Abstract
Purpose
Both tyrosine kinase inhibitors targeting the vascular endothelial growth factor (VEGF) receptor and bevacizumab, a monoclonal antibody targeting VEGF, have antitumor activity in neuroendocrine tumors (NETs). Temozolomide, an oral analog of dacarbazine, also has activity against NETs when administered alone or in combination with other agents. We performed a phase II study to evaluate the efficacy of temozolomide in combination with bevacizumab in patients with locally advanced or metastatic NETs.
Patients and Methods
Thirty-four patients (56% with carcinoid, 44% with pancreatic NETs) were treated with temozolomide 150 mg/m2 orally per day on days 1 through 7 and days 15 through 21, together with bevacizumab at a dose of 5 mg/kg per day intravenously on days 1 and 15 of each 28-day cycle. All patients received prophylaxis against Pneumocystis carinii and varicella zoster. Patients were followed for toxicity, biochemical and radiologic response, and survival.
Results
The combination of temozolomide and bevacizumab was associated with anticipated grade 3 to 4 toxicities, including lymphopenia (53%) and thrombocytopenia (18%). Although the overall radiographic response rate was 15% (five of 34), response rates differed between patients with pancreatic NETs (33%; five of 15) and those with carcinoid tumors (zero of 19). The median progression-free survival was 11.0 months (14.3 months for pancreatic NETs v 7.3 months for carcinoid tumors). The median overall survival was 33.3 months (41.7 months for pancreatic NETs v 18.8 months for carcinoid tumors).
Conclusion
Temozolomide and bevacizumab can be safely administered together in patients with advanced NETs, and the combination regimen appears promising for patients with pancreatic NETs. Studies evaluating the relative contributions of these two agents to the observed antitumor activity are warranted.
INTRODUCTION
Recent randomized studies1,2 have demonstrated improvements in progression-free survival (PFS) in patients with advanced pancreatic neuroendocrine tumors (NETs) treated with everolimus or sunitinib. Response rates associated with both of these agents, however, are relatively modest. In addition, there remains no standard treatment for patients with advanced carcinoid tumors.
Although cytotoxic chemotherapy regimens that use streptozocin and dacarbazine are associated with modest antitumor activity in patients with advanced carcinoid tumors and pancreatic NETs, their use has been limited because of toxicity concerns.3–7 Temozolomide was developed as a less toxic alternative to dacarbazine and has demonstrated activity in NETs in both retrospective and prospective studies.8–10 Overall response rates associated with temozolomide, administered alone or in combination with other agents, range from 8% to 70% in patients with pancreatic NETs; response rates in carcinoid tumors have generally been lower.
Bevacizumab, a monoclonal antibody targeting vascular endothelial growth factor, has been evaluated in advanced carcinoid tumors in two phase II studies. In a randomized phase II study of patients with advanced carcinoid tumors,11 treatment with bevacizumab was associated with an objective response rate of 18% and a trend toward improved PFS compared with interferon alfa. A subsequent phase II study12 combined bevacizumab with 2-methoxyestradiol, a putative angiogenesis inhibitor, in patients with advanced carcinoid tumors. Although no confirmed radiologic responses by Response Evaluation Criteria in Solid Tumors (RECIST) were observed, 68% of evaluable patients experienced some degree of tumor reduction with a promising median PFS duration of 11.3 months.
Given the reported activity of both agents in carcinoid tumors and pancreatic NETs, we conducted a multi-institutional phase II study to assess the safety and efficacy of temozolomide given with bevacizumab in patients with advanced NETs.
PATIENTS AND METHODS
The study population consisted of patients with histologically confirmed, metastatic or locally unresectable NETs, excluding small-cell carcinoma. Patients were required to have measurable disease by RECIST; Eastern Cooperative Oncology Group (ECOG) performance status of 2 or better; life expectancy of at least 12 weeks; and adequate hepatic, renal, and bone marrow function.
Prior systemic treatment (excluding temozolomide, dacarbazine, bevacizumab) and prior local therapy (chemoembolization, radiation, cryotherapy) were permitted if completed 4 or more weeks before initiation of the trial. Prior therapy with anti–vascular endothelial growth factor pathway inhibitors was permitted. Lesions previously treated with radiation, cryotherapy, or chemoembolization were not considered measurable disease; concurrent treatment with these modalities was not permitted. Exclusion criteria were proteinuria ≥ 2 g/24 hours, clinically significant cardiovascular disease, major surgery within 28 days before study initiation, clinically apparent CNS metastases, or other severe or uncontrolled medical or psychiatric illness. All patients provided signed, informed consent as required by the institutional review boards of their institutions. Participating centers were Beth Israel Deaconess Medical Center, Brigham and Women's Hospital, Dana-Farber Cancer Institute, and Massachusetts General Hospital (all tertiary care centers in Boston, MA).
Treatment Program
Temozolomide was administered orally at a starting dose of 150 mg/m2 per day on days 1 through 7 and days 15 through 21. Bevacizumab was administered intravenously at a dose of 5 mg/kg per day on days 1 and 15. This cycle was repeated every 28 days. Dose adjustments for temozolomide were made for hematologic toxicity. Temozolomide was held if patients developed an absolute neutrophil count less than 1,000/μL or a platelet count less than 50,000/μL. On recovery above these parameters, temozolomide was resumed with dose reduction by 50 mg/m2. Continued treatment with bevacizumab was permitted if temozolomide was delayed for hematologic toxicity. Treatment with temozolomide and bevacizumab was held for all nonhematologic treatment-related toxicities grade ≥ 3 according to the National Cancer Institute Common Toxicity Criteria, version 3. Treatment with both agents resumed with dose reduction if nonhematologic toxicities recovered to grade ≤ 1 within 3 weeks. If nonhematologic toxicity did not recover within 3 weeks or if the patient experienced an unacceptable toxicity, study treatment was discontinued.
To protect against temozolomide-related selective lymphopenia and risk of opportunistic infection, patients received prophylaxis against Pneumocystis carinii (PCP) with double-strength trimethoprim-sulfamethoxazole, one tablet orally every Monday, Wednesday, and Friday. Patients with allergies to trimethoprim-sulfamethoxazole received an alternate prophylaxis regimen. Patients also received prophylaxis against varicella zoster with acyclovir 400 mg orally three times per day while receiving protocol therapy.
Radiologic tumor assessments with computed tomography scan and biochemical assessments with plasma chromogranin A levels were performed at baseline and every two cycles after initiation of treatment. Radiologic response was classified according to RECIST. Biochemical response for patients with an increased baseline chromogranin A was defined as a decrease in chromogranin A by 50% or more from baseline.
Statistical Methods
The primary objective of this study was to determine the radiographic response rate for the combination of temozolomide and bevacizumab in patients with NETs. Secondary objectives included assessment of PFS, biochemical response, toxicity, and overall survival (OS).
Patients who underwent restaging scans after completing two cycles of therapy were evaluated for radiologic response. Patients with increased chromogranin A levels at baseline who had follow-up assessment of chromogranin A were evaluated for biochemical response. PFS was defined as the time between study enrollment and progression of disease by RECIST or death while on protocol; patients who withdrew from the study for reasons other than progression or death were censored at the time of discontinuation of study therapy. Patients were followed for survival through July 2010. OS was defined as the time between study enrollment and death. Both PFS and OS were estimated by the Kaplan-Meier method with intention-to-treat analysis. Toxicity assessments were based on reports of adverse events, physical examinations, and laboratory assessments.
Power calculations were based on a phase II two-stage design. A total of 34 eligible patients (defined as receiving at least one dose of therapy) were entered onto the study in a two-stage design. Seventeen patients were entered in the first stage; one response was required to enroll an additional 17 patients onto the second stage of the study. With this design, the probability of terminating the study after 17 patients were recruited was 0.42 if the true but unknown response rate was 5% but 0.06 if the true but unknown response rate was 15%. The study had an overall power of 0.87 and overall type I error of 0.22. The trial was designed for a combined analysis of patients with carcinoid tumors and pancreatic NETs on the basis of standard study design at the time the trial was initiated. Response rates and survival durations were analyzed for the entire cohort and separately in patients with carcinoid tumors and pancreatic NETs. However, the study was not powered for separate analysis of each group. Statistical analyses were conducted by using SAS version 9.2 (SAS Institute, Cary, NC). Statistical significance was determined by a P value less than .05.
RESULTS
Patient Characteristics
Between November 2004 and July 2005, a total of 34 patients enrolled onto the study. Baseline characteristics of the patients are listed in Table 1. The median age of the patient population was 60 years, and 56% were male. The majority of patients had an ECOG performance status of 0 or 1 (94%). Nineteen patients had carcinoid tumors (56%), and 15 (44%) had pancreatic NETs. Although patients with small-cell neuroendocrine carcinoma were excluded, three patients with pancreatic NETs had poorly differentiated or high-grade histology. All but seven patients had evidence of radiographic disease progression before initiation of therapy. Seven patients (21%) had received prior cytotoxic chemotherapy. Twenty patients (59%) had received prior therapy with octreotide, and 12 patients (35%) received octreotide concurrent with study therapy. Twenty patients (nine with pancreatic NETs and 11 with carcinoid tumors) had increased chromogranin A levels (> 39 ng/mL) at baseline. The median chromogranin A level at baseline was 175 ng/mL, with a range of 5 to 26,800 ng/mL. Nine patients with carcinoid tumors had increased 24-hour urinary 5-hydroxyindoleacetic acid levels at baseline (> 6 mg/24 hours); the median 5-hydroxyindoleacetic acid level of these nine patients was 122.3 mg/24 hours (range, 8.5 to 352.1 mg/24 hours).
Table 1.
Baseline Patient Characteristics
| Characteristic | No. of Patients (N = 34) | % |
|---|---|---|
| Age, years | ||
| Median | 60 | |
| Range | 36-74 | |
| Sex | ||
| Male | 19 | 56 |
| Female | 15 | 44 |
| ECOG performance status | ||
| 0 | 12 | 35 |
| 1 | 20 | 59 |
| 2 | 2 | 6 |
| Type of tumor | ||
| Carcinoid | 19 | 56 |
| Appendix | 1 | 3 |
| Small bowel/likely midgut | 7 | 21 |
| Bronchial | 4 | 12 |
| Unknown primary | 7 | 21 |
| Pancreatic neuroendocrine tumor | 15 | 44 |
| Concurrent octreotide use | 12 | 35 |
| Evidence of radiographic disease progression prior to treatment initiation | 27 | 79 |
| Prior treatment* | ||
| Octreotide | 20 | 59 |
| Embolization | 11 | 32 |
| Chemotherapy† | 7 | 21 |
| Sunitinib | 6 | 18 |
| Radiofrequency ablation | 3 | 9 |
| Interferon | 3 | 9 |
| Radiation | 4 | 12 |
| No. of prior systemic therapy regimens‡ | ||
| 0 | 19 | 56 |
| 1 | 13 | 38 |
| 2 or more | 2 | 6 |
| Patients with increased baseline chromogranin A (> 39 ng/mL) | 20 | 59 |
| Baseline chromogranin A, ng/mL | ||
| Median | 175 | |
| Range | 5-26,800 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Some patients received more than one prior therapy; therefore, percentages do not add to 100%.
Prior chemotherapy regimens: platinum-etoposide (n = 3), docetaxel (n = 2), capecitabine (n = 1), streptozocin-doxorubicin (n = 1), cisplatin-irinotecan (n = 1), carboplatin-etoposide-paclitaxel (n = 1).
Not including octreotide.
Duration of Therapy
Patients received a median of five 4-week treatment cycles (range, 1 to 39 cycles). Disease progression was the most common reason for treatment discontinuation; of the 16 patients who discontinued therapy because of progression, one died because of progressive disease, 10 had documented radiologic progression by RECIST, and five discontinued treatment because of clinical progression. An additional 10 patients discontinued treatment because of treatment-related toxicity: grade 3 thrombocytopenia (five patients), grade 3 neutropenia (two patients), infection (one patient), and fatigue (two patients). Treatment discontinuation for toxicity occurred after at least 3.4 months of therapy, with a median time to discontinuation for these patients of 6.5 months (range, 3.4 to 14.7 months). Eight patients discontinued treatment after withdrawing consent, including one patient who wished to continue treatment elsewhere and three who withdrew consent despite prolonged stable disease while on study.
Toxicity
All thirty-four treated patients were assessable for toxicity, as summarized in Table 2. The most common grade 3 or 4 toxicities were lymphopenia (53%) and thrombocytopenia (18%). Lymphopenia generally developed in the absence of significant leukopenia or neutropenia.
Table 2.
Treatment-Related Toxicity
| Toxicity | Maximum Toxicity Grade |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic | ||||||||
| Hemoglobin | 11 | 32 | 4 | 12 | ||||
| Leukocytes | 6 | 18 | 7 | 21 | 3 | 9 | ||
| Neutrophils | 3 | 9 | 5 | 15 | 1 | 3 | 1 | 3 |
| Lymphocytes | 1 | 3 | 2 | 6 | 14 | 41 | 4 | 12 |
| Platelets | 14 | 41 | 3 | 9 | 6 | 18 | ||
| Nonhematologic | ||||||||
| Nausea | 17 | 50 | 4 | 12 | 2 | 6 | ||
| Fatigue | 14 | 41 | 10 | 29 | 2 | 6 | ||
| Anorexia | 10 | 29 | 5 | 15 | ||||
| Vomiting | 11 | 32 | 8 | 24 | 3 | 9 | ||
| Diarrhea | 9 | 26 | 1 | 3 | ||||
| Increased alkaline phosphatase | 8 | 24 | 1 | 3 | ||||
| Constipation | 7 | 21 | 3 | 9 | 1 | 3 | ||
| Mucositis | 5 | 15 | ||||||
| Dyspnea | 5 | 15 | 2 | 6 | ||||
| Weight loss | 5 | 15 | 1 | 3 | ||||
| Headache | 5 | 15 | 2 | 6 | ||||
| Increased PTT | 5 | 15 | ||||||
| Proteinuria | 4 | 12 | 1 | 3 | 1 | 3 | ||
| Pruritis | 4 | 12 | 1 | 3 | ||||
| Fever | 4 | 12 | ||||||
| Epistaxis | 4 | 12 | 1 | 3 | ||||
| Anxiety | 3 | 9 | 1 | 3 | ||||
| Hypertension | 3 | 9 | 4 | 12 | 1 | 3 | ||
| Increased AST | 3 | 9 | 2 | 6 | ||||
| Increased ALT | 2 | 6 | 1 | 3 | 1 | 3 | ||
| Increased INR | 3 | 9 | ||||||
| Abdominal pain | 2 | 6 | 2 | 6 | ||||
| Elevated bilirubin | 1 | 3 | ||||||
| Skin rash | 1 | 3 | 2 | 6 | ||||
| Hyponatremia | 1 | 3 | 1 | 3 | ||||
| Dehydration | 1 | 3 | 1 | 3 | ||||
Abbreviations: INR, international normalized ratio; PTT, partial thromboplastin time.
A total of five patients developed infections while receiving study treatment. One patient who received concurrent steroids developed a Mycobacterium avium intracellulare complex opportunistic infection that was successfully treated. Four patients developed upper respiratory infections. However, no documented cases of PCP or varicella zoster were reported.
The most common nonhematologic adverse events were fatigue (76%), nausea (68%), vomiting (65%), anorexia (44%), constipation (32%), and diarrhea (29%). Most toxicities were relatively mild. Grade 3 or higher toxicities occurring in more than one patient were vomiting (three patients), nausea (two patients), and fatigue (two patients). Treatment-related hypertension developed in eight patients (24%). Proteinuria developed in six patients (18%), including one with grade 3 proteinuria.
Efficacy
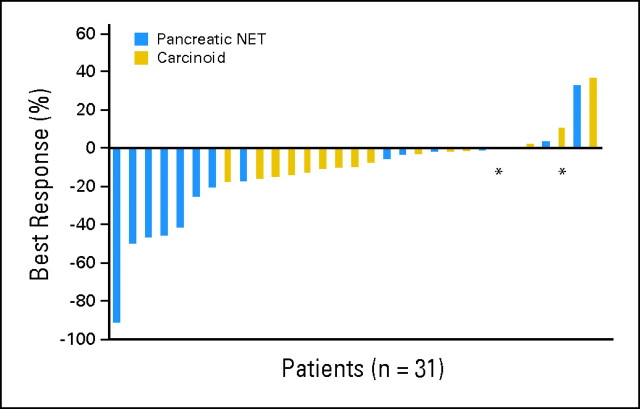
Of the 34 patients treated on the study, 31 were radiographically assessable for treatment response (Table 3). Using RECIST, five patients (15%; 95% CI, 7% to 30%) experienced partial radiographic responses as the best response to treatment, including one patient with a near complete response. All five of these patients had pancreatic NETs. Twenty-two patients (65%; 95% CI, 48% to 79%) experienced stable disease, and four (12%; 95% CI, 5% to 27%) experienced progressive disease as the best response (Fig 1). By using a waterfall plot analysis, 24 patients experienced some degree of tumor shrinkage as the best response to treatment, including 12 patients with pancreatic NETs (80%; 95% CI, 54% to 93%) and 12 patients with carcinoid tumors (63%; 95% CI, 41% to 81%).
Table 3.
Radiographic Tumor Response (RECIST)
| Disease Response | No. of Patients (N = 34) | % | 95% CI | No. Among Patients With Pancreatic NETs (n = 15) | % | 95% CI | No. Among Patients With Carcinoid Tumors (n = 19) | % | 95% CI |
|---|---|---|---|---|---|---|---|---|---|
| Partial response | 5 | 15 | 7 to 30 | 5 | 33 | 15 to 59 | 0 | 0 | |
| Stable disease | 22 | 65 | 48 to 79 | 8 | 53 | 30 to 75 | 14 | 74 | 51 to 88 |
| Progressive disease | 4 | 12 | 5 to 27 | 2 | 13 | 4 to 38 | 2 | 11 | 3 to 32 |
| Not evaluable* | 3 | 0 | 3 |
Abbreviations: NET, neuroendocrine tumor; RECIST, Response Evaluation Criteria in Solid Tumors.
Three patients with carcinoid tumor were not evaluable for response: one patient withdrew consent before restaging scans, one patient discontinued treatment to undergo surgery, and one patient died as a result of disease before restaging scans.
Fig 1.
Best objective radiographic tumor response. (*) Patients who experienced progressive disease on the basis of new lesions. NET, neuroendocrine tumor.
Seven patients with pancreatic NETs had increased baseline chromogranin A levels and were assessable for chromogranin A response (Table 4). Of these patients, the best biochemical response for four (57%) was a chromogranin A decrease of more than 50%; for two (29%), it was a stable chromogranin A level (< 50% decrease or < 25% increase); and for one (14%), it was progressive chromogranin A level. Three of the four patients with chromogranin A response had partial radiographic responses, including one patient with an insulin-producing tumor whose glucose levels improved after starting therapy. However, the patient was also receiving concurrent diazoxide and octreotide. The other patients with biochemical response did not have functional tumors. Nine patients with carcinoid tumors had increased baseline chromogranin A levels and were evaluable for chromogranin A response. None experienced more than 50% decrease in chromogranin A, seven (78%) experienced stable chromogranin A levels, and two (22%) experienced progressive chromogranin A levels as their best response to treatment.
Table 4.
Biochemical Response
| Disease Response | No. of Evaluable Patients* (n = 16) |
No. Among Patients With Pancreatic NETs (n = 7) |
No. Among Patients With Carcinoid Tumors (n = 9) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| > 50% decline in chromogranin A | 4 | 25 | 4 | 57 | 0 | |
| < 50% decline to < 25% increase in chromogranin A | 9 | 56 | 2 | 29 | 7 | 78 |
| > 25% increase in chromogranin A | 3 | 19 | 1 | 14 | 2 | 22 |
Abbreviation: NET, neuroendocrine tumor.
Twenty patients had elevated baseline chromogranin A levels. Four of these patients did not have follow-up assessment of chromogranin A. The 16 evaluable patients consisted of those with baseline increase in chromogranin A who were subsequently evaluable for biochemical response.
The median follow-up time for the patient cohort was 28.7 months (range, 1 to 65 months). Eleven patients (32%) developed progressive disease while receiving study therapy. The median PFS for the entire cohort was 11.0 months (95% CI, 7.3 months to not estimable [NE] upper limit). As we observed with tumor responses, we also observed a difference in PFS according to tumor type. The median PFS was 14.3 months (95% CI, 8.5 months to NE) for patients with pancreatic NETs and 7.3 months (95% CI, 3.9 months to NE) for patients with carcinoid tumors (P = .23; Fig 2A). The proportion of patients without progression at 6 months was 76% (95% CI, 67% to 84%) for the entire cohort; for pancreatic NETs, 6-month PFS was 87% (95% CI, 78% to 95%) compared with 66% (95% CI, 53% to 79%) for carcinoid tumors. The median OS was 33.3 months (95% CI, 13.4 to 41.7 months). The median OS also differed by subtype: 41.7 months for pancreatic NETs (95% CI, 23.6 months to NE) and 18.8 months for carcinoid tumors (95% CI, 8.5 to 36.1 months; P = .07; Fig 2B).There was no significant association between PFS and concurrent octreotide use. The median PFS for patients receiving concurrent octreotide was 25.3 months compared with 39.9 months for patients not receiving octreotide (P = .18). Similarly, there was no correlation between OS and concurrent octreotide use. We also found no significant difference in median PFS between patients who had and who had not experienced evidence of prior progression (14.3 months v 8.5 months, respectively; P = .51).
Fig 2.
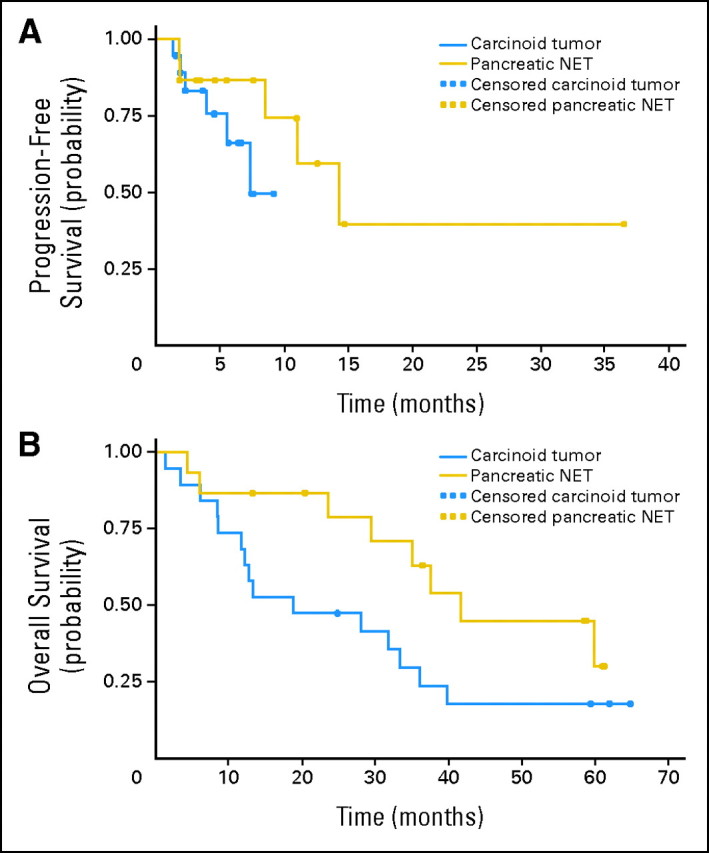
(A) Progression-free survival, and (B) overall survival. NET, neuroendocrine tumor.
DISCUSSION
This phase II study demonstrates that the combination of temozolomide and bevacizumab has antitumor activity in advanced NETs. Consistent with prior studies, response rates were higher among patients with pancreatic NETs (33%) than in those with carcinoid tumors (0%). Notably, PFS was also longer in patients with pancreatic NETs (14.3 months) than in those with carcinoid tumors (7.3 months). The shorter-than-expected PFS and OS durations in patients with carcinoid tumors may reflect a more heavily pretreated patient population.
Our observation of higher response rates in pancreatic NETs compared with carcinoid tumors is consistent with other studies of alkylating agents in NETs. Streptozocin-based therapy has been associated with responses of 39% to 69% in patients with pancreatic NETs.4,5 In contrast, radiographic response rates associated with streptozocin-based regimens in patients with carcinoid tumors have ranged between 16% and 33%.3,7,13 The combination of temozolomide and thalidomide was also found to be more active in pancreatic NETs than in carcinoid tumors.9 This observation was confirmed in a large retrospective series in which the radiographic response rate to temozolomide-based regimens was 34% in pancreatic NETs and only 2% in carcinoid tumors. In that study,14 the activity of temozolomide-based therapy in NETs was associated with the absence of the DNA repair protein O6-methylguanine methyltransferase.
In a recent retrospective series of patients with advanced pancreatic NETs, the combination of temozolomide and capecitabine was associated with radiographic response in 21 (70%) of 30 patients.10 Although the response rate reported in this study is higher than what we observed and what has been reported in other studies of temozolomide-based therapy in NETs,8,9 the findings warrant further evaluation in a prospective, randomized trial.
The relative contribution of bevacizumab to the activity observed in our study is uncertain. Bevacizumab has not been prospectively evaluated previously in pancreatic NETs but has demonstrated preliminary evidence of activity in carcinoid tumors. A recent study12 of bevacizumab and 2-methoxyestradiol reported a high rate of tumor stabilization and an encouraging PFS duration in carcinoid tumors but did not report any objective responses as defined by RECIST. Recent randomized studies1,2 of sunitinib or everolimus in pancreatic NETs showed a similar pattern of low radiographic response rates but high rates of disease stabilization translating into improved PFS. Although based on relatively small numbers, the PFS duration of 14.3 months observed in our study for pancreatic NETs compares favorably with the PFS observed in the randomized studies of everolimus and sunitinib: 11 and 11.4 months, respectively. Whether the encouraging PFS duration in pancreatic NETs in our study is attributable to temozolomide alone or to the addition of bevacizumab is uncertain; a randomized study investigating this question is warranted.
Temozolomide has been safely and effectively administered with bevacizumab in several different dosing regimens. Glioma studies have generally used a 5-day per every 4 weeks temozolomide dosing regimen,15 whereas a recent melanoma study used a dose-intense regimen similar to that in our study.16 There has also been variability in temozolomide regimens in trials for NETs. Although a prior phase II trial of temozolomide and thalidomide used the dose-intense regimen of temozolomide,9 a study of temozolomide with capecitabine used the 5-day per month regimen.10 Although no patients developed PCP or varicella zoster in our study, five patients developed other infections, typically after more than 6 months of therapy. Therefore, future studies may minimize infectious complications by limiting exposure to dose-intense temozolomide to ≤ 6 months. Alternatively, the less immunosuppressive nature of the monthly dosing regimen of temozolomide may allow therapy without interruption. Although 10 patients in this study discontinued therapy because of treatment-related adverse events, the toxicities leading to discontinuation were primarily reversible hematologic adverse events. Trials comparing dose-intense versus standard temozolomide regimens would help clarify the relative efficacy and safety of these dosing schedules for patients with NETs.
Several limitations of our study deserve comment. First, the number of patients limits statistical comparison between patients with pancreatic NETs and carcinoid tumors. However, our observations are consistent with prior reports demonstrating higher response rates to temozolomide-based therapy in pancreatic NETs compared with carcinoid tumors. Second, our type I error rate of 22% is somewhat higher than the 10% to 20% observed in many randomized phase II studies.17 Although this could lead to a relatively high false-positive rate, our results are consistent with the results of prior studies showing activity of temozolomide-based therapy in NETs. Nonetheless, future studies are warranted to extend these findings. Third, there were differences among patients in the use of concurrent octreotide. Given that objective tumor shrinkage with octreotide is rare, it is unlikely that differential octreotide use explains observed differences in radiographic tumor response rate. Although the antiproliferative effects of octreotide might have affected PFS, we found no statistically significant difference in PFS depending on octreotide use.18 Fourth, although the majority of patients had well-differentiated tumors, there was heterogeneity in tumor histology: three patients with pancreatic NETs had poorly differentiated or high-grade histology. Although the radiographic response rate was higher in patients with pancreatic NETs, all patients with higher-grade histology had either stable disease or progressive disease as the best response to therapy. Finally, there was heterogeneity in disease activity before study enrollment because 21% did not demonstrate radiographic progression of disease before study entry. However, we found no significant difference in PFS between patients who had and had not experienced evidence of prior progression.
In summary, the combination of temozolomide and bevacizumab has activity in patients with advanced pancreatic NETs. Further studies examining the optimal dose regimen for temozolomide and the relative contributions of temozolomide and bevacizumab to the antitumor activity and PFS are warranted.
Footnotes
Supported by Genentech and Schering-Plough/Merck.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00137774.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Peter C. Enzinger, Genentech (C); Charles S. Fuchs, Genentech (C), Infinity Pharmaceuticals (C), Pfizer (C), Roche (C), sanofi-aventis (C) Stock Ownership: Jennifer A. Chan, Merck Honoraria: Peter C. Enzinger, Genentech Research Funding: Jennifer A. Chan, Bayer, Merck, Novartis, Onyx Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Matthew H. Kulke
Administrative support: Rebecca Miksad
Provision of study materials or patients: Keith Stuart, Craig C. Earle, Jeffrey W. Clark, Pankaj Bhargava, Rebecca Miksad, Lawrence Blaszkowsky, Peter C. Enzinger, Jeffrey A. Meyerhardt, Charles S. Fuchs, Matthew H. Kulke
Collection and assembly of data: Jennifer A. Chan, Keith Stuart, Craig C. Earle, Jeffrey W. Clark, Pankaj Bhargava, Rebecca Miksad, Lawrence Blaszkowsky, Peter C. Enzinger, Jeffrey A. Meyerhardt, Charles S. Fuchs, Matthew H. Kulke
Data analysis and interpretation: Jennifer A. Chan, Rebecca Miksad, Hui Zheng, Matthew H. Kulke
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engstrom PF, Lavin PT, Moertel CG, et al. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol. 1984;2:1255–1259. doi: 10.1200/JCO.1984.2.11.1255. [DOI] [PubMed] [Google Scholar]
- 4.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, stretpozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 6.Ramanathan RK, Cnaan A, Hahn RG, et al. Phase II trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma: Study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139–1143. doi: 10.1023/a:1011632713360. [DOI] [PubMed] [Google Scholar]
- 7.Sun W, Lipsitz S, Catalano P, et al. Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol. 2005;23:4897–4904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- 8.Ekeblad S, Sundin A, Janson ET, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.CCR-06-2053. [DOI] [PubMed] [Google Scholar]
- 9.Kulke MH, Stuart K, Enzinger PC, et al. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401–406. doi: 10.1200/JCO.2005.03.6046. [DOI] [PubMed] [Google Scholar]
- 10.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268–275. doi: 10.1002/cncr.25425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: A random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 12.Kulke MH, Chan JA, Meyerhardt JA, et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68:293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moertel CG, Hanley JA. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin Trials. 1979;2:327–334. [PubMed] [Google Scholar]
- 14.Kulke MH, Hornick JL, Frauenhoffer C, et al. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338–345. doi: 10.1158/1078-0432.CCR-08-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Moos R, Seifert B, Simcock M, et al. First-line temozolomide combined with bevacizumab in metastatic melanoma: A multicentre phase II trial (SAKK 50/07) Ann Oncol. 2012;23:531–536. doi: 10.1093/annonc/mdr126. [DOI] [PubMed] [Google Scholar]
- 17.Cannistra SA. Phase II trials in Journal of Clinical Oncology. J Clin Oncol. 2009;27:3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 18.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]