Summary
Hysterosalpingography is an imaging method to evaluate the endometrial and uterine morphology and fallopian tube patency. Contrast intravasation implies backflow of injected contrast into the adjoining vessels mostly the veins and may be related to factors altering endometrial vascularity and permeability. Radiologists and gynaecologists should be well acquainted with the technique of hysterosalpingography, its interpretation, and intravasation of contrast agents for safer procedure and to minimize the associated complications.
MeSH Keywords: Contrast Media, Hysterosalpingography, Infertility
Hysterosalpingography (HSG), also known as uterosalpingography, is an imaging method that uses fluoroscopy and iodinated contrast media to evaluate the endometrial and uterine morphology and fallopian tube patency in women suffering from infertility and habitual abortions [1]. Indications for HSG include synechiae, hyperplasia, fibroids, polyps and Mullerian duct abnormalities [2]. Obstruction of the fallopian tubes due to infection, scarring, ectopic pregnancy, tubal ligation and recanalisation procedures can also be evaluated by HSG [2,3]. Peritoneal spillage provides an idea about the uterine contour and peritoneal adhesions.
It is an easy, safe and useful procedure with favourable outcomes [4]. However, complications [5] including infection, vaginal bleeding, exposure to radiation, vaso- vagal attack, uterine injury, intravasation and reaction to contrast agent might be observed during or after the procedure. In addition, complications may be accompanied by intravasation itself, which may involve hypersensitivity, bleeding, and infection. Venous intravasation can cause pulmonary embolism along with associated systemic side effects [6].
Intravasation implies backflow of injected contrast into the adjoining vessels mostly the veins. The contrast passes from the uterine cavity directly into the myometrial vessels with subsequent drainage to the pelvic veins. The prevalence of intravasation has been reported to be 0.4–6.9% [7]. On imaging, intravasation has varied in its appearance from a reticular pattern to a linear pattern seen as multiple thin lines (Figure 1A, 1B).
Figure 1.

(A, B) Contrast intravasation during HSG in a 32-year-old woman suffering from primary infertility. (A) Initial image acquired after intrauterine contrast injection shows peritoneal spill from the right fallopian tube; no spill from the left side was seen (B) image acquired later shows intravasation of the contrast agent resulting in the opacification of veins of the myometrium and the pelvis up to the iliac veins. The opacification reduced and subsequently disappeared with the cessation of further injection (level 2 intravasation).
Prevention of intravasation during HSG examination is of vital importance for procedural safety and may be related to predisposing factors altering endometrial vascularity and permeability. There is an increased risk of intravasation during HSG in women with certain clinical conditions like nonspecific pelvic pain, menometrorrhagia, secondary infertility, ectopic pregnancy, polycystic ovarian disease, endometriosis, hydatidiform mole, vaginal itching, and subclinical urinary infections [8]. Nevertheless, it may be seen in normal patients [9], as well as in those with a history of recent uterine surgery or increased intrauterine pressure due to tubal obstruction. It has been noted that women who experience pain during HSG procedure are more likely to develop intravasation [8]. Discomfort and a painful procedure may be associated with spasms due to cervical fixation and cannulation which can be traumatic and cause intravasation [6]. This is more commonly seen in women who are in the post-menstrual and preovulatory phase [9]. By eliminating predisposing factors, intravasation may be minimized or prevented and reduce further complications. There appears to be a relation between recent uterine intervention and intravasation as a result of increased permeability [8]. Recent uterine and endometrial interventions, repetitive curettage, and missed or medical abortion might be related to intravasation (Figure 2A, 2B).
Figure 2.
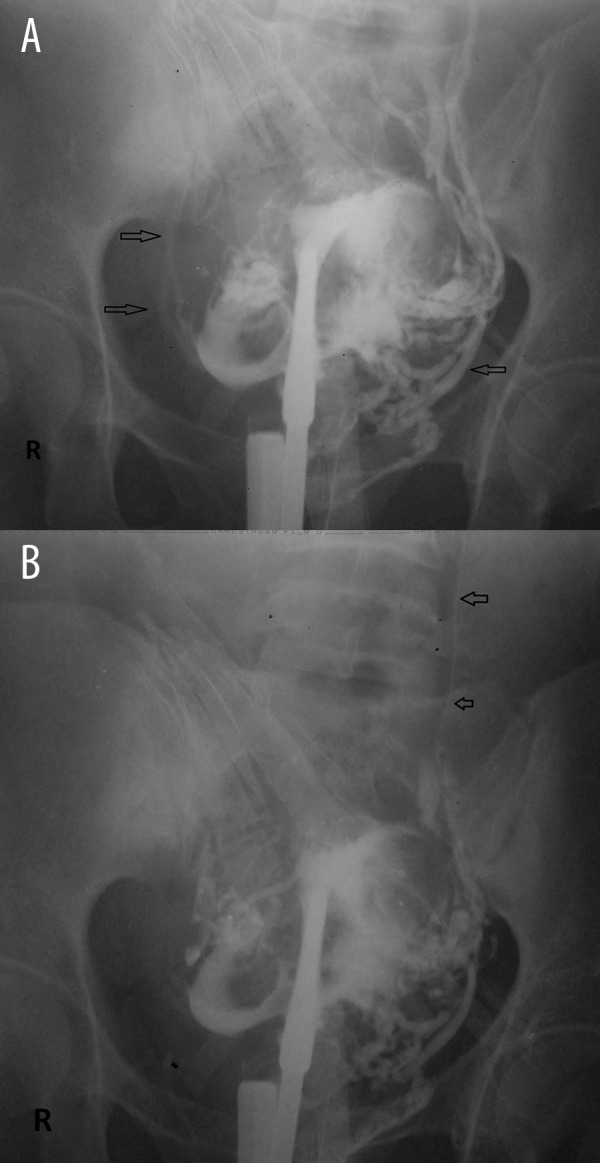
(A, B) Intravasation of contrast during HSG in a 24-year-old woman suffering from primary infertility who had undergone uterine curettage 5 months back for dysfunctional uterine bleeding. Free peritoneal spillage was noted on both sides indicating bilateral patent tubes; however, contrast had intravasated into the pelvic vessels almost instantaneously. (Arrows point to contrast in the vessels) (level 3 intravasation).
PID (pelvic inflammatory disease) is a contraindication for HSG [4]; as such it should be treated before the procedure to minimize the potential complications.
HSG should be scheduled between the cessation of menstruation and before ovulation as this is the time when pregnancy is least likely [2,5,6]. A classification has been proposed [8] to overcome the problem of discrepancy between clinical and basic research; intravasation of contrast agents may be classified accordingly into four levels: Level 0, no intravasation of contrast agent (Figures 3A, 3B, 4A–4C); Level 1, minimal intravasation which is limited to the myometrium (resulting in problems with diagnosis and confused with adenomyosis); Level 2, moderate intravasation involving the parametrial-adnexial veins and occurring slowly; and Level 3 severe intravasation extending from the myometrial-parametrial to the paracaval veins and occurring instantly.
Figure 3.
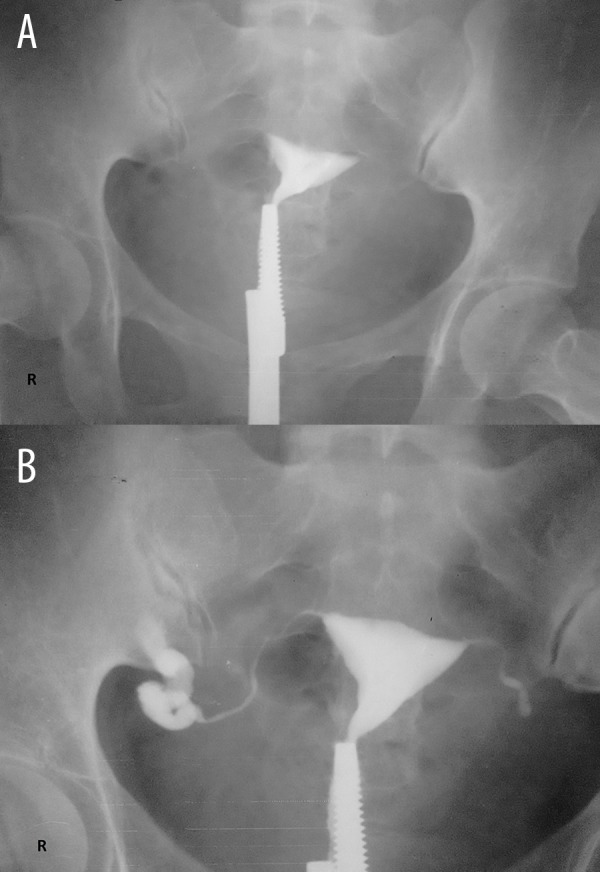
(A, B) HSG of a 28-year-old female suffering from primary infertility due to tuberculosis. No peritoneal spill of contrast was seen on either side indicating bilateral tubal blockage. However, no intravasation of contrast was seen (level 0 intravasation).
Figure 4.
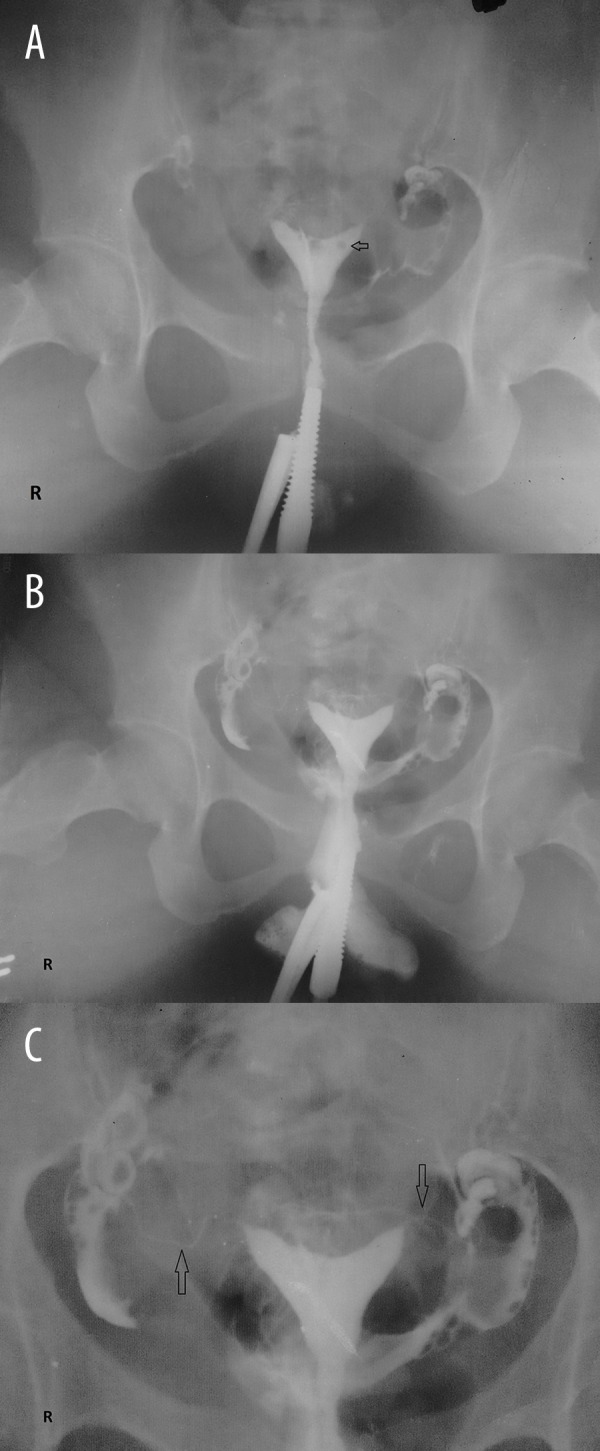
(A–C) HSG of a 36- year- old woman with a history of previous 3 abortions, showing a bicornuate uterus. Free peritoneal spill was seen on both sides indicating bilateral patent tubes. (arrow in ‘A’ points to a gas bubble erroneously introduced during the procedure; arrows in ‘C’ point to the fallopian tubes) (level 0 intravasation).
Water-soluble contrast agents are associated with decreased complications and better radiographic quality as compared to the lipo-soluble contrast media [6]. Venous intravasation is usually of no significance with water- soluble contrast material; however, oil- based contrast media may result in fat embolism along with systemic side effects if venous intravasation occurs. Although intravasation was historically associated with an increased risk of a venous embolus due to the used contrast agents, negative side effects have been reduced since HSGs are now performed with hydro-soluble contrast media. For this reason, the hydro-soluble media achieved popularity for use with HSG.
Intravasation may indirectly indicate tubal occlusion [6,10]. If the contrast medium is in the fallopian tubes, intravasation tends to persist; if not, it tends to be washed out. Intravasation may extend along the venous route. It has been postulated that tubal occlusion might be associated with intravasation because of increasing intrauterine pressure. Although it is a relatively rare event, it is important to distinguish venous intravasation from free intraperitoneal spillage of contrast (e.g. in case of patent fallopian tubes, or uterine perforation) [10]. This is a complication and potential pitfall during HSG procedure as the intravasation can mimic intraperitoneal spillage in the occluded tube.
Conclusions
In conclusion, scheduling of HSG during the middle follicular period, elimination of predisposing factors, and application of hydro-soluble contrast media minimizes or prevents intravasation. Radiologists and gynaecologists should be well acquainted with the technique of HSG, its interpretation, and intravasation of contrast agents for safer procedure and to minimize the associated complications.
Footnotes
Conflict of interest/financial support
None.
References
- 1.Livsey R. Hysterosalpingography. Australas Radiol. 2001;45:98–99. doi: 10.1046/j.1440-1673.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- 2.Simpson WL, Jr, Beitia LG, Mester J. Hysterosalpingography: A re-emerging study. Radiographics. 2006;26:419–31. doi: 10.1148/rg.262055109. [DOI] [PubMed] [Google Scholar]
- 3.Roma Dalfó A, Ubeda B, Ubeda A, et al. Diagnostic value of hysterosalpingography in the detection of intrauterine abnormalities: A comparison with hysteroscopy. Am J Roentgenol. 2004;183:1405–9. doi: 10.2214/ajr.183.5.1831405. [DOI] [PubMed] [Google Scholar]
- 4.Maheux-Lacroix S, Boutin A, Moore L, et al. Hysterosalpingosonography for diagnosing tubal occlusion in subfertile women: a systematic review with meta-analysis. Hum Reprod. 2014;29:953–63. doi: 10.1093/humrep/deu024. [DOI] [PubMed] [Google Scholar]
- 5.Ercole C, Cassel-Knipping N, Blanc B. [Complications of hysterosalpingography]. J Gynecol Obstet Biol Reprod. 1994;23:494–97. [PubMed] [Google Scholar]
- 6.Nunley WC, Jr, Bateman BG, Kitchin JD, III, Pope TL., Jr Intravasation during hysterosalpingography using oil-base contrast medium – a second look. Obstet Gynecol. 1987;70:309–12. [PubMed] [Google Scholar]
- 7.Gowin W, Fuchs P. Pericaval intravasation of contrast media in the changed indication for hysterosalpingography. Rontgenblatter. 1984;37:26–28. [PubMed] [Google Scholar]
- 8.Dusak A, Soydinc HE, Onder H, et al. Venous intravasation as a complication and potential pitfall during hysterosalpingography: Re-emerging study with a novel classification. J Clin Imaging Sci. 2013;3:67. doi: 10.4103/2156-7514.124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tur-Kaspa I, Seidman DS, Soriano D, et al. Hysterosalpingography with a balloon catheter versus a metal cannula: A prospective, randomized, blinded comparative study. Hum Reprod. 1998;13:75–77. doi: 10.1093/humrep/13.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Ubeda B, Paraira M, Alert E, Abuin RA. Hysterosalpingography: spectrum of normal variants and nonpathologic findings. Am J Roentgenol. 2001;177:131–35. doi: 10.2214/ajr.177.1.1770131. [DOI] [PubMed] [Google Scholar]


