Abstract
Background:
This pilot study was used to evaluate safety and subjective outcomes in a small series of Peyronie’s disease patients using a combination of autologous stromal vascular fraction (SVF) and penile shock wave treatments. SVF can be procured and deployed into Peyronie’s plaques, enabling the surgeons to procure and mobilize significant numbers of both adult mesenchymal stem cells and antiinflammatory cytokines released from the adipose collagen matrix after collagen digestion. Penile shock wave therapy stimulates targeted tissues and may activate stem cells found in the SVF and promote healing and fibrosis mitigation.
Methods:
SVF isolated from lipoaspirate was deployed by injection into 11 patients with Peyronie’s plaques in combination with a series of shock wave treatments. Subjective outcomes tests performed at baseline and at 6 months included the Erectile Hardness Grading Score and the Peyronie’s Disease Questionnaire (Questions 1–6).
Results:
All patients noted subjective improvement in curvature and subjective reduction in plaque size. Seven patients reported improvement in erectile function. Mean Erectile Hardness Grading Score increased from 2.7 to 3.5, and mean Peyronie’s Disease Questionnaire scores decreased from 15.0 to 8.7.
Conclusions:
SVF is known to have scar mitigation, antiinflammatory, immunomodulatory, and regenerative effects, and it has been used for a variety of conditions on an investigational basis. SVF containing mesenchymal stem cells can be procured in a closed surgical system from lipoaspirate in a same-day setting and deployed directly into Peyronie’s plaques in combination with penile shock wave therapy resulting in plaque mitigation.
Peyronie’s disease is considered a hypertrophied scar of the tunica albuginea and affects millions of American men. Surgical repair is associated with penile scarring, penile shortening, and worsening of erectile dysfunction (ED) in many cases. Nonsurgical therapeutic options are limited, and results do not exceed placebo for most remedies. Evidence shows that there is no benefit with respect to deformity reduction in Peyronie’s disease with any oral therapy, including vitamin E, potassium aminobenzoate, colchicine, tamoxifen, and carnitine.1 Double-blind studies on intralesional verapamil and interferon have failed to demonstrate any significant differences/improvements in penile deformity, pain, plaque softening, or sexual function, and intralesional steroids have not shown objective therapeutic benefit.1 Intralesional collagenase Clostridium histolyticum (Xiaflex, Auxilium Pharmaceuticals, Chesterbrook, Penn.) injections are the first Food and Drug Administration-approved treatment for Peyronie’s disease (PD), and the studies demonstrate efficacy of the treatment, but there have been documented cases of corporal rupture, penile hematoma, and penile pain.2 There has been recent anecdotal interest in biologic therapies including the use of platelet-rich plasma to treat Peyronie’s disease, but there is a paucity of outcomes data. Platelet-rich plasma contains many antiinflammatory cytokines, but it is relatively deficient in cellular components.
Stromal vascular fraction (SVF) obtained from the enzymatic digestion of liposuction fat is known to have regenerative, antiinflammatory, scar mitigating, and immunomodulatory properties. Pursuing an SVF-based cell therapy option for PD requires a deeper understanding of the cellular basis of Peyronie’s disease. Excessive amounts of fibrin were identified in these plaques in 1997 by Somers and Dawson.3 A deeper understanding of the cellular basis of Peyronie’s plaques came a few years later with the understanding that there was oversecretion of collagen associated with the excessive transforming growth factor β1 (TGF-β1)–mediated fibroblast conversion into myofibroblasts. These cells that are a normal part of healing are expected to undergo apoptosis when healing is complete, and loss of the apoptotic signal appears to be instrumental in Peyronie’s formation.4 These findings in addition to the presence of reactive oxygen species (ROS) contribute to the thick scarring, curvature, and ED associated with Peyronie’s disease.5
Through technologic advances, we are currently able to isolate autologous SVF from 50 cm3 of adipose tissue lipoaspirate in a sterile, closed system within 2 hours in the operating room. SVF isolated from the connective tissue associated with subcutaneous fat and blood vessels is known to contain adult mesenchymal stem cells (MSCs), T regulatory cells, endothelial precursor cells, preadipocytes, antiinflammatory M2 macrophages, and numerous cytokine growth factors.6 Recently, there is a plethora of anecdotal evidence-based information to suggest that MSCs may have significant beneficial use for a variety of autoimmune, inflammatory, and degenerative conditions.7–12 There is also a large veterinary experience using SVF showing safety and efficacy.13 The antiinflammatory cytokines in SVF may accelerate healing and mitigate collagen overdeposition activity associated with Peyronie’s disease. The literature regarding the cellular and molecular differences in inflammation between scar-free wound healing and scar-forming wound healing indicates that scar formation may be prevented by inflammatory regulation.14
Low-intensity acoustic shock waves have been used to treat Peyronie’s disease, and positive results have been well described in the urologic literature.15,16 Shock wave technology has been previously combined with mesenchymal cell therapy in the treatment of cardiac disease, and the combination has been shown to be superior to either therapy alone for improving ejection fraction and reducing infarct size.17 Regenerative cells in SVF can be activated by cytokine signals released from tissue that is diseased, damaged, or inflamed. Low-intensity shock waves create controlled microtrauma that is expected to be able to mimic these conditions and may help tropism homing and activation of stem cells and other resident progenitor cells.
MATERIALS AND METHODS
This study was performed as a part of the Safety and Clinical Outcomes Study: SVF for Urologic Conditions registered as CSN111 in clinicaltrials.gov. Under institutional review board (International Cell Surgical Society) approval for investigational use of SVF, 11 patients (age, 52–70 years; mean age, 61 year) who had documented chronic stable Peyronie’s disease were treated with autologous SVF. Curvature ranged from mild to 90 degrees. All of the patients suffered some degree of ED, and none complained of pain associated with their plaques. Patients underwent basic urologic evaluation all had palpable plaques on physical examination, and some had plaque imaging with ultrasound or MRI. Patients underwent instillation of local anesthetic, and a 50-cc miniliposuction was performed. Good manufacturing practices-grade collagenase (Roche, Indianapolis, Ind.) was used for enzymatic digestion of condensed fat. The Time Machine centrifuge and incubator from Medikan (Kangnam, South Korea) was used to isolate the SVF product. Under local penile block, patients underwent a shock wave treatment focused on softening the plaque using a Storz D-ACTOR 200 hand-held device (Storz Medical, Tagerwilen, Switzerland). SVF was then injected into the Peyronie’s plaque. All patients received a series of 1 to 6 shock wave treatments over the next few weeks. The D-ACTOR transforms the kinetic energy of a ballistically generated bullet into a radially expanding pressure wave.18
RESULTS
Clinically significant improvement was seen in all 11 patients at 6 months. There were no adverse events. All patients noted subjective straightening of the penis and some with complete response. All 11 patients reported subjective reduction in plaque size. All 11 patients described subjective improvement in curvature, and 7 reported improvement in erectile function. Mean Erectile Hardness Grading Score (Fig. 1) increased from 2.7 to 3.5 (on a scale from 1 to 4). Mean Peyronie’s Disease Questionnaire (Questions 1–6) scores (Fig. 2) decreased from 15 to 8.7. (Maximum score is 24, and no Peyronie’s symptoms equals 0.) There was only minimal abrasion and bruising of the penile skin at the time of treatment in most of the patients, but none experienced hematoma, laceration, postprocedure pain, or any other damage to the surrounding organs (testicles or urethra). In most cases, Peyronie’s plaques appeared to soften subjectively on physical examination performed before and immediately after shock wave therapy.
Fig. 1.
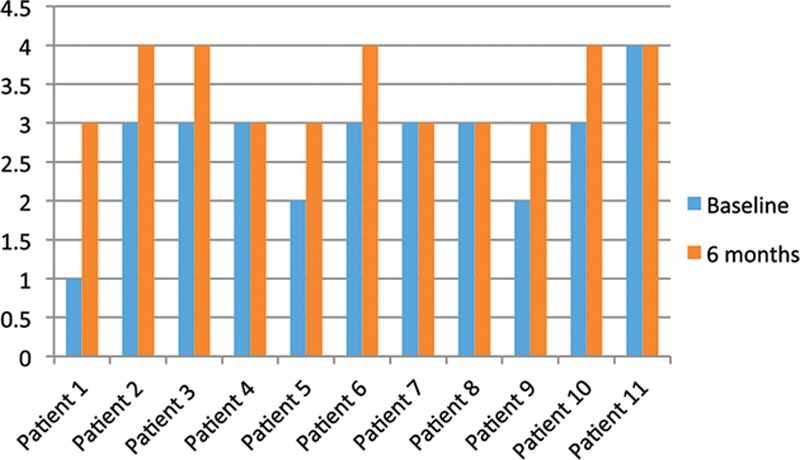
Seven patients demonstrated improvement and 4 patients showed no change in Erectile Hardness Grading Score at 6 months. No patient experienced worsening in erectile function.
Fig. 2.
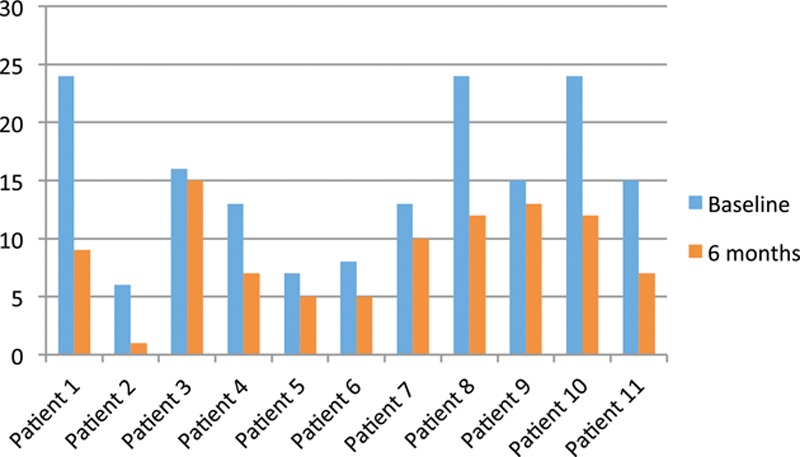
All patients reported improvement in subjective outcomes in questions 1 through 6 of the Peyronie’s Disease Questionnaire (PDQ; US version) at 6 months. The PDQ score ranges from 0 (no symptoms of Peyronie’s disease) to a maximum of 24.
DISCUSSION
SVF from lipoaspirate has been used clinically for a variety of autoimmune, inflammatory, and degenerative diseases and for scar mitigation as well. Intracavernosal and intraplaque deployment of autologous SVF in a small group of Peyronie’s patients seem to decrease curvature and improve sexual function in short-term follow-up. Penile shock wave therapy may contribute to disruption of Peyronie’s plaques, promote angiogenesis, and create a cytokine milieu that provides an opportunity for improved healing in combination with the introduction of exogenous autologous MSCs in high numbers from autologous SVF.
Mesenchymal adipose-derived stem cells have been shown to mitigate fibrosis of the tunica albuginea in a rat model.19,20 It is known that MSCs may mitigate ROS and the release of profibrotic (TGF-β1), which is involved in the conversion of fibroblasts to myofibroblasts, a prominent histologic feature of Peyronie’s disease.21 Other various inflammatory modulators secreted by MSCs include the following: nitric oxide (NO), indoleamine 2,3-dioxygenase, prostaglandin E2, interleukin-10, and TNF-α-stimulated gene/protein 6.22 In addition, MSCs substantially up-regulate the expression of inducible nitric oxide synthase in response to their interaction with T cells in a proinflammatory environment. NO is complementary to prostaglandin E2 for the inhibition of T-cell proliferation and can reduce oxidative damage associated with ROS.23 ROS including superoxide, hydrogen peroxide, and alkyl peroxides are secreted in healing wounds by neutrophils. They are highly cytotoxic compounds used to achieve wound sterility; however, these ROS are also intensifiers of collagen deposition.24 Prolonged exposure to ROS that occurs during wound healing is associated with enhanced fibrogenesis and fibrotic tissues accumulation through a mechanism that involves membrane lipid oxidation and the induction of TGF-β1.25 NO produced by MSCs in the wound can scavenge ROS to produce reactive nitrogen species, such as peroxynitrite.26 Although these reaction products are also oxidative and cytotoxic, they react more slowly than their associated ROS27 and prevent oxidative damage to DNA and membrane lipids.26 A recent study has demonstrated that inducible nitric oxide synthase expression is sufficient to alter the ROS/reactive nitrogen species balance to mitigate the formation of fibrotic tissue.28
In addition to the production of antifibrotic factors and NO associated with neutralization of ROS in wound healing, MSCs may mitigate scar formation by immunomodulation. The ability of MSCs to regulate T-cell function and recruitment, proliferation, and activity is well documented.29,30 There is also evidence that MSCs are capable of suppressing the proliferation of B cells and natural killer cells, thereby ameliorating the profibrotic acute immune response to tissue trauma and prolonged inflammation.31,32
This is the first report in the literature to combine cell therapy with shock wave therapy for human penile tissue. Shock wave therapy has been demonstrated to enhance the secretion and proliferation of MSCs by promoting angiogenesis and nerve regeneration in vitro.33 The use of shock waves has been described in the wound healing literature, and the biologic response to shock waves includes enhanced angiogenesis, as well as stem cell recruitment, proliferation, and differentiation.34 The urologic literature has abundant sources indicating that various forms of penile shock wave treatment may be helpful in mitigating symptoms of PD with some articles showing significant diminution in erections, pain, or curvature35–39 and others less supportive of shock wave therapy for PD.40–44 The 2010 International Consultation of Sexual Medicine evidence-based guidelines state that there is evidence that extracorporeal shock wave therapy does not improve PD-related deformity.45
Although most reports in the literature overall support the use of shock wave for various symptoms of PD, results are conflicting, and it is notable that each study used a different types of shock wave device making comparison very difficult. Very few studies used a radial hand-held shock wave device directly placed on the penile shaft, and most studies involved the use of large machines designed for stone lithotripsy. In general, higher energies delivered were associated with improvements in pain and curvature. This study utilizes the Storz D-ACTOR 200 shock wave generator, which is ideally suited for penile shock wave therapy, and radially focuses on the majority of the emitted energy deep to the penile skin and actively into the palpable plaque.
Safety in this study appears to have been adequately demonstrated by the lack of adverse events directly related to SVF deployment or the shock wave treatments. Only minimal and occasional complaints about the liposuction procedure or the occasional delayed healing at the liposuction site were noted. Mild penile swelling and superficial abrasions were noted in all of the patients. A recent evaluation of the first 1,000 patients in our research collaborative network showed minimal side effects with no severe adverse reactions to autologous SVF deployment for a number of degenerative, autoimmune, and inflammatory conditions (manuscript in preparation). There are evidence-based studies from the veterinary world that support the regenerative powers of adipose-derived adult MSCs.13 A recent study of 1178 patients receiving intraarticular SVF for deployment for osteoarthritis demonstrated excellent safety and efficacy.11
The Cell Surgical Network has developed a point of care completely closed sterile surgical procedure with an excellent safety profile that appears to provide an abundance of cells that can differentiate into the necessary tissue to reverse a variety of degenerative conditions. SVF is immunomodulatory,46 regenerative, and antiinflammatory, and it has scar mitigation capabilities. The addition of shock waves may enhance the efficacy of SVF in treating Peyronie’s plaques in this small series of patients. Shock wave therapy may contribute to disruption of Peyronie’s plaques and may result in improved healing under the antiinflammatory influence of exogenous MSCs in extremely high numbers from autologous SVF.47 Results of combined therapy exceeded those expected if shock waves were used alone to mitigate PD.48 Autologous cell-based therapy in combination with penile shock wave therapy may have a role in the treatment of Peyronie’s disease. Further long-term controlled studies that randomize patients into either the experimental group (SVF/penile shock wave) versus The current standard of care would be beneficial and should include penile shock wave administration using various treatment intervals and settings to better understand the benefits of combined treatment.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Larsen SM, Levine LA. Review of non-surgical treatment options for Peyronie’s disease. Int J Impot Res. 2012;24:1–10. doi: 10.1038/ijir.2011.45. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz LI, Goldstein I, Seftel AD, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie’s disease by subgroup: results from two large, double-blind, randomized, placebo-controlled, phase III studies. BJU Int. 2015;116:650–656. doi: 10.1111/bju.13096. [DOI] [PubMed] [Google Scholar]
- 3.Somers KD, Dawson DM. Fibrin deposition in Peyronie’s disease plaque. J Urol. 1997;157:311–315. [PubMed] [Google Scholar]
- 4.Hinz B, Phan SH, Thannickal VJ, et al. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vernet D, Ferrini MG, Valente EG, et al. Effect of nitric oxide on the differentiation of fibroblasts into myofibroblasts in the Peyronie’s fibrotic plaque and in its rat model. Nitric Oxide. 2002;7:262–276. doi: 10.1016/s1089-8603(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 6.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed Res Int. 2014;2014:436029. doi: 10.1155/2014/436029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayci C, Carlsen BT. Osteoarthritis of the wrist. Plast Reconstr Surg. 2014;133:605. doi: 10.1097/01.prs.0000438463.90968.d6. [DOI] [PubMed] [Google Scholar]
- 9.Chang H, Do BR, Che JH, et al. Safety of adipose-derived stem cells and collagenase in fat tissue preparation. Aesth Plast Surg. 2013;37:802–808. doi: 10.1007/s00266-013-0156-7. [DOI] [PubMed] [Google Scholar]
- 10.Pak J. Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep. 2011;5:296. doi: 10.1186/1752-1947-5-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalek J, Moster R, Lukac L, et al. Autologous adipose tissue-derived stromal vascular fraction cells application in patients with osteoarthritis. Cell Transplant. 2015 Jan 20; doi: 10.3727/096368915X686760. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez JP, Murphy MP, Hong S, et al. Autologous stromal vascular fraction therapy for rheumatoid arthritis: rationale and clinical safety. Int Arch Med. 2012;5:5. doi: 10.1186/1755-7682-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black LL, Gaynor J, Gahring D, et al. Effect of adipose-derived mesenchymal stem and regenerative cells on lameness in dogs with chronic osteoarthritis of the coxofemoral joints: a randomized, double-blinded, multicenter, controlled trial. Vet Ther. 2007;8:272–284. [PubMed] [Google Scholar]
- 14.Liu S, Jiang L, Li H, et al. Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J Invest Dermatol. 2014;134:2648–2657. doi: 10.1038/jid.2014.169. [DOI] [PubMed] [Google Scholar]
- 15.Guillot-Tantay C, Phé V, Chartier-Kastler E, et al. [Medical and surgical treatments of congenital and acquired penile curvatures: a review]. Prog Urol. 2014;24:203–211. doi: 10.1016/j.purol.2013.08.328. [DOI] [PubMed] [Google Scholar]
- 16.Paulis G, Brancato T. Inflammatory mechanisms and oxidative stress in Peyronie’s disease: therapeutic “rationale” and related emerging treatment strategies. Inflamm Allergy Drug Targets. 2012;11:48–57. doi: 10.2174/187152812798889321. [DOI] [PubMed] [Google Scholar]
- 17.Sheu JJ, Lee FY, Yuen CM, et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69–83. doi: 10.1016/j.ijcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Qin X, Lu YP. [Application of extracorporeal shockwave therapy in andrology]. Zhonghua Nan Ke Xue. 2012;18:1125–1129. [PubMed] [Google Scholar]
- 19.Gokce A, Abd Elmageed ZY, Lasker GF, et al. Intratunical injection of genetically modified adipose tissue-derived stem cells with human interferon α-2b for treatment of erectile dysfunction in a rat model of tunica albugineal fibrosis. J Sex Med. 2015;12:1533–1544. doi: 10.1111/jsm.12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castiglione F, Hedlund P, Van der Aa F, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie’s disease. Eur Urol. 2013;63:551–560. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bivalacqua TJ, Diner EK, Novak TE, et al. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J Urol. 2000;163:1992–1998. [PubMed] [Google Scholar]
- 22.Jackson WM, LJ, Tuan RS. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther. 2012;3:20. doi: 10.1186/scrt111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 24.Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000;21:49–98. doi: 10.1016/s0098-2997(00)00004-2. [DOI] [PubMed] [Google Scholar]
- 25.Muriel P. Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochem Pharmacol. 1998;56:773–779. doi: 10.1016/s0006-2952(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 26.Wink DA, Vodovotz Y, Grisham MB, et al. Antioxidant effects of nitric oxide. Methods Enzymol. 1999;301:413–424. doi: 10.1016/s0076-6879(99)01105-2. [DOI] [PubMed] [Google Scholar]
- 27.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 28.Ferrini MG, Vernet D, Magee TR, et al. Antifibrotic role of inducible nitric oxide synthase. Nitric Oxide. 2002;6:283–294. doi: 10.1006/niox.2001.0421. [DOI] [PubMed] [Google Scholar]
- 29.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 31.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 32.Sotiropoulou P, Perez S, Gritzapis A, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Wang J, Wang M, et al. Activation of bone marrow-derived mesenchymal stromal cells—a new mechanism of defocused low-energy shock wave in regenerative medicine. Cytotherapy. 2013;15:1449–1457. doi: 10.1016/j.jcyt.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Mittermayr R, Antonic V, Hartinger J, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair Regen. 2012;20:456–465. doi: 10.1111/j.1524-475X.2012.00796.x. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri A, Imbimbo C, Longo N, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie’s disease. Eur Urol. 2009;56:363–369. doi: 10.1016/j.eururo.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Srirangam SJ, Manikandan R, Hussain J, et al. Long-term results of extracorporeal shockwave therapy for Peyronie’s disease. J Endourol. 2006;20:880–884. doi: 10.1089/end.2006.20.880. [DOI] [PubMed] [Google Scholar]
- 37.Husain J, Lynn NN, Jones DK, et al. Extracorporeal shock wave therapy in the management of Peyronie’s disease: initial experience. BJU Int. 2000;86:466–468. doi: 10.1046/j.1464-410x.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Salam Y, Budair Z, Renner C, et al. Treatment of Peyronie’s disease by extracorporeal shockwave therapy: evaluation of our preliminary results. J Endourol. 1999;13:549–552. doi: 10.1089/end.1999.13.549. [DOI] [PubMed] [Google Scholar]
- 39.Lebret T, Loison G, Hervé JM, et al. Extracorporeal shock wave therapy in the treatment of Peyronie’s disease: experience with standard lithotriptor (siemens-multiline). Urology. 2002;59:657–661. doi: 10.1016/s0090-4295(02)01527-3. [DOI] [PubMed] [Google Scholar]
- 40.Manikandan R, Islam W, Srinivasan V, et al. Evaluation of extracorporeal shock wave therapy in Peyronie’s disease. Urology. 2002;60:795–799; discussion 799. doi: 10.1016/s0090-4295(02)01970-2. [DOI] [PubMed] [Google Scholar]
- 41.Colombo F, Nicola M. [Peyronie’s disease: ultrasonographic follow-up of ESWT]. Arch Ital Urol Androl. 2000;72:388–391. [PubMed] [Google Scholar]
- 42.Hatzichristodoulou G, Meisner C, Gschwend JE, et al. Extracorporeal shock wave therapy in Peyronie’s disease: results of a placebo-controlled, prospective, randomized, single-blind study. J Sex Med. 2013;10:2815–2821. doi: 10.1111/jsm.12275. [DOI] [PubMed] [Google Scholar]
- 43.Poulakis V, Skriapas K, de Vries R, et al. Extracorporeal shockwave therapy for Peyronie’s disease: an alternative treatment? Asian J Androl. 2006;8:361–366. doi: 10.1111/j.1745-7262.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- 44.Strebel RT, Suter S, Sautter T, et al. Extracorporeal shockwave therapy for Peyronie’s disease does not correct penile deformity. Int J Impot Res. 2004;16:448–451. doi: 10.1038/sj.ijir.3901192. [DOI] [PubMed] [Google Scholar]
- 45.Ralph D, Gonzalez-Cadavid N, Mirone V, et al. The management of Peyronie’s disease: evidence-based 2010 guidelines. J Sex Med. 2010;7:2359–2374. doi: 10.1111/j.1743-6109.2010.01850.x. [DOI] [PubMed] [Google Scholar]
- 46.Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129. doi: 10.1111/j.1365-2141.2005.05409.x. [DOI] [PubMed] [Google Scholar]
- 47.Lander E, Berman M, See J. Autologous Adipose Derived Stromal Vascular Fraction Combined with Low Intensity Shock Wave Therapy for the Treatment of Peyronie’s Disease—A Pilot Study. Western Section-AUA Annual Meeting; 2013. pp. 3–7. [Google Scholar]
- 48.Hauck EW, Mueller UO, Bschleipfer T, et al. Extracorporeal shock wave therapy for Peyronie’s disease: exploratory meta-analysis of clinical trials. J Urol. 2004;171(2 Pt 1):740–745. doi: 10.1097/01.ju.0000108060.30363.8d. [DOI] [PubMed] [Google Scholar]


