Abstract
Waldenström Macroglobulinemia (WM) is a proliferative disorder of IgM secreting, lymphoplasmacytoid cells that inhabit the lymph nodes and bone marrow. The disease carries a high prevalence of activating mutations in MyD88 (91%) and CXCR4 (28%). Because signaling through these pathways leads to Bcl-xL induction, we examined Bcl-2 family expression in WM patients and cell lines. Unlike other B-lymphocyte-derived malignancies, which become dependent on expression of anti-apoptotic proteins to counter expression of pro-apoptotic proteins, WM samples expressed both pro- and anti-apoptotic Bcl-2 proteins at low levels similar to their normal B-cell and plasma cell counterparts. Three WM cell lines expressed pro-apoptotic Bcl-2 family members Bim or Bax and Bak at low levels which determined their sensitivity to inducers of intrinsic apoptosis. In two cell lines, miR-155 upregulation, which is common in WM, was responsible for inhibition of FOXO3a and Bim expression. Both antagonizing miR-155 to induce Bim and proteasome inhibition increased the sensitivity to ABT-737 in these lines indicating a lowering of the apoptotic threshold. In this manner, treatments that increase pro-apoptotic protein expression increase the efficacy of agents treated in combination in addition to direct killing.
Keywords: Bcl-2 family, apoptosis, Waldenström Macroglobulinemia, miR-155
INTRODUCTION
Since the discovery of B-cell lymphoma-2 (Bcl-2), nearly three decades ago, the Bcl-2 family of proteins has been shown to regulate the process of apoptosis in all mammalian cells1. Bcl-2 family regulation of apoptosis culminates in the pro-apoptotic BH3-only activator proteins Bim, tBid and Puma transiently associating with and causing a conformational change in the multi-domain pro-apoptotic proteins Bax and Bak2. Upon their activation, Bax and Bak homo-oligomerize and initiate mitochondrial outer membrane permeabilization (MOMP)3. When Bim or tBid are present a cell must also express anti-apoptotic Bcl-2 family proteins to block apoptosis. The set of known anti-apoptotic Bcl-2 family proteins now includes Bcl-2, Bcl-xL, Mcl-1, Bcl-w, Bcl-b and A1/Bfl-1, which are capable of binding BH3-only activators as well as activated monomers of Bax and Bak4. Expression of these proteins is capable of blocking apoptosis provided they are expressed at levels in excess of pro-apoptotic proteins. This balance can be shifted, however, by the expression of pro-apoptotic BH3-only proteins termed sensitizers including Hrk, Bad, Bik, Bmf and Noxa. Sensitizers bind the anti-apoptotic Bcl-2 proteins with varying affinities and are capable of displacing Bim, tBid, Puma or activated Bax and Bak leading to MOMP and apoptosis5.
Processes of proliferation and differentiation induce death signals in the form of pro-apoptotic protein expression that are countered by survival signals that induce expression of anti-apoptotic proteins. For example, enforced expression of the proliferative oncogene c-myc leads to proliferation but also leads to apoptosis. However, co-expression of Bcl-2 or any other anti-apoptotic family member with c-myc rescues this cell death resulting in tumor formation6, 7. In this manner a cancer cell that breaks a differentiation or proliferation checkpoint must then compensate for the inherent activation of pro-apoptotic Bcl-2 family members with increased expression of anti-apoptotic family members. This has come to be known as mitochondrial priming in that cancer cells become primed for death by increased abundance of pro-apoptotic protein being sequestered by anti-apoptotic proteins5. In this way the apoptotic threshold of a cancer cell is lowered because it requires less death signaling to engage mitochondrial-dependent apoptosis. Furthermore, it has been shown that the level of priming of a variety of cancers and healthy tissues determines their response to various anti-cancer agents illustrating a basis for the therapeutic index seen in-vivo8.
Waldenström Macroglobulinemia (WM) is a low grade lymphoproliferative disorder characterized by clonal, lymphoplasmacytoid, IgM-secreting cells9, 10. The clonal cancer cells exist at the point of differentiation between a B-cell and plasma cell. Two activating mutations have been shown to be common in WM. The MyD88 (L265P) mutation is found in 91% of WM cases11, 12 and the CXCR4 (S338X) mutation is found in nearly a third of WM cases. Since both MyD88 and CXCR4 signaling lead to downstream activation of NF-κB which induces Bcl-xL, and since we have shown that differentiating plasma cells proceed through a Bcl-xL-dependent intermediate13, we hypothesized that WM cells are dependent on Bcl-xL for survival. In this study we examined the Bcl-2 protein expression in WM patient samples and observed that WM cells are characterized by low expression of both pro- and anti-apoptotic Bcl-2 family proteins. This is in sharp contrast with the plasma cell tumor, multiple myeloma (MM), which is characterized by increased expression of anti-apoptotic Bcl-2 family members to compensate for increased expression of Bim. These data provide evidence that the apoptotic threshold in WM cells is high due to low expression of pro-apoptotic Bcl-2 family members not due to high expression of anti-apoptotic proteins.
RESULTS
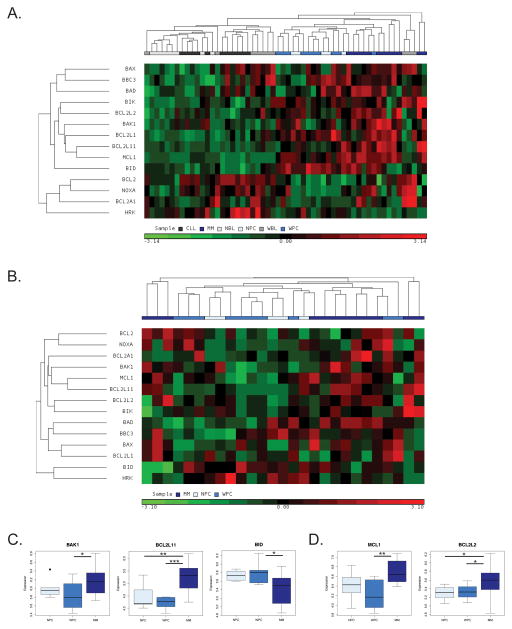
We examined Bcl-2 protein expression in a published expression database containing 10 WM patients along with 11 chronic lymphocytic leukemia (CLL) patients, 12 multiple myeloma (MM) patients, 8 normal B-cell (NBL) donors and 5 normal plasma cell (NPC) donors14. All patients in the study were newly diagnosed and untreated. The WM cells were separated pairwise by patient based on their B-cell-like (WBL) or plasma cell-like (WPC) phenotype. We performed an unsupervised hierarchical clustering of 14 Bcl-2 family genes in all samples (Figure 1A). Interestingly, these Bcl-2 family genes alone were sufficient to cluster the various cell types14. The greatest separation based on gene expression of the cell types was between the B-cell-like (NBL, CLL, WBL), and plasma cell-like (WPC, NPC, MM) groups indicating that Bcl-2 family expression is primarily driven by the state of differentiation, not transformation. We therefore split these groups and performed an unsupervised hierarchical clustering of these same 14 genes on the set of B-cell like or plasma cell like groups separately. In the B-cell-like group, we observed a pattern where NBL samples expressed lower levels of Bcl-2 proteins than CLL samples and WBL samples were split between being similar to NBL and CLL samples (Figure S1A). An exception to this was Bak which was underexpressed in WBL samples compared to CLL samples and Bid which was overexpressed in WBL samples compared to CLL samples (Figure S1B). Anti-apoptotic Bcl-2 was expressed at higher levels in both CLL and WBL samples compared to NBL samples while, interestingly, Bcl2A1 was overexpressed in WBL samples compared with both NBL and CLL samples (Figure S1C)
Figure 1.
Bcl-2 family clustering of B cell malignancies including WM. (A) Unsupervised hierarchical clustering of B-cell (CLL, WBL, and NBL) and Plasma-cell (MM, WPC, and NPC) based on expression data for 14 Bcl-2 family genes 14. (B) Unsupervised hierarchical clustering of plasma cell phenotype samples. (C) Pro-apoptotic Bcl-2 family proteins Bak (BAK1), Bim (BCL2L11) and Bid (BID) are differentially expressed in WPC samples compared to MM samples. (D) Anti-apoptotic Bcl-2 family members Mcl-1 (MCL-1) and Bcl-w (BCL2L2) are differentially expressed in WPC samples compared to MM samples. *p-value is calculated by Wilcoxon rank-sum test. (*p < .05, **p < .01, *p < .001)
We observed that WPC samples clustered more closely with NPC samples not MM samples and that in general WPC and NPC samples expressed lower levels of Bcl-2 family proteins than MM samples (Figure 1B). Analysis of specific genes revealed that pro-apoptotic Bcl-2 family members Bak and Bim were expressed at significantly lower levels in WPC samples compared to MM samples while Bid was expressed at higher levels in WPC than MM samples (Figure 1C). Furthermore, anti-apoptotic Bcl-2 family members Mcl-1 and Bcl-w were both expressed at significantly lower levels in WPC samples than MM samples (Figure 1D). All other Bcl-2 family proteins including Bcl-xL were expressed at levels not significantly different in WPC samples compared with either NPC or MM samples (Figure 1A and data not shown). These data demonstrate that plasma cell phenotype WM cells express Bcl-2 family proteins at levels more similar to normal plasma cells than MM which is indicative of WM cells having a higher apoptotic threshold.
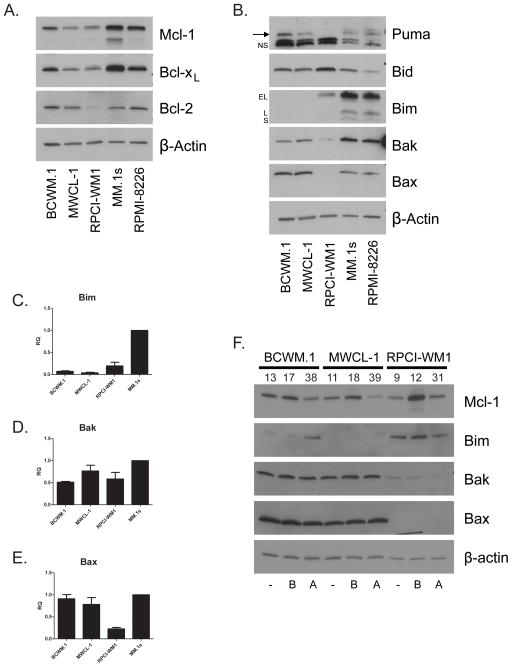
In order to determine the effects of Bcl-2 family protein expression on WM cell survival, we obtained three previously described, plasma cell phenotype WM cell lines, BCWM.1, MWCL-1 and RPCI-WM115–17. We then assessed Bcl-2 family protein expression in three WM cell lines compared to two MM cell lines. We observed low to moderate levels of the anti-apoptotic proteins Bcl-xL, Mcl-1 and Bcl-2, the last of which was nearly undetectable in RPCI-WM1 cells (Figure 2A). All three WM cell lines expressed Bid at higher levels than the MM cell lines and both BCWM.1 and MWCL-1 cells expressed moderate levels of Puma (Figure 2B). Strikingly, we observed marked defects in the expression of Bak, Bax and Bim in these cell lines (Figure 2B). BCWM.1 cells expressed Bim at a very low level and MWCL-1 cells expressed no detectable Bim. RPCI-WM1 cells expressed Bim at moderate levels compared to MM cell lines however expressed very little Bak and no detectable Bax. We then examined the mRNA expression of these genes in the cell lines and observed very low levels of Bim mRNA in both BCWM.1 and MWCL-1 cells (Figure 2C). While RPCI-WM1 cells displayed levels of Bak mRNA expression comparable to the other cell lines, only very low levels of Bax mRNA was detected indicating distinct mechanisms of down regulation of these two proteins (Figure 2D, E). We then examined the expression of Bim, Bak and Bax with bortezomib and arsenic trioxide (ATO) treatment. Bortezomib induces apoptosis in B cell malignancies and is used in the treatment of WM. ATO is not routinely used in the treatment of WM, and at the concentrations tested, activates apoptosis in a Bim-dependent manner in MM18. We observed that Bim was induced by ATO treatment in BCWM.1 but not MWCL-1 cells (Figure 2F). Bortezomib treatment did not increase protein expression of Bim, Bax or Bak in these cell lines indicating that the low expression was not due to increased proteasomal degredation. In contrast the proteasome substrate Mcl-1 was up-regulated by proteasome inhibition (Figure 2F). The expression of Bcl-2 family members in these WM cell lines indicate distinct mechanisms leading to a common defect in the intrinsic apoptotic pathway at the point of Bim activation of Bax and Bak.
Figure 2.
WM cell lines aberrantly express pro-apoptotic BH3 proteins. (A, B) Western blots were prepared from lysates of WM and MM cell lines. In (B), “→” denotes Puma band, “NS” denotes two non-specific bands. “EL”, “L” and “S” denote isoforms of Bim. (C – E) qRT-PCR was performed on RNA isolated from untreated WM cells. Data is presented as relative quantity (RQ) compared to MM.1s cells (1) normalized to GAPDH endogenous control. (F) WM cell lines were left untreated [−], treated with bortezomib [B] at 5 nM for BCWM.1 and MWCL-1 cells and 20 nM for RPCI-WM1 cells or arsenic trioxide [A] at 5 μM for BCWM.1 and MWCL-1 cells and 10 μM for RPCI-WM1 cells. Western Blots were prepared from lysates of the treated cells at 24 h. Numbers above individual lanes indicate % cell death of the sample.
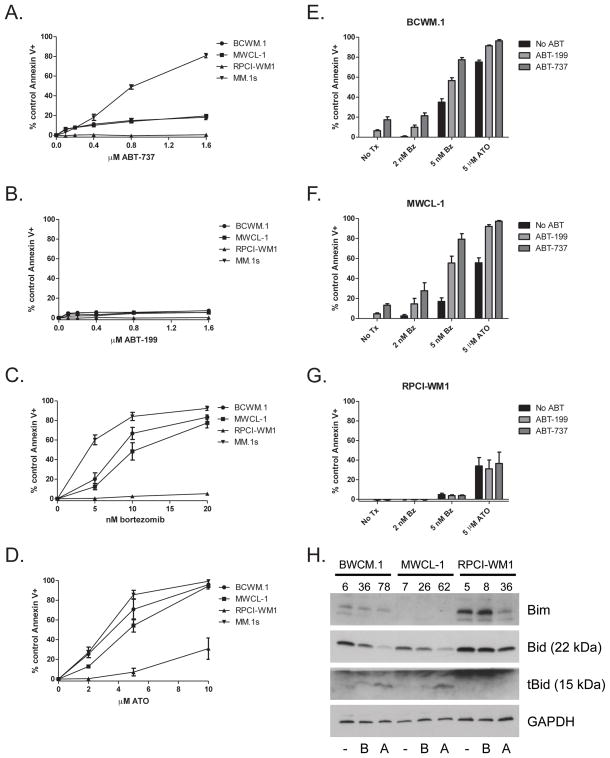
Given the deficiencies in proteins required to activate mitochondria-dependent apoptosis, we then determined the sensitivity of these cells to agents known to activate this pathway. We first examined sensitivity to the Bcl-2 and Bcl-xL inhibitor, ABT-73719. Importantly, treatment with ABT-737 alone, which relies on the priming of a cell to activate apoptosis, induced only very low levels of apoptosis in BCWM.1 and MWCL-1 cells as compared to the ABT-737-sensitive MM cell line MM.1s (Figure 3A). No detectable apoptosis was induced by ABT-737 treatment in RPCI-WM1 cells (Figure 3A). We also treated WM cells with ABT-199 which inhibits Bcl-2 alone19 and observed no sensitivity (Figure 3B). Treatment with the proteasome inhibitor, bortezomib induced moderate apoptosis in both BWCM.1 and MWCL-1 cells compared with MM.1s but once again induced no apoptosis in RPCI-WM1 cells (Figure 3C). Treatment with ATO, which induces mitochondria-dependent apoptosis at low concentrations, induced moderate levels of apoptosis in BCWM.1 and MWCL-1 cells and no apoptosis in RPCI-WM1 unless the concentration was increased to levels of toxicity (Figure 3D). Treatment with Dexamethasone induced no cell death in all three cell lines (not shown). Interestingly, treatment with bortezomib or ATO alone at a concentration that induced moderate apoptosis, induced more than additive amounts of apoptosis with co-treatment with ABT-737 or ABT-199 in both BCWM.1 and MWCL-1 cells yet induced no additional apoptosis in RPCI-WM1 cells (Figure 3E–G). Western blot analysis of BCWM.1 and MWCL-1 cell treated with either bortezomib or ATO displayed no changes in the expression of Bim, however the BH3 activator protein Bid is cleaved to tBid (Figure 3H).
Figure 3.
WM cell lines are insensitive to inducers of intrinsic apoptosis. WM cell lines were exposed to varying concentrations of ABT-737 (A), ABT-199 (B), bortezomib (C) or arsenic trioxide (ATO) (D) for 24 hours. Apoptosis was measured by annexin V and PI staining and flow cytometry. Bortezomib and ATO sensitize BCWM.1 and MWCL-1 but not RPCI-WM1 cells to ABT-737- and ABT-199-induced apoptosis. BCWM.1 (E), MWCL-1 (F) and RPCI-WM1 (G) cell lines were treated with bortezomib or ATO in the presence or absence of ABT-737 or ABT-199 for 24 hours. Apoptosis was measured by annexin V and PI staining and flow cytometry. Data are presented as the mean ± SE of three independent experiments. Western blots were performed on lysates prepared from cells treated for 24 hours with bortezomib [B], ATO [A] or left untreated [−]. Numbers above individual lanes indicate % cell death of the sample.
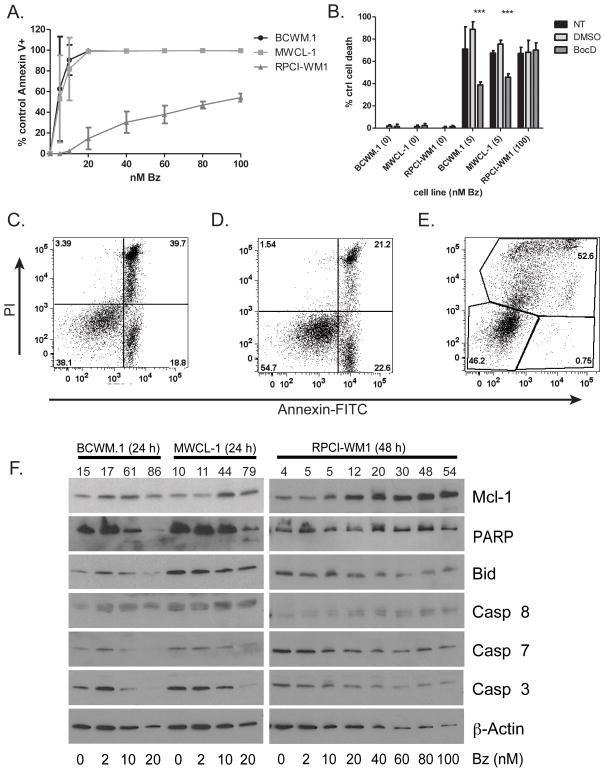
Since RPCI-WM1 cells were insensitive to agents that induced death in BCWM.1 and MWCL-1 cells, we treated RPCI-WM1 cells with up to 100 nM bortezomib for 48 hours and observed cell death (Figure 4A). We then treated the cells with bortezomib in the presence of the pan-caspase inhibitor BocD-FMK (BocD) in order to determine if this death was caspase dependent. In BCWM.1 and MWCL-1 cells the presence of BocD abrogated approximately 50% of the cell death whereas it was unable to block any cell death induced by high dose bortezomib in RPCI-WM1 cells (Figure 4B). Furthermore, the annexin-V and PI staining pattern of both bortezomib treated BCWM.1 and MWCL-1 cells is indicative of apoptosis with cells becoming annexin-V positive and then annexin-V and PI double positive, while the staining pattern for RPCI-WM1 cells treated with bortezomib is not indicative of apoptosis with cells becoming PI positive and then PI and annexin-V double positive (Figure 4C–E). Examination of apoptosis-related proteins by western blot indicated the loss of full length Caspases 3 and 7 and Bid as well as PARP cleavage in BCWM.1 and MWCL-1 cells but not in RPCI-WM1 cells (Figure 4F). Together these data show BCWM.1 and MWCL-1 cells, though insensitive to agents acting through a Bim-dependent mechanism such as ABT-737 and Dexamethasone, are capable of undergoing apoptosis with bortezomib treatment while RPCI-WM1 cells are insensitive to all these agents and are completely apoptosis deficient.
Figure 4.
BCWM.1 and MWCL-1 cells but not RPCI-WM1 cells activate apoptosis during cell death. (A) WM cell lines were treated with various concentrations of bortezomib for 48 h. (B) WM cell lines were treated with high dose bortezomib in the presence of BocD-fmk or vehicle for 48 hours. Cell death was measured by annexin V and PI staining and flow cytometry. Data in A and B are presented as the mean ± SE of three independent experiments. (***p < .001) (C–E) Representative FACS plots from WM cells treated with bortezomib. Apoptosis was determined for BCWM.1 (C) and MWCL-1 (D) cells as percent of control annexin-V+ events after treatment with 10 nm bortezomib for 24 hours. Cell death was determined in RPCI-WM1 (E) cells as percent of control PI+ events after treatment with 100 nM bortezomib for 48 h. (F) Western Blots were prepared from lysates of WM cell lines treated with indicated concentrations of bortezomib sufficient to induce cell death for 24 h (BCWM.1 and MWCL-1) or 48 h (RPCI-WM1). Numbers above individual lanes indicate % cell death of the sample.
In order to further investigate the mechanism of dysregulation of Bim, Bax and Bak in the WM cell lines, we first examined the genomic copy number data available in WM patient samples and the cell lines. Array-CGH analysis of the RPCI-WM1 cell line, the original donor patient sample and an age and sex-matched normal donor showed no genomic copy number variation of either Bak, Bax or Bim (Table S1 and not shown). We then examined a SNP copy number array of 46 WM patients at the loci for Bak, Bax and Bim. This data set contained only one copy number gain for each Bak and Bax and no losses in any of the three genes nor did it show evidence of uniparental disomy in these genes (Table S2). These data provide no evidence that low expression of Bax, Bak or Bim protein in either the cell lines or WM patients is due to genomic copy number variation.
With no evidence or changes in genomic copy number variation and no evidence of modulation in protein stability as proteasome inhibition did not cause a recovery of Bim, Bax or Bak protein in the cell lines (Figure 2F), we then examined mechanisms of epigenetic regulation of expression. We first examined the methylation state of promoter CpG islands in Bax, Bak and Bim in BCWM.1, MWCL-1 and RPCI-WM1 cells. PCR amplification of bisulfite treated samples from all three cell lines yielded only products of primers targeted to unmethylated DNA and therefore converted promoters (Figure S2A). Similarly, bisulfite sequencing of Bax and Bim promoters displayed no evidence of hypermethylation of CpG islands in the promoters of these genes in these WM cell lines (Figure S2B, C).
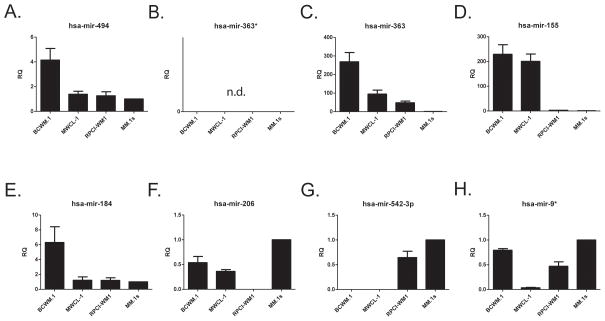
We next examined the microRNA expression in these cell lines. We first analyzed the set of seven commonly dysregulated microRNAs in WM20 for miRs targeting Bim, Bax and Bak and found that only miR-494 had a low probability 7mer site in the 3′ UTR of Bim21 (Figure S3A). While it was reported that miR-363* is upregulated in WM, its sister mature microRNA, miR-363 had two moderately strong target sites in the 3′ UTR of Bim so we added it to our analysis (Figure S3B). miR-494 was not expressed at higher levels in MWCL-1 cells than RPCI-WM1 cells and only slightly higher levels in BCWM.1 cells were detected (Figure 5A). Though miR-363* was undetectable in all three WM cell lines, miR-363 was expressed at high levels in BCWM.1 and to a lesser degree in both MWCL.1 and RPCI-WM1 (Figure 5B, C). Since the pattern of these two possible Bim targeting microRNAs did not fit the pattern of Bim expression in the three WM cell lines, we examined the rest of the commonly upregulated microRNAs (Figure 5D–G) and one downregulated microRNA (Figure 5H). Only the miR-155 expression pattern was consistent with suppression of Bim (Figure 5D). None of the set of commonly upregulated microRNAs had predicted targets in the 3′ UTRs of Bax or Bak (not shown) and none were expressed at higher levels in RPCI-WM1 than in Bax/Bak expressing cells (Figure 5).
Figure 5.
miR-155 is upregulated in BCWM.1 and MWCL-1 cells but not RPCI-WM1 cells. microRNA enriched RNA was prepared from untreated WM and MM cells and cDNA was prepared using a primer pool containing 8 microRNA and 4 small nuclear or non-coding RNA primers (endogenous controls). qPCR was then performed using probes for these 12 targets. Cycles to threshold were first normalized to the mean of the 4 endogenous controls and then to the MM.1s cell line. Data are presented as the mean ± SE of three independent experiments.
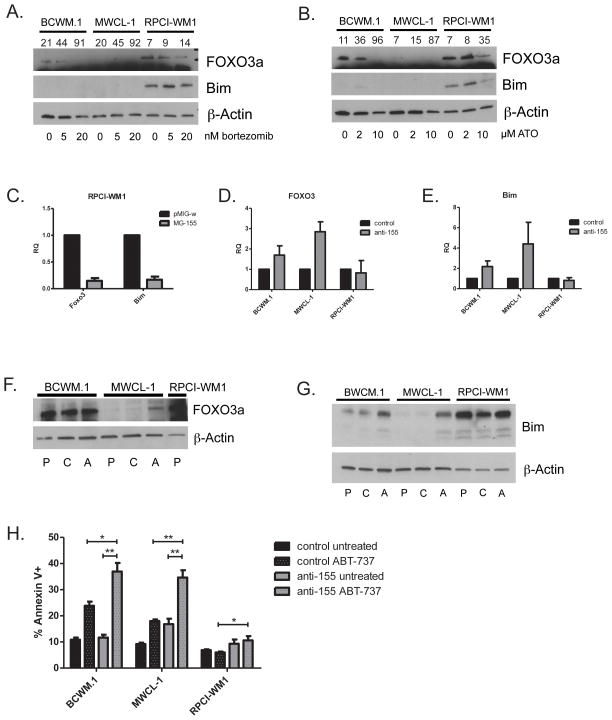
miR-155 has been shown to both directly and indirectly target the transcription factor FOXO3a of which Bim is a downstream target (Figure S3C)21–24. Furthermore, miR-155 has been shown to be important in WM xenograft survival, in addition to being overexpressed in WM patients20, 24. We therefore examined the expression of FOXO3a protein in the WM cell lines, with and without treatment with bortezomib and ATO, and observed that FOXO3a expression mirrored the Bim expression pattern (Figure 6A, B). To investigate miR-155 regulation of FOXO3a and Bim we retrovirally transduced RPCI-WM1 cells, which do not overexpress miR-155, with a miR-155 expression construct and observed greater than 80% reduction in expression of FOXO3a and Bim compared to the vector backbone (Figure 6C). Having observed miR-155 regulation of FOXO3a and Bim, we then used a lentiviral vector to stably deliver either an anti-miR targeting miR-155 or a control anti-miR to the three WM cell lines in order to determine its role in Bim expression control. In both BCWM.1 and MWCL-1 cells, along with increased doubling time (data not shown), we observed an increase in mRNA for FOXO3 and Bim in BWCM.1 and MWCL-1 cells (Figure 6D, E). While increased FOXO3a protein was observed only in MWCL-1 cells expressing anti-miR-155 (Figure 6F), incresed Bim protein expression was observed in both BCWM.1 and MWCL-1 cells expressing the anti-miR (Figure 6G). Expression of anti-miR-155 had no significant effect on expression of other direct activator BH3-only, multi-domain effector or anti-apoptotic Bcl-2 family members (Figure S4A, B). While there was no increased apoptosis in untreated anti-miR-155 expressing BCWM.1 or RPCI-WM1 cells there was a small but significant increase in apoptosis in MWCL-1 cells expressing anti-miR-155 (Figure 6H). We then treated these cells with ABT-737 and observed an increase in apoptosis in both BCWM.1 and MWCL-1 cells in the absence of any other death signal (Figure 6H). These data demonstrate that increased expression of miR-155 in BCWM.1 and MWCL-1 cells decreases expression of Bim and its transcription factor FOXO3a abrogating activation of intrinsic apoptosis.
Figure 6.
miR-155 regulates FOXO3a and Bim expression in WM cells. WM cell lines were treated with indicated doses of bortezomib (A) and ATO (B) for 24 hours. Western blots were prepared from samples collected at 24 h post treatment. miR-155 inhibits Bim expression. Numbers above individual lanes indicate % cell death of the sample. (C) RPCI-WM1 cells infected with retrovirus containing a miR-155 expression construct [MG-155] or empty vector backbone [pMIG-w] were sorted for GFP positivity. qRT-PCR was performed on RNA prepared from these cells. Data is presented as relative quantity (RQ) compared to the empty vector control (1) normalized to GAPDH endogenous control. (D, E) qRT-PCR was performed on RNA prepared from WM cell lines stably expressing either anti-miR-155 or control anti-miR. Data is presented as relative quantity (RQ) compared to control anti-miR (1) normalized to GAPDH endogenous control. (F, G) Western blots performed on protein prepared from untreated parental [P], control anti-miR [C] or anti-miR-155 [A] cells. (H) WM cells stably expressing either control anti-miR or anti-miR-155 were treated with 0.4 μM ABT-737 for 24 hours. Apoptosis was measured by annexin-V and PI staining and flow cytometry. Data in C, D, E and H are presented as the mean ± SE of three independent experiments. (*p < .05, **p < .01, *p < .001)
DISCUSSION
All cancer treatment requires a therapeutic index that dictates that the tumor cells will die before, and in greater numbers than the patient’s healthy tissues. It is sometimes acceptable to use therapy that targets the normal biology of the tumor as in the case of rituximab targeting CD20-expressing cells. Since the normal cells that express CD20 are not essential for life, targeting this biology has limited toxicity. However, most chemotherapeutic agents target all cells, yet kill cancer cells at lower concentrations than healthy cells. In the case of these treatments, the therapeutic index relies on the concept of mitochondrial priming8. This priming is the load of pro-apoptotic protein being sequestered in a given cell. The more primed a cell is the less death signaling it needs to cross the apoptotic threshold. On the whole, cancer cells exhibit higher priming due to breaking proliferative and differentiation checkpoints. In this study we examined the expression of Bcl-2 family proteins in Waldenström Macroglobulinemia and its effect on apoptotic threshold.
Based on our studies of plasma cell differentiation, we had hypothesized that a disease harboring the MyD88 (L265P) mutation would have increased expression of Bcl-xL acting as a protective oncogene13. At the very outset we observed that this was not the case in WM as patient gene expression data showed that Bcl-xL expression was at levels similar to normal tissues (Figure 1). Furthermore, treatment with ABT-737 or ABT-199 did not induce significant apoptosis in WM cell lines (Figure 2A) which would also suggest that there is not pro-apoptotic protein being sequestered by either Bcl-xL or Bcl-2. Both in patient expression data and in BCWM.1 and MWCL-1 cells we observed low expression of Bim (Figure 3, 4). In contrast, the RPCI-WM1 cell line expressed Bim at higher levels yet was deficient in both Bax and Bak which rendered it apoptosis-deficient in all conditions tested (Figure 3 – 5). Thus all three cell lines resist apoptosis through loss of pro-apoptotic proteins not upregulation of anti-apoptotic Bcl-2 family members. However, both BCWM.1 and MWCL-1 cells did undergo apoptosis provided that it was Bim-independent. Bortezomib treatment has been shown to induce Caspase-8-mediated cleavage of Bid which then synergizes with ABT-737 treatment25. We observed a reduction in full length Bid along with expression of tBid in both bortezomib and ATO treated cells which coincided with increased sensitivity to co-treatment with ABT-737 and ABT-199 (Figure 3) suggesting induction of dependence on both Bcl-xL and Bcl-2 for survival. In line with this observation, WM patient samples displayed Bid expression at levels similar to normal plasma cells and higher than MM patient samples (Figure 1B, D). However, Bid expressed in WM and normal plasma cell requires cleavage by Caspase-8 for full activity and thus does not contribute to mitochondrial priming without treatment.
Low levels of mitochondrial priming in WM implies that treatments that require differential priming for efficacy may not be active against the disease unless priming can be induced. It has been previsously published that miR-155 antagonism also slows growth of WM cells and leads to xenograft regression24. We observed that while antagonizing miR-155 slowed growth it also induced Bim priming in BCWM.1 and MWCL-1 which increased their sensitivity to ABT-737 in absence of any other death signal (not shown and Figure 6). We also observed that inducing cleavage of Bid with proteasome inhibition increased sensitivity to ABT-737 and ABT-199 suggesting an increase in priming through a tBid-dependent mechanism (Figure 2). Therefore, though the mitochondria of WM cells may not be primed with pro-apoptotic protein, there are treatments that can induce priming which in turn increases their sensitivity to a variety of anti-cancer agents.
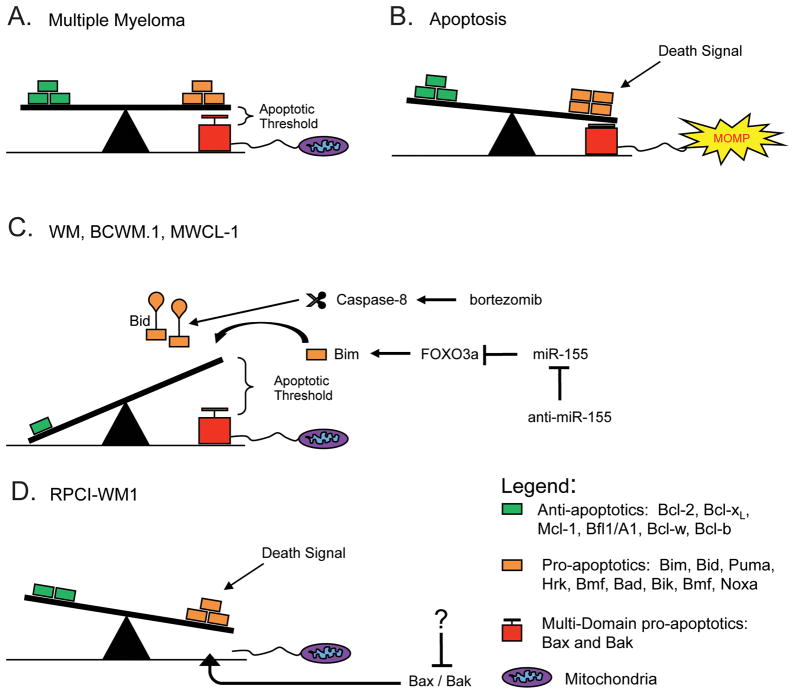
In Figure 7 we present a model for apoptotic threshold in WM. MM cells express both pro- and anti-apoptotic Bcl-2 proteins at higher levels than healthy cells (Figure 7A). This sets a lower apoptotic threshold as it takes less death signaling to induce pro-apoptotic proteins sufficient to shift the balance and activate Bax and Bak and induce apoptosis (Figure 7B). In the case of WM, both pro- and anti-apoptotic proteins are expressed at low levels similar to normal plasma cells which sets a higher apoptotic threshold (Figure 7C). Mechanistically this occurs through the expression of miR-155 which inhibits expression of Bim resulting in decreased priming. The pro-apoptotic Bcl-2 protein Bid is expressed in WM (Figure 1B, D), but it requires processing before becoming fully able to activate Bak. This means that more death signaling is required to cause MOMP and therefore apoptosis in WM. However, activation of Caspase-8 by bortezomib treatment, or antagonizing miR-155 to relieve inhibition of Bim lowers the apoptotic threshold which allows activation of apoptosis with less death signaling (Figure 7C). Finally, the RPCI-WM1 line, which does not conform to the pattern of WM expression seen in the patient data or the other two cell lines, expresses Bim at moderate levels but is unable to activate mitochondria-dependent apoptosis as it lacks Bax and Bak (Figure 7D). Therefore its apoptotic threshold is essentially infinite. Though the mechanism of Bax and Bak inhibition in these cells remains to be determined, in the case of Bak, low expression is consistent with patient data (Figure 1B, S1B) and restoring Bak or Bax expression in these cells should be sufficient to allow for the induction of apoptosis (Figure 7D).
Figure 7.
A model of apoptotic threshold in Waldenström Macroglobulinemia. See Discussion text.
The current standard of care in WM includes rituximab which targets the B-cell biology of the WM cell and depends more on complement and immune killer cell-mediated mechanisms than differential mitochondrial priming for action26. However, rituximab as a single agent offers no advantage over other single agent treatments in WM patients26. Virtually all WM patients are now treated with some form of combination therapy. Knowledge of the state of mitochondrial priming in WM can provide direction in choosing the combination therapies likely to be effective in treatment. Treatment modalities showing promise in WM are those combinations including a proteasome inhibitor27. Targeting the proteasome in WM allows for the induction of mitochondrial priming because of both the Bcl-2 family expression and the biology of the cell. Proteasome inhibitors attack the WM cell’s dependency on both the constitutive and immunoproteasome causing both proteotoxic stress and inhibiting immune functions of the cell in addition to more broadly targeted effects like NF-κB inhibition and cell cycle modulation28, 29. The data presented in this study show that WM cells express pro-apoptotic Bcl-2 family members at low levels which makes them less sensitive to apoptosis-inducing agents. However we provide evidence that mitochondrial priming can be induced in WM by antagonism of miR-155 or cleavage of Bid, which is expressed in WM. In this way, including agents that are capable of inducing priming and therefore lowering the apoptotic threshold as part of a combination therapy will increase the efficacy of a variety of other co-treatments.
MATERIALS AND METHODS
Gene Expression Array Analysis
Normalized (Affymetrix HG-U133A Plus 2.0 Microarray) expression data was obtained from GEO database (GSE6691; PMID: 17252022) for the study14. The expression data was RMA normalized and log2 transformed, to examine the gene expression differences between B-cell like and plasma-cell like patient groups. Gene expression was calculated as the mean signal of all available probes for each gene. Unsupervised hierarchical clustering of 14 BCL-2 genes microarray derived expression was performed to demonstrate the up- and down- regulated expression differences at the gene level between B-cell like and plasma-cell like patient samples. Euclidean distance measure with agglomerative average-linkage was used for clustering row normalized expression data.
Cell Culture
BCWM.1, MWCL-1 and RPCI-WM1 cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 I.U. penicillin/streptomycin, 1 mM sodium pyruvate and non-essential amino acids. Bortezomib was purchased from LC Laboratories (Woburn, MA). ATO was purchased from Sigma Aldrich (St. Louis, MO). ABT-737 and ABT-199 were generous gifts of Abbvie (North Chicago, IL).
Lentiviral anti-mIR expression
Virus encoding anti-mIR-155 or control Arabidopsis thaliana anti-mIR was purchased from Sigma Aldrich. WM cell lines were then infected and selected with puromycin. Transductants were kept in selection during passage and verified by western blot and qRT-PCR of downstream targets. Experiments were carried out in the absence of puromycin.
Retroviral miR-155 expression
Virus was prepared using the phoenix cell packaging system and either MG-155 (Addgene #26529) or pMIG-w (Addgene #12282) empty vector. Cells were infected with three rounds of virus over 24 hours. Cells were then sorted for high GFP (EGFP) expression on a BD FACSAria and propagated prior to collection and assessment of expression.
Apoptosis was assayed with Annexin-V-FITC (Biovision, Milpitas, CA) and propidium iodide (2 μg/mL Sigma) staining and measured with a BD FACScanto II as previously described18.
Gene Expression
cDNA was prepared from RNA harvested at specified time points using ABI high capacity cDNA kit (Life Technologies, Grand Island, NY). Realtime PCR was performed using Taqman gene expression master mix (ABI 4368814) with an ABI 9600 Fast thermocycler as previously described30. Probes: Bim (bcl2l11) Hs00708019_s1, Bak (bak1) Hs00832876_g1, Bax (bax) Hs00180269_m1, FOXO3a (foxo3) Hs00818121_m1, GAPDH 4333764.
MicroRNA Expression
RNA was extracted and enriched for small RNA using the Ambion Mirvanna kit (Life Technologies AM1561) according to protocol. cDNA was prepared using a specific primer pool of 8 target microRNAs plus 4 small control RNAs (Applied Biosystems). Realtime PCR was performed using TaqMan Universal Master Mix II (4440040) according to protocol. Endogenous control CTec was determined by average of the four control small RNA CT values. RQ was determined by the CT value of the experimental miR normalized to the endogenous control (ΔCT=CT-CTec) then normalized to the experimental control (ΔΔCT=ΔCT-ΔCTc) and expressed as the relative quantity (RQ=2−ΔΔCT). Probe and primer sets: (PN4427975) hsa-mir-9*, hsa-mir-155, hsa-mir-184, hsa-mir-206, hsa-mir-363, hsa-mir-363*, hsa-mir-494, hsa-mir-542-3p, RNU6B, RNU44, RNU48, U47.
Immunoblotting
Western blotting was performed on lysates collected at the various time points and treatments as previously described13, 18. Antibodies: Rabbit IgG-HRP donkey and Mouse-IgG-HRP sheep were purchased from GE Healthcare (Piscataway, NJ). Mcl-1 rabbit polyclonal was purchased from Enzo Life Sciences (Farmingdale, NY). Bcl-2 hamster (6C8) was purchased from BD Pharmingen (San Jose, CA). Bax, Caspase-3, Caspase-7, Bid and FOXO3a rabbit polyclonal and Caspase-8 mouse (1C12) were purchased from Cell Signaling Technology (Danvers, MA). Bim and Bak rabbit polyclonal were purchased from Milipore (Darmstadt, Germany). β-Actin mouse (AC-15) and Parp rabbit polyclonal were purchased from Sigma Aldrich. Bcl-xL mouse (2A1) and Bcl-xL rabbit polyclonal (13.6) have been previously described31.
Supplementary Material
Acknowledgments
We thank Stephen Ansell and Kelvin Lee for cell lines and reagents. This work was supported by R01 CA127910 and R01 CA129968 as well as funding from the TJ Martell Foundation (LHB), the Leukemia & Lymphoma Society (AAC), the International Waldenstrom Macroglobulinemia (AAC) and the Comité du Septentrion de la Ligue contre le Cancer (XL). LHB is a GRA Distinguished Cancer Scientist.
ABT-737 and ABT-199 were a generous gift of Abbvie, (North Chicago, IL)
Footnotes
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest to disclose.
REFFERENCES
- 1.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010 Feb 12;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010 Dec 3;330(6009):1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001 Apr 27;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006 May;9(5):351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990 Nov 22;348(6299):331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- 7.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009 Mar 5;28(9):1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, del Moore VG, et al. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011 Nov 25;334(6059):1129–1133. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herrinton LJ, Weiss NS. Incidence of Waldenstrom’s macroglobulinemia. Blood. 1993 Nov 15;82(10):3148–3150. [PubMed] [Google Scholar]
- 10.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003 Nov;4(11):679–685. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- 11.Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med. 2012 Aug 30;367(9):826–833. doi: 10.1056/NEJMoa1200710. [DOI] [PubMed] [Google Scholar]
- 12.Poulain S, Roumier C, Decambron A, Renneville A, Herbaux C, Bertrand E, et al. MYD88 L265P mutation in Waldenstrom macroglobulinemia. Blood. 2013 May 30;121(22):4504–4511. doi: 10.1182/blood-2012-06-436329. [DOI] [PubMed] [Google Scholar]
- 13.Gaudette BT, Iwakoshi NN, Boise LH. Bcl-xL Protein Protects from C/EBP Homologous Protein (CHOP)-dependent Apoptosis during Plasma Cell Differentiation. J Biol Chem. 2014 Aug 22;289(34):23629–23640. doi: 10.1074/jbc.M114.569376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez NC, Ocio EM, de Las Rivas J, Maiso P, Delgado M, Ferminan E, et al. Gene expression profiling of B lymphocytes and plasma cells from Waldenstrom’s macroglobulinemia: comparison with expression patterns of the same cell counterparts from chronic lymphocytic leukemia, multiple myeloma and normal individuals. Leukemia. 2007 Mar;21(3):541–549. doi: 10.1038/sj.leu.2404520. [DOI] [PubMed] [Google Scholar]
- 15.Ditzel Santos D, Ho AW, Tournilhac O, Hatjiharissi E, Leleu X, Xu L, et al. Establishment of BCWM.1 cell line for Waldenstrom’s macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp Hematol. 2007 Sep;35(9):1366–1375. doi: 10.1016/j.exphem.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Hodge LS, Novak AJ, Grote DM, Braggio E, Ketterling RP, Manske MK, et al. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL-1. Blood. 2011 May 12;117(19):e190–197. doi: 10.1182/blood-2010-12-326868. [DOI] [PubMed] [Google Scholar]
- 17.Chitta KS, Paulus A, Ailawadhi S, Foster BA, Moser MT, Starostik P, et al. Development and characterization of a novel human Waldenstrom macroglobulinemia cell line: RPCI-WM1, Roswell Park Cancer Institute - Waldenstrom Macroglobulinemia 1. Leuk Lymphoma. 2013 Feb;54(2):387–396. doi: 10.3109/10428194.2012.713481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales AA, Gutman D, Lee KP, Boise LH. BH3-only proteins Noxa, Bmf, and Bim are necessary for arsenic trioxide-induced cell death in myeloma. Blood. 2008 May 15;111(10):5152–5162. doi: 10.1182/blood-2007-10-116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 20.Roccaro AM, Sacco A, Chen C, Runnels J, Leleu X, Azab F, et al. microRNA expression in the biology, prognosis, and therapy of Waldenstrom macroglobulinemia. Blood. 2009 Apr 30;113(18):4391–4402. doi: 10.1182/blood-2008-09-178228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis BP, Burge CB, Bartel DP. Cell. Vol. 120. United States: 2005. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets; pp. 15–20. [DOI] [PubMed] [Google Scholar]
- 22.Kong W, He L, Coppola M, Guo J, Esposito NN, Coppola D, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010 Jun 4;285(23):17869–17879. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Yamanaka Y, Tagawa H, Takahashi N, Watanabe A, Guo YM, Iwamoto K, et al. Aberrant overexpression of microRNAs activate AKT signaling via down-regulation of tumor suppressors in natural killer-cell lymphoma/leukemia. Blood. 2009 Oct 8;114(15):3265–3275. doi: 10.1182/blood-2009-06-222794. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Roccaro AM, Rombaoa C, Flores L, Obad S, Fernandes SM, et al. LNA-mediated anti-miR-155 silencing in low-grade B-cell lymphomas. Blood. 2012 Aug 23;120(8):1678–1686. doi: 10.1182/blood-2012-02-410647. [DOI] [PubMed] [Google Scholar]
- 25.Premkumar DR, Jane EP, DiDomenico JD, Vukmer NA, Agostino NR, Pollack IF. ABT-737 synergizes with bortezomib to induce apoptosis, mediated by Bid cleavage, Bax activation, and mitochondrial dysfunction in an Akt-dependent context in malignant human glioma cell lines. J Pharmacol Exp Ther. 2012 Jun;341(3):859–872. doi: 10.1124/jpet.112.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gertz MA. Waldenstrom macroglobulinemia: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013 Aug;88(8):703–711. doi: 10.1002/ajh.23472. [DOI] [PubMed] [Google Scholar]
- 27.Treon SP, Tripsas CK, Meid K, Kanan S, Sheehy P, Chuma S, et al. Carfilzomib, rituximab and dexamethasone (CaRD) is active and offers a neuropathy-sparing approach for proteasome-inhibitor based therapy in Waldenstrom’s macroglobulinemia. Blood. 2014 May 23; doi: 10.1182/blood-2014-03-566273. [DOI] [PubMed] [Google Scholar]
- 28.Roccaro AM, Sacco A, Aujay M, Ngo HT, Azab AK, Azab F, et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood. 2010 May 20;115(20):4051–4060. doi: 10.1182/blood-2009-09-243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boise LH. Aiming at WM with both barrels blocked. Blood. 2010 May 20;115(20):4007–4008. doi: 10.1182/blood-2010-02-270231. [DOI] [PubMed] [Google Scholar]
- 30.Morales AA, Kurtoglu M, Matulis SM, Liu J, Siefker D, Gutman DM, et al. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood. 2011 Aug 4;118(5):1329–1339. doi: 10.1182/blood-2011-01-327197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-xL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]; J Immunol. 2010 Oct 1;185(7):3788–3799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.