Abstract
Background:
Autologous adipose tissue injection is used in plastic surgery for correction of localized tissue atrophy and has also been successfully offered for treatment of localized scleroderma. We aimed to evaluate whether patients with systemic sclerosis (SSc) and facial handicap could also benefit from this therapy.
Methods:
We included 14 patients (mean age of 53.8 ± 9.6 years) suffering from SSc with facial handicap defined by Mouth Handicap in Systemic Sclerosis Scale (MHISS) score more than or equal to 20, a Rodnan skin score on the face more than or equal to 1, and maximal mouth opening of less than 55 mm. Autologous adipose tissue injection was performed under local anesthesia using the technique of subcutaneous microinjection. The main objective of this study was an improvement of the MHISS score 6 months after the surgical treatment.
Results:
The procedure was well tolerated. We observed a mean decrease in the MHISS score of 10.7 points (±5.1; P < 0.0001) at 6 months (35% improvement). Secondary efficacy parameters assessing perioral skin sclerosis, maximum mouth opening, sicca syndrome, and facial pain significantly improved at 3 and 6 months postsurgery. At a 6-month follow-up, 75% of patients were satisfied or very satisfied of the adipose tissue microinjection therapy.
Conclusions:
Our study suggests that subcutaneous perioral microfat injection in patients with SSc is beneficial in the treatment of facial handicap, skin sclerosis, mouth opening limitation, sicca syndrome, and facial pain. Thus, this minimally invasive approach offers a new hope for face therapy for patients with SSc.
Facial manifestations have been described in systemic sclerosis (SSc) since the end of the 1980s.1 Patients with SSc often have perioral skin sclerosis leading to mouth opening limitation (MOL), interfering considerably with eating, speaking, oral hygiene, intubation, and dental treatment.1 In the study by Vincent et al2 on 30 patients with SSc, face cutaneous hardening was found in more than 90% of cases, with facial cutaneous atrophy (93%), perioral wrinkles with disappearance of the frontal folds (83%), and MOL (70%). Xerostomia is also a frequent symptom that concerns 70% of the patients.1,3 Temporomandibular pain can be observed in 30% of cases.2 It is estimated that orofacial manifestations concern almost 80% of patients with SSc.2,4 To date, face involvement can be considered by physicians as a minor consequence of the disease and can often be overlooked. Nevertheless, this facial transformation can be disfiguring and be a source of functional, aesthetic, and social discomfort with a significant psychological stress for patients.
In recent decades, autologous adipose tissue (AT) grafting has been successfully used to correct volume and contour defects. In addition to a filling effect, grafted fat seems to influence dynamic tissue regeneration at the recipient site5,6 and is commonly used for the regeneration of the skin for different indications such as radiodermitis,7 wound healing,8 and localized forms of scleroderma such as “en coup de sabre scleroderma.”9–11
When compared with Coleman fat grafting, microfat grafting12 that involves the harvest and injection of only microlobules of AT (around 500 μm) was able to decrease skin fibrosis and improve peripheral vascularization in mice with bleomycin-induced skin sclerosis.13 It was recently demonstrated that in vitro viability and migration of isolated adipose stromal cells (ASCs) obtained from micro-harvested lipoaspirates were significantly higher than the conventional fat harvesting by the Coleman cannula (3 mm, 1-hole blunt tip). Moreover, a significant high adherence rate of isolated ASCs from the microfat harvesting technique onto matrices was observed.14 Thus, we suggested that microfat grafting may be more suitable for tissue-regenerative approach in SSc. In this study, we aimed to assess for the first time the safety and efficacy of autologous microfat grafting in the face of patients with SSc and facial handicap.
PATIENTS AND METHODS
Study Design, Subjects, and Eligibility Criteria
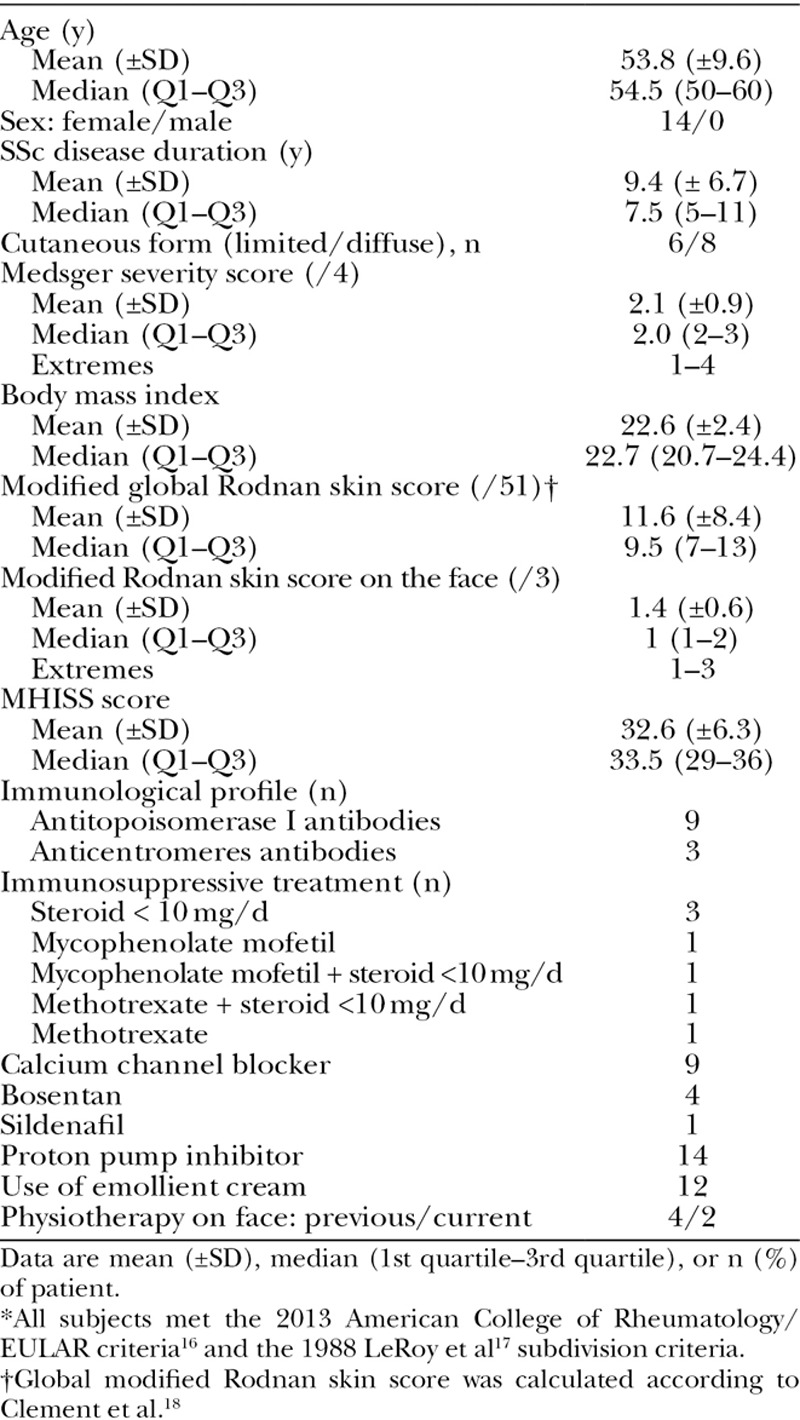
This was a single-center, open-label study performed in Marseille (France). Longitudinal intrasubject analyses were performed. Inclusion criteria were as follows: (1) Mouth Handicap in Systemic Sclerosis Scale (MHISS) score more than or equal to 20 [(0–48)15; Table 1], (2) Rodnan skin score on the face more than or equal to 1 (ie, mild skin sclerosis on a scale from 0 to 3), and (3) maximal mouth opening of less than 55 mm. The main characteristics of 14 patients are shown (Table 2). No patient was treated with a dose of steroids more than 10 mg/day or with cyclophosphamide on entering the study. Two patients were treated with mycophenolate mofetil and 2 others with methotrexate. One patient died 44 days after the surgical treatment from an acute disease unrelated to the autologous fat grafting. One patient refused the 6-month follow-up. Thus, only 12 patients were assessed at 6 months. Patients were asked not to change their regular medications and physical therapy of the face during the trial.
Table 1.
The Mouth Handicap in Systemic Sclerosis scale15
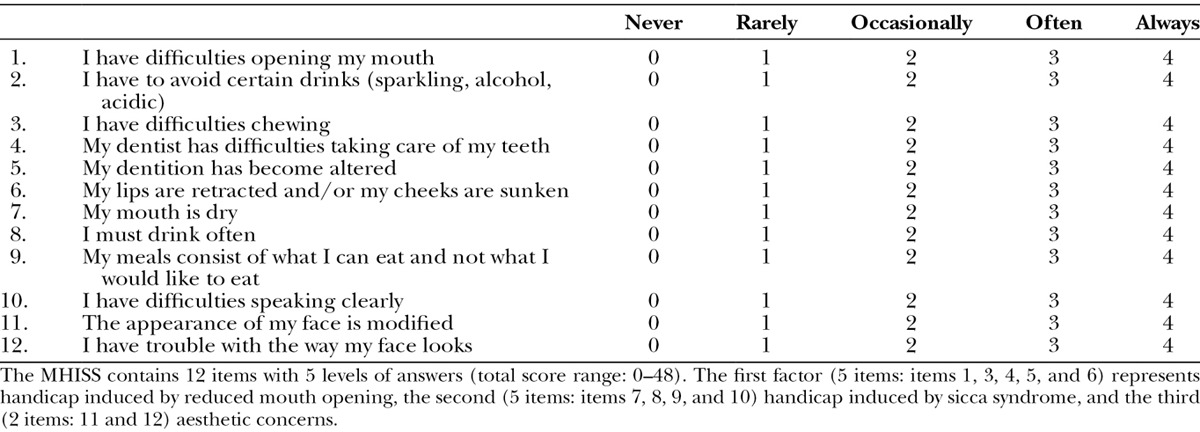
Table 2.
Characteristics of Systemic Sclerosis Patients* (n = 14)
This study was registered with ClinicalTrials.gov number NCT02206672. The Local Patient Protection Committee expressed a favorable opinion, and informed consent for patients’ participation in this study was obtained.
Tissue Collection and Fat Preparation
Surgery was performed by the same surgeon under local anesthesia using the technique of subcutaneous microinjection of autologous fat.12 The zones of liposuction were marked before surgery, with a priority for the inner side of the knees, followed by the abdomen and hips. The entry points for the infiltration cannula were anesthetized with pure 1% adrenaline and lidocaine with a 30-gauge (0.25 mm) needle. An infiltration was then carried out in the area with a 14-gauge cannula with 10 ml of 1% adrenaline and lidocaine diluted in 20 ml of physiological salt solution, with an injected volume at each entry point of 0.5 ml. Liposuction was performed with a 14-gauge (2-mm cannula height holes of less than 1-mm blunt tip) connected to a faucet in 3 ways with 2 valves antireturn, with a 10-ml syringe maintaining the plunger in contact with the fat tissue and with a negative pressure less than 350 mm Hg. The number of sites of fat harvest was between 2 and 4 per patient.
The harvested fat was directly injected into a closed-circuit PureGraft 50 ml system filtration pocket (Puregraft, San Diego, Calif.). The PureGraft system allows the purification of AT by elimination of fluid excess, lipid phase, blood cells, and fragments through filtration by membrane in a sterile environment. The quantity of harvested fat was about 50 ml per patient, which allowed obtaining a final volume of pure fat ranging from 20 to 25 ml. The cleaned tissue was directly retrieved by connecting the PureGraft system to syringes of 1 ml to allow a precise reinjection into the face.
Fat Delivery
Entry points were anesthetized with 0.5 ml of pure 1% adrenaline and lidocaine using a 30-gauge (0.25 mm) needle. Four entry points were determined in the perioral region: 2 at the level of nasogenien grooves (1 cm from the corner of the mouth) and 2 at the level of the lower lip (1 cm below each corner of the mouth).
The penetration of the skin and the dermis was carried out using a 21-gauge (0.8 mm) needle, which was then replaced by a cannula of the same diameter. Subcutaneous injections were performed into different directions from each injection site. The quantity of fat to be injected was determined based on the morphology of patient’s face.
Assessment of Safety and Efficacy
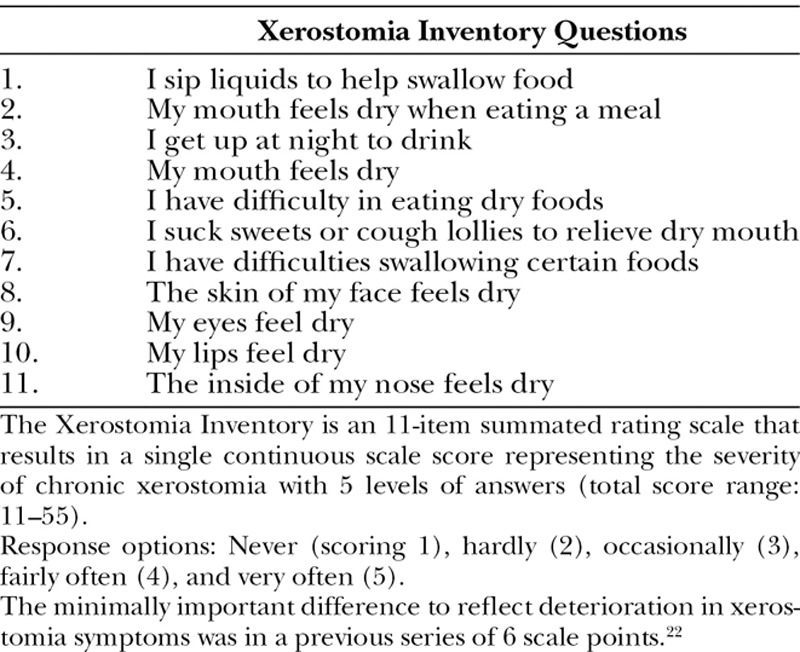
Safety was assessed at day +7, day +21, and 3 and 6 months after the procedure. Facial handicap was assessed by the MHISS,15 which includes 12 items (score 0–48) and estimates 3 domains: handicap induced by MOL and sicca syndrome, and aesthetic facial concerns. The following secondary efficacy parameters were assessed at 3 and 6 months. (1) Skin sclerosis: Rodnan skin score on face (0 = normal skin; 1 = mild thickness; 2 = moderate thickness; and 3 = severe thickness with inability to pinch the skin into a fold), maximal mouth opening between the incisive edge of the upper and lower first incisors measuring in millimeters,19 visual analog scale (VAS; 0–100) for mouth opening, and skin elasticity assessed by a suction skin elasticity meter (Cutometer dual MPA 580, Courage & Khazaka Electronic GmbH, Cologne, Germany) at 4 locations (2 points in the nasolabial region and 2 points in the cheekbone region) using Dobrev’s parameters with a 2-mm diameter measuring probe.20 (2) Sicca syndrome: Xerostomia Inventory questionnaire (11 = no discomfort to 55 = severe discomfort; Table 3),21,22 sugar test (time required to melt a sugar without crunching it), and VAS (0–100) for sicca syndrome. (3) Pain induced by the palpation of masseters and temporal muscles (VAS 0–100) and facial pain (VAS 0–100). (4) Volumizing and aesthetic effect: standard photographs performed by the same operator and with the same incidences and light. (5) Satisfaction assessment: Patients were asked to fill out a 4-point scale from very satisfied to unsatisfied, 6 months after fat microinjection. (6) Global disability: The Health Assessment Questionnaire (HAQ) adapted to SSc (SSc-HAQ) was used. It includes the HAQ disease index and 5 specific VAS (scale 0–3).23
Table 3.
The Xerostomia Inventory21
Statistical Analysis
Adverse events were coded by the dictionary MedDRA (Medical Dictionary for Regulatory Activities). Efficacy endpoints were continuous or ordinal variables. Data are shown as mean ± SD and median and range (1st quartile to 3rd quartile). Results of the assessment at 3 and 6 months of the procedure were compared from baseline using a single sample test on mean of 0. Normal distribution was examined by Shapiro-Wilk test. Normally distributed were analyzed by 1 sample t test. Nonparametric data were analyzed by Wilcoxon signed-rank test. Spearman r coefficient for correlation test was used. A nominal P ≤ 0.05 (2 sided) was considered significant. Results were not corrected for multiple comparisons. Statistical analyses were performed using SAS (SAS Institute Inc., version 9.2, Cary, N.C.).
RESULTS
Fourteen patients with SSc, all women, with mean age of 53.8 years (±9.6) were enrolled and treated in the same month (Table 2).
Safety Assessment
Mean quantity of purified fat injection was 16.3 ml (±4.7 ml) and median quantity was 17 ml with extremes from 6.2 to 23 ml. The surgeon noticed difficulties in fat reinjection for 5 patients because of skin fibrosis but without preventing the good progress of surgery. The duration of the procedure from skin disinfection to bandages was 60 to 90 minutes.
No infectious complication related to the procedure was recorded. As classically reported after liposuction, small areas of bruising at the zones of fat harvest were observed in 8 patients and local pain in 3 patients. Concerning perioral fat reinjection, injection site bruising (n = 3), pain (n = 3), perioral sensitive manifestation (n = 1), and trigeminal neuralgia (n = 1) were observed. All symptoms were of mild to moderate intensity and spontaneously resolved in a few days.
Improvement of MHISS Score in Systemic Sclerosis Patients Treated by Autologous Fat Microinjection
At baseline, the mean MHISS score was 32.6 (±6.3) and significantly decreased to 25.3 (±11.7) at 3 months and to 21.8 (±8.9) at 6 months. The reduction of the MHISS score at 6 months corresponded to a 34.6% of improvement from baseline, with a mean decrease in the MHISS score of 10.7 points (±5.1; P < 0.001). A 50% improvement of the MHISS score was observed for 3 patients 3 months after the procedure, and lasted until the 6-month follow-up (Table 4). Contribution of each domain of the MHISS score showed improvement. For “oral opening” domain (on 24 points), we noticed a significant improvement of 3.2 points (±6.3) at 3 months and 5.9 points (±3.9) at 6 months. For “dry syndrome” domain (on 16 points), a significant improvement of 2.4 points (±2.7) at 3 months, which was lasting at 6 months (2.4 points ±1.7), was also observed. Finally, for “aesthetic concerns” domain (on 8 points), a significant improvement of 1.5 (±1.8) at 3 months and 2.3 (±1.9) at 6 months was noted.
Table 4.
MHISS Outcome After Microinjection of Autologous Adipose Tissue in the Face of Patients with SSc (Total Score and Details on the 3 Domains of the Score)

The MHISS score was not different according to the cutaneous limited or diffuse subgroup, respectively, at baseline of 33.7 (±3.9) and 31.8 (±7.8; P = 0.593). Both subgroups improved at 3 months to 25.2 (±13.0) and 25.4 (±11.8; P = 0.980) and at 6 months, respectively, to 21.0 (±7.0) and 22.3 (±10.2; P = 0.832). No correlation between the MHISS score improvement and the volume of injected fat was observed at both tested periods (month 3: r = −0.12, P = 0.707 and month 6: r = −0.11, P = 0.729).
Evaluation of Facial Skin Fibrosis
Although the global modified Rodnan skin score did not change during the study, a 57.7% and 54.2% reduction of the face-specific modified Rodnan skin score was observed at 3 (P = 0.008) and 6 months (P = 0.016), respectively (Table 5). When focused on the perioral area, a 79.5% and 65.3% improvement of the skin sclerosis was observed at 3 (P < 0.001) and 6 months (P = 0.002), respectively.
Table 5.
Outcome of Several Parameters Related to Sclerosis, Oral Opening, Sicca Syndrome, Pain, and Quality of Life
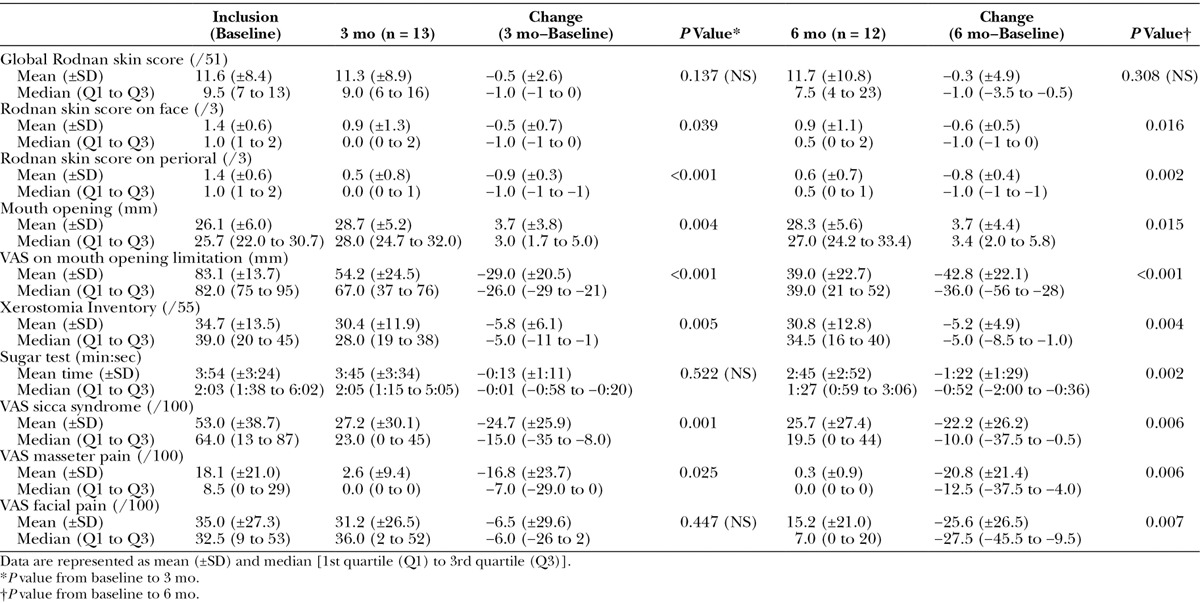
Mouth opening significantly increased at 3 (+3.7 ± 3.8 mm) and 6 months (+3.7 mm ± 4.4 mm). There was a significant decrease in the VAS assessing discomfort related to MOL at 3 (35.7%) and 6 months (52.2%; P < 0.001). Cutometer measurement of skin elasticity showed that there was no significant change at 3 and 6 months of the procedure (data not shown).
Evaluation of the Oral Sicca Syndrome
At 3 and 6 months after fat grafting, the Xerostomia Inventory score significantly improved with a reduction of 5.2 points (±4.9) at 6 months. A significant decrease in the time to melt a sugar on the tongue of 1 minute 22 seconds (±1 minute 29 seconds) in comparison with baseline was observed at 6 months. In this context, a 57.7% and 53% reduction of the VAS focused on handicap related to dry mouth was observed at 3 (P < 0.001) and 6 months (P = 0.003), respectively.
Evaluation of Facial Pain
Pain induced by the palpation of the muscles of the face was mild at inclusion, but it significantly improved at 3 and 6 months (P = 0.025 and P = 0.006, respectively). Similarly, VAS for facial pain showed a 39.4% reduction at 3 months (P = 0.027) and a 62.8% reduction at 6 months (P = 0.010).
Volumizing and Aesthetic Effects Assessed on Standard Photographs
Improvement of perioral folds and mouth opening was clinically obvious for some patients as illustrated in Figures 1 through 6.
Fig. 1.

A 58-year-old patient with a diffuse cutaneous form of systemic sclerosis before (M0) surgery.
Fig. 6.
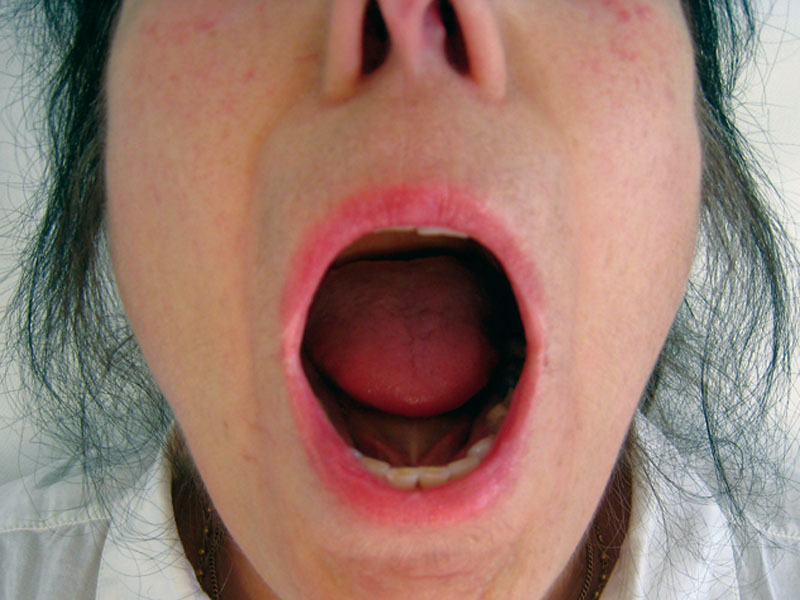
The same patient after 6 months (M6). Mouth opening was of 25 mm at inclusion and 35 mm at M6.
Fig. 2.
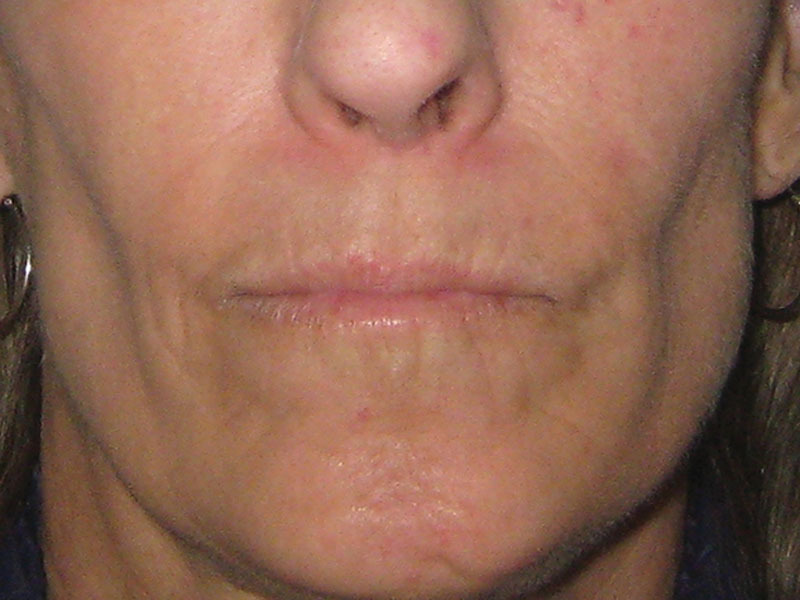
The same patient at 6 months (M6) of the reinjection of 16 ml of adipose tissue on the face.
Fig. 3.
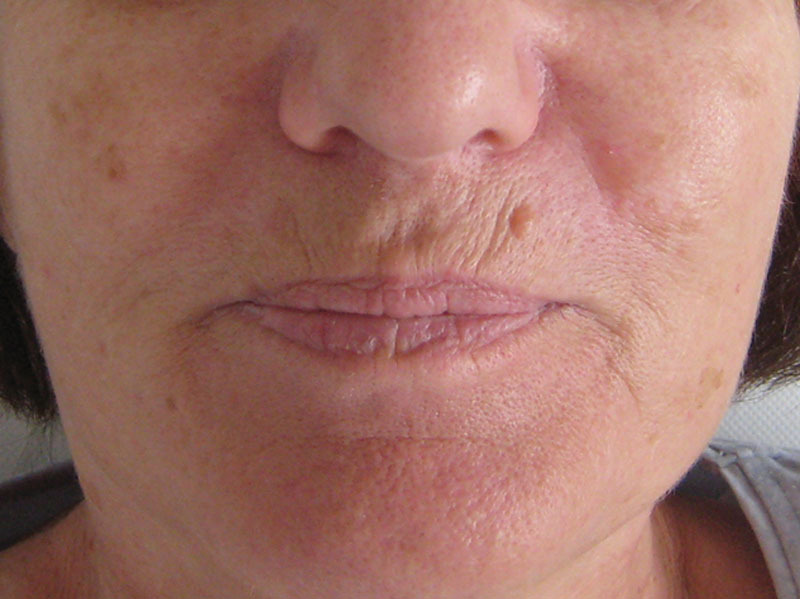
A 67-year-old patient with a limited cutaneous form before (M0) surgery.
Fig. 4.
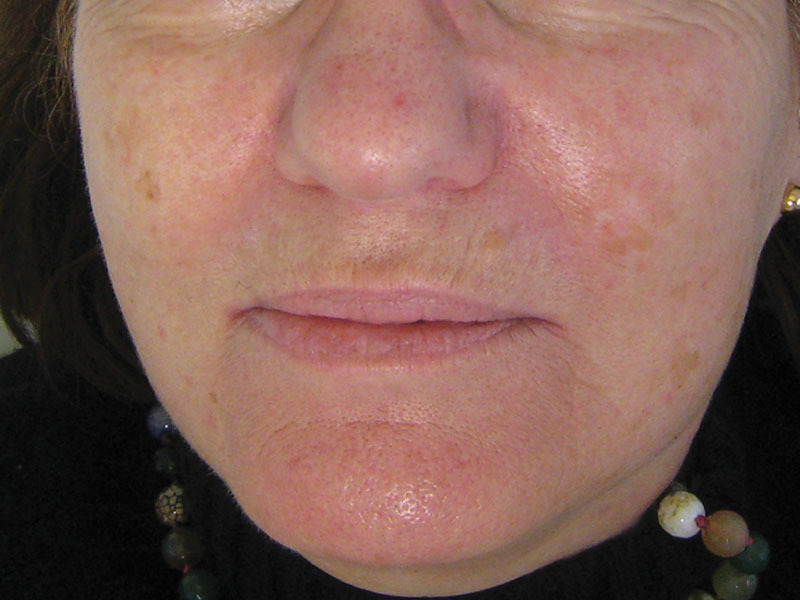
The same patient after 6 months (M6) of the microreinjection of 11.6 ml of adipose tissue.
Fig. 5.
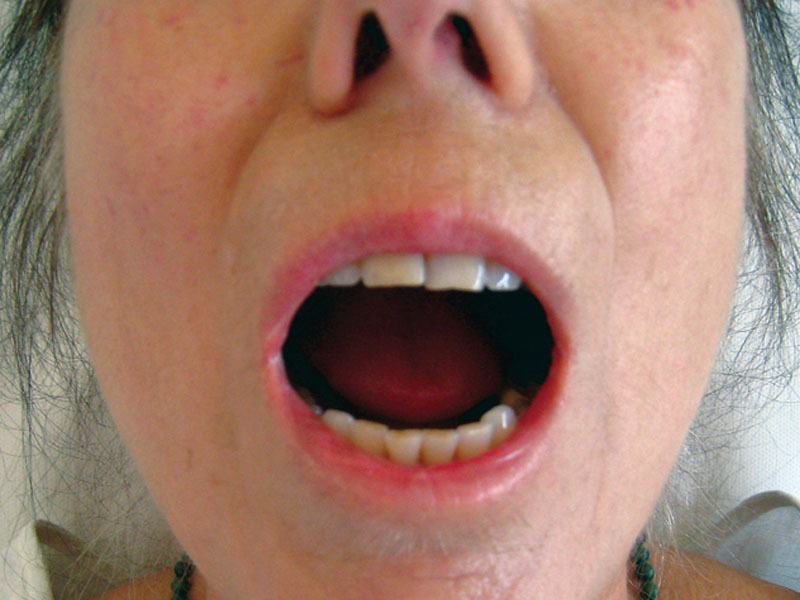
A 60-year-old patient with a diffuse cutaneous form of the disease when opening the mouth before surgery (M0).
Patient’s Satisfaction
At 6 months of the procedure, 9 of 12 patients declared to be either very satisfied (n = 4) or satisfied (n = 5), 2 patients moderately satisfied, and 1 patient remained unsatisfied.
Impact on Global Disability
When assessing the impact of the therapy on global disability related to the disease, no significant change was observed: mean SSc-HAQ score at inclusion was of 1.26 points (±0.38) and remained at 3 and 6 months, respectively, of 1.07 (±0.53) and 1.25 (±0.74). No significant correlation between the MHISS score and the SSc-HAQ score was observed whatever the tested period (month 0: r = 0.23, P = 0.133; month 3: r = 0.54, P = 0.053; and month 6: r = 0.30, P = 0.336).
DISCUSSION
Facial handicap in scleroderma patients is often overlooked, but it is highly important for the patient’s quality of life, and therapeutic approaches are lacking. Until now, standard treatment has relied on a facial stretching program approach, but the observed functional improvement seems to be largely dependent on the continuity and repetition of exercises.24
The goal of our study was to assess whether autologous fat microinjection can improve facial sclerosis, sicca syndrome, aesthetic aspects, and quality of life. Our study shows that autologous AT microinjection is safe in patients with SSc and improves facial handicap, as shown by the significant reduction of the MHISS score at 3 and 6 months after surgery including a significant improvement for each domain (mouth opening, sicca syndrome, and aesthetic discomfort). Complementary parameters specifically assessing mouth opening (such as maximal mouth opening measure) and sicca syndrome (such as Xerostomia Inventory and sugar test time) confirmed the significant improvement at 6 months. Thus, both subjective and objective parameters confirmed the beneficial results.
Del Papa et al25 recently reported a significant clinical improvement in oral opening in 20 patients with SSc using the Coleman method. This study showed safety and feasibility of a different surgical procedure and a significant improvement of the mouth opening capacity after perioral fat grafting, with a benefit obtained as early as 3 months after the procedure. In comparison with our study, a similar mean quantity of purified fat reinjection was performed (16 ml), and the statistically significant increase of the maximum interincisive distance at 3 months in the study by Del Papa et al (mean, +2.63 mm) was very close to the one observed in this study (+3.7 mm).
To note, only patients with SSc with the diffuse cutaneous form were included in the study by Del Papa et al, whereas limited and diffuse cutaneous forms with facial skin sclerosis were included in this study. Follow-up was only 3 months in Del Papa et al’s study design, whereas we performed a longer follow-up of 6 months. Coleman’s technique was used in the study by Del Papa et al, whereas we performed the microinjection protocol. Then, we used the PureGraft filtration technique that offers a better fat quality by reducing blood cell and free lipid content, with a greater AT viability when compared with gravity separation and centrifugation methods.14 We used the MHISS questionnaire validated in patients15 with SSc that allowed us to appreciate the positive effect on facial handicap in 3 domains: reduction in mouth opening, sicca syndrome, and aesthetic concerns. We also specifically analyzed the sicca syndrome and showed an improvement of the Xerostomia Inventory questionnaire and the delay to melt a sugar on the tongue and the VAS focused on handicap related to sicca syndrome. Thus, this study demonstrates that not only autologous fat graft has a beneficial effect on skin sclerosis in both limited and diffuse cutaneous forms of the disease but also that the effect persists up to 6 months and has benefits extending to facial handicap, sicca syndrome, and facial pain.
The beneficial effect of autologous AT microinjection cannot be ascribed solely to the filling effect because no correlation between the MHISS scores’ improvement and the volume of fat reinjection was shown in our study.
The trophic properties of AT are mainly because of the stromal vascular fraction (SVF) of AT, which appears to have angiogenic, antiinflammatory, and immunomodulatory effects.26 SVF contains mesenchymal stem cells (called ASCs), endothelial progenitor cells, hematopoietic cells, preadipocytes, and pericytes.26,27 ASCs are able to differentiate into the cells of various lineages,26,27 to secrete angiogenic factors, and to have an immunomodulatory effect that appear to involve different mechanisms.28,29 Hematopoietic and endothelial progenitor cells27,30 are able to promote the vascular growth or vascular repair by the secretion of major growth factors and cytokines involved in wound healing and neovascularization and could limit tissue damage by the production of antiapoptotic and antiinflammatory factors.26,27,31,32 Innate immunity cells and cells of adaptive immunity are likely to participate in an important immunoregulatory effect but are still poorly documented. The role of these subpopulations in the beneficial effect observed in fat graft is still unknown. It is hypothesized that AT-derived cells fraction of the unprocessed AT including adipocytes and SVF may create an optimal environment that protect the perivascular niche and support the regenerative effect of cells and growth factors within host tissues. ASC-based therapy have recently shown interest in localized scleroderma: Scuderi et al33 assessed the effect of local injections of a combination of ex vivo expanded ASC and hyaluronic acid solution in 6 patients with morphea. They reported an arrest of local disease progression, a regression of dyschromia, and an improvement of skin softening and sensitivity.
One limit of current and previous studies is the time frame of the follow-up. Although convention would suggest that the maximal effects of fat grafting can be assessed as early as 3 months after injection, there is a need for long-term follow-up on the background of the SSc phenotype. For that, we planned to evaluate patients 12 months postsurgery. Faced with these encouraging results obtained in open-labeled studies, larger and controlled studies in scleroderma disease are required.
ACKNOWLEDGMENTS
We thank Doctor Christine Serratrice and Professor Jean Gabriel Fuzibet for the recruitment of 3 patients with SSc who participated in the study. We thank the Centre d’Investigation Clinique for having welcomed the patients, and Joelle Micallef and Estelle Charles-Baumel from the Centre d’Investigation Clinique - Centre de Pharmacologie Clinique et d’Evaluations Thérapeutiques (CIC-CPCET), Assistance Publique Hôpitaux de Marseilles.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Wood RE, Lee P. Analysis of the oral manifestations of systemic sclerosis (scleroderma). Oral Surg Oral Med Oral Pathol. 1988;65:172–178. doi: 10.1016/0030-4220(88)90161-2. [DOI] [PubMed] [Google Scholar]
- 2.Vincent C, Agard C, Barbarot S, et al. [Orofacial manifestations of systemic sclerosis: a study of 30 consecutive patients]. Rev Med Interne. 2009;30:5–11. doi: 10.1016/j.revmed.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Avouac J, Sordet C, Depinay C, et al. Systemic sclerosis-associated Sjögren’s syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum. 2006;54:2243–2249. doi: 10.1002/art.21922. [DOI] [PubMed] [Google Scholar]
- 4.Scardina GA, Pizzigatti ME, Messina P. Periodontal microcirculatory abnormalities in patients with systemic sclerosis. J Periodontol. 2005;76:1991–1995. doi: 10.1902/jop.2005.76.11.1991. [DOI] [PubMed] [Google Scholar]
- 5.Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S–120S. doi: 10.1097/01.prs.0000234610.81672.e7. [DOI] [PubMed] [Google Scholar]
- 6.Meruane MA, Rojas M, Marcelain K. The use of adipose tissue-derived stem cells within a dermal substitute improves skin regeneration by increasing neoangiogenesis and collagen synthesis. Plast Reconstr Surg. 2012;130:53–63. doi: 10.1097/PRS.0b013e3182547e04. [DOI] [PubMed] [Google Scholar]
- 7.Sultan SM, Stern CS, Allen RJ, Jr, et al. Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg. 2011;128:363–372. doi: 10.1097/PRS.0b013e31821e6e90. [DOI] [PubMed] [Google Scholar]
- 8.Klinger M, Caviggioli F, Vinci V, et al. Treatment of chronic posttraumatic ulcers using autologous fat graft. Plast Reconstr Surg. 2010;126:154e–155e. doi: 10.1097/PRS.0b013e3181e3b585. [DOI] [PubMed] [Google Scholar]
- 9.Oh CK, Lee J, Jang BS, et al. Treatment of atrophies secondary to trilinear scleroderma en coup de sabre by autologous tissue cocktail injection. Dermatol Surg. 2003;29:1073–1075. doi: 10.1046/j.1524-4725.2003.29307.x. [DOI] [PubMed] [Google Scholar]
- 10.Palmero ML, Uziel Y, Laxer RM, et al. En coup de sabre scleroderma and Parry-Romberg syndrome in adolescents: surgical options and patient-related outcomes. J Rheumatol. 2010;37:2174–2179. doi: 10.3899/jrheum.100062. [DOI] [PubMed] [Google Scholar]
- 11.Consorti G, Tieghi R, Clauser LC. Frontal linear scleroderma: long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J Craniofac Surg. 2012;23:e263–e265. doi: 10.1097/SCS.0b013e31824ef7e8. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen PS, Desouches C, Gay AM, et al. Development of micro-injection as an innovative autologous fat graft technique: the use of adipose tissue as dermal filler. J Plast Reconstr Aesthet Surg. 2012;65:1692–1699. doi: 10.1016/j.bjps.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Djaffar O-A. Bleomycin-induced scleroderma in nude mice can be reversed by injection of adipose tissue: evidence for a novel therapeutic intervention in systemic sclerosis. J Clin Exp Dermatol Res. 2012;3:164–168. [Google Scholar]
- 14.Alharbi Z, Opländer C, Almakadi S, et al. Conventional vs. micro-fat harvesting: how fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J Plast Reconstr Aesthet Surg. 2013;66:1271–1278. doi: 10.1016/j.bjps.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Mouthon L, Rannou F, Bérezné A, et al. Development and validation of a scale for mouth handicap in systemic sclerosis: the Mouth Handicap in Systemic Sclerosis scale. Ann Rheum Dis. 2007;66:1651–1655. doi: 10.1136/ard.2007.070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 17.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 18.Clements PJ, Lachenbruch PA, Seibold JR, et al. Skin thickness score in systemic sclerosis: an assessment of interobserver variability in 3 independent studies. J Rheumatol. 1993;20:1892–1896. [PubMed] [Google Scholar]
- 19.Placko G, Bellot-Samson V, Brunet S, et al. [Normal mouth opening in the adult French population]. Rev Stomatol Chir Maxillofac. 2005;106:267–271. doi: 10.1016/s0035-1768(05)86038-3. [DOI] [PubMed] [Google Scholar]
- 20.Dobrev HP. In vivo study of skin mechanical properties in patients with systemic sclerosis. J Am Acad Dermatol. 1999;40:436–442. doi: 10.1016/s0190-9622(99)70494-9. [DOI] [PubMed] [Google Scholar]
- 21.Thomson WM, Chalmers JM, Spencer AJ, et al. The Xerostomia Inventory: a multi-item approach to measuring dry mouth. Community Dent Health. 1999;16:12–17. [PubMed] [Google Scholar]
- 22.Thomson WM. Measuring change in dry-mouth symptoms over time using the Xerostomia Inventory. Gerodontology. 2007;24:30–35. doi: 10.1111/j.1741-2358.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 23.Poole JL, Steen VD. The use of the Health Assessment Questionnaire (HAQ) to determine physical disability in systemic sclerosis. Arthritis Care Res. 1991;4:27–31. doi: 10.1002/art.1790040106. [DOI] [PubMed] [Google Scholar]
- 24.Maddali-Bongi S, Landi G, Galluccio F, et al. The rehabilitation of facial involvement in systemic sclerosis: efficacy of the combination of connective tissue massage, Kabat’s technique and kinesitherapy: a randomized controlled trial. Rheumatol Int. 2011;31:895–901. doi: 10.1007/s00296-010-1382-9. [DOI] [PubMed] [Google Scholar]
- 25.Del Papa N, Caviggioli F, Sambataro D, et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transplant. 2015;24:63–72. doi: 10.3727/096368914X674062. [DOI] [PubMed] [Google Scholar]
- 26.Zuk PA. Adipose-derived stem cells in tissue regeneration: a review. ISRN Stem Cells. 2013:35. [Google Scholar]
- 27.Ong WK, Sugii S. Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol. 2013;45:1083–1086. doi: 10.1016/j.biocel.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Leto Barone AA, Khalifian S, Lee WP, et al. Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int. 2013;2013:383685. doi: 10.1155/2013/383685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichim TE, Alexandrescu DT, Solano F, et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol. 2010;260:75–82. doi: 10.1016/j.cellimm.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y. Tumor angiogenesis and therapy. Biomed Pharmacother. 2005;59(suppl 2):S340–S343. doi: 10.1016/s0753-3322(05)80070-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakagami H, Morishita R, Maeda K, et al. Adipose tissue-derived stromal cells as a novel option for regenerative cell therapy. J Atheroscler Thromb. 2006;13:77–81. doi: 10.5551/jat.13.77. [DOI] [PubMed] [Google Scholar]
- 32.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 33.Scuderi N, Anniboletti T, Carlesimo B, et al. Clinical application of autologous three-cellular cultured skin substitutes based on esterified hyaluronic acid scaffold: our experience. In Vivo. 2009;23:991–1003. [PubMed] [Google Scholar]


