leukemia 1–positive [CLL-1+], and so on).3–6 However, more recent data indicate that the phenotype of LSCs may be somewhat variable from patient to patient and that, in some cases, more than one phenotypically distinct subpopulation may possess LSC activity.7,8 Nonetheless, the overall heterogeneity of AML and the presence of LSCs are still strongly supported by multiple lines of evidence.
From a clinical perspective, it has been demonstrated that LSCs are substantially more resistant to standard forms of chemotherapy than bulk leukemia populations. Laboratory studies have specifically examined challenge with cytarabine and daunorubicin and have demonstrated preferential survival of functionally defined LSCs.9–11 Thus, elucidating the specific molecular and cellular properties that mediate survival of LSCs is an extremely important step toward the goal of creating improved therapeutic regimens for leukemia. In this article, we review both the intrinsic and extrinsic components that are known to influence the survival of human LSCs.
INTRINSIC PROPERTIES REGULATING THE SURVIVAL OF LSCs
Perhaps the most fundamental property of LSCs that may influence relative drug sensitivity is cell cycle status. Like normal hematopoietic stem cells (HSCs), LSCs reside in a mostly quiescent state. Indeed, studies by Guan et al12 showed that quiescent cells isolated from primary human AML specimens possessed most of their repopulating potential on transplantation into immune-deficient mice. Similarly, primary human chronic myelogenous leukemia (CML) specimens also demonstrate a mostly quiescent LSC population.13,14 As a consequence, the overall activity of many chemotherapeutic agents that function by targeting cycling cells is likely diminished. Thus, as a general rule, agents or regimens that can selectively eradicate LSCs independent of cell cycle status are almost certainly preferable. Alternatively, recent studies have also proposed that inducing cell cycle activity of LSCs before treatment with conventional chemotherapy may also be feasible.15
Because of the unique biology of LSCs, multiple studies have investigated the molecular properties of such cells, with a particular emphasis on those genes and pathways that distinguish leukemia from normal stem cells. For example, Guzman et al9,16 reported constitutive activation of the nuclear factor kappa B (NF-κB) pathway in primary human AML stem cells and provided evidence that NF-κB plays a significant role in the overall survival of LSCs as well as AML cell types in general. Furthermore, most of the compounds that have been shown to successfully eradicate LSCs are known to be inhibitors of NF-κB.17 Hence, this pathway is strongly implicated as a central target in developing LSC-specific therapies. Constitutive activation of Akt is also commonly observed in primary AML specimens, and inhibition of Akt via the use of phosphatidylinositol-3 kinase (PI3K) inhibitors such as wortmanin and LY294002 have been shown to augment therapies designed to target LSCs.18,19 Interestingly, aside from its known role as a survival signal (via modulation of the proapoptotic factor Bad), the activity of the PI3K pathway has also recently been implicated in antioxidant defenses. Increased activity of the PI3K pathway was associated with induction of the Nrf2 pathway and downstream activation of heme oxygenase (HMOX-1), a well-known component of the antioxidant defense machinery.20 Furthermore, treatment with PI3K or mammalian target of rapamycin pathway inhibitors effectively blocked induction of HMOX-1. Thus, agents that inhibit PI3K activity may act to increased oxidative stress in AML cells by inhibiting cellular antioxidant mechanisms.
Agents that induce oxidative stress have also been strongly implicated in targeting of LSCs, but this activity alone is clearly not sufficient, since many pro-oxidants do not affect the viability of malignant stem cells. Nonetheless, in the absence of oxidative stress, there are few if any well-documented cases in which LSCs are targeted, with the exception of some antibody-based strategies. Interestingly, aside from a direct role in the cell death process, oxidative stress may also inhibit self-renewal of LSCs. Indeed, normal HSCs, as well as neuronal progenitors cells, have been shown to respond strongly to changes in oxidative state.21–23 Increased oxidation is clearly associated with differentiation, whereas more reduced conditions promote self-renewal. If the self-renewal of LSCs is regulated by molecular mechanisms similar to those of normal HSCs, then it stands to reason that increased oxidative stress may reduce self-renewal, which in turn should lead to either differentiation or death of primitive AML cells.
Specific molecular components of self-renewal have also been implicated in the survival of LSCs. For example, recent studies by Wang et al24 demonstrated that in murine LSCs derived from MLL-AF9–induced leukemias, signaling via the Wnt/beta-catenin pathway was required for self-renewal. Similarly, Zhao et al25 demonstrated a central role for beta-catenin signaling in the survival of CML stem cells. More recent studies26 have also shown a role for the embryonic self-renewal regulator Musashi in blast crisis CML. Taken together, the data indicate that strategies targeting several of the known regulators of stem-cell self-renewal may be a promising approach to improved therapy for AML.
Selective upregulation of the components of apoptotic machinery in leukemic versus normal progenitor cells may provide additional tools for sensitizing LSCs to conventional chemotherapeutics. Mcl-1–deficient mice succumb secondary to hematopoieic failure, indicating the key role of this protein in normal HSCs.27 In turn, Bcl-XL and Bcl-2 antiapoptotic proteins are highly expressed in leukemic progenitor cells and are redundant for normal hematopoiesis. This differential use of Bcl-2 family members can be exploited through use of compounds such as BH3-mimetic ABT-737, which was shown to preferentially inhibit survival of LSCs in preclinical AML models.28 The caveat of this approach is lack of efficacy in leukemias with high levels of Mcl-1 and potential hematologic toxicity of other BH3 mimetics capable of targeting Mcl-1.
Collectively, the findings outlined above indicate the most likely regimens for targeting intrinsic components of LSC biology: (1) exhibit cell cycle independence (ie, be as efficacious for quiescent cells as for cycling cells), (2) inhibit NF-κB signaling and/or related survival pathways, (3) induce oxidative stress, and (4) inhibit self-renewal mechanisms. Agents known to modulate one or more of these properties are currently approved or in various stages of clinical evaluation. For example, arsenic trioxide and bortezomib are strong inhibitors of the NF-κB pathway and also induce high levels of oxidative stress. The clinical analog of Bcl-2 inhibitor ABT-737, ABT-263, is undergoing phase I and II clinical trials as a single agent or in combination with monoclonal antibodies in CLL and acute lymphocytic leukemia (ALL). Multiple agents that inhibit PI3K pathway components are in development, and derivatives of the mammalian target of rapamycin inhibitor are also available (eg, temsirolimus). In addition, there is strong interest in targeting the Wnt/beta-catenin pathway, with several types of agents currently under investigation.29,30 Taken together with strategies that target extrinsic components of the tumor microenvironment (see below), it appears that several exciting new therapeutic options will be available in the near future.
ENVIRONMENT-MEDIATED DRUG RESISTANCE: ROLE OF EXTRINSIC FACTORS IN LSC SURVIVAL AND CHEMORESISTANCE
The majority of leukemias respond to initial treatment; however, relapse is common, indicating resistance of LSCs to current therapies. There is emerging evidence that extrinsic components mediated by the microenvironment play a pivotal role in survival and drug resistance of LSCs. It is believed that environment-mediated drug resistance is a transient state whereby LSCs are protected through signals from the niche, which eventually leads to the selection of secondary genetic changes and outgrowth of cells that acquired multiple mechanisms of pharmacologic resistance.31 These findings have generated novel approaches targeting the microenvironment supporting the LSC phenotype.
MICROENVIRONMENTAL NICHES OF NORMAL HSCs
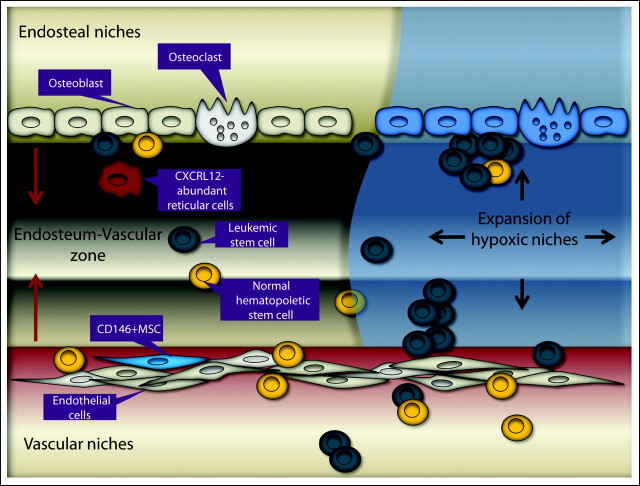
To understand the role of the microenvironment in leukemia, it is important to characterize the normal physiologic mechanisms of niche-mediated support of HSC maintenance (Fig 1). HSCs reside within specialized areas of the bone marrow (BM) microenvironment, defined as two distinct microenvironmental niches: osteoblastic (endosteal) and vascular.32 The osteoblastic niche, localized at the inner surface of the bone cavity and with abundant bone-forming osteoblasts, provides a microenvironment for long-term HSCs which are capable of contributing to hematopoiesis as quiescent or slow-cycling cells.32–35 The vascular niche, which consists of sinusoidal endothelial cells lining blood vessels, is thought to promote proliferation and differentiation of actively cycling, short-term HSCs.36 Recent studies indicate that these niches work in concert. Xie et al37 demonstrated that the endosteum forms a well-vascularized special zone that frequently is localized near N-cadherin–positive preosteoblastic cells and that this special niche promotes expansion of HSCs in response to BM damage.
Fig 1.
Mechanisms of normal and acute myelogenous leukemia stem cell interactions with the niche. The normal and leukemic stem cells (LSCs) reside in either the osteoblastic or vascular niche. In the osteoblastic niche at or near the endosteum, osteoblasts, octeoclasts, and stromal cells may provide a microenvironment for normal cells and LCSs. In the vascular niche around sinusoids, CD146+ mesenchymal progenitors facilitate transendothelial migration, homing, proliferation, and differentiation of normal cells and LSCs. Oxygen tension gradually declines from the vascular niche to the osteoblastic niche, and LSC proliferation results in expansion of hypoxic microenvironmental niches. MSC, mesenchymal stem or stromal cell.
Hematopoietic cell development is tightly regulated by BM stromal cells (BMSCs) through production of cytokines, chemokines, and intracellular signals initiated by cellular adhesion. BMSCs encompass a variety of cell types, including osteoblasts, osteoclasts, endothelial cells, perivascular reticular cells, and mesenchymal stem or stromal cells (MSCs), all of which are critical for the regulation of HSC maintenance and localization.38 Although the nature of the true MSC remains enigmatic, CXC chemokine ligand 12 (CXCL12) –expressing CD146+ MSCs were recently reported to be self-renewing progenitors that reside on the sinusoidal surfaces and contribute to organization of the sinusoidal wall structure,39 produce angiopoietin-1 (Ang-1), and are capable of generating osteoblasts that form the endosteal niche. These CXCL12-abundant reticular cells may serve as a transit pathway for shuttling HSCs between the osteoblastic and vascular niches where essential but different maintenance signals are provided.32 Cytokines and chemokines produced by BMSCs concentrate in particular niches secondary to varying local production and through the effects of cytokine-binding glycosaminoglycans. Of these, CXCL12/stromal cell–derived factor-1 alpha positively regulates HSC homing, while transforming growth factors FMS-like tyrosine kinase 3 (Flt3) ligand and Ang-1 function as quiescence factors. CXCL12-CXCR4 signaling is involved in homing of HSCs into BM during ontogeny as well as survival and proliferation of colony-forming progenitor cells.40,41 The CXCR4-selective antagonist–induced mobilization of HSCs into the peripheral blood further indicates a role for CXCL12 in retaining HSCs in hematopoietic organs.42 BM engraftment involves subsequent cell-to-cell interactions through the BMSC-produced complex extracellular matrix. Thus, vascular cell adhesion molecule-1 (VCAM-1) or fibronectin is critical for adhesion to the BMSCs.
LEUKEMIC MICROENVIRONMENT: NICHE RETREATS FOR LSCs
Recent data indicate that, in parallel with leukemogenic events in the hematopoietic system, the niche is converted into an environment with dominant signals that favor cell proliferation and growth. The microenvironment may have a role in determining the lineage commitment of acute leukemia. MLL-AF9–transduced cord blood cells on transplantation into immunodeficient mice generated AML, ALL, or biphenotypic leukemia, depending on the mouse strain or cytokine medium, hence demonstrating the influence of microenvironmental cues for lineage differentiation.43
The molecular mechanisms for maintaining quiescence of normal stem cells may also facilitate LSC survival. For LSC survival, proliferation, and differentiation, both the osteoblastic and vascular niches are critical.33–35,39,44,45 Ninomiya et al46 modeled the homing, proliferation, and survival sites of human leukemia cells and of cord blood CD34+ cells. The transplanted leukemia cells initially localized on the surface of osteoblasts in the epiphysial region and then expanded to the inner vascular and diaphysial regions. Eight weeks after transplantation, the number of leukemia cells transiently increased by as much as 50%, predominantly in the epiphysial region. After administration of high-dose cytarabine, residual leukemia cells clustered and adhered to the blood vessels as well as to the endosteum, suggesting that leukemia cells receive antiapoptotic signals not only from osteoblasts but also from vascular endothelium.46
Suppression of normal hematopoiesis is observed frequently in leukemia patients with relatively low tumor burden, which does not necessarily reflect occupancy of the anatomic space by leukemic cells. It has been demonstrated that leukemic cell growth disrupts normal hematopoietic progenitor cell (HPC) BM niches and creates a tumor microenvironment.47 Leukemic cells initially migrate toward the CXCL12-positive vascular niches in the BM, which in the murine model overlap with normal HPC niches.47,48 After 1 month of leukemia growth in vivo, CXCL12 production in the tumor vascular niche was markedly downregulated, and the normal human CD34+ cells transplanted in leukemic mice migrated to tumor niches through a CXCL12-independent mechanism, by virtue of stem-cell factor abundantly secreted by leukemic cells in the tumor niches. These findings indicate that the alteration of signaling mechanisms of BM niches used by normal HSC homing may be hijacked by LSCs.
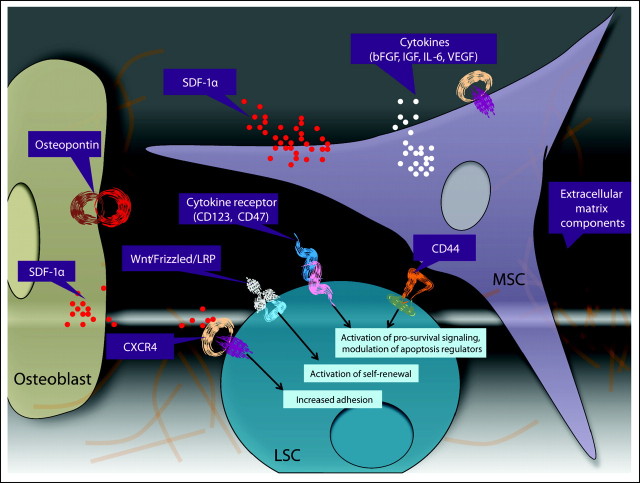
One of the key initial steps of leukemia-stroma interactions in vivo is homing and subsequent adhesion of LSCs to the protective areas of BM microenvironment (Fig 2). The interaction between CXCL12 (stromal cell–derived factor-1 alpha) and its receptor CXCR4 on leukemic progenitor cells contributes to their homing to the BM microenvironment. CXCR4 levels are significantly elevated in leukemic cells from patients with AML,49 and CXCR4 expression is associated with poor outcome.50,51 Administration of anti-CXCR4 antibody to mice engrafted with primary AML cells resulted in a dramatic decrease in the levels of human AML cells in the BM, blood, and spleen but did not significantly affect the levels of normal human progenitor cells engrafted into NOD/SCID mice.52 Significantly increased CXCR4 expression has been reported in Flt3/internal tandem duplication AML compared with FLT3/wild-type AML.50 This finding and additional preclinical data indicate that the Flt3 axis participates in the trafficking of transformed hematopoietic cells through CXCR4. In turn, integrins are required for LSCs to lodge in the BM niche. The attachment of AML cells to the BM microenvironment through interaction between very late antigen-4 on leukemic cells and fibronectin on MSCs has been shown to be crucial for the persistence of minimal residual disease in AML.53 Integrin ligation triggers activation of prosurvival signaling cascades. As such, integrin-linked kinase directly interacts with β integrins, phosphorylates Akt in a PI3K-dependent manner, and promotes survival of leukemic cells.54 Another adhesion molecule, CD44, has been demonstrated to be a key regulator of AML LSCs homing to microenvironmental niches and maintaining a primitive state.55 CD44 mediates adhesive cell-cell and cell–complex extracellular matrix interactions through binding to its main ligand, hyaluronan, a glycosaminoglycan highly concentrated in the endosteal region.56 Other ligands include osteopontin, fibronectin, and selectin, all of which are involved in cell trafficking and lodgment. Beyond its adhesion function, CD44 can also transduce multiple intracellular signal transduction pathways when ligated with hyaluronan or specific function-activating monoclonal antibodies.57
Fig 2.
Regulators of leukemic stem cell (LSC) niche interactions in microenvironmental niches. Within bone marrow niches, a complex interplay of cells, extracellular matrix components, and secreted factors may modulate the biology of LSCs. Osteoblasts provide a source of osteopontin and stromal cell–derived factor-1 alpha (SDF-1α), which may induce migration of CXCR4-expressing LSCs toward the osteoblastic niche. Similarly, mesenchymal stem cells (MSCs) also secrete SDF-1α, as well as cytokines, which induce cell proliferation, activate prosurvival signaling cascades, and modulate the expression of the antiapoptotic molecules, potentially resulting in drug resistance. In addition, activation of the self-renewal pathways (such as Wnt) has been postulated to result in enhanced LSC survival24 and may, in part, be mediated through the niche.100 bFGF, basic fibroblast growth factor; IGF, insulin-like growth factor; IL-6, interleukin-6; VEGF, vascular endothelial growth factor; LRP, leukocyte common antigen-related phosphatase.
It has been shown that progression of leukemia in a rat model was associated with marked expansion of hypoxia,58 compared with distinct subendosteal hypoxic areas of normal BM.59 Leukemic cells are able to proliferate even under hypoxic conditions, indicating that the cells are able to adapt to hypoxic conditions.58,60 In addition, overexpression of the key hypoxia mediator hypoxia-inducible transcription factor-1 alpha (HIF-1α) has been observed in clusters of leukemic cells in BM specimens from patients with primary ALL.61 Recently, mutations in the gene encoding isocitrate dehydrogenase-1 and isocitrate dehydrogenase-2 (IDH1, IDH2) were described in approximately 16% of AML.62,63 It remains to be established whether these mutations may constitute a poor prognostic factor in distinct subtypes of AML and may be associated with induction of the HIF-1α pathway as described in glioma.64 Notably, CXCR4 expression was upregulated under hypoxic conditions in AML cells.65 Consistent with the findings that HIF-1α regulates CXCR4,66 these data suggest that a hypoxic BM microenvironment represents a conditional stem and progenitor cell niche in which HIF-1α–induced stabilization and activation of both the trafficking stimulus (CXCL1267) and receptor (CXCR4) facilitate recruitment and retention of leukemic progenitor cells. Hypoxia is the major stimulus for angiogenesis through HIF-1α–mediated upregulation of vascular endothelial growth factor (VEGF). Formation of new vessels by angiogenesis represents an adaptive response to hypoxia and involves endothelial cell proliferation, a process stimulated by hypoxia-inducible growth factors, such as VEGF. Increased angiogenesis is observed in myelodysplastic syndrome (MDS),68 AML,69 and ALL.70 The levels of circulating endothelial progenitor cells were found to be increased in AML patients and, remarkably, endothelial progenitor cells shared the genetic abnormalities with the leukemic clone in AML,71 MDS,72 and CML.73 In turn, monoblasts from AML patients from AML-M5 leukemias were capable of differentiating into endothelial cells in vitro when cultured in the presence of VEGF and Ang-1.74 These findings underscore the need for combining the strategies aimed at elimination of both leukemic progenitor cells and endothelial precursors. Hypoxia promotes a switch to glycolytic metabolism via the activation of HIF-1α,75 and additional microenvironmental cues may be instrumental for the establishment of the proglycolytic phenotype.76,77 In solid tumors, hypoxia induces a metabolic shift causing acidosis78 and promotes autophagy, which may mediate cell survival response.79 Because these metabolic changes have been linked to chemoresistance, it is tempting to speculate that targeting glycolytic pathways may provide additional therapeutic tools for the treatment of hematopoietic malignancies.80
MOLECULAR AND GENETIC CHANGES WITHIN THE BM MICROENVIRONMENT THAT CONTRIBUTE TO LEUKEMOGENESIS
The significance of the hematopoietic microenvironment to disease initiation has been suggested by studies81 with mice deficient in phosphatase and tensin homolog (PTEN). PTEN deficiency in both hematopoietic cells and the microenvironment resulted in myeloproliferation that progressed to overt leukemia/lymphoma. However, inducible PTEN deletion in hematopoietic cells in the presence of a wild-type BM microenvironment promoted HSC depletion without evidence of myeloproliferation or leukemic development.81 These results suggest that PTEN deficiency in hematopoietic cells alone is not sufficient for malignant transformation. Likewise, activation of NF-κB in myelopoietic cells and the absence of its inhibitor IκBα are not sufficient for induction of hypergranulopoiesis, but these changes in the nonhematopoietic compartment, such as fetal liver, resulted in increased numbers of dysplastic hematopoietic cells with progression into secondary AML.82 Walkley et al83 have further demonstrated that dysfunction of retinoblastoma protein or retinoic acid receptor gamma84 in the BM microenvironment can contribute to development of preleukemic myeloproliferative disease. Both retinoblastoma protein and retinoic acid receptor gamma deficiency–induced expansion of HSCs and progenitor cells may result from loss of inhibitory signals normally provided by the osteoblastic niche. These findings critically underscore the importance of interactions between hematopoietic cells and the BM niche/microenvironment and indicate that additional genetic mutations within the BM microenvironment may be necessary for leukemic transformation.
In confirmation of genetic aberrations in BM microenvironment in leukemia, structural chromosomal changes have been found in BMSCs from 44% of patients with MDS and 54% of those with AML.85 Lopez-Villar et al86 reported the presence of cytogenetic aberrations on MSCs from MDS patients by array-based comparative genomic hybridization and fluorescence in situ hybridization, some of them specially linked to a particular MDS subtype, the 5q- syndrome. These findings suggest enhanced genetic instability of BMSCs in MDS and AML and indicate the potential involvement of BMSCs in the pathophysiology of these conditions.
In a recent study,87 mice with osteoblast-specific deletion of Dicer1, a gene required for RNA and microRNA processing, showed cytopenia, multilineage dysplasia, and increased proliferation and apoptosis of primitive hematopoietic progenitors, highly resembling a clinical picture of MDS. Importantly, in some animals, the dyspoietic changes lead to the development of clonal myeloid sarcomas and AML, which provided the first proof that perturbations in niche signaling may select for the acquisition of secondary genetic changes in the neighboring HSCs.
Additional evidence of microenvironmental dysfunction comes from recent findings in MDS. Marrow stroma from patients with early-stage MDS, in contrast to that from more advanced stages of MDS, such as chronic myelomonocytic leukemia, expressed 14- to 17-fold higher levels of interleukin-32 (IL-32) mRNA than healthy controls, and this constitutive IL-32 expression promoted apoptosis in MDS cells, reproducing the inefficient hematopoiesis and extensive apoptosis in MDS marrows.88 These findings indicate two independent mechanisms of stromal abnormalities in tumors: genetic changes in stromal cells and secondary epigenetic changes arising in the niche in response to tumor cells. These early preliminary studies require further confirmatory investigations of the suspected genetic abnormalities in leukemic stroma by using stringent cell separation techniques and modern high-throughput sequencing technologies.
THERAPEUTIC TARGETING OF LEUKEMIC MICROENVIRONMENT: CONCEPTS AND APPROACHES
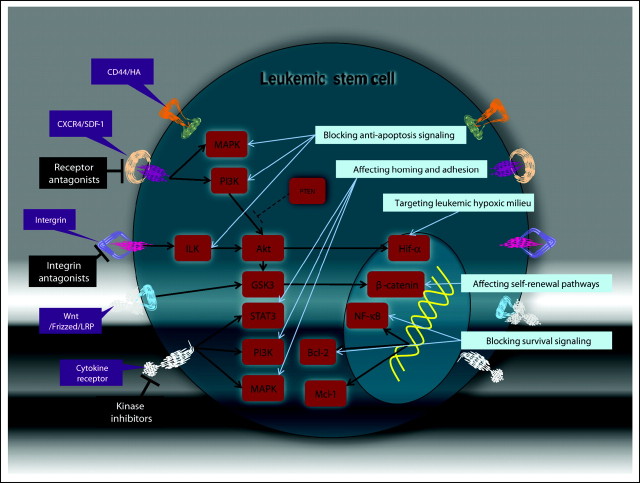
By elucidating the role of the BM microenvironment in the pathogenesis of hematologic tumors, recent studies have provided the framework for identifying and validating novel therapies that target both leukemic cells and cells in their surrounding microenvironment. Targeting the leukemia microenvironment can be implemented through several strategies: (1) affecting the key self-renewal pathways in LSCs that are promoted by the niche; (2) blockading the prosurvival signaling pathways induced by stromal cells in LSCs; (3) affecting homing and adhesion through interference with chemokines and adhesion molecules; (4) targeting the hypoxic milieu of leukemic microenvironment, and (5) inhibiting abnormally activated pathways within the cells of niche (Fig 3). Activation of the principal self-renewal pathways through Wnt/beta-catenin and notch signaling can be caused by microenvironmental stimuli and is amenable to therapeutic interventions. Upregulation of the prosurvival Bcl-289 and Mcl-190 pathways is a frequent event in leukemic cells growing in contact with BM-derived stromal cells and is potentially treatable with drugs such as BH3 mimetics (Bcl-2, ABT-737) or kinase/Cdk inhibitors (Mcl-1, MEK inhibitors/flavoperidol). Disruption of migratory and adhesion signals represents the strategy of blocking LSC homing to a BM niche and/or sensitizing leukemic cells to chemotherapy or kinase inhibitors. Targeting CXCR4 with small-molecule pharmacologic inhibitors has been shown to be efficacious in preclinical models of CLL,91 ALL,92 and AML,93,94 presumably through recruitment of leukemic cells out of their protective microenvironmental niches. In support of the role of VLA-4 in the sensitivity of AML cells to chemotherapy, a neutralizing VLA-4 antibody, in conjunction with cytarabine, prevented the development of AML in a xenograft model.53 Although the small-molecule inhibitors of VLA-4/VCAM-1 interactions caused impressive mobilization of normal HPCs, alone or in combination with granulocyte colony-stimulating factor or CXCR4 inhibitors,95 this approach has yet to be explored in leukemias. Likewise, ligation of CD44 with the H90 monoclonal antibody resulted in marked reduction of the leukemic burden in NOD-SCID mice transplanted with primary AML cells through alteration of AML LSC fate and abrogation of AML LSC homing.55 In addition, specific antibodies against CD123 and CD47 have recently been reported to reduce the growth of AML LSCs in xenograft models.96,97 The mechanism of CD123-mediated targeting appears to be complex, potentially acting through inhibition of homing, activation of innate immunity, and/or inhibition of intracellular signaling events. In contrast, antibodies that mask CD47 can trigger innate immune responses via activation of phagocytosis.
Fig 3.
Therapeutic targeting leukemic stem cell (LSC) niche interactions. Cytokines, chemokines, and the extracellular matrix activate the prosurvival signaling pathways (phosphatidylinositol-3 kinase [PI3K]/Akt, mitogen-activated protein kinase [MAPK], signal transducer and activator of transcription 3 [STAT3], and nuclear factor kappa B [NF-κB]) that regulate downstream components likely promoting survival and proliferation of LSCs. The therapeutic strategies designed to target the LSC within their surrounding microenvironment include adhesion molecule and cytokine antagonists as well as inhibitors of intracellular prosurvival and self-renewal pathways. These approaches may more selectively eradicate LSCs without adversely affecting normal stem-cell self-renewal. HA, hyaluronic acid; SDF-1, stromal cell–derived factor 1; PTEN, phosphatase and tensin homolog; ILK, integrin-linked kinase; HIF-1α, hypoxia-inducible transcription factor-1 alpha; GSK3, glycogen synthase kinase 3; LRP, leukocyte common antigen-related phosphatase; Bcl-2, B-cell lymphoma 2; Mcl-1, myeloid cell leukemia-1.
Concerns have been raised over the potential toxicity of some approaches outlined above, in particular, when combined with cytotoxic drugs, because mobilized normal HPCs that are usually protected in the BM microenvironment would be potentially exposed to the toxicity of chemotherapy. A leukemia cell–targeted approach, such as monoclonal antibodies (eg, anti-CD33, anti–CLL-1, anti-CD96, anti-CD25, or anti-CD32) or selective kinase inhibitors (sorafenib,94 or imatinib98) in combination with CXCR4 antagonists could avoid these potential adverse effects. In turn, targeting of stem-cell factor may inhibit HPC interaction with tumor niches and conceivably maintain normal progenitor cell function in the setting of malignancy.47 Since normal stem cells and LSCs appear to frequently use identical pathways of microenvironmental protection, it remains to be established in future prospective clinical trials whether these approaches will provide a sufficient therapeutic window in targeting LSCs.
Targeting angiogenesis is arguably the most clinically advanced approach for influencing the tumor/leukemia microenvironment. The anti-VEGF monoclonal antibody that neutralizes VEGF-A— bevacizumab—is the first antiangiogenic agent that has been validated as a cancer therapy. Bevacizumab combined with cytarabine and mitoxantrone has been demonstrated to improve the overall response rate of 48% in the phase II study.99 Other types of antiangiogenic agents, such as tyrosine kinase inhibitors (sunitinib and sorafenib) and anticytokine drugs (thalidomide and lenalidomide) have now entered clinical practice. Although these agents may affect endothelial BM niches, they may, in turn, enforce expansion of hypoxic niches and possibly promote chemoresistance. In this context, HIF-1α may represent an important molecular target within the tumor microenvironment. Several strategies specifically targeting HIF-1α are being explored in solid tumor models. These include a novel antisense oligonucleotide against HIF-1α and small-molecule HIF-1α inhibitors. The applicability of these reagents to LSC niche biology remains to be established.
In conclusion, we note that recent studies have elucidated multiple intrinsic and extrinsic factors that provide potential opportunities to improve therapeutic targeting of LSCs. Going forward, the challenge will be to identify the optimal combinations of drugs to appropriately modulate such factors in the most selective fashion possible. Although the potential algorithm for creating better regimens is not entirely clear, a few key principles appear to be important. First, new regimens must take into consideration the cell cycle status of LSCs, which is mostly quiescent. Thus, therapies either must be capable of killing quiescent cells or must include cell cycle activation of LSCs as part of the overall strategy. Second, improved approaches should target those properties that are most consistently aberrant in LSCs. To date, studies show that increased oxidative state, constitutive activation of NF-κB, constitutive activation of PI3K signaling, and increased reliance on Bcl-2 activity appear to be promising activities to target. Third, new strategies should exploit potentially unique properties of the LSC microenvironment to permit more selective and efficient eradication of primitive cells. For example, targeting of the mechanisms that mediate LSC adhesion within BM niches and stimulation of niche-induced prosurvival and self-renewal pathways both appear to be useful strategies. We propose that by profiling current and new experimental agents with regard to the parameters outlined above, it should be possible to design rational drug combinations that will more effectively eradicate LSCs.
Acknowledgment
We thank Zhihong Zeng, MD, for editorial assistance with the figures.
Footnotes
Supported by Grants No. RSG-06-054-01-LIB from the American Cancer Society (M.Y.K.) and No. W81XWH-07-1-0601 from the Department of Defense (C.T.J.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marina Y. Konopleva, Craig T. Jordan
Manuscript writing: Marina Y. Konopleva, Craig T. Jordan
Final approval of manuscript: Marina Y. Konopleva, Craig T. Jordan
REFERENCES
- 1.Dick JE. Acute myeloid leukemia stem cells. Ann N Y Acad Sci. 2005;1044:1–5. doi: 10.1196/annals.1349.001. [DOI] [PubMed] [Google Scholar]
- 2.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Jordan CT, Upchurch D, Szilvassy SJ, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 6.van Rhenen A, van Dongen GA, Kelder A, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659–2666. doi: 10.1182/blood-2007-03-083048. [DOI] [PubMed] [Google Scholar]
- 7.Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(-) fraction. Blood. 2010;115:1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taussig DC, Miraki-Moud F, Anjos-Afonso F, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 9.Guzman ML, Neering SJ, Upchurch D, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 10.Costello RT, Mallet F, Gaugler B, et al. Human acute myeloid leukemia CD34+/CD38- progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- 11.Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2009;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Gerhard B, Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML) Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- 13.Holyoake TL, Jiang X, Drummond MW, et al. Elucidating critical mechanisms of deregulated stem cell turnover in the chronic phase of chronic myeloid leukemia. Leukemia. 2002;16:549–558. doi: 10.1038/sj.leu.2402444. [DOI] [PubMed] [Google Scholar]
- 14.Holyoake TL, Jiang X, Jorgensen HG, et al. Primitive quiescent leukemic cells from patients with chronic myeloid leukemia spontaneously initiate factor-independent growth in vitro in association with up-regulation of expression of interleukin-3. Blood. 2001;97:720–728. doi: 10.1182/blood.v97.3.720. [DOI] [PubMed] [Google Scholar]
- 15.Saito Y, Uchida N, Tanaka S, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzman ML, Swiderski CF, Howard DS, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassane DC, Guzman ML, Corbett C, et al. Discovery of agents that eradicate leukemia stem cells using an in silico screen of public gene expression data. Blood. 2008;111:5654–5662. doi: 10.1182/blood-2007-11-126003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, Simpson SE, Scialla TJ, et al. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 20.Hassane D, Sen S, Corbett CA, et al. Chemical genomic screening reveals that PI3K/mTOR inhibition enhances activity of the anti-leukemia stem cell compound parthenolide. Blood. 2009;114:388. abstr 388. [Google Scholar]
- 21.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 22.Tothova Z, Kollipara R, Huntly BJ, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Smith J, Ladi E, Mayer-Proschel M, et al. Redox state is a central modulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao C, Blum J, Chen A, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Kwon HY, Zimdahl B, et al. Regulation of myeloid leukaemia by the cell-fate determinant Musashi. Nature. 2010;466:765–768. doi: 10.1038/nature09171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 28.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B, Dodge ME, Tang W, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 32.Perry JM, Li L. Disrupting the stem cell niche: Good seeds in bad soil. Cell. 2007;129:1045–1047. doi: 10.1016/j.cell.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 33.Arai F, Hirao A, Ohmura M, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 36.Kopp HG, Avecilla ST, Hooper AT, et al. The bone marrow vascular niche: Home of HSC differentiation and mobilization. Physiology (Bethesda) 2005;20:349–356. doi: 10.1152/physiol.00025.2005. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, Yin T, Wiegraebe W, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 38.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 41.Broxmeyer HE, Cooper S, Kohli L, et al. Transgenic expression of stromal cell-derived factor-1/CXC chemokine ligand 12 enhances myeloid progenitor cell survival/antiapoptosis in vitro in response to growth factor withdrawal and enhances myelopoiesis in vivo. J Immunol. 2003;170:421–429. doi: 10.4049/jimmunol.170.1.421. [DOI] [PubMed] [Google Scholar]
- 42.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, Wunderlich M, Fox C, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13:483–495. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson SK, Johnston HM, Whitty GA, et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 45.Naveiras O, Daley GQ. Stem cells and their niche: A matter of fate. Cell Mol Life Sci. 2006;63:760–766. doi: 10.1007/s00018-005-5469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ninomiya M, Abe A, Katsumi A, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]
- 47.Colmone A, Amorim M, Pontier AL, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 48.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Möhle R, Schittenhelm M, Failenschmid C, et al. Functional response of leukaemic blasts to stromal cell-derived factor-1 correlates with preferential expression of the chemokine receptor CXCR4 in acute myelomonocytic and lymphoblastic leukemia. Br J Haematol. 2000;110:563–572. doi: 10.1046/j.1365-2141.2000.02157.x. [DOI] [PubMed] [Google Scholar]
- 50.Rombouts EJ, Pavic B, Löwenberg B, et al. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550–557. doi: 10.1182/blood-2004-02-0566. [DOI] [PubMed] [Google Scholar]
- 51.Konoplev S, Rassidakis GZ, Estey E, et al. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109:1152–1156. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- 52.Tavor S, Petit I, Porozov S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 53.Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158–1165. doi: 10.1038/nm909. [DOI] [PubMed] [Google Scholar]
- 54.Tabe Y, Jin L, Tsutsumi-Ishii Y, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67:684–694. doi: 10.1158/0008-5472.CAN-06-3166. [DOI] [PubMed] [Google Scholar]
- 55.Jin L, Hope KJ, Zhai Q, et al. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 56.Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 57.Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- 58.Mortensen BT, Jensen PO, Helledie N, et al. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br J Haematol. 1998;102:458–464. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 59.Parmar K, Mauch P, Vergilio JA, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen PO, Mortensen BT, Hodgkiss RJ, et al. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif. 2000;33:381–395. doi: 10.1046/j.1365-2184.2000.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wellmann S, Guschmann M, Griethe W, et al. Activation of the HIF pathway in childhood ALL, prognostic implications of VEGF. Leukemia. 2004;18:926–933. doi: 10.1038/sj.leu.2403332. [DOI] [PubMed] [Google Scholar]
- 62.Marcucci G, Maharry K, Wu YZ, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–3643. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 64.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1 alpha. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiegl M, Samudio I, Clise-Dwyer K, et al. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staller P, Sulitkova J, Lisztwan J, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 67.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 68.Korkolopoulou P, Apostolidou E, Pavlopoulos PM, et al. Prognostic evaluation of the microvascular network in myelodysplastic syndromes. Leukemia. 2001;15:1369–1376. doi: 10.1038/sj.leu.2402220. [DOI] [PubMed] [Google Scholar]
- 69.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–313. [PubMed] [Google Scholar]
- 70.Koomagi R, Zintl F, Sauerbrey A, et al. Vascular endothelial growth factor in newly diagnosed and recurrent childhood acute lymphoblastic leukemia as measured by real-time quantitative polymerase chain reaction. Clin Cancer Res. 2001;7:3381–3384. [PubMed] [Google Scholar]
- 71.Rigolin GM, Mauro E, Ciccone M, et al. Neoplastic circulating endothelial-like cells in patients with acute myeloid leukaemia. Eur J Haematol. 2007;78:365–373. doi: 10.1111/j.1600-0609.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 72.Della Porta MG, Malcovati L, Rigolin GM, et al. Immunophenotypic, cytogenetic and functional characterization of circulating endothelial cells in myelodysplastic syndromes. Leukemia. 2008;22:530–537. doi: 10.1038/sj.leu.2405069. [DOI] [PubMed] [Google Scholar]
- 73.Gunsilius E, Duba HC, Petzer AL, et al. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 74.Rehman J, Li J, Orschell CM, et al. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 75.Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–234. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 76.Lum JJ, Bui T, Gruber M, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003;63:3847–3854. [PubMed] [Google Scholar]
- 78.Chiche J, Brahimi-Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J Cell Mol Med. 2010;14:771–794. doi: 10.1111/j.1582-4934.2009.00994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mazure NM, Pouysségur J. Hypoxia-induced autophagy: Cell death or cell survival? Curr Opin Cell Biol. 2010;22:177–180. doi: 10.1016/j.ceb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 80.Hulleman E, Kazemier KM, Holleman A, et al. Inhibition of glycolysis modulates prednisolone resistance in acute lymphoblastic leukemia cells. Blood. 2009;113:2014–2021. doi: 10.1182/blood-2008-05-157842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 82.Rupec RA, Jundt F, Rebholz B, et al. Stroma-mediated dysregulation of myelopoiesis in mice lacking I kappa B alpha. Immunity. 2005;22:479–491. doi: 10.1016/j.immuni.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 83.Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Blau O, Hofmann WK, Baldus CD, et al. Chromosomal aberrations in bone marrow mesenchymal stroma cells from patients with myelodysplastic syndrome and acute myeloblastic leukemia. Exp Hematol. 2007;35:221–229. doi: 10.1016/j.exphem.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23:664–672. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 87.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marcondes AM, Mhyre AJ, Stirewalt DL, et al. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105:2865–2870. doi: 10.1073/pnas.0712391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konopleva M, Konoplev S, Hu W, et al. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 90.Balakrishnan K, Burger JA, Wierda WG, et al. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–153. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burger M, Hartmann T, Krome M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 92.Juarez J, Bradstock KF, Gottlieb DJ, et al. Effects of inhibitors of the chemokine receptor CXCR4 on acute lymphoblastic leukemia cells in vitro. Leukemia. 2003;17:1294–1300. doi: 10.1038/sj.leu.2402998. [DOI] [PubMed] [Google Scholar]
- 93.Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 97.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin L, Tabe Y, Konoplev S, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7:48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- 99.Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: Therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 100.Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]