Key Points
cTFH are activated and skewed toward a Th2/Th17 phenotype promoting their B-cell help function during cGVHD.
cTFH activation signature correlates with memory B-cell and plasmablast phenotype in cGVHD patients.
Abstract
Chronic graft-versus-host disease (cGVHD) remains a major late complication of allogeneic hematopoietic stem cell transplantation (HSCT). Previous studies have established that both donor B and T cells contribute to immune pathology in cGVHD but the mechanisms responsible for coordinated B- and T-cell responses directed against recipient antigens have not been understood. T follicular helper cells (TFH) play an important role in the regulation of B-cell immunity. We performed extensive phenotypic and functional analysis of circulating TFH (cTFH) and B cells in 66 patients after HSCT. Patients with active cGVHD had a significantly lower frequency of cTFH compared with patients without cGVHD. This was associated with higher CXCL13 plasma levels suggesting increased homing of TFH to secondary lymphoid organs. In patients with active cGVHD, cTFH phenotype was skewed toward a highly activated profile with predominance of T helper 2 (Th2)/Th17 subsets. Activated cTFH in patients with cGVHD demonstrated increased functional ability to promote B-cell immunoglobulin secretion and maturation. Moreover, the activation signature of cTFH was highly correlated with increased B-cell activation and plasmablast maturation in patients after transplant. These studies provide new insights into the immune pathogenesis of human cGVHD and identify TFH as a key coordinating element supporting B-cell involvement in this disease.
Introduction
Chronic graft-versus-host disease (cGVHD) remains a major long-term complication of allogeneic hematopoietic stem cell transplantation (HSCT), occurring in ∼50% of patients.1 Donor T cells targeting alloantigens in the recipient play a critical role in the initiation of tissue injury, and depletion of mature T cells from the stem cell graft markedly reduces the subsequent incidence of cGVHD.2,3 However, B cells also play a key role in the immune pathology of cGVHD.4-6 Both murine models and clinical studies have shown that cGVHD is associated with multiple abnormalities of B-cell reconstitution including decreased numbers of B-cell progenitors, increased representation of memory B cells, and decreased frequency of regulatory B cells.4,7-11 cGVHD is also associated with high levels of B-cell activating factor (BAFF), high BAFF-to-B-cell ratios and aberrant B-cell activation.4,6,10,12,13 The frequent detection of auto- and alloantibodies after HSCT and the clinical benefit of B-cell–targeted therapy also emphasize the important role of B cells in the development of cGVHD.14-18
T and B cells represent 2 distinct arms of the adaptive immune system and both arms interact at many levels to coordinate effective and specific immune responses. For example, T-cell–mediated activation of B cells in the germinal center (GC) reaction promotes differentiation of naive B cells into memory B cells and class switching of immunoglobulin genes. These specific helper functions are mediated by a distinct CD4 T-cell subset in the GC, termed the T follicular helper cells (TFH). TFH characteristically expresses the BCL-6 transcription factor in association with surface expression of CXCR5 and programmed cell death protein 1 (PD-1). TFH interactions with B cells are mediated through surface molecules such as inducible T-cell costimulator (ICOS) and CD40L and secretion of interleukin-21 (IL-21). Through these mechanisms, TFH promotes immunoglobulin class-switch and somatic hypermutation leading to production of high-affinity antibodies as well as differentiation into long-lived plasma cells.19
Although TFH function primarily in secondary lymphoid structures, recent studies have identified a TFH subset that normally circulates in the peripheral blood (circulating TFH [cTFH]).20,21 cTFH can be identified within the CD4+CD45RA− memory T-cell compartment by expression of CXCR5 but unlike GC TFH, cTFH do not express BCL-6. Nevertheless, resident GC TFH and cTFH share functional capacities for providing B-cell help and the identification of cTFH in peripheral blood has provided investigators with the opportunity to better understand interactions between T cells and B cells that occur in the GC during autoimmune diseases,22,23 infections,24,25 and vaccine responses.26,27
The present studies were undertaken to examine the phenotype and function of cTFH in patients with cGVHD. Although cTFH are reduced in cGVHD, cTFH express high levels of activation markers and exhibit high levels of functional helper activity for both autologous B cells and allogeneic B cells from healthy donors. In patient samples, phenotypic measures of cTFH activation were significantly correlated with markers of B-cell activation in vivo. These findings suggest that cTFH contribute to sustained activation of B cells and therefore play an important role coordinating T- and B-cell responses in patients with cGVHD.
Material and methods
Patient characteristics
Laboratory studies were performed on fresh samples from 66 patients who underwent allogeneic HSCT at the Dana-Farber Cancer Institute and Brigham and Women’s Hospital (Boston, MA). Clinical characteristics of these patients are summarized in Table 1. cGVHD grade was established at the time of sample collection using National Institutes of Health guidelines.28 Clinical manifestations of cGVHD are summarized in Table 2. Resolved cGVHD was defined as the absence of any clinically active disease without steroid therapy at the time of sample collection. Among 12 patients with resolved cGVHD, 2 patients were receiving immunosuppressive treatment (1 tacrolimus [Tac], 1 sirolimus [Sir]). Peripheral blood samples were also obtained from 22 healthy stem cell transplant donors. All patients and healthy donors were enrolled in clinical research protocols approved by the Human Subjects Protection Committee of the Dana-Farber/Harvard Cancer Center. Written informed consent was obtained prior to sample collection, in accordance with the Declaration of Helsinki. Boston Children’s Hospital Institutional Review Board approval was obtained to receive discarded fresh tonsil tissue after elective tonsillectomy.
Table 1.
Clinical characteristics of HSCT patients
| HSCT patients, N = 66 |
P |
|||
|---|---|---|---|---|
| No cGVHD, n = 16 | Active cGVHD, n = 38 | Resolved cGVHD, n = 12 | No vs Active | |
| Age, y | ||||
| Median | 56.6 | 61.7 | 63.1 | ns |
| Range | 25-74.9 | 25.9-75.6 | 32.6-69.5 | |
| Sex (%) | ||||
| M | 9 (56) | 23 (60) | 7 (58) | ns |
| F | 7 (44) | 15 (40) | 5 (42) | |
| Disease (%) | ||||
| Acute leukemia | 6 (38) | 15 (40) | 3 (25) | |
| HD, NHL, CLL, MM | 8 (50) | 15 (40) | 6 (50) | |
| MDS, MPN | 1 (6) | 7 (18) | 2 (17) | |
| Nonmalignant | 1 (6) | 1 (2) | 1 (8) | |
| Conditioning regimen (%) | ||||
| MAC | 4 (25) | 8 (21) | 6 (50) | ns |
| RIC | 12 (75) | 30 (79) | 6 (50) | |
| Donor type (%) | ||||
| MRD | 9 (56) | 10 (27) | 4 (33) | |
| MUD | 5 (31) | 24 (63) | 7 (58) | |
| mmUD | 2 (13) | 4 (10) | 1 (9) | |
| Graft source (%) | ||||
| PBSC | 13 (81) | 37 (97) | 9 (75) | |
| BM | 3 (19) | 1 (3) | 3 (25) | |
| GVHD prophylaxis (%) | ||||
| Tac-MTX | 6 (37) | 15 (40) | 6 (50) | |
| Tac-Sir | 3 (19) | 5 (13) | 1 (8) | |
| Tac-Sir-MTX | 7 (44) | 18 (47) | 5 (42) | |
| Months from HSCT to sample collection | ||||
| Median | 35.8 | 28.9 | 52.8 | .09 |
| Range | 12-87.9 | 9.6-123.8 | 11.7-140.9 | |
| Grade II-IV aGVHD (%) | ||||
| Yes | 0 | 17 (45) | 3 (25) | |
aGVHD, acute GVHD; BM, bone marrow; CLL, chronic lymphocytic leukemia; F, female; HD, Hodgkin disease; M, male; MAC, myeloablative conditioning; MDS, myelodysplastic syndrome; MM, multiple myeloma; mmUD, mismatched unrelated donor; MPN, myeloproliferative neoplasm; MRD, matched related donor; MUD, matched unrelated donor; NHL, non-Hodgkin lymphoma; ns, not significant; PBSC, peripheral blood stem cell; RIC, reduced-intensity conditioning; Tac-MTX, tacrolimus-methotrexate; Tac-Sir, tacrolimus-sirolimus; Tac-Sir-MTX, tacrolimus-sirolimus-methotrexate.
Table 2.
Clinical characteristics of cGVHD in the study population
| Mild |
Moderate |
Severe |
Resolved |
|
|---|---|---|---|---|
| N = 15 | N = 14 | N = 9 | N = 12 | |
| Mucosal, oral and/or genital (%) | 8 (53) | 11 (78) | 7 (77) | |
| Scleroderma (%) | 0 | 3 (21) | 3 (33) | |
| Lung (%) | 0 | 0 | 5 (55) | |
| Immunosuppressive therapy at sample collection (%) | 7 (47) | 10 (71) | 7 (77) | 1 (8) |
| Steroid treatment (%) | 5 (33) | 5 (35) | 6 (66) | 0 |
| Mean steroid dose, mg/kg/d | 0.108 | 0.142 | 0.276 | 0 |
| Mean time between resolution and sample collection, mo | — | — | — | 28.9 |
Sample processing
Blood samples anticoagulated with EDTA were processed within 6 hours of collection. Samples were centrifuged for 15 minutes at 2000 rpm to collect plasma which was stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation (Ficoll-Hypaque; GE Healthcare). Fresh tonsils were cut into 5-mm tissue blocks and mechanically disrupted through a 70-μm cell strainer. Tonsil mononuclear cells were separated by density gradient centrifugation and washed twice before being cryopreserved.
Flow cytometry
PBMCs were stained at 4°C for 20 minutes with fluorochrome-conjugated antibodies to characterize cTFH and B cells. The following reagents were used: CD3 (clone UCHT1), CD4 (SK3), CXCR5 (RF8B2), CXCR3 (1C6/CXCR3), CCR6 (11A9), CD38 (HIT2) from BD Biosciences; CD45RA (IMU2711) from Beckman; ICOS (C398.4A), PD-1 (EH12.2H7), CD19 (HIB19), immunoglobulin D (IgD; IA6-2) from BioLegend; and CD27 (O323) from eBioscience. To measure proliferation, PBMCs were stained with antibodies directed against intracellular antigen Ki67 (B56; BD) using the FoxP3 kit (eBioscience). Susceptibility to apoptosis was evaluated by staining for BCL-2 (BCL-2/100; BD) and CD95/Fas (DX2; BioLegend). For cytokine flow cytometry, sorted subsets were stimulated with phorbol myristate acetate (50 ng/mL), ionomycin (1 μM; from Sigma-Aldrich) in the presence of Brefeldin A (BD) for 4 hours. Cells were then fixed and permeabilized with the BD Cytofix/Cytoperm kit and stained with cytokine-specific antibodies: interferon-γ (IFN-γ; clone B27), and IL-17 (clone N49-653) from BD and IL-4 (clone MP4-25D2) from BioLegend. Flow cytometry standards and quality controls were run regularly to ensure stability of the analytical instruments. A minimum of 200 000 events were recorded in the lymphocyte gate. Cells were analyzed using LSR Fortessa (BD Bioscience) and FlowJo v.10 software (Treestar).
T-cell–B-cell coculture
PBMCs were enriched for either memory CD4+ T cells or B cells using the MACS kit (Miltenyi Biotec) and subsequently stained for flow sorting using the FACSAria (BD Biosciences). Cells were purified based on the following markers: cTFH (CD19−CXCR5+CD4+CD45RA− T cells), non-TFH (CD19−CXCR5−CD4+CD45RA− T cells), naive B cells (CD3−IgD+CD27−CD19+), and memory B cells (CD3−CD27+CD19+). Sorted populations were confirmed to be >95% pure. Subsets were plated at 20 000 cells per well, at a 1:1 ratio, in RPMI 1640 medium containing L-glutamine, penicillin, streptomycin, 10% fetal bovine serum, and 1 µg/mL staphylococcal enterotoxin reduced (SEB; Toxin Technology) for up to 12 days. Plasmablast differentiation (CD27hiCD38hi B cells) was assessed at day 6. IgG and IgM were measured at day 10.22
ELISA
CXCL13 was measured in plasma by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s protocols (R&D Systems). IgG and IgM were measured in culture supernatant using ELISA kits (Bethyl Laboratories). Samples were tested in duplicate along with standards and calibrator controls provided by the manufacturer.
Statistical analysis
Baseline clinical characteristics were compared between groups using either the Fisher exact test or the χ2 test for categorical variables or the Wilcoxon rank-sum test for continuous variables. Phenotypic and functional data were compared using the (exact) Wilcoxon rank-sum test for unpaired group comparison and the Wilcoxon signed-rank test for paired comparison. Univariable linear regression analysis was performed for factors listed in Tables 1-2 to identify those that were associated with the frequency of cTFH. Factors that were significant at the 0.1 level from the univariable analysis were included in the multivariable analysis. Prior to analysis, the normality assumption was examined and absolute cTFH values were natural log-transformed to meet the normality assumption. Due to the colinearity between epithelial cGVHD and cGVHD status at sample collection, these variables were included separately in 2 multivariable models. Correlation studies were performed using the nonparametric Spearman-rank test and various smoothing techniques were used in graphical presentation of correlation.29 All tests were 2-sided at the significance level of 0.05 and multiple comparisons were not considered. All statistical analyses were performed using SAS version 9.2 (SAS Institute) and R version 3.1.3 (the Comprehensive R Archive Network [CRAN] Project). All graphs were made using either R or Prism software (GraphPad).
Results
Altered cTFH frequency and numbers during cGVHD
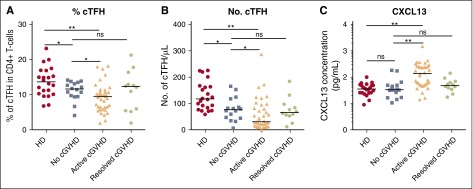
To characterize cTFH in cGVHD, we examined fresh blood samples from 22 healthy donors and a cohort of 66 patients at least 9 months after HSCT (Tables 1-2). Within the patient cohort, 16 had no cGVHD, 12 had resolved cGVHD, and 38 had active cGVHD at the time of testing. Overall, the frequency of cTFH was significantly decreased after HSCT compared with healthy donors (median, 9.87% vs 13.65% of CD4 T cells; P = .001). The frequency of cTFH was decreased in patients with active cGVHD compared with patients without cGVHD (median, 9.44% vs 11.65% of CD4 T cells; P = .03) (Figure 1A). Comparisons based on absolute numbers of cTFH showed a median twofold difference (30.65 vs 76.45 cTFH per μL, respectively) that was also significant (P = .038) (Figure 1B). In contrast, cTFH in patients with resolved cGVHD was similar to patients without cGVHD. Further multivariable linear regression analysis confirmed that active cGVHD was a significant factor for low percentage of cTFH after adjusting for other transplant characteristics (P = .046) (Table 3). When cGVHD site was examined, cGVHD involving the gastrointestinal track, skin, and sclerodermatous cGVHD were each significantly associated with lower percentage of cTFH. Combining these 3 sites as “epithelial cGVHD” was the strongest predictor of low percentage of cTFH in univariable and multivariable analysis (P = .001). The other factor that affected percentage of cTFH in multivariable analysis was acute GVHD prophylaxis (P = .03). Patients who received Tac/Sir ± methotrexate (MTX) had a significantly lower percentage of cTFH (9% vs 11.52%, P = .015 from univariable analysis) and absolute numbers of cTFH (32.4 vs 82.1, P = .0003) compared with those who received Tac/MTX.
Figure 1.
Abnormal cTFH values and CXCL13 levels in patients with active cGVHD. (A) Frequency of cTFH (CXCR5+CD45RA− within CD4+ T cells) in healthy donors (HD), patients with no cGVHD, active cGVHD, and resolved cGVHD. (B) Absolute number of cTFH (CXCR5+CD45RA−CD4+ T cells) in the different clinical groups. (C) CXCL13 plasma concentration (pg/mL) was measured by ELISA and plotted for each patient group. Data were log10 transformed. Black bar represents median value for each group. The Wilcoxon rank-sum test was used. *P < .05; **P < 10−4. ns, not significant.
Table 3.
LS linear regression analysis for factors associated with percentage of cTFH in all HSCT patients
| Clinical factors | Contrast | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|---|
| LS mean difference | STDERR | P | LS mean difference | STDERR | P | ||
| Age | ≥50 vs <50 y old | −1.85 | 1.09 | .09 | −1.33 | 1.08 | .22 |
| Conditioning intensity | RIC vs MAC | −2.54 | 1.12 | .026 | −1.61 | 1.27 | .21 |
| GVHD prophylaxis | (Tac/Sir or Tac/Sir/MTX) vs Tac/MTX | −2.52 | 1.00 | .015 | −2.14 | 0.97 | .03 |
| cGVHD status at sample collection | Active vs (none or resolved) | −1.98 | 1.02 | .055 | −1.99 | 0.98 | .046 |
| “Epithelial” cGVHD | Gut/scleroderma/skin vs others | −3.35 | 0.97 | .001 | −3.19 | 0.94 | .001 |
Other variables that were examined in univariable regression analysis were patient sex, donor type, graft source, and aGVHD grade II-IV. P values for these variables were >.1. In multivariable analysis, variables with P < .1 from the univariable analysis were included to avoid overfitting the model. P values shown in bold represent significant values.
LS, least squares; LS mean difference, least squares (marginal) mean difference between 2 groups; STDERR, standard error of the LS mean difference.
cTFH are characterized by expression of CXCR5, a receptor for CXCL13 chemokine. CXCL13-CXCR5 interaction promotes homing of TFH to lymphoid follicles, facilitating contact between TFH and B cells. CXCL13 levels in our cohort were significantly increased in patients with active cGVHD compared with patients with no cGVHD (137.7 pg/mL vs 33.74 pg/mL, P < 10−4) (Figure 1C). CXCL13 levels in patients with resolved cGVHD were similar to levels in patients without cGVHD and healthy donors.
cTFH are activated during cGVHD
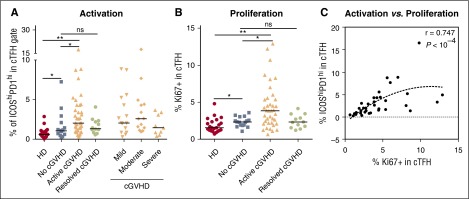
Functionally active TFH in lymphoid tissues express high levels of ICOS and PD-1 (supplemental Figure 1A, available on the Blood Web site). However, few activated cTFH are present in peripheral blood in healthy donors (supplemental Figure 1C).24,25 In contrast, ICOShiPD-1hi cTFH are increased in patients with active cGVHD compared with healthy donors (2.035% vs 0.59% for HD, P < 10−4) (Figure 2A). The frequency of ICOShiPD-1hi cTFH in patients with active cGVHD was also increased compared with patients with no cGVHD (2.035% vs 1.065%, respectively, P = .028) but was similar in patients with resolved cGVHD and no cGVHD. The frequency of ICOShiPD-1hi cTFH did not correlate with the clinical grade of cGVHD. This may reflect the impact of steroid use, which was more frequent in patients with severe cGVHD (Table 2).30 As shown in Figure 2B, the activation profile of cTFH in patients with active cGVHD is associated with higher proliferative activity measured by Ki67 (3.83% active cGVHD vs 2.31% no cGVHD, P = .01). Proliferation of cTFH in patients with no cGVHD was increased compared with healthy donors (P = .033), but cTFH proliferation in resolved cGVHD was not significantly increased (P = .057). Notably, proliferative activity is highly correlated with the frequency of ICOShiPD-1hi cTFH (Spearman, r = 0.747; P < 10−4) in the active cGVHD cohort (Figure 2C).
Figure 2.
cTFH are activated and proliferate in patients with active cGVHD. (A) Frequency of ICOShiPD-1hi in cTFH (CXCR5+CD45RA−CD4+ T cells) was determined in different patient groups. (B) Ki67 expression within cTFH (CXCR5+CD45RA−CD4+ T cells) was determined in different patient groups. (C) Positive correlation between proliferation and frequency of highly activated ICOShiPD-1hi cTFH in the active cGVHD group. Black bar represents median value for each group. The Wilcoxon rank-sum test was used in panels A and B. The Spearman test was used in panel C. *P < .05; **P < 10−4. The gating strategy for identifying ICOShiPD-1hi in cTFH is shown in supplemental Figure 1.
cTFH T helper subset homeostasis is altered in cGVHD
Previous studies have defined distinct CD4 T-cell subsets based on expression of CXCR3 and CCR6 as follows: T helper 1 (Th1) (CXCR3+CCR6−), Th2 (CXCR3−CCR6−), Th17 (CXCR3−CCR6+), and Th++ (CXCR3+CCR6+).22 Our studies confirmed that Th1, Th2, and Th17 subsets within cTFH are each characterized by a distinct cytokine expression profile. Th1 cTFH express high levels of IFN-γ; Th2 cTFH express IL-4; and Th17 cTFH express IL-17 (supplemental Figure 2). The functional capacity of cTFH and non-cTFH subsets was examined by evaluating the ability of each subset to induce secretion of IgM and support class-switch secretion of IgG by naive B cells. As expected, non-cTFH (CXCR5−) provided little or no B-cell help (Figure 3A-B). The Th17 cTFH subset was the primary subset capable of inducing IgM secretion, immunoglobulin heavy chain class switching, and IgG secretion by naive B cells. The Th2 cTFH subset also demonstrated functional capacity for induction of IgG secretion (Figure 3B) but the Th1 cTFH subset provided no support for either IgM or IgG secretion.
Figure 3.
Function of cTFH subsets and relative distribution after HSCT. (A) IgM production by naive B cells (IgD+CD27−) after in vitro coculture with different subsets of cTFH (CXCR5+) and non-TFH (CXCR5−) cells. (B) IgG production by naive B cells (IgD+CD27−) after in vitro coculture with different subsets of cTFH (CXCR5+) and non-TFH (CXCR5−) cells. N = 3. Graphs represent mean and standard deviation (SD). (C) Ratio of (Th2+Th17)/Th1 cTFH subsets in different patient groups. Black bars represent median values for each group. (D) Whisker plots represent frequency of Th1 (blue) and Th17 (red) cTFH subsets in patients with different severity of cGVHD. Exact Wilcoxon rank-sum test was used. ns, not significant; *P < .05; **P < 10−4.
After transplant, the relative distribution of functional cTFH subsets was altered in patients with cGVHD. Within cTFH, we used the ratio (Th2+Th17) to Th1 to evaluate the capacity for B-cell help in each patient sample. This ratio was not significantly different among active, none, and resolved cGVHD groups and was similar to healthy donors (Figure 3C). However, when frequencies of Th1 and Th17 cTFH were examined among cGVHD patients, there was an inverse linear relationship between Th1 cTFH (P = .0011) and clinical grade of cGVHD and a positive linear relationship between Th17 cTFH and clinical grade (P = .0013) (Figure 3D). Furthermore, the (Th2+Th17)-to-Th1 ratio was significantly higher in severe cGVHD compared with no cGVHD (3.59 vs 1.365, P = .0006) (Figure 3C). We also found that highly activated ICOShiPD-1hi cTFH were significantly increased within the CXCR3− fraction (Th2+Th17) in patients with active cGVHD compared with the no cGVHD group (1.47% vs 0.83%; P = .01) (data not shown). Taken together, these results show that cTFH homeostasis is skewed toward predominance of activated Th2 and Th17 subsets in patients with active severe cGVHD.
Enhanced function of cTFH in patients with cGVHD
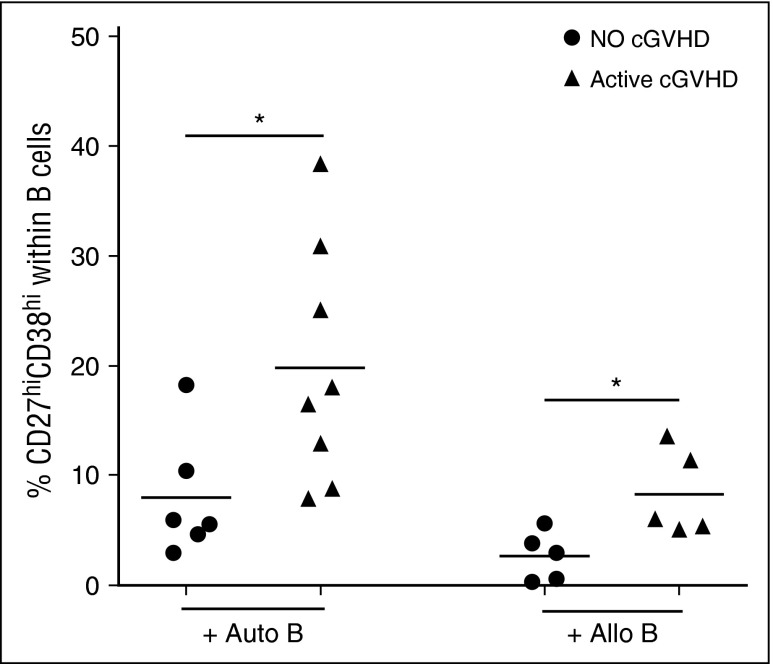
To examine the functional capacity of cTFH after transplant, we established a functional assay to measure the ability of cTFH to induce naive B-cell differentiation to mature plasmablasts. In this assay, cTFH are purified by cell sorting and incubated with naive B cells purified from the same sample. As shown in Figure 4, cTFH from patients with active cGVHD induced significantly greater plasmablast differentiation than cTFH from patients without cGVHD when cultured with autologous naive B cells (P = .029). Because previous studies demonstrated that B cells are constitutively activated in cGVHD, this finding may simply reflect increased sensitivity of B cells to external stimulation in these patients.12 We therefore also examined the functional capacity of patient cTFH to induce plasmablast differentiation of naive B cells from healthy donors. In these experiments, cTFH from patients with active cGVHD also induced greater plasmablast differentiation from normal naive B cells (P = .032). Clinical characteristics of patients used for functional experiments are summarized in supplemental Table 1. Taken together, these data indicate that cTFH in patients with cGVHD exhibit increased functional activity for B-cell help.
Figure 4.
Increased cTFH function in patients with active cGVHD. Frequency of plasmablasts (CD27hiCD38hi B cells) was measured after naive B cells were cultured with cTFH in the presence of SEB for 6 days. Circulating TFH were purified by cell sorting (purity >95%) from fresh patient samples and cultured with autologous naive (IgD+CD27−) B cells (Auto B) or naive B cells from an allogeneic healthy donor (Allo B). Results are compared for cTFH obtained from patients with no cGVHD (n = 6, for the autologous condition; n = 5 for the allogeneic condition) or active cGVHD (n = 8 for the autologous condition; n = 5 for the allogeneic condition). Black bars represent median values for each group. Exact Wilcoxon rank-sum test was used. *P < .05.
Altered phenotype of cTFH correlates with B-cell phenotype during cGVHD
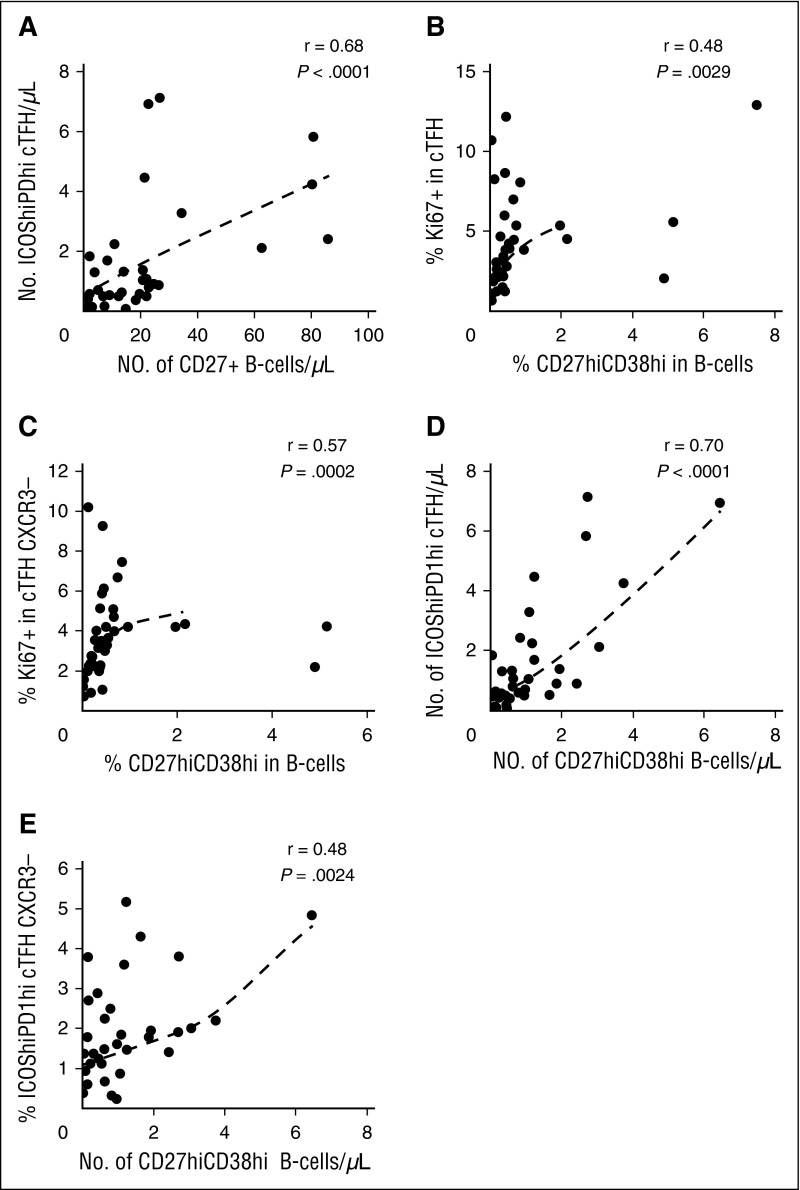
Previous studies have demonstrated that active cGVHD is associated with higher levels of CD27+ B cells including both IgD+CD38hi and IgD−CD38hi subsets.4 TFH support differentiation of B cells into plasmablasts, and we therefore examined whether the altered cTFH signature we identified in active cGVHD was correlated with B-cell phenotype in these patients. In this analysis, the total number of CD27+ B cells was strongly correlated with the number of highly activated ICOShiPD-1hi cTFH (r = 0.68, P < 10−4) (Figure 5A). Within cTFH, the Th1 subset is CXCR3+ whereas the Th2 and Th17 subsets are CXCR3−. Thus, cTFH subsets with increased B-cell help capacity can be phenotypically defined as CXCR3−. As shown in Figure 5B-C, the proliferative activity (Ki67) of cTFH and CXCR3− cTFH correlated with the generation of CD27hiCD38hi B cells (r = 0.48, P = .0029 and r = 0.57, P = .0002, respectively). Similarly, the number of highly activated ICOShiPD-1hi cTFH was strongly correlated with the generation of CD27hiCD38hi B cells (r = 0.70, P < 10−4) (Figure 5D) and the frequency of CXCR3−ICOShiPD-1hi cTFH with high B-cell help capacity correlated well with blood plasmablast count (r = 0.48, P = .0024) (Figure 5E).
Figure 5.
cTFH signature correlates with plasmablast generation in patients with cGVHD (N = 38). (A) Correlation between number of activated cTFH (ICOShiPD-1hi CXCR5+CD45RA−CD4+ T cells) and number of circulating CD27+ B cells in patient samples. (B) Correlation between cTFH proliferation (%Ki67) and percentage of plasmablasts (CD27hiCD38hi) in patient samples. (C) Correlation between proliferation (%Ki67) of CXCR3− cTFH and percentage of plasmablasts (CD27hiCD38hi) in patient samples. (D) Correlation between number of activated cTFH (ICOShiPD-1hi) and number of plasmablasts (CD27hiCD38hi) in patient samples. (E) Correlation between percentage of activated CXCR3− cTFH (ICOShiPD-1hi) and number of plasmablasts (CD27hiCD38hi) in patient samples. The Spearman test was used.
Discussion
Patients with cGVHD frequently develop IgG antibodies directed at minor histocompatibility antigens in the recipient.14,15,31 In individual patients, these specific B-cell responses have been associated with clonal T-cell responses directed against different peptide epitopes contained within the same antigen.32,33 These observations suggest that cGVHD is associated with coordinated T- and B-cell responses directed against allogeneic target antigens. Coordinated T- and B-cell responses have also been observed in murine models, where TFH cells are required for the development of immune pathology characteristic of cGVHD.8 Lymphoid TFH play a key role in promoting B-cell maturation leading to the generation of long-lived plasma cells that produce high-affinity IgG antibodies. Because cTFH exhibit many phenotypic and functional characteristics of resident TFH it was possible to examine the potential role of these cells in promoting B-cell immunity in cGVHD.
After HSCT, the percentage of cTFH was significantly lower in patients with active cGVHD compared with patients without cGVHD. Abnormal homing and trafficking of cTFH in patients with cGVHD could explain this decrease and led us to measure levels of CXCL13 in the same patients. In previous studies, increased levels of CXCL13 were found in patients with autoimmune diseases and high levels correlated with disease activity.34,35 After HSCT, increased plasma levels of CXCL13 were only present in patients with active cGVHD. CXCL13 levels in patients without cGVHD or resolved cGVHD were similar to levels in healthy donors. These results suggest that the lower frequency of cTFH in active cGVHD could reflect increased trafficking and retention of cTFH in lymphoid organs induced by CXCL13.
Activated TFH in secondary lymphoid organs express high levels of ICOS (CD278) and PD-1 (CD279). These immune-regulatory molecules are also expressed on cTFH, and their level of expression reflects the activation status of cTFH.36,37 ICOS, a CD28-superfamily costimulatory molecule, has been shown to play a key role in TFH commitment.38 PD-1 is a negative regulator of TFH activity.39 However, PD-1hi cTFH displays high levels of “helper” function.37 In our analysis, some cTFH exhibited a highly activated ICOShiPD-1hi phenotype that was similar to activated TFH in GC. This ICOShiPD-1hi subset within cTFH was 2 times more prevalent in patients with active cGVHD compared with patients without cGVHD. These cTFH were also highly proliferative and the level of activation correlated with their level of proliferation.
In healthy donors, cTFH are composed of Th1, Th2, and Th17 subsets, each with distinct functional capacity. In our study, both Th2 and Th17 cTFH subsets were able to promote class switching and induce IgG secretion from naive B cells. Th2 and Th17 subsets are therefore the primary cTFH subsets that provide B-cell “help”. In patients with active cGVHD, the ratio of (Th2+Th17)-to-Th1 cTFH was increased in severe cGVHD and the proportional increase in Th17 cTFH was positively correlated with clinical disease activity. In contrast, Th1 cTFH are proportionally increased in patients without cGVHD. Upon in vitro stimulation, Th1 cTFH secrete IFN-γ but do not secrete IL-4 or IL-17, the primary cytokines that support B-cell function and immunoglobulin class switching.22,25,26,40 This finding explains why Th1 cTFH provide little B-cell help. However, Th1 cTFH may also negatively regulate B-cell function. Notably, IFN-γ rapidly induces expression of PD-L1 on antigen-presenting cells, thus providing a negative costimulatory signal for TFH that expresses high levels of PD-1.39,41 Thus, Th1 cTFH may represent an important regulatory subset, and increased levels of this subset after HSCT may prevent B-cell activation by other cTFH subsets and suppress cGVHD.
When coculturing cTFH with autologous naive B cells, we found greater levels of plasmablast generation in patients with active cGVHD compared with patients without cGVHD. Because B cells are aberrantly activated in cGVHD,10,12,13 this observation could reflect in vivo priming and increased sensitivity of patient B cells to cTFH. To exclude the effect of in vivo B-cell priming, we carried out cocultures with patient cTFH and naive B cells from a healthy donor. Moreover, the same B cells were used in assays with different patient cTFH to normalize the evaluation of cTFH activity. Although the magnitude of B-cell maturation was less, cTFH from patients with active cGVHD also induced greater plasmablast differentiation from normal B cells.
The maintenance of T-cell alloreactivity is a dynamic process, which is influenced by the ability of activated cells to resist apoptosis. Cell death mechanisms through either extrinsic (CD95/Fas) or intrinsic (BCL-2) apoptosis pathways have thus been shown to play a role in the differential regulation of different T-cell subsets.42,43 BCL-2 is a critical antiapoptotic molecule that protects mitochondrial integrity during activation of the intrinsic apoptosis pathway. In murine models, BCL-2 helps to protect activated TFH from negative regulation.44 In healthy donors, we observed that Th2 and Th17 cTFH subsets express higher levels of BCL-2 than Th1 cTFH. Although differences were relatively small, the expression of BCL-2 in Th2 and Th17 cTFH was significantly increased in patients with active cGVHD compared with patients without cGVHD (data not shown). Consistent with results in murine models, these observations suggest that BCL-2 preferentially promotes the survival of cTFH subsets that provide increased levels of B-cell help.44 In contrast to BCL-2, CD95/Fas initiates death receptor–induced apoptosis through the extrinsic pathway. In healthy donors, CD95 is highly expressed on memory T cells, and these subsets are more susceptible to extrinsic pathway apoptosis. Similar to other memory T cells, cTFH express relatively high levels of CD95. CD95 expression is increased with cTFH activation and Th17 cTFH express higher levels of CD95 than other cTFH subsets. Nevertheless, CD95 expression in cTFH was similar in patients with and without cGVHD (data not shown). Although high level expression of CD95 likely serves to limit the survival of activated cTFH, expression of CD95 is not altered in cGVHD.
The altered cTFH signature we observed in patients with active cGVHD reflects significantly increased levels of cTFH activation, survival, and functional capacity for B-cell help. To examine whether these phenotypic measures of cTFH activity were translated into an actual effect on B cells in vivo, we examined our data for correlations between measures of cTFH activity and B-cell phenotypes. This analysis confirmed that several measures of cTFH activity were significantly correlated with either the number or proportion of memory B cells in patient samples. In particular, the proportion of ICOShiPD-1hicTFH and CXCR3−ICOShiPD-1hi cTFH subsets was highly correlated with the percentage and absolute numbers of peripheral plasmablasts, respectively. These correlations provide further evidence that increased levels of cTFH activation and functional activity result in altered B-cell homeostasis and promote increased B-cell maturation in patients with cGVHD. Interestingly, patients with cGVHD often present with hypogammaglobulinemia. However, B cells from patients with cGVHD patients produce greater amounts of IgG when stimulated ex vivo when compared with B cells from patients without cGVHD.4 In murine models IgG antibodies are deposited in tissues associated with cGVHD and this may explain the lower levels of immunoglobulin in plasma in patients with active disease.7 Taken together, these data suggest that increased cTFH activity promotes B-cell help leading to antibody production and deposition in damaged tissues. Alternatively, hypogammaglobulinemia may be due to chronic immune-suppressive therapy in these patients.
In summary, our studies provide new evidence that TFH play an important role in the coordination of T- and B-cell immunity in cGVHD. Although previous studies established that both donor T cells and B cells contribute to immune pathology associated with cGVHD, it was not known how these 2 arms of the adaptive immune system coordinated their attack on recipient cells. The identification of TFH as a key component of this coordinated response also suggests that known mediators of TFH functions play important roles in the regulation of these TFH–B-cell interactions. More specifically, TFH functions rely heavily on molecules that mediate homing to lymphoid organs (CXCL13/CXCR5) and specific signaling pathways and cytokines (ICOS, PD-1, CD40L, IL-21) that mediate T–B-cell collaborations. Specific targeting of these molecules may offer new therapeutic opportunities that may improve upon current strategies that rely primarily on pan-B-cell depletion. Although pan-B-cell depletion offers some benefit for patients with cGVHD, clinical improvement is often limited to only a fraction of patients.16,18 Targeting TFH–B-cell interactions is being developed as a therapy for autoimmune diseases and this may also be an effective strategy in patients with cGVHD.44-47
Acknowledgments
The authors thank Kristen Cowens, Steven Paula, Suzan Lazo-Kallanian, and John Daley for excellent assistance with flow cytometry studies and cell sorting, Lauren Gaffny and Doreen Hearsey for their support in obtaining human samples, and M. Fleming and H. Kozakewich for providing human tonsil specimens.
This work was supported by National Institutes of Health, National Cancer Institute grants P01CA142106, CA183559, CA183560, Leukemia and Lymphoma Translational Research Grant 6462-15, and the MD-PhD Program (Bordeaux Teaching Hospital, France).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.F. performed experiments; E.F. and J.R. designed the study, analyzed data, and wrote the manuscript; H.T.K. performed statistical analysis; K.W., A.C.A., and B.R.B. analyzed and interpreted data; C.C., S.N., J.K., V.T.H., P.A., E.P.A., R.J.S., and J.H.A. provided samples; and all authors contributed to editing and reviewed the manuscript.
Conflict-of-interest disclosure: J.K. received research funding from Prometheus Labs, Otsuka Pharmaceuticals, Millennium Pharmaceuticals; served on advisory boards for Kadmon Corp, Takeda Pharmaceuticals; and received honoraria from Miltenyi Biotec GmbH. The remaining authors declare no competing financial interests.
Correspondence: Jerome Ritz, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: jerome_ritz@dfci.harvard.edu.
References
- 1.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. doi: 10.1182/blood-2014-01-514752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17(9):1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soiffer RJ, Lerademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117(25):6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121(10):1886–1895. doi: 10.1182/blood-2012-06-435008. [DOI] [PubMed] [Google Scholar]
- 6.Sarantopoulos S, Blazar BR, Cutler C, Ritz J. B cells in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(1):16–23. doi: 10.1016/j.bbmt.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119(6):1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson CA, Sun L, Kim HT, et al. Post-transplantation B cell activating factor and B cell recovery before onset of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(5):668–675. doi: 10.1016/j.bbmt.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarantopoulos S, Ritz J. Aberrant B-cell homeostasis in chronic GVHD. Blood. 2015;125(11):1703–1707. doi: 10.1182/blood-2014-12-567834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Masson A, Bouaziz JD, Le Buanec H, et al. CD24(hi)CD27+ and plasmablast-like regulatory B cells in human chronic graft-versus-host disease. Blood. 2015;125(11):1830–1839. doi: 10.1182/blood-2014-09-599159. [DOI] [PubMed] [Google Scholar]
- 12.Allen JL, Fore MS, Wooten J, et al. B cells from patients with chronic GVHD are activated and primed for survival via BAFF-mediated pathways. Blood. 2012;120(12):2529–2536. doi: 10.1182/blood-2012-06-438911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JL, Tata PV, Fore MS, et al. Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. 2014;123(13):2108–2115. doi: 10.1182/blood-2013-10-533562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103(1):353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105(7):2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108(2):756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur R, Ebeling S, Hagenbeek A. B-cell involvement in chronic graft-versus-host disease. Haematologica. 2008;93(11):1702–1711. doi: 10.3324/haematol.13311. [DOI] [PubMed] [Google Scholar]
- 18.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122(8):1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192(11):1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192(11):1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34(1):108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62(1):234–244. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 24.Boswell KL, Paris R, Boritz E, et al. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathog. 2014;10(1):e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locci M, Havenar-Daughton C, Landais E, et al. International AIDS Vaccine Initiative Protocol C Principal Investigators. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39(4):758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bentebibel SE, Lopez S, Obermoser G, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5(176):176ra32. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herati RS, Reuter MA, Dolfi DV, et al. Circulating CXCR5+PD-1+ response predicts influenza vaccine antibody responses in young adults but not elderly adults. J Immunol. 2014;193(7):3528–3537. doi: 10.4049/jimmunol.1302503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Venables WN, Ripley BD. Modern Applied Statistics with S-Plus. 2nd ed. New York, NY: Springer; 1998. [Google Scholar]
- 30.Feng X, Wang D, Chen J, et al. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLoS One. 2012;7(12):e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakasone H, Tian L, Sahaf B, et al. Allogeneic HY antibodies detected 3 months after female-to-male HCT predict chronic GVHD and nonrelapse mortality in humans. Blood. 2015;125(20):3193–3201. doi: 10.1182/blood-2014-11-613323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199(8):1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porcheray F, Miklos DB, Floyd BH, et al. Combined CD4 T-cell and antibody response to human minor histocompatibility antigen DBY after allogeneic stem-cell transplantation. Transplantation. 2011;92(3):359–365. doi: 10.1097/TP.0b013e3182244cc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Festa ED, Hankiewicz K, Kim S, et al. Serum levels of CXCL13 are elevated in active multiple sclerosis. Mult Scler. 2009;15(11):1271–1279. doi: 10.1177/1352458509107017. [DOI] [PubMed] [Google Scholar]
- 35.Schiffer L, Kümpers P, Davalos-Misslitz AM, et al. B-cell-attracting chemokine CXCL13 as a marker of disease activity and renal involvement in systemic lupus erythematosus (SLE). Nephrol Dial Transplant. 2009;24(12):3708–3712. doi: 10.1093/ndt/gfp343. [DOI] [PubMed] [Google Scholar]
- 36.Schmitt N, Ueno H. Blood Tfh cells come with colors. Immunity. 2013;39(4):629–630. doi: 10.1016/j.immuni.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5⁺ CD4⁺ T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39(4):770–781. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Choi YS, Kageyama R, Eto D, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34(6):932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cubas RA, Mudd JC, Savoye AL, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med. 2013;19(4):494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chevalier N, Jarrossay D, Ho E, et al. CXCR5 expressing human central memory CD4 T cells and their relevance for humoral immune responses. J Immunol. 2011;186(10):5556–5568. doi: 10.4049/jimmunol.1002828. [DOI] [PubMed] [Google Scholar]
- 41.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26(1):677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murase K, Kim HT, Bascug OR, et al. Increased mitochondrial apoptotic priming of human regulatory T cells after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99(9):1499–1508. doi: 10.3324/haematol.2014.104166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarron MJ, Marie JC. TGF-β prevents T follicular helper cell accumulation and B cell autoreactivity. J Clin Invest. 2014;124(10):4375–4386. doi: 10.1172/JCI76179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klimatcheva E, Pandina T, Reilly C, et al. CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16(1):6. doi: 10.1186/s12865-015-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra43. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]