Abstract
A revision of the nearly 8-year-old World Health Organization classification of the lymphoid neoplasms and the accompanying monograph is being published. It reflects a consensus among hematopathologists, geneticists, and clinicians regarding both updates to current entities as well as the addition of a limited number of new provisional entities. The revision clarifies the diagnosis and management of lesions at the very early stages of lymphomagenesis, refines the diagnostic criteria for some entities, details the expanding genetic/molecular landscape of numerous lymphoid neoplasms and their clinical correlates, and refers to investigations leading to more targeted therapeutic strategies. The major changes are reviewed with an emphasis on the most important advances in our understanding that impact our diagnostic approach, clinical expectations, and therapeutic strategies for the lymphoid neoplasms.
Introduction
The 2008 World Health Organization (WHO) classification of hematopoietic and lymphoid tumors and the associated monograph represent the established guidelines for the diagnosis of malignant lymphomas; however, subsequently there have been major advances with significant clinical and biologic implications.1 A major revision is therefore being published that will be an update of the current fourth edition and not a truly new fifth edition as there are still other volumes pending in the fourth edition of the WHO tumor monograph series. Because it is considered a part of the fourth edition, while some provisional entities will be promoted to definite entities and a small number of new provisional entities added, there will be no new definite entities.
As with the 2001 and 2008 classifications, an all-important Clinical Advisory Committee meeting was held in 2014 to obtain the advice and consent of clinical hematologists/oncologists and other physicians critical to the revision (supplemental Appendix, available on the Blood Web site). Additional editorial meetings and consultations followed leading to the updated classification (Table 1).2 Although there are only limited alterations in the classification compared with 2008, the revised monograph will incorporate a large body of information published over the last 8 years relating to existing entities with some important diagnostic, prognostic, and therapeutic implications. The classification maintains the goals of helping to identify homogeneous groups of well-defined entities and facilitating the recognition of uncommon diseases that require further clarification.3 This manuscript will review the major areas in lymphoid, histiocytic, and dendritic neoplasms where changes from the prior edition are foreseen as well as emphasize conceptual themes (Table 2).
Table 1.
2016 WHO classification of mature lymphoid, histiocytic, and dendritic neoplasms
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| Monoclonal B-cell lymphocytosis* |
| B-cell prolymphocytic leukemia |
| Splenic marginal zone lymphoma |
| Hairy cell leukemia |
| Splenic B-cell lymphoma/leukemia, unclassifiable |
| Splenic diffuse red pulp small B-cell lymphoma |
| Hairy cell leukemia-variant |
| Lymphoplasmacytic lymphoma |
| Waldenström macroglobulinemia |
| Monoclonal gammopathy of undetermined significance (MGUS), IgM* |
| μ heavy-chain disease |
| γ heavy-chain disease |
| α heavy-chain disease |
| Monoclonal gammopathy of undetermined significance (MGUS), IgG/A* |
| Plasma cell myeloma |
| Solitary plasmacytoma of bone |
| Extraosseous plasmacytoma |
| Monoclonal immunoglobulin deposition diseases* |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) |
| Nodal marginal zone lymphoma |
| Pediatric nodal marginal zone lymphoma |
| Follicular lymphoma |
| In situ follicular neoplasia* |
| Duodenal-type follicular lymphoma* |
| Pediatric-type follicular lymphoma* |
| Large B-cell lymphoma with IRF4 rearrangement* |
| Primary cutaneous follicle center lymphoma |
| Mantle cell lymphoma |
| In situ mantle cell neoplasia* |
| Diffuse large B-cell lymphoma (DLBCL), NOS |
| Germinal center B-cell type* |
| Activated B-cell type* |
| T-cell/histiocyte-rich large B-cell lymphoma |
| Primary DLBCL of the central nervous system (CNS) |
| Primary cutaneous DLBCL, leg type |
| EBV+ DLBCL, NOS* |
| EBV+ mucocutaneous ulcer* |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| ALK+ large B-cell lymphoma |
| Plasmablastic lymphoma |
| Primary effusion lymphoma |
| HHV8+ DLBCL, NOS* |
| Burkitt lymphoma |
| Burkitt-like lymphoma with 11q aberration* |
| High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 rearrangements* |
| High-grade B-cell lymphoma, NOS* |
| B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma |
| Mature T and NK neoplasms |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK cells |
| Aggressive NK-cell leukemia |
| Systemic EBV+ T-cell lymphoma of childhood* |
| Hydroa vacciniforme–like lymphoproliferative disorder* |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK-/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Monomorphic epitheliotropic intestinal T-cell lymphoma* |
| Indolent T-cell lymphoproliferative disorder of the GI tract* |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30+ T-cell lymphoproliferative disorders |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous γδ T-cell lymphoma |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma |
| Primary cutaneous acral CD8+ T-cell lymphoma* |
| Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder* |
| Peripheral T-cell lymphoma, NOS |
| Angioimmunoblastic T-cell lymphoma |
| Follicular T-cell lymphoma* |
| Nodal peripheral T-cell lymphoma with TFH phenotype* |
| Anaplastic large-cell lymphoma, ALK+ |
| Anaplastic large-cell lymphoma, ALK−* |
| Breast implant–associated anaplastic large-cell lymphoma* |
| Hodgkin lymphoma |
| Nodular lymphocyte predominant Hodgkin lymphoma |
| Classical Hodgkin lymphoma |
| Nodular sclerosis classical Hodgkin lymphoma |
| Lymphocyte-rich classical Hodgkin lymphoma |
| Mixed cellularity classical Hodgkin lymphoma |
| Lymphocyte-depleted classical Hodgkin lymphoma |
| Posttransplant lymphoproliferative disorders (PTLD) |
| Plasmacytic hyperplasia PTLD |
| Infectious mononucleosis PTLD |
| Florid follicular hyperplasia PTLD* |
| Polymorphic PTLD |
| Monomorphic PTLD (B- and T-/NK-cell types) |
| Classical Hodgkin lymphoma PTLD |
| Histiocytic and dendritic cell neoplasms |
| Histiocytic sarcoma |
| Langerhans cell histiocytosis |
| Langerhans cell sarcoma |
| Indeterminate dendritic cell tumor |
| Interdigitating dendritic cell sarcoma |
| Follicular dendritic cell sarcoma |
| Fibroblastic reticular cell tumor |
| Disseminated juvenile xanthogranuloma |
| Erdheim-Chester disease* |
Provisional entities are listed in italics.
Changes from the 2008 classification.
Table 2.
Highlights of changes in 2016 WHO classification of lymphoid, histiocytic, and dendritic neoplasms
| Entity/category | Change |
|---|---|
| CLL/SLL | • Cytopenias or disease-related symptoms are now insufficient to make a diagnosis of CLL with <5 × 109/L PB CLL cells. |
| • Large/confluent and/or highly proliferative proliferation centers are adverse prognostic indicators. | |
| • Mutations of potential clinical relevance, such as TP53, NOTCH1, SF3B1, ATM, and BIRC3, have been recognized. | |
| Monoclonal B-cell lymphocytosis | • Must distinguish low-count from high-count MBL. |
| • A lymph node equivalent of MBL exists. | |
| Hairy cell leukemia | • BRAF V600E mutations in vast majority of cases with MAP2K1 mutations in most cases that use IGHV4-34 and lack BRAF mutation. |
| Lymphoplasmacytic lymphoma (LPL) | • MYD88 L265P mutation in vast majority of cases impacting diagnostic criteria even though finding is not specific for LPL. |
| • IgM MGUS is more closely related to LPL and other B-cell lymphomas than to myeloma. | |
| Follicular lymphoma (FL) | • Mutational landscape better understood but clinical impact remains to be determined. |
| In situ follicular neoplasia | • New name for in situ follicular lymphoma reflects low risk of progression to lymphoma. |
| Pediatric-type FL | • A localized clonal proliferation with excellent prognosis; conservative therapeutic approach may be sufficient. |
| • Occurs in children and young adults, rarely in older individuals. | |
| Large B-cell lymphoma with IRF4 rearrangement | • New provisional entity to distinguish from pediatric-type FL and other DLBCL. |
| • Localized disease, often involves cervical lymph nodes or Waldeyer ring. | |
| Duodenal-type FL | • Localized process with low risk for dissemination. |
| Predominantly diffuse FL with 1p36 deletion | • Accounts for some cases of diffuse FL, lacks BCL2 rearrangement; presents as localized mass, often inguinal. |
| Mantle cell lymphoma (MCL) | • Two MCL subtypes recognized with different clinicopathological manifestations and molecular pathogenetic pathways: one largely with unmutated/minimally mutated IGHV and mostly SOX11+ and the other largely with mutated IGHV and mostly SOX11− (indolent leukemic nonnodal MCL with PB, bone marrow (BM), ±splenic involvement, may become more aggressive). |
| • Mutations of potential clinical importance, such as TP53, NOTCH 1/2, recognized in small proportion of cases. | |
| • CCND2 rearrangements in approximately half of cyclin D1− MCL. | |
| In situ mantle cell neoplasia | • New name for in situ MCL, reflecting low clinical risk. |
| Diffuse large B-cell lymphoma, NOS | • Distinction of GCB vs ABC/non-GC type required with use of immunohistochemical algorithm acceptable, may affect therapy. |
| • Coexpression of MYC and BCL2 considered new prognostic marker (double-expressor lymphoma). | |
| • Mutational landscape better understood but clinical impact remains to be determined. | |
| EBV+ DLBCL, NOS | • This term replaces EBV+ DLBCL of the elderly because it may occur in younger patients. |
| • Does not include EBV+ B-cell lymphomas that can be given a more specific diagnosis. | |
| EBV+ mucocutaneous ulcer | • Newly recognized entity associated with iatrogenic immunosuppression or age-related immunosenescence. |
| Burkitt lymphoma | • TCF3 or ID3 mutations in up to ∼70% of cases. |
| Burkitt-like lymphoma with 11q aberration | • New provisional entity that closely resembles Burkitt lymphoma but lacks MYC rearrangement and has some other distinctive features. |
| High-grade B-cell lymphoma, with MYC and BCL2 and/or BCL6 translocations | • New category for all “double-/triple-hit” lymphomas other than FL or lymphoblastic lymphomas. |
| High-grade B-cell lymphoma, NOS | • Together with the new category for the “double-/triple-hit” lymphomas, replaces the 2008 category of B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BCLU). |
| • Includes blastoid-appearing large B-cell lymphomas and cases lacking MYC and BCL2 or BCL6 translocations that would formerly have been called BCLU. | |
| T-cell large granular lymphocyte leukemia | • New subtypes recognized with clinicopathologic associations. |
| • STAT3 and STAT5B mutations in a subset, latter associated with more clinically aggressive disease. | |
| Systemic EBV+ T-cell lymphoma of childhood | • Name changed from lymphoproliferative disorder to lymphoma due to its fulminant clinical course and desire to clearly distinguish it from chronic active EBV infection. |
| Hydroa vacciniforme–like lymphoproliferative disorder | • Name changed from lymphoma to lymphoproliferative disorder due to its relationship with chronic active EBV infection and a spectrum in terms of its clinical course. |
| Enteropathy-associated T-cell lymphoma (EATL) | • Diagnosis only to be used for cases formerly known as type I EATL, typically associated with celiac disease. |
| Monomorphic epitheliotropic intestinal T-cell lymphoma | • Formerly type II EATL; segregated from type I EATL and given a new name due to its distinctive nature and lack of association with celiac disease. |
| Indolent T-cell lymphoproliferative disorder of the GI tract | • New indolent provisional entity with superficial monoclonal intestinal T-cell infiltrate, some cases show progression. |
| Lymphomatoid papulosis | • New subtypes described with similar clinical behavior but atypical histologic/immunophenotypic features. |
| Primary cutaneous γ δ T-cell lymphoma | • Important to exclude other cutaneous T-cell lymphomas/lymphoproliferative disorders that may also be derived from γ δ T cells such as mycosis fungoides or lymphomatoid papulosis. |
| Primary cutaneous acral CD8+ T-cell lymphoma | • New indolent provisional entity, originally described as originating in the ear. |
| Primary cutaneous CD4+ small/medium T-cell lymphoproliferative disorder | • No longer to be diagnosed as an overt lymphoma due to limited clinical risk, localized disease, and similarity to clonal drug reactions. |
| • Remains a provisional entity. | |
| Peripheral T-cell lymphoma (PTCL), NOS | • Subsets based on phenotype and molecular abnormalities being recognized that may have clinical implications but are mostly not a part of routine practice at this time. |
| Nodal T-cell lymphomas with T-follicular helper (TFH) phenotype | • An umbrella category created to highlight the spectrum of nodal lymphomas with a TFH phenotype including angioimmunoblastic T-cell lymphoma, follicular T-cell lymphoma, and other nodal PTCL with a TFH phenotype (specific diagnoses to be used due to clinicopathologic differences). |
| • Overlapping recurrent molecular/cytogenetic abnormalities recognized that potentially could impact therapy. | |
| ALK− anaplastic large-cell lymphoma | • Now a definite entity that includes cytogenetic subsets that appear to have prognostic implications (eg, 6p25 rearrangments at IRF4/DUSP22 locus). |
| Breast implant–associated anaplastic large cell lymphoma | • New provisional entity distinguished from other ALK− ALCL; noninvasive disease associated with excellent outcome. |
| Nodular lymphocyte–predominant Hodgkin lymphoma | • Variant growth patterns, if present, should be noted in diagnostic report, due to their clinicopathologic associations. |
| • Cases associated with synchronous or subsequent sites that are indistinguishable from T-cell histiocyte-rich large B-cell lymphoma (THRLBCL) without a nodular component should be designated THRLBCL-like transformation. | |
| Lymphocyte-rich classical Hodgkin lymphoma | • Features recognized that are intermediate between NLPHL and other types of classical Hodgkin lymphoma. |
| Erdheim-Chester disease | • Should be distinguished from other members of the juvenile xanthogranuloma family; often associated with BRAF mutations. |
| Other histiocytic/dendritic neoplasms | • Clonal relationship to lymphoid neoplasms recognized in some cases. |
Mature B-cell lymphoid neoplasms
An important element that pervades many parts of the new monograph derives from an explosion of new clinical, pathological, and genetic/molecular data concerning the “small B-cell” lymphomas. The concept that there are lymphoid proliferations that we used to diagnose as overt lymphoid neoplasms but which are not considered as such in 2016 will be further emphasized. Among the aggressive B-cell lymphomas, there are major changes that impact how these cases should be evaluated and diagnosed that have important therapeutic implications as well as being of biologic interest.
Chronic lymphocytic leukemia/small lymphocytic lymphoma and monoclonal B-cell lymphocytosis
The 2008 monograph recognized monoclonal B-cell lymphocytosis (MBL) as the presence of monoclonal B-cell populations in the peripheral blood (PB) of up to 5 × 109/L either with the phenotype of chronic lymphocytic leukemia (CLL), atypical CLL, or non-CLL (CD5−) B cells in the absence of other lymphomatous features. Found in up to 12% of healthy individuals, in some it may be an extremely small population, but in others associated with a lymphocytosis.4 Whereas in 2008 it was unknown whether MBL was a precursor of CLL, we now know that MBL precedes virtually all cases of CLL/small lymphocytic lymphoma (SLL).5 The updated WHO will retain the current criteria for MBL, but will emphasize that “low-count” MBL, defined as a PB CLL count of <0.5 × 109/L, must be distinguished from “high-count” MBL because low count MBL has significant differences from CLL, an extremely limited, if any, chance of progression, and, until new evidence is provided, does not require routine follow-up outside of standard medical care.6,7 In contrast, high-count MBL requires routine/yearly follow-up, and has very similar phenotypic and genetic/molecular features as Rai stage 0 CLL, although immunoglobulin heavy chain variable region (IGHV)-mutated cases are more frequent in MBL.8 Also impacting our diagnostic criteria, the revision will eliminate the option to diagnose CLL with <5 × 109/L PB CLL cells in the absence of extramedullary disease even if there are cytopenias or disease-related symptoms. Non-CLL type MBL, at least some of which may be closely related to splenic marginal zone lymphoma, is also recognized.9,10
In addition, although other confirmatory studies are necessary, the concept of tissue-based MBL of CLL type will be discussed as there are a subset of cases with lymph node involvement by “SLL” that also do not seem to have a significant rate of progression. In 1 retrospective study, lymph nodes with CLL/SLL in which proliferation centers were not observed and patients in whom adenopathy was <1.5 cm based on computed tomography scans were the best candidates for tissue-based MBL.11
Also related to CLL/SLL, there is increasing interest in proliferation centers (PCs) in overt CLL/SLL. We have learned that: PCs can have cyclin D1 expression in up to about 30% of CLL/SLL, they express MYC protein, and, based on 3 of 4 studies, PCs which are large/confluent and/or have a high proliferative fraction are a significant and independent adverse prognostic indicator.12-16
Follicular lymphoma, in situ follicular neoplasia, pediatric-type follicular lymphoma, and other related lymphomas
Consistent with the growing conservatism in lymphoma diagnosis, in situ follicular lymphoma (FL) will be renamed in situ follicular neoplasia (ISFN) with the criteria remaining those described previously. Much has been learned about these neoplasms, which have a low rate of progression, but are more often associated with prior or synchronous overt lymphomas, thus requiring additional clinical assessment.17,18 They must be distinguished from partial involvement by FL that is more likely to progress.17 Unfortunately, the extent of the in situ lesions, such as the number or proportion of abnormal follicles or the degree of involvement within the abnormal follicles, cannot be used to predict which patients have isolated ISFN or who are least likely to progress to an overt lymphoma.17,18 ISFN does have fewer chromosomal copy-number abnormalities than focal and especially overt FL, although secondary genetic abnormalities are present even in the earliest lesions in addition to BCL2 rearrangements.19,20 At the very lowest end of this spectrum, cells similar to those with t(14;18)(q32;q21) IGH/BCL2 translocation that circulate in many healthy individuals may reside in the germinal centers as nonproliferative centrocytes even in the absence of recognizable ISFN.21 However, higher levels of circulating t(14;18)+ lymphocytes (>10−4 of total cells) indicate a higher risk for FL.22 It is also important to recognize that flow cytometric studies demonstrate populations of B cells with a FL-type phenotype in about half of all lymph nodes with ISFN.18 This is of particular importance in the evaluation of fine-needle aspirations in which architectural features cannot be evaluated.
Pediatric FL will become a definite entity in the 2016 classification but are now known as pediatric-type FL because similar lymphomas may occur in adults.23,24 It is a nodal disease characterized by large expansile highly proliferative follicles that often have prominent blastoid follicular center cells rather than classic centroblasts (or centrocytes).23 Some have reported a moderate number of cases as grade 1-2 of 3. BCL2 rearrangements must not be present, but there may be some BCL2 protein expression. They also lack BCL6 and MYC rearrangements with ongoing investigations of their genetic/molecular landscape.25 Nearly all cases are localized and may not require treatment other than excision. The criteria for pediatric-type FL, however, must be strictly applied to avoid underdiagnosing conventional grade 3 FL, with particular caution required before making this diagnosis in an adult. This category also excludes cases with diffuse areas (ie, foci of diffuse large B-cell lymphoma [DLBCL]). Some studies have raised the possibility that pediatric-type FL might be a “benign clonal proliferation with low malignant potential.”23,24
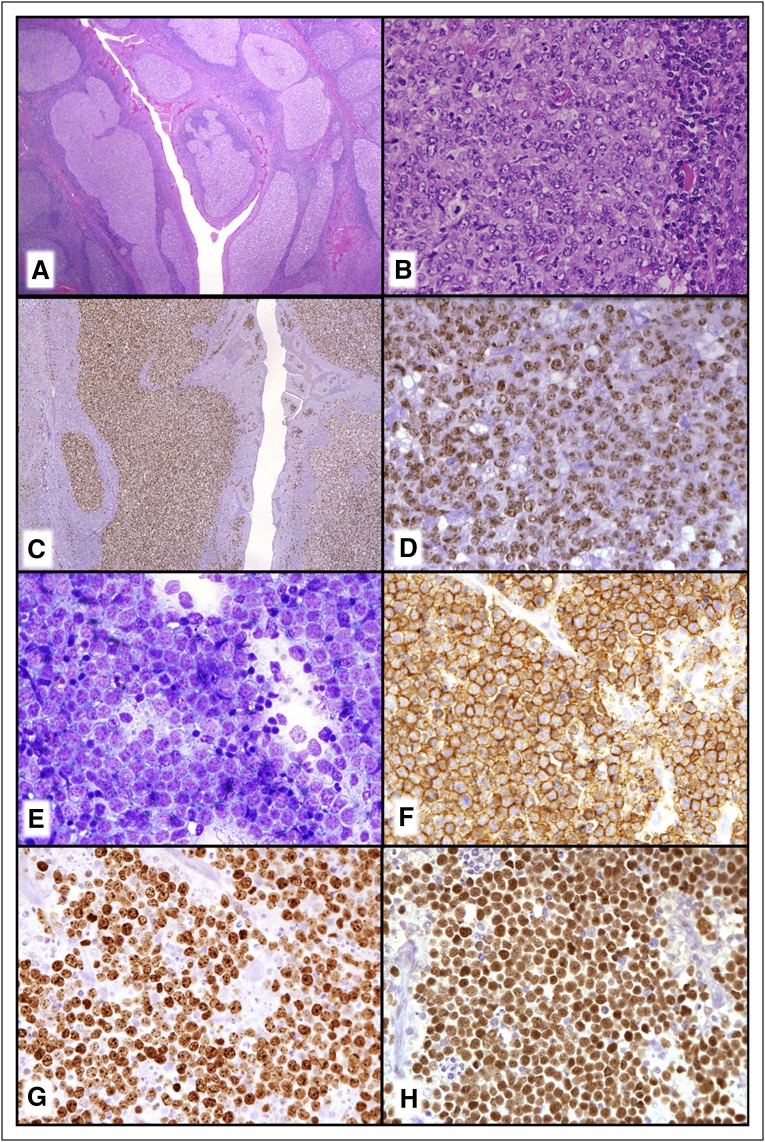
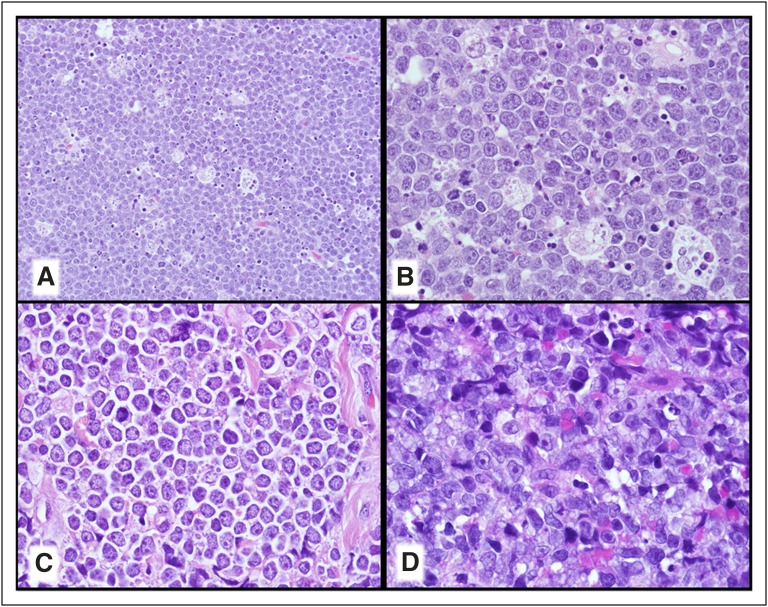
Large B-cell lymphoma (LBCL) with IRF4 rearrangement, which also occurs most commonly in children and young adults, will be considered a distinct new provisional entity (Figure 1A-D).23,26 These lymphomas most typically occur in Waldeyer ring and/or cervical lymph nodes and are low stage. They may have a follicular, follicular and diffuse, or pure diffuse growth pattern resembling FL grade 3B or a DLBCL. Strong IRF4/MUM1 expression is seen usually with BCL6 and a high proliferative fraction. BCL2 and CD10 are also expressed in more than half of the cases with a minority CD5+. They are most often of germinal center type, particularly based on gene expression profiling (GEP) studies.26 Most cases have IG/IRF4 rearrangements sometimes together with BCL6 rearrangements but they uniformly lack BCL2 rearrangements. Some cases that also seem to belong in this category lack a demonstrable IRF4 rearrangement but have strong IRF4/MUM1 expression.23 This lymphoma is considered to be more aggressive than other pediatric-type FL but patients, at least when treated, have done very well.26 These cases must be distinguished from the CD10−, IRF4/MUM1+ FL which are often associated with DLBCL and occur in older individuals.27
Figure 1.
New provisional B-cell lymphoma entities. (A-D) LBCL with IRF4 rearrangement. (A) Note the very large abnormal-appearing follicles in the central portion of this tonsil. (B) The neoplastic follicles have numerous transformed cells that are (C) IRF4/MUM-1+ and (D) BCL6+. (E-H) Burkitt-like lymphoma with 11q aberration. (E) The touch imprint demonstrates a monotonous population of transformed cells with basophilic cytoplasm that are (F) CD20+, (G) have a very high MIB1/Ki-67 proliferation fraction, and are (H) BCL6+. (A-B) Hematoxylin and eosin stain; (C,D-H) immunoperoxidase stains as specified; (E) Romanowsky-type stain.
The current monograph recognizes gastrointestinal (GI) tract FL as a variant. The revision will emphasize the distinctive nature specifically of duodenal-type FL, which although having features of a localized overt low-grade FL, is distinct from other GI tract FL, and has many features that overlap with ISFN as well as some features resembling an extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue.19,28,29 These patients appear to have an excellent outcome, including some cases managed with a watch-and-wait strategy.28
The new monograph will also recognize that although some diffuse-appearing FL may simply reflect a sampling issue, there is a group of distinctive largely diffuse low-grade FL that typically present as large localized inguinal masses, lack BCL2 rearrangements, and have 1p36 deletions.30 It should be noted that the latter is not a specific finding and can be seen in other lymphomas including conventional FL.
Mantle cell lymphoma, leukemic nonnodal mantle cell lymphoma, and in situ mantle cell neoplasia
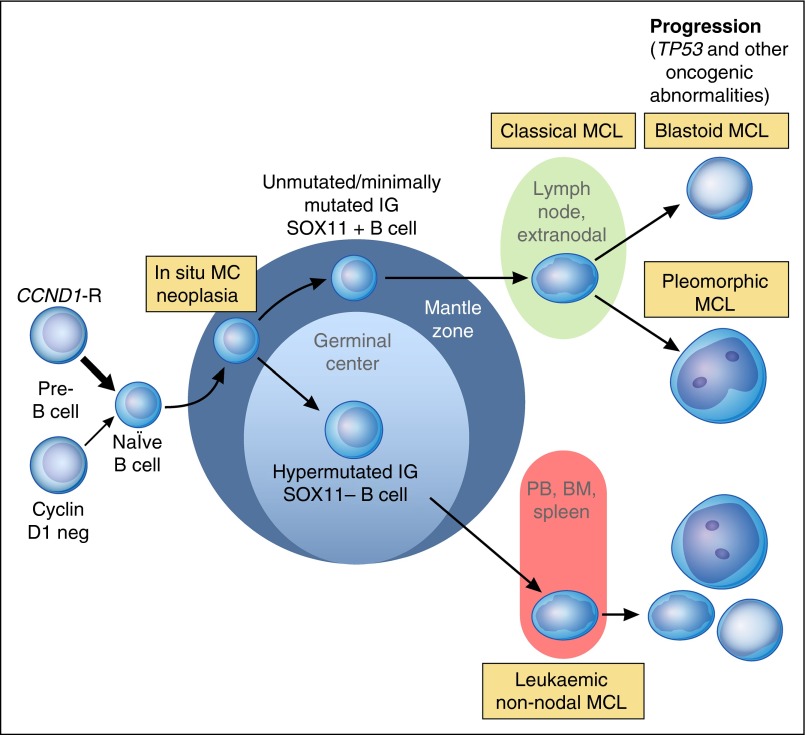
Mantle cell lymphoma (MCL) classically has been recognized as an aggressive but incurable small B-cell lymphoma that developed in a linear fashion from naïve B cells. Two types of clinically indolent variants are now recognized and reflect, in part, that MCL develops along 2 very different pathways (Figure 2).31 Classical MCL is usually composed of IGHV-unmutated or minimally mutated B cells that usually express SOX11 and typically involves lymph nodes and other extranodal sites. Acquisition of additional molecular/cytogenetic abnormalities can lead to even more aggressive blastoid or pleomorphic MCL. Other MCL develop from IGHV-mutated SOX11− B cells which leads to leukemic nonnodal MCL, usually involving the PB, bone marrow, and often spleen. These cases are frequently clinically indolent; however, secondary abnormalities, often involving TP53, may occur and lead to very aggressive disease. In situ MCL is now to be called in situ mantle cell neoplasia (ISMCN), again emphasizing a more conservative approach for lymphoid neoplasms with a low rate of progression. It is characterized by the presence of cyclin D1+ cells, most typically in the inner mantle zones of follicles, in lymphoid tissues that do not otherwise suggest the diagnosis of a MCL, and is often found incidentally, sometimes in association with other lymphomas.32 They are much less common than ISFN and although they may be disseminated, appear to have a low rate of progression.32 ISMCN should be distinguished from overt MCL with a mantle zone growth pattern. Nevertheless, these latter cases as well as other classical MCL with a low proliferative fraction may also be relatively indolent.
Figure 2.
Proposed model of molecular pathogenesis in the development and progression of major subtypes of MCL. Precursor B cells usually with but sometimes without a CCND1 rearrangement mature to abnormal naïve B cells which may initially colonize, often the inner portion of the mantle zones, representing ISMCN. These cells already have additional molecular genetic abnormalities, such as inactivating ATM mutations. They may progress to classical MCL which most frequently is SOX11+, has no evidence of transit through the germinal center, and is genetically unstable acquiring additional abnormalities related to cell cycle dysregulation, the DNA damage response pathway, cell survival, and other pathways. Ultimately, progression to blastoid or pleomorphic MCL may occur. A smaller proportion of neoplastic mantle cells may undergo somatic hypermutation, presumably in germinal centers, leading to SOX11− MCL that are more genetically stable for long periods of time and which preferentially involve the PB, bone marrow (BM), and sometimes the spleen. Even these MCL, however, may undergo additional molecular/cytogenetic abnormalities, particularly TP53 abnormalities, leading to clinical and sometime morphological progression. Adapted from Jares et al31 and Swerdlow et al.2 Professional illustration by Patrick Lane, ScEYEnce Studios.
Impact of newer molecular/cytogenetic studies related to the small B-cell lymphoid neoplasms
Next-generation sequencing (NGS) studies have led not only to major advances in better understanding the small B-cell lymphoid neoplasms, but also to discoveries of diagnostic importance. Whereas the 2008 monograph reported that “No cytogenetic abnormality is specific for [hairy cell leukemia]”, we now know that BRAF V600E mutations are found in almost all cases of hairy cell leukemia (HCL) but not in HCL-variant (HCL-v) or other small B-cell lymphoid neoplasms.33 More recently, mutations in MAP2K1 which encodes MEK1 (which is downstream of BRAF) have been reported in almost half of HCL-v and in the majority of HCL that use IGHV4-34 and which, like HCL-v, lack BRAF V600E mutations.34
Similarly, the 2008 monograph noted that “no specific chromosomal or oncogene abnormalities are recognized” in lymphoplasmacytic lymphoma (LPL); however, we now know that about 90% of LPL or Waldenström macroglobulinemia (LPL plus an immunoglobulin M [IgM] paraprotein) have MYD88 L265P mutations.35 This mutation also is found in a significant proportion of IgM but not IgG or IgA monoclonal gammopathy of undetermined significance (MGUS) cases, a small proportion of other small B-cell lymphomas even after careful review, in ∼30% of non–germinal center-type DLBCL, more than half of primary cutaneous DLBCL, leg type, and many DLBCL at immune-privileged sites but not in plasma cell myeloma, even of IgM type.36 Review of cases with and without the mutation have led to revised criteria for LPL, emphasizing the monotony of the lymphoplasmacytic proliferation in cases other than those undergoing transformation, total architectural effacement in some cases, and allowing for significant follicular colonization.37 Although seeming to be more inclusive, these studies also suggest that cases previously described as polymorphic LPL and some LPL-like cases of γ heavy chain disease be excluded from the LPL category.37,38 These studies also have led to IgM MGUS being thought of as more closely related to LPL or other B-cell lymphomas and segregated from the uniformly wild-type MYD88 IgG and IgA MGUS cases that are more closely related to plasma cell myeloma. Consistent with this approach, CXCR4 mutations are found in about 30% of LPL and 20% of IgM MGUS but are not found in IgG or IgA MGUS cases.39-41
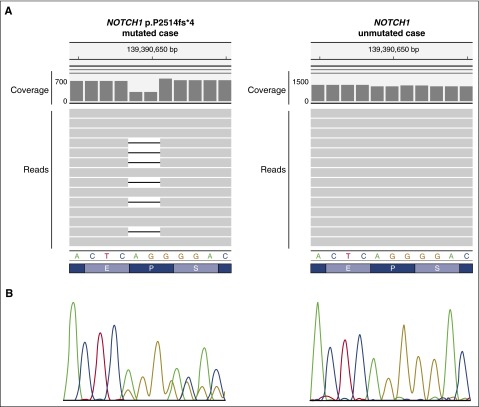
The situation with CLL/SLL is quite different and more complex because although there are no recognized disease-defining mutations, there are a large number of mutations that occur with a relatively low frequency.42-51 In addition to their biological implications, at least some, such as TP53, NOTCH1, SF3B1, and BIRC3, are of clinical interest because of their adverse prognostic implications and with some being potential direct or indirect therapeutic targets (Figure 3). It has been suggested that some of these could be integrated into an updated cytogenetic risk profile that also includes the well-known recurrent chromosomal abnormalities typically identified with fluorescence in situ hybridization studies50,52; however, although interest remains in this concept, the literature is inconsistent regarding the clinical implications of some of the mutations and combined risk profile, and it will not be recommended in the revised monograph.49,51
Figure 3.
NOTCH1 mutation detected by NGS and Sanger sequencing. (A) NOTCH1 p.P2514fs*4 (NP_060087.3) (c.7541-7542delCT, NM_017617.3) mutation detected by NGS (MiSeq, Illumina) as visualized in the Integrative Genomics Viewer (IGV, www.broadinstitute.org/igv, human reference genome GRCh37/hg19) (left, mutated case) and the same region of a NOTCH1 unmutated sample (right, unmutated case). In each case, the nucleotide coverage as well as a few representative NGS reads are shown. A deletion of AG (CT if considering the reverse strand) is observed in the mutated case. By NGS, each read is represented by a gray horizontal bar and the deletion is represented as a black line within those reads carrying the mutation. A decrease in 50% of the coverage can be observed for the 2 nucleotides affected showing that the mutation is present in half of the reads. (B) Sanger sequencing results are shown under the reference nucleotide and amino acid sequences.
In addition to having many nonrandom secondary chromosomal gains and losses as well as recurrent copy-neutral loss of heterozygosity that often involves the same regions where the losses occur such as TP53, MCL also are characterized by having mutations affecting many different genes with ATM (40%-75%) and CCND1 (35%) the most frequent.53 Other mutations are present in <15% of cases, including some such as NOTCH1 and NOTCH2 that are of prognostic and potential therapeutic importance.53,54 It has also been learned that about half of MCL that lack cyclin D1 expression/CCND1 rearrangements have CCND2 translocations, often with IGK or IGL as a partner locus, a finding that can be of diagnostic utility.55
Much has also been learned about the mutational landscape in terms of the development and progression in FL. Mutations in chromatin regulator/modifier genes, such as CREBBP and KMT2D (MLL2), are extremely common early events and may be potential therapeutic targets.56-59 EZH2 mutations, present in about 20% to 25% of FL, are another early event and potential therapeutic target.57,58,60 Mutations are present in a large number of other genes, including some seen predominantly with transformation, but in significantly lower proportions of patients. A new prognostic model integrating gene mutations with clinical parameters has been proposed, but, although conceptually interesting, requires validation.58 Whether mutational analysis should be performed routinely for diagnostic, prognostic, or therapeutic purposes and whether it should be integrated with other pathological and clinical prognostic factors remains to be determined.
Diffuse large B-cell lymphoma
Cell-of-origin classification
The 2008 classification recognized germinal center B-cell-like (GCB) and activated B-cell-like (ABC) molecular “subgroups” of DLBCL based on GEP as well as a group of cases that could not be put into either category (unclassifiable). The GCB and ABC subgroups differed in their chromosomal alterations, activation of signaling pathways, and clinical outcome.61 It separately recognized GCB and non-GCB immunohistochemical subgroups based on the Hans algorithm which used antibodies to CD10, BCL6, and IRF4/MUM1 but noted that these groups did not “exactly correlate” with the molecular categories and that these subgroups did not determine therapy. However, because GEP was not available as a routine clinical test, and because there were issues of reproducibility and reliability of immunohistochemical algorithms, subclassification of DLBCL, not otherwise specified (NOS) was considered optional in the 2008 classification. The better understanding of the molecular pathogenesis of these 2 subgroups since 2008, however, has led to the investigation of more specific therapeutic strategies to mitigate the worse outcome among those with ABC/non-GCB type DLBCL reported in most studies; prospective trials are ongoing to determine whether these therapies should be incorporated into clinical practice.62 For this reason, the revised classification will require the identification of these 2 subtypes. With GEP still not a routine clinical test, the use of immunohistochemistry (IHC) algorithms will be considered acceptable. Although the Hans algorithm remains the most popular and has a reasonable correlation with the GEP, other algorithms also may be used. It is acknowledged that the IHC algorithms do not recognize the 10% to 15% of tumors unclassified by GEP, have reproducibility issues, and are not uniformly reported to have prognostic utility. Newer methods based on quantification of RNA transcripts extracted from formalin-fixed paraffin-embedded tissues provide concordant results with conventional microarray GEP, are reproducible between laboratories, and capture the prognostic impact of the cell-of-origin classification.63-66 These methods are still not accessible to most laboratories but may represent a promising alternative to the current IHC-based algorithms.
Other phenotypic and molecular/cytogenetic features of clinical importance
A significant advance in recent years has been the better understanding of MYC alterations in LBCLs.67 MYC is rearranged in 5% to 15% of DLBCL, NOS, and is frequently associated with BCL2 or, to a lesser extent, BCL6 translocation, in the so-called “double-hit” or “triple-hit” lymphomas that are included in the updated WHO classification in the new category of high-grade B-cell lymphoma (HGBL), with rearrangements of MYC and BCL2 and/or BCL6 (see detailed discussion under “High-grade B-cell lymphomas, with and without MYC and BCL2 or BCL6 translocations”).68
MYC protein expression is detected in a much higher proportion of DLBCL (30%-50%) and is associated with concomitant expression of BCL2 in 20% to 35% of cases.67 Most of these tumors do not carry MYC/BCL2 chromosomal alterations and have been named “double-expressor lymphoma.” Most studies use a cutoff of 40% MYC-expressing cells to define these cases; the cutoff for BCL2 expression has varied considerably in the literature, but a figure of >50% is recommended. In several but not all studies, the double-expressor lymphomas have a worse outcome than other DLBCL, NOS but they are not as aggressive as the HGBL, with rearrangements of MYC and BCL2 and/or BCL6.69,70 These observations have suggested that double expression of MYC and BCL2 proteins without gene aberrations should be considered a prognostic indicator in DLBCL, NOS but not a separate category. CD30 expression in DLBCL, NOS is also of potential interest as it may be a potential target for new antibody-based therapies.
Recent NGS studies have identified common somatic mutations in all subgroups of DLBCL but also a profile of alterations differentially represented in both GCB and ABC subtypes.71 Somatic mutations common in both DLBCL subtypes are inactivating mutations of TP53 and genes involved in immunosurveillance (B2M, CD58), alterations in epigenetic regulators (CREBBP/EP300, KMT2D/C [MLL2/3], MEF2B), and oncogenic activation of BCL6. GCB-DLBCL carry frequent alteration in the histone methyl transferase EZH2, BCL2 translocations, and mutations in the cell motility regulator GNA13, whereas ABC-DLBCL have mutations in genes (MYD88, CD79A, CARD11, TNFAIP3) activating the B-cell receptor/Toll-like receptor and NF-κB pathways. Although the clinical implications of these mutations are not fully understood, there are increasing expectations that they will become important in guiding future targeted therapies.62,72
EBV+ large B-cell lymphomas and EBV+ mucocutaneous ulcer
The 2008 monograph included “EBV-positive DLBCL of the elderly” as a provisional entity. These tumors occur in apparently immunocompetent patients usually >50 years old and have a worse prognosis than Epstein-Barr virus–negative (EBV−) tumors. Epstein-Barr virus–positive (EBV+) DLBCL, however, have been increasingly recognized in younger patients, with a broader morphological spectrum and better survival than initially thought.73-75 This new information has led to substitution of the modifier “elderly” with “not otherwise specified” (EBV+ DLBCL, NOS) in the updated classification. The NOS is to highlight that there are other more specific entities with neoplastic EBV+ large B cells, such as lymphomatoid granulomatosis. In addition, the new category EBV+ mucocutaneous ulcer has been segregated from EBV+ DLBCL as a provisional entity due to its self-limited growth potential and response to conservative management. These lesions may present in advanced age or with iatrogenic immunosuppression.76,77
Burkitt lymphoma
Recent NGS studies of Burkitt lymphoma (BL) have improved our understanding of the pathogenesis of these tumors. Mutations in the transcription factor TCF3 or its negative regulator ID3 occur in about 70% of sporadic and immunodeficiency-related BL and 40% of endemic cases. TCF3 promotes survival and proliferation in lymphoid cells by activating the B-cell receptor/phosphatidylinositol 3-kinase signaling pathways and modulating the expression of cyclin D3, which is also mutated in 30% of BL.78-81
One controversial issue not fully resolved is whether true BL without MYC translocations really exist. Some recent studies have identified a subset of lymphomas that resemble BL morphologically, to a large extent phenotypically and by GEP, but which lack MYC rearrangements. Instead, they have a chromosome 11q alteration characterized by proximal gains and telomeric losses.82,83 Compared with BL, these lymphomas have more complex karyotypes, lower levels of MYC expression, a certain degree of cytological pleomorphism, occasionally a follicular pattern, and frequently a nodal presentation. The clinical course seems to be similar to BL, but the number of cases reported is still limited. Although more studies are needed, the consensus for the revised WHO classification was to consider these a new provisional entity designated Burkitt-like lymphoma with 11q aberration (Figure 1E-H).
High-grade B-cell lymphomas, with and without MYC and BCL2 or BCL6 translocations
The 2008 WHO classification introduced the category of “B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and BL” (BCLU) to recognize a subset of very aggressive tumors in which the distinction between DLBCL and BL was very difficult. Lymphomas with a GEP intermediate between that of molecular BL and molecular non-BL (mostly DLBCL), also lends support to the existence of these intermediate-type cases, which were not, however, considered a specific entity.84,85 Segregation of these cases was also necessary to better define these clinically problematic tumors.3 Additional studies followed that demonstrated that BCLU and other LBCL, with rearrangements of MYC and BCL2 and/or BCL6, had mutational features intermediate between DLBCL and BL. They also better characterized the double-/triple-hit lymphomas, including identifying features that might mitigate the adverse clinical impact of MYC translocations.68,86-88 The criteria for BCLU, however, are vague and the diagnosis has not been used uniformly, limiting its utility as a diagnostic category.68,86,87
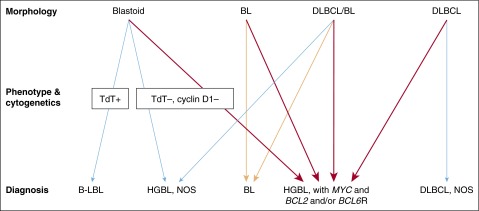
All LBCL with MYC and BCL2 and/or BCL6 rearrangements will be included in a single category to be designated HGBL, with MYC and BCL2 and/or BCL6 rearrangements, except for cases that fulfill the criteria for a follicular or lymphoblastic lymphoma (Figures 4 and 5).89 The morphologic appearance should be noted in a comment. The category of BCLU will be eliminated. Cases that appear blastoid or cases intermediate between DLBCL and BL, but which lack a MYC and BCL2 and/or BCL6 rearrangement, will be placed in the category of HGBL, NOS. A consensus has not yet been reached to provide specific guidelines as to which LBCL should have fluorescence in situ hybridization studies. Some believe that all DLBCL should have genetic studies for the detection of MYC, BCL2, and BCL6 rearrangements, whereas others would limit them, for example, to cases with a GCB phenotype and/or high-grade morphology or to cases with >40% MYC+ cells.
Figure 4.
Diagnostic approach to HBCLs. Lymphomas that potentially fall into the HGBL categories can morphologically resemble B-lymphoblastic leukemia/lymphoma (B-LBL), BL, and DLBCL as well as lymphomas that are intermediate between DLBCL and BL (DLBCL/BL). These distinctions can be very subjective. The orange arrows indicate cases with a BL phenotype and a MYC rearrangement without BCL2 or BCL6 rearrangements (“single hit”). The red arrows indicate cases with MYC and BCL2 and/or BCL6 rearrangements (“double or triple hit”). Neither MCLs, subtypes of LBCLs, nor Burkitt-like lymphoma with 11q aberration are indicated in this diagram. Adapted from Kluin et al89 with permission. Professional illustration by Patrick Lane, ScEYEnce Studios.
Figure 5.
Cytologic spectrum of HGBL, with MYC and BCL2 and/or BCL6 rearrangements. (A-B) This HGBL with MYC and BCL6 rearrangements closely resembles a BL including a starry sky with tingible body macrophages and many intermediate-sized transformed cells although there are some subtle cytologic differences from a classic BL. (C) This HGBL with MYC, BCL2, and BCL6 rearrangements appears more blastoid but was TdT−. (D) This HGBL with MYC and BCL2 rearrangements would otherwise have been considered a DLBCL that included many immunoblastic-type cells with single prominent central nucleoli. (A-D) Hematoxylin and eosin stain.
Mature T- and NK-cell neoplasms
Nodal T-cell lymphomas: angioimmunoblastic T-cell lymphoma, follicular T-cell lymphoma, and peripheral T-cell lymphoma, not otherwise specified
Significant advances have occurred in the classification of both nodal and extranodal T-cell and natural killer (NK)-cell neoplasms, which have led to revisions in the classification and introduction of new provisional entities. Many of these changes are the result of genomic studies using approaches to examine GEP and the genetic landscape of T-cell and NK-cell neoplasms.
There have been new insights into the complexity of nodal peripheral T-cell lymphoma (PTCL). Genetic studies have shown recurrent mutations that affect a significant proportion of cases of angioimmunoblastic T-cell lymphoma (AITL). Importantly, many of the same genetic changes are observed in cases of PTCL, NOS that manifest a T follicular helper (TFH) phenotype.90-92 For this designation, the neoplastic cells should express at least 2 or 3 TFH-related antigens, including CD279/PD1, CD10, BCL6, CXCL13, ICOS, SAP, and CCR5. This common phenotype has led to follicular T-cell lymphoma (FTCL) and AITL being unified under a common heading. Cases of nodal PTCL with TFH phenotype will be included here as well. Recurrent genetic abnormalities include TET2, IDH2, DNMT3A, RHOA, and CD28 mutations, as well as gene fusions such as ITK-SYK or CTLA4-CD28. All these lesions can take part in the process of lymphomagenesis and may represent the target of tailored therapies (eg, epigenetic modifiers). These cases also share many features by GEP.
Both AITL and FTCL may contain B-cell blasts, often EBV+, in addition to the neoplastic TFH cells. In some cases, the atypical B-cell blasts simulate Hodgkin–Reed-Sternberg cells, leading to a mistaken diagnosis of classical Hodgkin lymphoma (CHL).93,94 Progression to EBV+, and more rarely EBV−, B-cell neoplasms may occur in a subset of cases.95 Nevertheless, although the neoplastic cells share a TFH phenotype and share many genetic changes, clinical and pathological differences remain, so that both diagnoses are retained in the classification. FTCL more often presents with localized disease, with fewer systemic symptoms.
The cases remaining in the PTCL, NOS category still show extreme cytological and phenotypic heterogeneity. GEP studies display a global signature close to the one of activated T-lymphocytes. GEP analysis of 372 cryopreserved PTCLs identified at least 3 subtypes characterized by overexpression of GATA3, TBX21, and cytotoxic genes, as well as expression of the corresponding molecules using IHC.96 These subtypes are associated with a different clinical behavior and response to therapy. The GATA3 subtype has an inferior prognosis, shows high levels of Th2 cytokines, and can be identified by IHC.97 Studies using NGS are at an early stage in PTCL, NOS, but have provided new insights, which may lead to further refinement in the classification or new targets for therapy. These studies have identified mutations of epigenetic mediators (KMT2D [MLL2], TET2, KDM6A, ARID1B, DNMT3A, CREBBP, MLL, and ARID2), genes involved in signaling pathways (TNFAIP3, APC, CHD8, ZAP70, NF1, TNFRSF14, TRAF3), and tumor suppressors (TP53, FOXO1, BCORL1, ATM).98
Anaplastic large-cell lymphomas: ALK+, ALK−, and breast implant–associated
GEP studies also have provided insights into the distinction of CD30-expressing T-cell lymphomas (TCLs), and have facilitated the distinction of PTCL with high CD30 expression from ALK− anaplastic large-cell lymphoma (ALCL), the latter having a superior prognosis.99,100 ALK+ and ALK− ALCL were both recognized in the 2008 classification, although ALK− ALCL was considered a provisional entity, as criteria for distinguishing ALK− ALCL from CD30+ PTCL were imperfect. Improved criteria now exist for the recognition of ALK− ALCL in daily practice, and it is no longer considered provisional.101
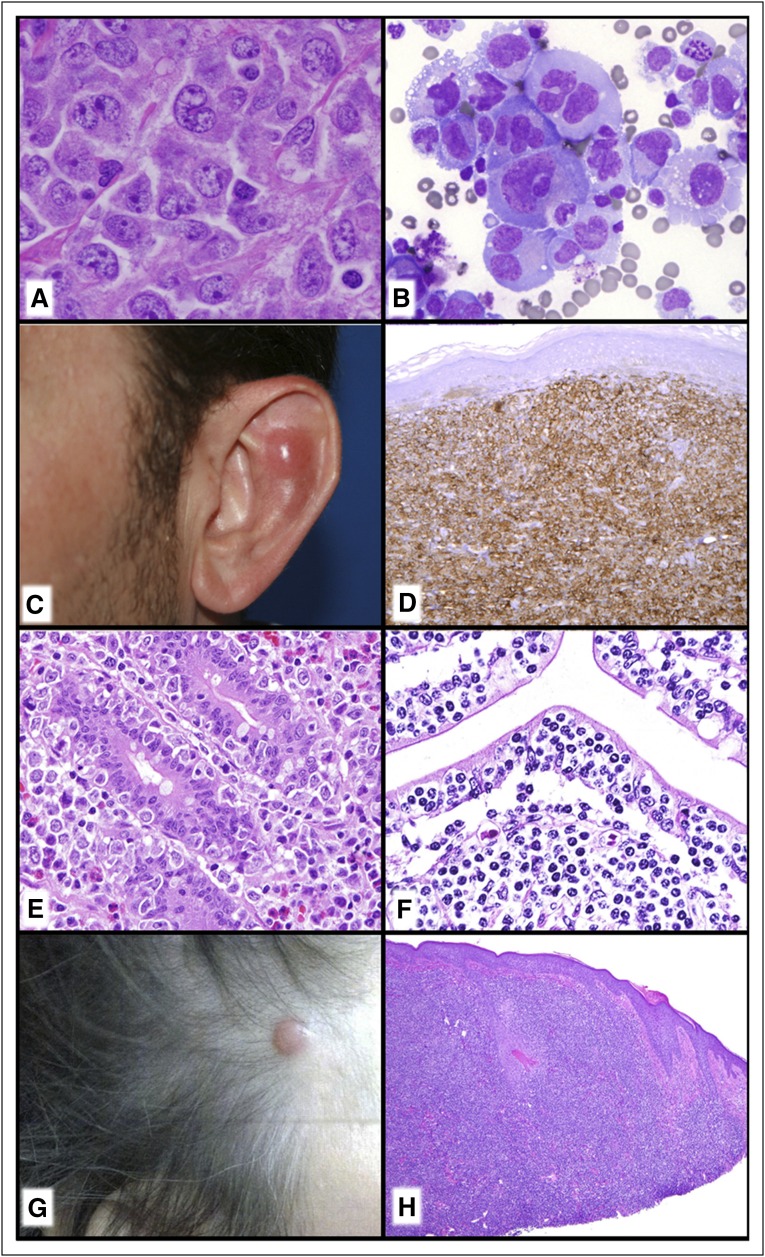
GEP studies have shown that ALK− ALCL has a signature quite close to that of ALK+ ALCL and distinct from other NK/TCLs. More recent studies illuminating the genetic landscape of ALK− ALCL have shown convergent mutations and kinase fusions that lead to constitutive activation of the JAK/STAT3 pathway.102 These studies provide a genetic rationale for the morphologic and phenotypic similarities between ALK+ and ALK− ALCL. However, not all cases of ALK− ALCLs are created equal. A subset with rearrangements at the locus containing DUSP22 and IRF4 in chromosome 6p25 tends to be relatively monomorphic, usually lack cytotoxic granules, and have been reported to have a superior prognosis, whereas a small subset with TP63 rearrangements are very aggressive (Figure 6A).103-105 Interestingly, the same locus in 6p25 has also been implicated in lymphomatoid papulosis (LYP) and primary cutaneous ALCL.106,107 LYP is a clinically diverse disorder, and in recent years a number of new pathological and clinical variants have been described. The WHO classification recognizes the original variants, types A, B, and C; as well as the more recently described types D (mimics primary cutaneous aggressive epidermotropic CD8+ cytotoxic TCL),108 E (angioinvasive),109 and LyP with chromosome 6p25 rearrangement,107 as well as some even more rare variants. Appreciation of these variants is important, as histologically they can mimic very aggressive TCLs, but they are clinically similar to other forms of LYP.
Figure 6.
TCLs. (A) ALK− ALCL with DUSP22 rearrangement. There is a relatively monotonous proliferation of large transformed cells and classic “Hallmark” cells. (B) Breast implant–associated ALCL. The seroma cavity demonstrates numerous very large anaplastic-appearing lymphoid cells. (C-D) Primary cutaneous acral CD8+ TCL. (C) Nodule on the ear. (D) There is a diffuse monotonous infiltrate of CD8+ T cells. (E) EATL. The somewhat pleomorphic intestinal infiltrate extends into the epithelium and would be associated with enteropathic changes elsewhere in the intestine. (F) MEITL. The monotonous intestinal infiltrate is very epitheliotropic. (G-H) Primary cutaneous CD4+ small/medium T-cell LPD. (G) Small nodule on scalp. (H) Although the infiltrate is dense and lymphoma-like, this is now to be considered a lymphoproliferative disorder rather than a “lymphoma.” (A,E,F,H) Hematoxylin and eosin stain; (B) Romanowsky-type stain; (D) CD8 immunoperoxidase stain.
A number of studies in recent years have identified a unique form of ALK− ALCL arising in association with breast implants designated as breast implant–associated ALCL (Figure 6B).110 First described in 1997, it usually presents as an accumulation of seroma fluid between the implant itself and the surrounding fibrous capsule.111 Both saline- and silicone-filled implants have been implicated, with a median interval from the time of the implant to the lymphoma of about 10 years.112 In most cases, the neoplastic cells are confined to the seroma fluid, without invasion of the capsule. In such cases, conservative management is recommended, with removal of the implant and capsule.113 If there is invasion through the capsule, there is risk of lymph node involvement and systemic spread, warranting systemic chemotherapy.114 The factors leading to progression have not been delineated.
Cytotoxic T-cell lymphomas and leukemias
Mature T-cell and NK-cell lymphomas and leukemias expressing cytotoxic molecules constitute a heterogeneous group of diseases with variations in clinical behavior and prognosis.115 Other than ALCL, most of these neoplasms present with extranodal disease, or are systemic with involvement of liver, spleen, and bone marrow. Since the publication of the 2008 monograph, several entities have received greater recognition, and the revised classification reflects the new data. Besides breast implant–associated ALCL, we have the addition of indolent T-cell lymphoproliferative disorder (LPD) of the GI tract and primary cutaneous acral CD8+ TCL as provisional entities.116,117 Both are clonal disorders, usually composed of CD8+ T cells, with an indolent clinical course. The cutaneous acral lesions, first recognized affecting the ear, are nearly always localized to a single site and can be managed conservatively (Figure 6C-D). Indolent T-cell LPD of the GI tract can be derived from either CD8 or less often CD4+ T cells, affects many sites in the GI tract, but has an indolent clinical course. Their optimal management is not yet determined.
The desire to categorize lymphomas according to the precise cellular origin is attractive, but among the mature TCLs, promiscuity is observed. Some years ago, it was recognized that although hepatosplenic TCL is usually of γδ T-cell derivation, some cases have an αβ phenotype,118 yet are otherwise clinically and genetically similar. Furthermore, among cutaneous TCLs, although γδ TCL are generally aggressive,119,120 γδ variants of mycosis fungoides or other TCLs with an indolent clinical course have been described.121,122
Recent studies have identified recurrent mutations affecting the JAK/STAT pathway in many T-cell and NK-cell malignancies, further emphasizing the overlapping biology in many of these malignancies.123-126 STAT3 mutations are common in large granular lymphocyte leukemias of both T-cell and NK-cell types.123,124 STAT5B mutations are more uncommon and are associated with more clinically aggressive disease.127 Recurrent mutations of STAT5B and less often STAT3 are seen in hepatosplenic TCL of γδ origin128 and a similar pattern was observed in primary cutaneous TCL.129 Additionally, STAT5B mutations were reported in 36% of cases of what has been known as enteropathy-associated TCL (EATL), type II, all of which were of γδ T-cell origin.129
These data and others have led to changes in the categorization of intestinal TCLs. It has become apparent that the 2 subtypes of EATL are distinct, and will be more clearly distinguished in the revised WHO classification.130 EATL, type I, now simply designated as enteropathy-associated TCL, is closely linked to celiac disease, and is primarily a disease of individuals of northern European origin. EATL, type II, now formally designated as monomorphic epitheliotropic intestinal TCL (MEITL), shows no association with celiac disease, and appears increased in incidence in Asians, and Hispanic populations (Figure 6E-F). Although EATL generally has a polymorphic cellular composition and wide range in cytology, MEITL is monomorphic, and usually positive for CD8, CD56, and MATK.131 Gains in chromosome 8q24 involving MYC are seen in a high proportion of cases.132 Many cases of MEITL are derived from γδ T cells, but exceptions exist; some cases are T-cell receptor (TCR) silent and some cases express TCR αβ.133 Likewise, most cases of EATL express TCR αβ, but γδ variants exist. As noted in the previous paragraph, mutations in STAT5B were only associated with γδ MEITL, but investigation of classical EATL or αβ cases was limited.
Cutaneous T-cell lymphomas
Primary cutaneous acral CD8+ TCL and primary cutaneous γδ TCL are discussed in the previous section. Primary cutaneous CD4+ small/medium TCL was included as a provisional entity in the 2008 classification (Figure 6G-H). Since then, several clinical series have been reported, further elucidating its cellular origin and clinical behavior. The cells have a TFH phenotype,134 but recurrent mutations as seen in nodal TFH lymphoma have not been reported. The clinical behavior is almost always indolent, with most patients presenting with localized disease. Systemic disease is rare, and conservative local management is sufficient in most patients.135-137 It has been suggested that this may represent a limited clonal response to an unknown stimulus, not fulfilling criteria for malignancy. The terminology in the revised classification has been modified to reflect this uncertain malignant potential, designating these cases as primary cutaneous CD4+ small/medium T-cell LPD.
EBV+ T-cell and NK-cell lymphomas
The most common EBV+ NK-cell lymphoma or TCL is extranodal NK/T-cell lymphoma, nasal type, which usually presents in the upper aerodigestive tract. However, there are less common EBV+ TCLs and leukemias with different clinical presentations and biology. These are delineated in the upcoming revision of the WHO classification, and somewhat modified from the 2008 monograph. EBV-associated T- and NK-cell lymphoproliferative disorders in the pediatric age group include 2 major groups: chronic active EBV infection and systemic EBV+ TCL of childhood.138,139 Both occur with increased frequency in Asians, and in indigenous populations from Central and South America and Mexico. Chronic active EBV infection of T/NK type shows a broad range of clinical manifestations from indolent, localized forms like hydroa vacciniforme–like LPD and severe mosquito bite allergy to a more systemic form characterized by fever, hepatosplenomegaly, and lymphadenopathy with or without cutaneous manifestations.140,141 Systemic EBV+ TCL of childhood, no longer referred to as a “lymphoproliferative disorder,” has a fulminant clinical course usually associated with a hemophagocytic syndrome. The differential diagnosis includes acute EBV-associated hemophagocytic lymphohistiocytosis (HLH), which can present acutely, but in some patients responds well to the HLH 94 protocol, and is not considered neoplastic. Node-based EBV+ PTCL, defined as demonstrating EBV in the majority of the neoplastic cells, are uncommon and included under the broad heading of PTCL, NOS. They are generally monomorphic and lack the angioinvasion and necrosis of extranodal NK/T-cell lymphoma. They most often present in older adults, and also can be seen in the posttransplant setting and other immunodeficiency states.101,142,143
Hodgkin lymphomas
Although the classification of Hodgkin lymphomas (HLs) has not changed, the revision will include updates concerning nodular lymphocyte–predominant HL (NLPHL). It has long been recognized that NLPHL can have varied growth patterns, including some with diffuse areas and/or numerous T cells.144 Additionally, cases manifesting one of the variant patterns have been reported to be associated with advanced disease and a higher relapse rate, although they still have good survival.144-146 Thus, it is useful to note these features in the diagnostic report.
NLPHL may evolve to a completely diffuse T-cell–rich proliferation lacking any follicular dendritic cells which would be consistent with a T-cell histiocyte-rich LBCL (THRLBCL) or can be associated with such a proliferation at a separate site. Whereas the 2008 monograph said, “It is probably good practice to label cases of NLPHL that progress to a diffuse T-cell–rich pattern as NLPHL, THRLBCL-like…,” the revision will recommend the designation of THRLBCL-like transformation of NLPHL, with inclusion of the word “like” due to some remaining uncertainties. This consensus was based on the conclusion from the Clinical Advisory Committee that transformation of NLPHL to DLBCL should be based on WHO criteria (with THRLBCL being a type of LBCL). Recent data indicate that progression to a process with features of THRLBCL is associated with a more aggressive clinical course, and requires different management, such that the term NLPHL in this setting may not be sufficient.147,148 However, cases with only focal diffuse areas are not considered transformation. It is also of interest that aside from their immunomorphologic appearance, GEP and array comparative genomic hybridization studies have shown similarities between NLPHL and THRLBCL, suggesting a relationship to each other, in spite of other major differences.147,149 The revision will also acknowledge that lymphocyte-rich CHL has some features that are intermediate between other CHL and NLPHL.150
Histiocytic and dendritic cell neoplasms
The classification of the histiocytic and dendritic cell neoplasm is similar to that from 2008 except that the order of the entities is minimally altered and Erdheim-Chester disease has been added, as it should be distinguished from other members of the juvenile xanthogranuloma family.1,151 Histiocytic and dendritic cell neoplasms are grouped together based on the functional properties of their normal counterpart (ie, phagocytosis and/or processing and presentation of antigens) rather than their cell of origin. Although most arise from a common myeloid precursor, a few are of mesenchymal origin (ie, follicular dendritic cell sarcoma and fibroblastic reticular cell tumor).
During the last few years, several publications highlighted that, irrespective of their myeloid or mesenchymal origin, some of these neoplasms are associated with or preceded by FL, CLL, B- or T-lymphoblastic neoplasms, or PTCL.152-157 These cases carry the same TCR or IGHV rearrangements and chromosomal aberrations as the associated lymphoid neoplasms, suggesting a process of transdifferentiation.152-157 Moreover, the BRAF V600E mutation has been reported in the setting of Langerhans cell histiocytosis, histiocytic sarcoma, disseminated juvenile xanthogranuloma, Erdheim-Chester disease, and even follicular dendritic cell sarcoma.158
Summary
There have been major advances in our knowledge of the lymphoid neoplasms and how they should best be treated over the last 8 years. We have seen new insights into the biology and management of both clonal proliferations with limited malignant potential, as well as the aggressive lymphoid neoplasms where more targeted and effective therapies are being investigated. The 2016 WHO classification and associated monograph aim to provide updated diagnostic categories and criteria, together with biological and clinical correlates, and facilitate state-of-the-art patient care, future therapeutic advances, and basic research in this field.
Acknowledgments
The Clinical Advisory Committee meeting would not have been possible without the major support from James W. Vardiman, lead organizer, and the staff at the University of Chicago.
S.A.P. was supported in part through the Italian Association for Cancer Research (AIRC, Milan) grant 5x1000 number 10007. A.D.Z. was supported in part through the National Institutes of Health (NIH)/National Cancer Institute (NCI) Cancer Center Support Grant P30 CA008748. The authors acknowledge the unrestricted financial support of the Clinical Advisory Committee meeting held in Chicago, IL, March 31-April 1, 2014 from the following organizations: American Society of Hematology, Josep Carreras Foundation, Fondazione Italiana Linfomi (FIL), Leukemia Clinical Research Foundation, University of Chicago Comprehensive Cancer Center, Beckman Coulter Corporation, Celgene Corporation, Dako, Genentech Corporation, Incyte Corporation, Leica Corporation, Millennium Pharmaceuticals, Pharmacyclics, Seattle Genetics Corporation, Sysmex Corporation, and Ventana Medical Systems, Inc, a member of the Roche Group.
Footnotes
The online version of this article contains a data supplement.
Authorship
Contribution: All authors contributed to the contents of this manuscript which was written by S.H.S., E.C., S.A.P., and E.S.J. and reviewed and edited by all authors.
Conflict-of-interest disclosure: S.A.P. is member of the Scientific Advisory Board of Takeda/Millennium. M.G. has received honoraria from Roche, Celgene, Janssen, Gilead, Millenium, and Mundipharma. The remaining authors declare no competing financial interests.
The current affiliation for R.S. is Institute of Human Genetics, Ulm University, Ulm, Germany.
Correspondence: Steven H. Swerdlow, Division of Hematopathology, Department of Pathology, University of Pittsburgh School of Medicine, 200 Lothrop St, Pittsburgh, PA 15213; e-mail: swerdlowsh@upmc.edu; Elias Campo, Department of Pathology, Hospital Clinic, University of Barcelona, Villarroel 170, 08036 Barcelona, Spain; e-mail: ecampo@clinic.cat; and Elaine S. Jaffe, Hematopathology Section, Laboratory of Pathology, CCR, NCI, Building 10, Room 3s235, Bethesda, MD 20892-1500; e-mail: elainejaffe@nih.gov.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008. [Google Scholar]
- 2.Swerdlow SH, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. In: World Health Organization Classification of Tumours. Lyon, France: IARC. In press. [Google Scholar]
- 3.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto WG, Almeida J, Romero A, et al. Primary Health Care Group of Salamanca for the Study of MBL. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114(1):33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 5.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–667. doi: 10.1056/NEJMoa0806122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawstron AC, Shanafelt T, Lanasa MC, et al. Different biology and clinical outcome according to the absolute numbers of clonal B-cells in monoclonal B-cell lymphocytosis (MBL). Cytometry B Clin Cytom. 2010;78(suppl 1):S19–S23. doi: 10.1002/cyto.b.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardi A, Dagklis A, Scarfò L, et al. Immunogenetics shows that not all MBL are equal: the larger the clone, the more similar to CLL. Blood. 2013;121(22):4521–4528. doi: 10.1182/blood-2012-12-471698. [DOI] [PubMed] [Google Scholar]
- 8.Morabito F, Mosca L, Cutrona G, et al. Clinical monoclonal B lymphocytosis versus Rai 0 chronic lymphocytic leukemia: a comparison of cellular, cytogenetic, molecular, and clinical features. Clin Cancer Res. 2013;19(21):5890–5900. doi: 10.1158/1078-0432.CCR-13-0622. [DOI] [PubMed] [Google Scholar]
- 9.Xochelli A, Kalpadakis C, Gardiner A, et al. Clonal B-cell lymphocytosis exhibiting immunophenotypic features consistent with a marginal-zone origin: is this a distinct entity? Blood. 2014;123(8):1199–1206. doi: 10.1182/blood-2013-07-515155. [DOI] [PubMed] [Google Scholar]
- 10.Bruscaggin A, Monti S, Arcaini L, et al. Molecular lesions of signalling pathway genes in clonal B-cell lymphocytosis with marginal zone features. Br J Haematol. 2014;167(5):718–720. doi: 10.1111/bjh.13052. [DOI] [PubMed] [Google Scholar]
- 11.Gibson SE, Swerdlow SH, Ferry JA, et al. Reassessment of small lymphocytic lymphoma in the era of monoclonal B-cell lymphocytosis. Haematologica. 2011;96(8):1144–1152. doi: 10.3324/haematol.2011.042333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gradowski JF, Sargent RL, Craig FE, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma with cyclin D1 positive proliferation centers do not have CCND1 translocations or gains and lack SOX11 expression. Am J Clin Pathol. 2012;138(1):132–139. doi: 10.1309/AJCPIVKZRMPF93ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson SE, Leeman-Neill RJ, Jain S, Piao W, Cieply KM, Swerdlow SH. Proliferation centres of chronic lymphocytic leukaemia/small lymphocytic lymphoma have enhanced expression of MYC protein, which does not result from rearrangement or gain of the MYC gene [published online ahead of print November 16, 2015]. Br J Haematol. doi: 10.1111/bjh.13844. [DOI] [PubMed] [Google Scholar]
- 14.Giné E, Martinez A, Villamor N, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. 2010;95(9):1526–1533. doi: 10.3324/haematol.2010.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciccone M, Agostinelli C, Rigolin GM, et al. Proliferation centers in chronic lymphocytic leukemia: correlation with cytogenetic and clinicobiological features in consecutive patients analyzed on tissue microarrays. Leukemia. 2012;26(3):499–508. doi: 10.1038/leu.2011.247. [DOI] [PubMed] [Google Scholar]
- 16.Falchi L, Keating MJ, Marom EM, et al. Correlation between FDG/PET, histology, clinical characteristics, and survival in 332 patients with chronic lymphocytic leukemia. Blood. 2014;123(18):2783–2790. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jegalian AG, Eberle FC, Pack SD, et al. Follicular lymphoma in situ: clinical implications and comparisons with partial involvement by follicular lymphoma. Blood. 2011;118(11):2976–2984. doi: 10.1182/blood-2011-05-355255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai RK, Surti U, Swerdlow SH. Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica. 2013;98(10):1571–1580. doi: 10.3324/haematol.2013.085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamessier E, Song JY, Eberle FC, et al. Early lesions of follicular lymphoma: a genetic perspective. Haematologica. 2014;99(3):481–488. doi: 10.3324/haematol.2013.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt J, Salaverria I, Haake A, et al. Increasing genomic and epigenomic complexity in the clonal evolution from in situ to manifest t(14;18)-positive follicular lymphoma. Leukemia. 2014;28(5):1103–1112. doi: 10.1038/leu.2013.307. [DOI] [PubMed] [Google Scholar]
- 21.Tellier J, Menard C, Roulland S, et al. Human t(14;18)positive germinal center B cells: a new step in follicular lymphoma pathogenesis? Blood. 2014;123(22):3462–3465. doi: 10.1182/blood-2013-12-545954. [DOI] [PubMed] [Google Scholar]
- 22.Roulland S, Kelly RS, Morgado E, et al. t(14;18) translocation: a predictive blood biomarker for follicular lymphoma. J Clin Oncol. 2014;32(13):1347–1355. doi: 10.1200/JCO.2013.52.8190. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Salaverria I, Pittaluga S, et al. Follicular lymphomas in children and young adults: a comparison of the pediatric variant with usual follicular lymphoma. Am J Surg Pathol. 2013;37(3):333–343. doi: 10.1097/PAS.0b013e31826b9b57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louissaint A, Jr, Ackerman AM, Dias-Santagata D, et al. Pediatric-type nodal follicular lymphoma: an indolent clonal proliferation in children and adults with high proliferation index and no BCL2 rearrangement. Blood. 2012;120(12):2395–2404. doi: 10.1182/blood-2012-05-429514. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Guerrero I, Salaverria I, Burkhardt B, et al. Recurrent loss of heterozygosity in 1p36 associated with TNFRSF14 mutations in IRF4 translocation negative pediatric follicular lymphomas. Haematologica. 2013;98(8):1237–1241. doi: 10.3324/haematol.2012.073916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salaverria I, Philipp C, Oschlies I, et al. Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe; German High-Grade Lymphoma Study Group; Berlin-Frankfurt-Münster-NHL Trial Group. Translocations activating IRF4 identify a subtype of germinal center-derived B-cell lymphoma affecting predominantly children and young adults. Blood. 2011;118(1):139–147. doi: 10.1182/blood-2011-01-330795. [DOI] [PubMed] [Google Scholar]
- 27.Karube K, Guo Y, Suzumiya J, et al. CD10-MUM1+ follicular lymphoma lacks BCL2 gene translocation and shows characteristic biologic and clinical features. Blood. 2007;109(7):3076–3079. doi: 10.1182/blood-2006-09-045989. [DOI] [PubMed] [Google Scholar]
- 28.Schmatz AI, Streubel B, Kretschmer-Chott E, et al. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29(11):1445–1451. doi: 10.1200/JCO.2010.32.9193. [DOI] [PubMed] [Google Scholar]
- 29.Takata K, Sato Y, Nakamura N, et al. Duodenal follicular lymphoma lacks AID but expresses BACH2 and has memory B-cell characteristics [published correction appears in Mod Pathol. 2013;26(8):1152]. Mod Pathol. 2013;26(1):22–31. doi: 10.1038/modpathol.2012.127. [DOI] [PubMed] [Google Scholar]
- 30.Katzenberger T, Kalla J, Leich E, et al. A distinctive subtype of t(14;18)-negative nodal follicular non-Hodgkin lymphoma characterized by a predominantly diffuse growth pattern and deletions in the chromosomal region 1p36. Blood. 2009;113(5):1053–1061. doi: 10.1182/blood-2008-07-168682. [DOI] [PubMed] [Google Scholar]
- 31.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122(10):3416–3423. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvajal-Cuenca A, Sua LF, Silva NM, et al. In situ mantle cell lymphoma: clinical implications of an incidental finding with indolent clinical behavior. Haematologica. 2012;97(2):270–278. doi: 10.3324/haematol.2011.052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiacci E, Trifonov V, Schiavoni G, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. 2011;364(24):2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet. 2014;46(1):8–10. doi: 10.1038/ng.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 36.Swerdlow SH, Kuzu I, Dogan A, et al. The many faces of small B cell lymphomas with plasmacytic differentiation and the contribution of MYD88 testing. Virchows Arch. doi: 10.1007/s00428-015-1858-9. 2016;468(3):259-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamadeh F, MacNamara SP, Aguilera NS, Swerdlow SH, Cook JR. MYD88 L265P mutation analysis helps define nodal lymphoplasmacytic lymphoma. Mod Pathol. 2015;28(4):564–574. doi: 10.1038/modpathol.2014.120. [DOI] [PubMed] [Google Scholar]
- 38.Hamadeh F, MacNamara S, Bacon CM, Sohani AR, Swerdlow SH, Cook JR. Gamma heavy chain disease lacks the MYD88 L265p mutation associated with lymphoplasmacytic lymphoma. Haematologica. 2014;99(9):e154–e155. doi: 10.3324/haematol.2014.108688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123(18):2791–2796. doi: 10.1182/blood-2014-01-550905. [DOI] [PubMed] [Google Scholar]
- 40.Roccaro AM, Sacco A, Jimenez C, et al. C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma. Blood. 2014;123(26):4120–4131. doi: 10.1182/blood-2014-03-564583. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt J, Federmann B, Schindler N, et al. MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol. 2015;169(6):795–803. doi: 10.1111/bjh.13361. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365(26):2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quesada V, Conde L, Villamor N, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2011;44(1):47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 45.Villamor N, Conde L, Martinez-Trillos A, et al. NOTCH1 mutations identify a genetic subgroup of chronic lymphocytic leukemia patients with high risk of transformation and poor outcome. Leukemia. 2013;27(5):1100–1106. doi: 10.1038/leu.2012.357. [DOI] [PubMed] [Google Scholar]
- 46.Puente XS, Beà S, Valdés-Mas R, et al. Non-coding recurrent mutations in chronic lymphocytic leukaemia. Nature. 2015;526(7574):519–524. doi: 10.1038/nature14666. [DOI] [PubMed] [Google Scholar]
- 47.Queirós AC, Villamor N, Clot G, et al. A B-cell epigenetic signature defines three biologic subgroups of chronic lymphocytic leukemia with clinical impact. Leukemia. 2015;29(3):598–605. doi: 10.1038/leu.2014.252. [DOI] [PubMed] [Google Scholar]
- 48.Fabbri G, Rasi S, Rossi D, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208(7):1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28(1):108–117. doi: 10.1038/leu.2013.263. [DOI] [PubMed] [Google Scholar]
- 50.Rossi D, Rasi S, Spina V, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121(8):1403–1412. doi: 10.1182/blood-2012-09-458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliakas P, Hadzidimitriou A, Sutton LA, et al. European Research Initiative on CLL (ERIC) Recurrent mutations refine prognosis in chronic lymphocytic leukemia. Leukemia. 2015;29(2):329–336. doi: 10.1038/leu.2014.196. [DOI] [PubMed] [Google Scholar]
- 52.Foà R, Guarini A. A mechanism-driven treatment for chronic lymphocytic leukemia? N Engl J Med. 2013;369(1):85–87. doi: 10.1056/NEJMe1303054. [DOI] [PubMed] [Google Scholar]
- 53.Beà S, Valdés-Mas R, Navarro A, et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc Natl Acad Sci USA. 2013;110(45):18250–18255. doi: 10.1073/pnas.1314608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kridel R, Meissner B, Rogic S, et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood. 2012;119(9):1963–1971. doi: 10.1182/blood-2011-11-391474. [DOI] [PubMed] [Google Scholar]
- 55.Salaverria I, Royo C, Carvajal-Cuenca A, et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1(-) mantle cell lymphoma. Blood. 2013;121(8):1394–1402. doi: 10.1182/blood-2012-08-452284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green MR, Kihira S, Liu CL, et al. Mutations in early follicular lymphoma progenitors are associated with suppressed antigen presentation. Proc Natl Acad Sci USA. 2015;112(10):E1116–E1125. doi: 10.1073/pnas.1501199112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okosun J, Bödör C, Wang J, et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46(2):176–181. doi: 10.1038/ng.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111–1122. doi: 10.1016/S1470-2045(15)00169-2. [DOI] [PubMed] [Google Scholar]
- 59.Loeffler M, Kreuz M, Haake A, et al. HaematoSys-Project. Genomic and epigenomic co-evolution in follicular lymphomas. Leukemia. 2015;29(2):456–463. doi: 10.1038/leu.2014.209. [DOI] [PubMed] [Google Scholar]
- 60.Bödör C, Grossmann V, Popov N, et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122(18):3165–3168. doi: 10.1182/blood-2013-04-496893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young RM, Shaffer AL, 3rd, Phelan JD, Staudt LM. B-cell receptor signaling in diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):77–85. doi: 10.1053/j.seminhematol.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roschewski M, Staudt LM, Wilson WH. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat Rev Clin Oncol. 2014;11(1):12–23. doi: 10.1038/nrclinonc.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123(8):1214–1217. doi: 10.1182/blood-2013-11-536433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scott DW, Mottok A, Ennishi D, et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol. 2015;33(26):2848–2856. doi: 10.1200/JCO.2014.60.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masqué-Soler N, Szczepanowski M, Kohler CW, Spang R, Klapper W. Molecular classification of mature aggressive B-cell lymphoma using digital multiplexed gene expression on formalin-fixed paraffin-embedded biopsy specimens. Blood. 2013;122(11):1985–1986. doi: 10.1182/blood-2013-06-508937. [DOI] [PubMed] [Google Scholar]
- 66.Mareschal S, Ruminy P, Bagacean C, et al. Accurate classification of germinal center B-cell-like/activated B-cell-like diffuse large B-cell lymphoma using a simple and rapid reverse transcriptase-multiplex ligation-dependent probe amplification assay: a CALYM Study. J Mol Diagn. doi: 10.1016/j.jmoldx.2015.01.007. 2015;17(3):273-283. [DOI] [PubMed] [Google Scholar]
- 67.Karube K, Campo E. MYC alterations in diffuse large B-cell lymphomas. Semin Hematol. 2015;52(2):97–106. doi: 10.1053/j.seminhematol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 68.Swerdlow SH. Diagnosis of ‘double hit’ diffuse large B-cell lymphoma and B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma: when and how, FISH versus IHC. Hematology Am Soc Hematol Educ Program. 2014;2014(1):90–99. doi: 10.1182/asheducation-2014.1.90. [DOI] [PubMed] [Google Scholar]
- 69.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Molina TJ, Canioni D, Copie-Bergman C, et al. Young patients with non-germinal center B-cell-like diffuse large B-cell lymphoma benefit from intensified chemotherapy with ACVBP plus rituximab compared with CHOP plus rituximab: analysis of data from the Groupe d’Etudes des Lymphomes de l’Adulte/Lymphoma Study Association phase III trial LNH 03-2B. J Clin Oncol. 2014;32(35):3996–4003. doi: 10.1200/JCO.2013.54.9493. [DOI] [PubMed] [Google Scholar]
- 71.Pasqualucci L, Dalla-Favera R. The genetic landscape of diffuse large B-cell lymphoma. Semin Hematol. 2015;52(2):67–76. doi: 10.1053/j.seminhematol.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Intlekofer AM, Younes A. Precision therapy for lymphoma--current state and future directions. Nat Rev Clin Oncol. 2014;11(10):585–596. doi: 10.1038/nrclinonc.2014.137. [DOI] [PubMed] [Google Scholar]
- 73.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117(18):4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nicolae A, Pittaluga S, Abdullah S, et al. EBV-positive large B-cell lymphomas in young patients: a nodal lymphoma with evidence for a tolerogenic immune environment. Blood. 2015;126(7):863–872. doi: 10.1182/blood-2015-02-630632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Said J. The expanding spectrum of EBV+ lymphomas. Blood. 2015;126(7):827–828. doi: 10.1182/blood-2015-06-648097. [DOI] [PubMed] [Google Scholar]
- 76.Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34(3):405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hart M, Thakral B, Yohe S, et al. EBV-positive mucocutaneous ulcer in organ transplant recipients: a localized indolent posttransplant lymphoproliferative disorder. Am J Surg Pathol. 2014;38(11):1522–1529. doi: 10.1097/PAS.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 78.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44(12):1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richter J, Schlesner M, Hoffmann S, et al. ICGC MMML-Seq Project. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet. 2012;44(12):1316–1320. doi: 10.1038/ng.2469. [DOI] [PubMed] [Google Scholar]
- 80.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22(2):167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Salaverria I, Martin-Guerrero I, Wagener R, et al. Molecular Mechanisms in Malignant Lymphoma Network Project; Berlin-Frankfurt-Münster Non-Hodgkin Lymphoma Group. A recurrent 11q aberration pattern characterizes a subset of MYC-negative high-grade B-cell lymphomas resembling Burkitt lymphoma. Blood. 2014;123(8):1187–1198. doi: 10.1182/blood-2013-06-507996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferreiro JF, Morscio J, Dierickx D, et al. Post-transplant molecularly defined Burkitt lymphomas are frequently MYC-negative and characterized by the 11q-gain/loss pattern. Haematologica. 2015;100(7):e275–e279. doi: 10.3324/haematol.2015.124305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dave SS, Fu K, Wright GW, et al. Lymphoma/Leukemia Molecular Profiling Project. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354(23):2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 85.Hummel M, Bentink S, Berger H, et al. Molecular Mechanisms in Malignant Lymphomas Network Project of the Deutsche Krebshilfe. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med. 2006;354(23):2419–2430. doi: 10.1056/NEJMoa055351. [DOI] [PubMed] [Google Scholar]
- 86.Campo E. MYC in DLBCL: partners matter. Blood. 2015;126(22):2439–2440. doi: 10.1182/blood-2015-10-671362. [DOI] [PubMed] [Google Scholar]
- 87.Momose S, Weißbach S, Pischimarov J, et al. The diagnostic gray zone between Burkitt lymphoma and diffuse large B-cell lymphoma is also a gray zone of the mutational spectrum. Leukemia. 2015;29(8):1789–1791. doi: 10.1038/leu.2015.34. [DOI] [PubMed] [Google Scholar]
- 88.Gebauer N, Bernard V, Feller AC, Merz H. ID3 mutations are recurrent events in double-hit B-cell lymphomas. Anticancer Res. 2013;33(11):4771–4778. [PubMed] [Google Scholar]
- 89.Kluin P, et al. High grade B-cell lymphoma. In: Swerdlow SH, et al. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumours. Lyon, France: IARC. In press. [Google Scholar]
- 90.Lemonnier F, Couronné L, Parrens M, et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood. 2012;120(7):1466–1469. doi: 10.1182/blood-2012-02-408542. [DOI] [PubMed] [Google Scholar]
- 91.Sakata-Yanagimoto M, Enami T, Yoshida K, et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet. 2014;46(2):171–175. doi: 10.1038/ng.2872. [DOI] [PubMed] [Google Scholar]
- 92.Cairns RA, Iqbal J, Lemonnier F, et al. IDH2 mutations are frequent in angioimmunoblastic T-cell lymphoma. Blood. 2012;119(8):1901–1903. doi: 10.1182/blood-2011-11-391748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nicolae A, Pittaluga S, Venkataraman G, et al. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: both EBV-positive and EBV-negative variants exist. Am J Surg Pathol. 2013;37(6):816–826. doi: 10.1097/PAS.0b013e3182785610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moroch J, Copie-Bergman C, de Leval L, et al. Follicular peripheral T-cell lymphoma expands the spectrum of classical Hodgkin lymphoma mimics. Am J Surg Pathol. 2012;36(11):1636–1646. doi: 10.1097/PAS.0b013e318268d9ff. [DOI] [PubMed] [Google Scholar]
- 95.Balagué O, Martínez A, Colomo L, et al. Epstein-Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T-cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol. 2007;31(9):1310–1322. doi: 10.1097/PAS.0b013e3180339f18. [DOI] [PubMed] [Google Scholar]
- 96.Iqbal J, Wright G, Wang C, et al. Lymphoma Leukemia Molecular Profiling Project and the International Peripheral T-cell Lymphoma Project. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood. 2014;123(19):2915–2923. doi: 10.1182/blood-2013-11-536359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang T, Feldman AL, Wada DA, et al. GATA-3 expression identifies a high-risk subset of PTCL, NOS with distinct molecular and clinical features. Blood. 2014;123(19):3007–3015. doi: 10.1182/blood-2013-12-544809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Palomero T, Couronné L, Khiabanian H, et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet. 2014;46(2):166–170. doi: 10.1038/ng.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agnelli L, Mereu E, Pellegrino E, et al. European T-Cell Lymphoma Study Group. Identification of a 3-gene model as a powerful diagnostic tool for the recognition of ALK-negative anaplastic large-cell lymphoma. Blood. 2012;120(6):1274–1281. doi: 10.1182/blood-2012-01-405555. [DOI] [PubMed] [Google Scholar]
- 100.Piccaluga PP, Fuligni F, De Leo A, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31(24):3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]
- 101.Attygalle AD, Cabeçadas J, Gaulard P, et al. Peripheral T-cell and NK-cell lymphomas and their mimics; taking a step forward - report on the Lymphoma Workshop of the XVIth meeting of the European Association for Haematopathology and the Society for Hematopathology. Histopathology. 2014;64(2):171–199. doi: 10.1111/his.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Crescenzo R, Abate F, Lasorsa E, et al. European T-Cell Lymphoma Study Group, T-Cell Project: Prospective Collection of Data in Patients with Peripheral T-Cell Lymphoma and the AIRC 5xMille Consortium “Genetics-Driven Targeted Management of Lymphoid Malignancies”. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma [published correction appears in Cancer Cell. 2015;27(5):744]. Cancer Cell. 2015;27(4):516–532. doi: 10.1016/j.ccell.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Parilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.King RL, Dao LN, McPhail ED, et al. Morphologic features of ALK-negative anaplastic large cell lymphomas with DUSP22 rearrangements. Am J Surg Pathol. 2016;40(1):36–43. doi: 10.1097/PAS.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parrilla Castellar ER, Jaffe ES, Said JW, et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood. 2014;124(9):1473–1480. doi: 10.1182/blood-2014-04-571091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feldman AL, Dogan A, Smith DI, et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood. 2011;117(3):915–919. doi: 10.1182/blood-2010-08-303305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Karai LJ, Kadin ME, Hsi ED, et al. Chromosomal rearrangements of 6p25.3 define a new subtype of lymphomatoid papulosis. Am J Surg Pathol. 2013;37(8):1173–1181. doi: 10.1097/PAS.0b013e318282d01e. [DOI] [PubMed] [Google Scholar]
- 108.Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Description of 9 cases. Am J Surg Pathol. 2010;34(8):1168–1175. doi: 10.1097/PAS.0b013e3181e75356. [DOI] [PubMed] [Google Scholar]
- 109.Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37(1):1–13. doi: 10.1097/PAS.0b013e3182648596. [DOI] [PubMed] [Google Scholar]
- 110.Thompson PA, Lade S, Webster H, Ryan G, Prince HM. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinico-pathological entity. Haematologica. 2010;95(11):1977–1979. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100(2):554–555. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 112.Miranda RN, Aladily TN, Prince HM, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014;32(2):114–120. doi: 10.1200/JCO.2013.52.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Clemens MW, Medeiros LJ, Butler CE, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34(2):160–168. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laurent C, Delas A, Gaulard P, et al. Breast implant associated anaplastic large cell lymphoma: two distinct clinicopathological variants with different outcomes. Ann Oncol. 2016;27(2):306–314. doi: 10.1093/annonc/mdv575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Swerdlow SH, Jaffe ES, Brousset P, et al. International Lymphoma Study Group. Cytotoxic T-cell and NK-cell lymphomas: current questions and controversies. Am J Surg Pathol. 2014;38(10):e60–e71. doi: 10.1097/PAS.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perry AM, Warnke RA, Hu Q, et al. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122(22):3599–3606. doi: 10.1182/blood-2013-07-512830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Petrella T, Maubec E, Cornillet-Lefebvre P, et al. Indolent CD8-positive lymphoid proliferation of the ear: a distinct primary cutaneous T-cell lymphoma? Am J Surg Pathol. 2007;31(12):1887–1892. doi: 10.1097/PAS.0b013e318068b527. [DOI] [PubMed] [Google Scholar]
- 118.Macon WR, Levy NB, Kurtin PJ, et al. Hepatosplenic alphabeta T-cell lymphomas: a report of 14 cases and comparison with hepatosplenic gammadelta T-cell lymphomas. Am J Surg Pathol. 2001;25(3):285–296. doi: 10.1097/00000478-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Toro JR, Liewehr DJ, Pabby N, et al. Gamma-delta T-cell phenotype is associated with significantly decreased survival in cutaneous T-cell lymphoma. Blood. 2003;101(9):3407–3412. doi: 10.1182/blood-2002-05-1597. [DOI] [PubMed] [Google Scholar]
- 120.Guitart J, Weisenburger DD, Subtil A, et al. Cutaneous γδ T-cell lymphomas: a spectrum of presentations with overlap with other cytotoxic lymphomas. Am J Surg Pathol. 2012;36(11):1656–1665. doi: 10.1097/PAS.0b013e31826a5038. [DOI] [PubMed] [Google Scholar]
- 121.Rodríguez-Pinilla SM, Ortiz-Romero PL, Monsalvez V, et al. TCR-γ expression in primary cutaneous T-cell lymphomas. Am J Surg Pathol. 2013;37(3):375–384. doi: 10.1097/PAS.0b013e318275d1a2. [DOI] [PubMed] [Google Scholar]
- 122.Endly DC, Weenig RH, Peters MS, Viswanatha DS, Comfere NI. Indolent course of cutaneous gamma-delta T-cell lymphoma. J Cutan Pathol. 2013;40(10):896–902. doi: 10.1111/cup.12091. [DOI] [PubMed] [Google Scholar]
- 123.Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366(20):1905–1913. doi: 10.1056/NEJMoa1114885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jerez A, Clemente MJ, Makishima H, et al. STAT3 mutations unify the pathogenesis of chronic lymphoproliferative disorders of NK cells and T-cell large granular lymphocyte leukemia. Blood. 2012;120(15):3048–3057. doi: 10.1182/blood-2012-06-435297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bergmann AK, Schneppenheim S, Seifert M, et al. Recurrent mutation of JAK3 in T-cell prolymphocytic leukemia. Genes Chromosomes Cancer. 2014;53(4):309–316. doi: 10.1002/gcc.22141. [DOI] [PubMed] [Google Scholar]
- 126.Kiel MJ, Velusamy T, Rolland D, et al. Integrated genomic sequencing reveals mutational landscape of T-cell prolymphocytic leukemia. Blood. 2014;124(9):1460–1472. doi: 10.1182/blood-2014-03-559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rajala HL, Eldfors S, Kuusanmäki H, et al. Discovery of somatic STAT5b mutations in large granular lymphocytic leukemia. Blood. 2013;121(22):4541–4550. doi: 10.1182/blood-2012-12-474577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nicolae A, Xi L, Pittaluga S, et al. Frequent STAT5B mutations in γδ hepatosplenic T-cell lymphomas. Leukemia. 2014;28(11):2244–2248. doi: 10.1038/leu.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Küçük C, Jiang B, Hu X, et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat Commun. 2015;6:6025. doi: 10.1038/ncomms7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deleeuw RJ, Zettl A, Klinker E, et al. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132(5):1902–1911. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 131.Tan SY, Ooi AS, Ang MK, et al. Nuclear expression of MATK is a novel marker of type II enteropathy-associated T-cell lymphoma. Leukemia. 2011;25(3):555–557. doi: 10.1038/leu.2010.295. [DOI] [PubMed] [Google Scholar]
- 132.Tomita S, Kikuti YY, Carreras J, et al. Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod Pathol. 2015;28(10):1286–1296. doi: 10.1038/modpathol.2015.85. [DOI] [PubMed] [Google Scholar]
- 133.Chan JK, Chan AC, Cheuk W, et al. Type II enteropathy-associated T-cell lymphoma: a distinct aggressive lymphoma with frequent γδ T-cell receptor expression. Am J Surg Pathol. 2011;35(10):1557–1569. doi: 10.1097/PAS.0b013e318222dfcd. [DOI] [PubMed] [Google Scholar]
- 134.Rodríguez Pinilla SM, Roncador G, Rodríguez-Peralto JL, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol. 2009;33(1):81–90. doi: 10.1097/PAS.0b013e31818e52fe. [DOI] [PubMed] [Google Scholar]
- 135.Garcia-Herrera A, Colomo L, Camós M, et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol. 2008;26(20):3364–3371. doi: 10.1200/JCO.2008.16.1307. [DOI] [PubMed] [Google Scholar]
- 136.Grogg KL, Jung S, Erickson LA, McClure RF, Dogan A. Primary cutaneous CD4-positive small/medium-sized pleomorphic T-cell lymphoma: a clonal T-cell lymphoproliferative disorder with indolent behavior. Mod Pathol. 2008;21(6):708–715. doi: 10.1038/modpathol.2008.40. [DOI] [PubMed] [Google Scholar]
- 137.Beltraminelli H, Leinweber B, Kerl H, Cerroni L. Primary cutaneous CD4+ small-/medium-sized pleomorphic T-cell lymphoma: a cutaneous nodular proliferation of pleomorphic T lymphocytes of undetermined significance? A study of 136 cases. Am J Dermatopathol. 2009;31(4):317–322. doi: 10.1097/DAD.0b013e31819f19bb. [DOI] [PubMed] [Google Scholar]
- 138.Cohen JI, Kimura H, Nakamura S, Ko YH, Jaffe ES. Epstein-Barr virus-associated lymphoproliferative disease in non-immunocompromised hosts: a status report and summary of an international meeting, 8-9 September 2008. Ann Oncol. 2009;20(9):1472–1482. doi: 10.1093/annonc/mdp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kimura H, Ito Y, Kawabe S, et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised hosts: prospective analysis of 108 cases. Blood. 2012;119(3):673–686. doi: 10.1182/blood-2011-10-381921. [DOI] [PubMed] [Google Scholar]
- 140.Ohshima K, Kimura H, Yoshino T, et al. CAEBV Study Group. Proposed categorization of pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant EBV T-LPD. Pathol Int. 2008;58(4):209–217. doi: 10.1111/j.1440-1827.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 141.Quintanilla-Martinez L, Ridaura C, Nagl F, et al. Hydroa vacciniforme-like lymphoma: a chronic EBV+ lymphoproliferative disorder with risk to develop a systemic lymphoma. Blood. 2013;122(18):3101–3110. doi: 10.1182/blood-2013-05-502203. [DOI] [PubMed] [Google Scholar]
- 142.Swerdlow SH. T-cell and NK-cell posttransplantation lymphoproliferative disorders. Am J Clin Pathol. 2007;127(6):887–895. doi: 10.1309/LYXN3RGF7D7KPYG0. [DOI] [PubMed] [Google Scholar]
- 143.Kato S, Asano N, Miyata-Takata T, et al. T-cell receptor (TCR) phenotype of nodal Epstein-Barr virus (EBV)-positive cytotoxic T-cell lymphoma (CTL): a clinicopathologic study of 39 cases. Am J Surg Pathol. 2015;39(4):462–471. doi: 10.1097/PAS.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 144.Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27(10):1346–1356. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 145.Hartmann S, Eichenauer DA, Plütschow A, et al. The prognostic impact of variant histology in nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group (GHSG). Blood. 2013;122(26):4246–4252. doi: 10.1182/blood-2013-07-515825. [DOI] [PubMed] [Google Scholar]
- 146.Boudová L, Torlakovic E, Delabie J, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood. 2003;102(10):3753–3758. doi: 10.1182/blood-2003-02-0626. [DOI] [PubMed] [Google Scholar]
- 147.Hartmann S, Döring C, Jakobus C, et al. Nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma--endpoints of a spectrum of one disease? PLoS One. 2013;8(11):e78812. doi: 10.1371/journal.pone.0078812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood. 2014;123(23):3567–3573. doi: 10.1182/blood-2013-12-541078. [DOI] [PubMed] [Google Scholar]
- 149.Hartmann S, Döring C, Vucic E, et al. Array comparative genomic hybridization reveals similarities between nodular lymphocyte predominant Hodgkin lymphoma and T cell/histiocyte rich large B cell lymphoma. Br J Haematol. 2015;169(3):415–422. doi: 10.1111/bjh.13310. [DOI] [PubMed] [Google Scholar]
- 150.Nam-Cha SH, Montes-Moreno S, Salcedo MT, Sanjuan J, Garcia JF, Piris MA. Lymphocyte-rich classical Hodgkin’s lymphoma: distinctive tumor and microenvironment markers. Mod Pathol. 2009;22(8):1006–1015. doi: 10.1038/modpathol.2009.54. [DOI] [PubMed] [Google Scholar]
- 151.Haroche J, Abla O. Uncommon histiocytic disorders: Rosai-Dorfman, juvenile xanthogranuloma, and Erdheim-Chester disease. Hematology Am Soc Hematol Educ Program. 2015;2015(1):571–578. doi: 10.1182/asheducation-2015.1.571. [DOI] [PubMed] [Google Scholar]
- 152.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111(12):5433–5439. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shao H, Xi L, Raffeld M, et al. Clonally related histiocytic/dendritic cell sarcoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: a study of seven cases. Mod Pathol. 2011;24(11):1421–1432. doi: 10.1038/modpathol.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chen W, Jaffe R, Zhang L, et al. Langerhans cell sarcoma arising from chronic lymphocytic lymphoma/small lymphocytic leukemia: lineage analysis and BRAF V600E Mutation Study. N Am J Med Sci. 2013;5(6):386–391. doi: 10.4103/1947-2714.114172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ratei R, Hummel M, Anagnostopoulos I, et al. Common clonal origin of an acute B-lymphoblastic leukemia and a Langerhans’ cell sarcoma: evidence for hematopoietic plasticity. Haematologica. 2010;95(9):1461–1466. doi: 10.3324/haematol.2009.021212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dalia S, Jaglal M, Chervenick P, Cualing H, Sokol L. Clinicopathologic characteristics and outcomes of histiocytic and dendritic cell neoplasms: the Moffitt Cancer Center experience over the last twenty five years. Cancers (Basel) 2014;6(4):2275–2295. doi: 10.3390/cancers6042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dalia S, Shao H, Sagatys E, Cualing H, Sokol L. Dendritic cell and histiocytic neoplasms: biology, diagnosis, and treatment. Cancer Control. 2014;21(4):290–300. doi: 10.1177/107327481402100405. [DOI] [PubMed] [Google Scholar]
- 158.Go H, Jeon YK, Huh J, et al. Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology. 2014;65(2):261–272. doi: 10.1111/his.12416. [DOI] [PubMed] [Google Scholar]