Abstract
Importance
Anti-type VII collagen autoantibodies are often detectable in patients with bullous systemic lupus erythematosus (BSLE); however their timing of appearance preceding onset of disease is unknown.
Observations
We report the case of a 50-year-old female with a history of systemic lupus erythematosus who presented with vesicles and bullae around her lips, trunk, axillae, arms, and thighs. Histologic analysis as well as immunofluorescence and immunoblot studies confirmed the diagnosis of BSLE. Immunoblotting and ELISA studies of the patient’s serum obtained three months prior to the onset of BSLE showed presence of anti-type VII collagen autoantibodies. Levels of anti-type VII collagen IgG increased after bullous lesions appeared. Within one month after initiating dapsone and increasing the dose of prednisone, skin lesions promptly resolved. A year after onset of BSLE, her anti-type VII collagen IgG decreased below levels observed prior to the inception of her bullous lesions.
Conclusions and Relevance
This study shows that anti-type VII collagen autoantibodies can precede the clinical appearance of BSLE. The subsequent increase and decrease in the levels of circulating anti-type VII collagen autoantibodies, which mirrored skin disease activity, support a potential role in their initiation of disease.
Keywords: bullous systemic lupus erythematosus, systemic lupus erythematosus, type VII collagen autoantibodies
INTRODUCTION
Bullous systemic lupus erythematosus (BSLE) is a rare vesiculobullous eruption favoring photoexposed areas and mucous membranes. Vesicles and bullae of varying sizes can appear with crusting, and resolve as hyperpigmented patches. The absence of milia and scarring as well as the prominence in trauma-prone areas distinguishes this entity from epidermolysis bullosa acquisita (EBA). The histology of BSLE primarily shows subepidermal blisters and neutrophilic upper dermal infiltrates; direct immunofluorescence studies of normal appearing perilesional skin display immunoglobulin and complement deposition at the basement membrane zone.1
While other antigenic targets such as bullous pemphigoid antigen 1, laminin-5, and laminin-6, have been reported in cases with BSLE,2 anti-type VII collagen autoantibodies have been detected in the sera of many patients with BSLE.3 As the major component of the anchoring fibrils, type VII collagen links the lamina densa to the underlying dermis.4 While autoantibodies in the sera of patients with SLE prior to their diagnosis have been previously observed,5 whether or not circulating anti-type VII collagen autoantibodies are present prior to the appearance of BSLE is unknown. We describe a SLE patient whose serum contained IgG anti-type VII collagen autoantibodies before BSLE onset. Moreover, after her BSLE resolved, her anti-type VII collagen IgG levels diminished below those documented prior to the onset of her immunobullous disease.
REPORT OF A CASE
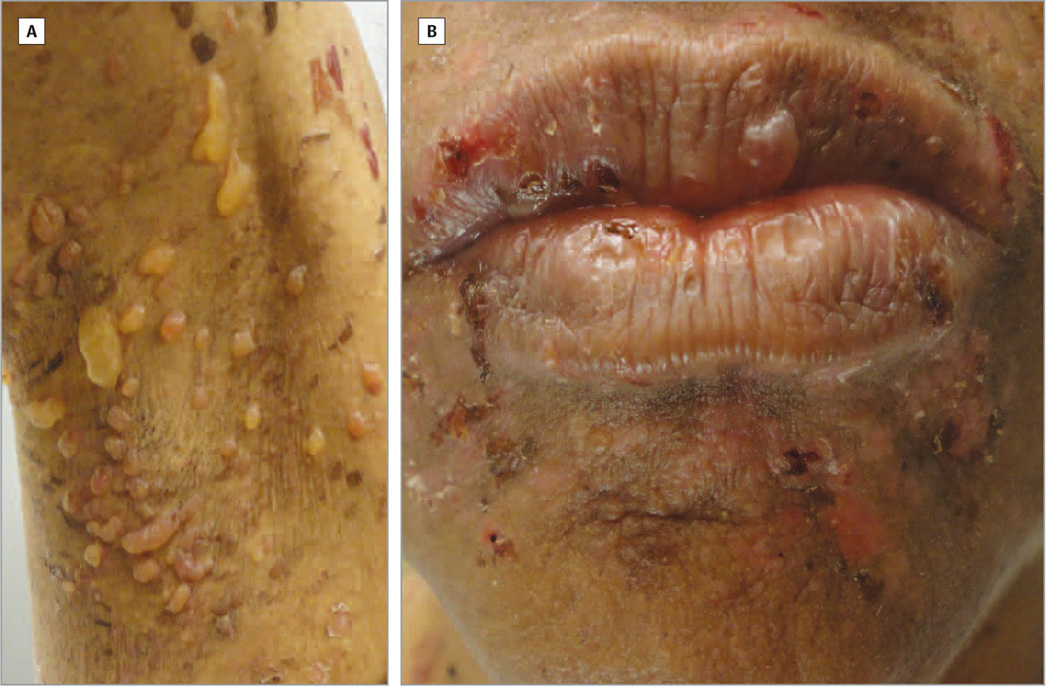
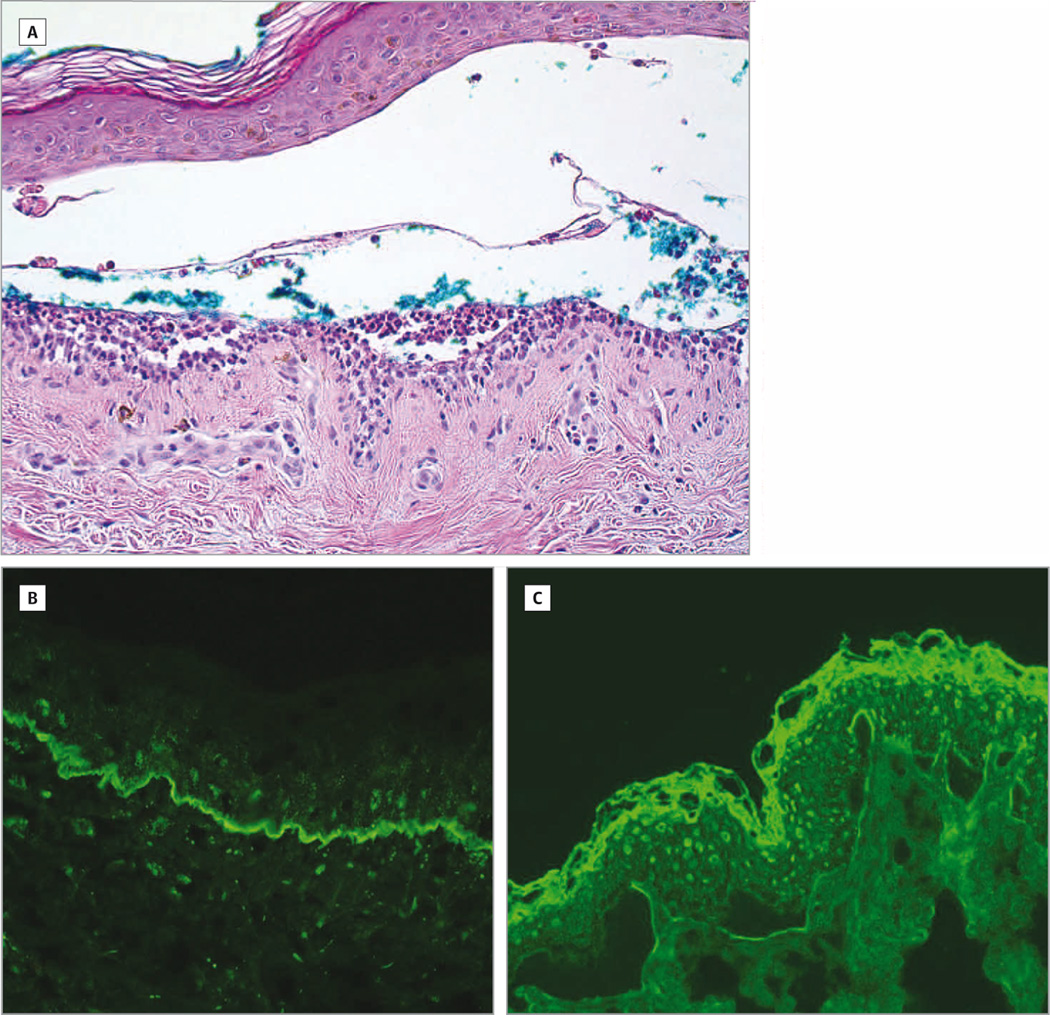
A 50 year-old African American female with a six-month history of SLE (with the following positive American College of Rheumatology SLE criteria: discoid lupus erythematosus (DLE), photosensitivity, oral ulcers, arthritis, positive anti-nuclear antibody test, and immunologic disorder (positive anti-Smith antibodies)) and type II diabetes presented to the University of Texas Southwestern (UTSW) Dermatology outpatient clinic with a three-week history of a pruritic, vesiculobullous eruption covering her perioral area, trunk, axillae, arms, and inner thighs. At the onset of the eruption, the patient was on prednisone 7.5 mg daily, which was tapered from 15 mg daily a month ago. She was also taking chloroquine 250 mg daily on weekdays and 125 mg daily on weekends, and mycophenolate mofetil 500 mg twice daily for the past three months. In response to the rash and presumed lupus flare due to her arthritis, elevated double-stranded DNA titers and low complement levels, her rheumatologist increased her prednisone dose to 30 mg daily. The patient also stopped her mycophenolate mofetil and chloroquine herself because she was concerned about drug reactions. On physical examination, multiple tense vesicles and bullae with hemorrhagic crusting and annular erythematous plaques were observed on her upper arms, forearms, axillae (Figure 1A), eyebrows, perioral area (Figure 1B), chest, abdomen, back, and inner thighs. The patient had diffuse scarring alopecia on her scalp with hypopigmented patches and underlying erythema on the crown consistent with DLE. A biopsy from the edge of a bulla in the right upper arm showed a subepidermal vesiculobullous dermatosis with neutrophils, occasional lymphocytes, and red blood cells within the blister cavity and a sparse perivascular infiltrate with lymphocytes and neutrophils (Figure 2A). Direct immunofluorescence showed a linear pattern of IgG (Figure 2B), C3, and IgA along the basement membrane zone. Indirect immunofluorescence studies of the BSLE patient’s serum on salt-split skin showed positive IgG binding to the dermal side at a titer of 1:10 (Figure 2C). Immunoblot studies of a recombinant of the noncollagenous (NC1) domain of type VII collagen confirmed that this patient had IgG autoantibodies directed against this signature autoantigen (Figure 3A). After the diagnosis of BSLE was made, the patient was treated with dapsone 50 mg twice daily and started on a tapering dose of prednisone starting at 40 mg daily. One month later, the patient experienced a significant decrease in vesicles. After three months of skin inactivity, the patient’s prednisone was discontinued, and the dapsone was discontinued four months later. Twelve months after the eruption, the patient had hyperpigmented macules and patches in her perioral area, axillae, trunk, and arms with no bullae and vesicles.
Figure 1.
BSLE in an African American female. A, Multiple small and large tense vesicles and bullae in the patient’s right axilla; B, Vesicles and bullae with surrounding erythematous plaques with hemorrhagic crusting in the perioral area.
Figure 2.
Pathology studies supporting BSLE diagnosis. A, Histologic analysis of the biopsy from the right upper arm showed subepidermal bulla with separation of the epidermis from the underlying dermis. Within the blister cavity and near the dermal-epidermal junction, there were neutrophils with occasional lymphocytes, red blood cells and a sparse perivascular infiltrate with lymphocytes and neutrophils. Magnification: 200×; B, Direct immunofluorescence studies showed IgG with a strong linear deposition along the basement membrane zone. Magnification: 200×; C, Indirect immunofluorescence studies of sera taken from the patient during her active BSLE flare showed IgG at a 1:10 titer binding to the dermal side of 1 M NaCl-split skin. Magnification: 200×.
Figure 3.
Anti-type VII collagen autoantibodies before, during, and after BSLE onset. A, Immunoblot studies on a recombinant of the NC1 domain of type VII collagen showed IgG binding to the signature autoantigen (arrow) in sera from patients with epidermolysis bullosa acquisita (lane 2) or BSLE prior to (lane 3) and during active skin disease (lane 4). Lane 1, normal control sera; lane 2, sera from EBA patient; lane 3, sera from BSLE patient at 1:40 dilution three months before eruption; lane 4, sera from BSLE patient at 1:40 dilution three weeks after rash onset; B, Indirect immunofluorescence study of the BSLE patient’s serum three months before disease onset showed IgG binding to the dermal side of the dermal-epidermal junction in salt-split skin test at a 1:5 titer. Magnification: 200×.
In addition, at three months prior to her BSLE onset, serum was collected from this patient, who was on a stable dose of prednisone 15 mg daily for two months, because the patient enrolled in the UTSW Cutaneous Lupus Registry, which is a longitudinal observational study of the disease course of cutaneous lupus patients. Twelve months after her BSLE appeared, another serum sample was drawn from the patient, who was taking chloroquine 250 mg daily for four months. Immunofluorescence studies of the serum drawn before BSLE onset on salt-split skin showed IgG bound to the dermal side at a titer of 1:5 (Figure 3B). Immunoblot studies of the NC1 type VII collagen recombinant again demonstrated IgG autoantibodies against this particular antigen in sera drawn three months before and three weeks after the eruption started (Figure 3A). Quantitation of IgG anti-type VII collagen autoantibodies by ELISAs (MBL International, Woburn, MA) revealed sequential unit values of 9.23 U/mL (3 months prior to disease onset), 71.74 U/mL (3 weeks after), and 2.30 U/mL (12 months after) during the course of this patient’s immunobullous lesions.
DISCUSSION
This report describes novel findings of a patient with BSLE with anti-type VII collagen autoantibodies in her serum three months prior to her development of the disease. After these autoantibodies increased three weeks after her eruption started, resolution of her rash was accompanied by a subsequent decrease in these autoantibodies.
Found in the lamina densa and the sub-lamina densa fibrillar area of the dermal-epidermal junction, type VII collagen is composed of three alpha chains containing a central collagenous triple helix and noncollagenous domains in the amino-terminal (NC1) and carboxy-terminal (NC2) ends.6 The immunodominant domains of type VII collagen recognized by IgG autoantibodies from patients with BSLE (and EBA) reside in the NC1 domain.3,7 Passive transfer of purified rabbit anti-type VII collagen IgG in adult nude, Balb/c, and C57BL/6 mice resulted in the formation of skin blisters and erosions.8 Similarly, purified anti-type VII collagen antibodies from EBA patients' sera injected in hairless mice showed that these antibodies can induce EBA-like skin lesions.9 Based on our observations that anti-type VII collagen autoantibodies were present before and increased after her bullous lesions appeared, we hypothesize that there is a critical threshold of these antibodies in circulation that is surpassed before the skin eruption occurs in BSLE.
The presence and accumulation of circulating autoantibodies have been previously observed in lupus patients. A large prospective study of 130 military recruits who were followed before their SLE diagnosis showed that multiple autoantibodies such as anti-nuclear antibodies and anti-double-stranded DNA antibodies were present in their blood years prior to their diagnosis and onset of systemic symptoms.5 Moreover, 58% of patients with SLE showed escalating dsDNA antibody levels leading up to their SLE diagnosis.10 A case for the pathogenic potential of autoantibodies in lupus can be made with neonatal lupus, in which transplacental transfer of Ro, La, and RNP autoantibodies occurs from mother to fetus. The disease wanes as these autoantibodies level off and/or decline.11 Moreover, Sjögren’s syndrome patients with positive Ro autoantibodies have been observed to later develop subacute cutaneous lupus (SCLE).12
Utilizing the sera repository from the UTSW Cutaneous Lupus Registry, we also measured anti-type VII collagen IgG by ELISAs in age- and gender-matched SLE patients (N=13), DLE patients (N=13), and normal controls (N=14). All of these serum samples had levels of anti-type VII collagen IgG below the established positive threshold. They were lower than those observed in our patient’s sera before and during the BSLE eruption and sera from two patients with EBA, serving as positive controls (Table 1). We were able to confirm findings from a previous study that sera from SLE patients without BSLE do not contain significant levels of anti-type VII collagen autoantibodies.13
Table 1.
Anti-type VII collagen IgG levels in sera of BSLE patient 3 months prior, 3 weeks after, and 12 months after disease onset, EBA, normal, DLE, and SLE patients.
| Sample | Anti-type VII collagen IgG (U/mL) |
|---|---|
| BSLE patient 3 months before disease onset | 9.23 |
| BSLE patient 3 weeks after disease onset | 71.74 |
| BSLE patient 12 months after disease onset | 2.30 |
| EBA patients (N=2), mean±SD | 141.46±67.01 |
| Normal controls (N=14)*, mean±SD | 0.11±0.40 |
| DLE patients (N=13)*, mean±SD | 0.36±0.93 |
| SLE patients (N=13)*, mean±SD | 0.23±0.37 |
Compared with EBA patients, normal, DLE, and SLE patients had significantly lower anti-type VII collagen IgG (p<0.01). Sera for age- and gender-matched normal controls, DLE patients, and SLE patients were obtained from the UTSW Cutaneous Lupus Registry. None of the normal, DLE, and SLE samples was positive.
Twelve months after the initial eruption, our patient’s serum showed markedly decreased levels of anti-type VII collagen IgG. At that time, the patient’s BSLE was quiescent. A similar finding was also previously reported in a patient with Sjögren’s syndrome/SLE overlap with BSLE whose anti-type VII collagen IgG were undetectable at the time of BSLE remission.14 The rise and fall in levels of anti-type VII collagen autoantibodies during the course of the patient’s BSLE disease implies that they have possible utility as biomarkers, which can be used to assess disease activity and guide treatment.
Limitations of this study include small sample size, as these findings were observed from one patient, and a limited follow-up of one year. In addition, concurrent immunosuppressant medications may alter autoantibody levels. However, the patient was on stable doses of her immunosuppressants at times before and after her BSLE eruption.
In summary, anti-type VII collagen autoantibodies were detected three months prior to the inception of BSLE in a patient with SLE. Their levels subsequently increased after disease onset and decreased with disease resolution. We hypothesize that surpassing a critical level of anti-type VII collagen autoantibodies may be an important event in the evolution of BSLE. An alternative explanation would be that the patient had non-pathogenic autoantibodies prior to her BSLE. Epitope spreading may be responsible for the appearance of pathogenic autoantibodies resulting in epidermal-dermal separation and eventual onset of her BSLE.2,15 Larger prospective studies in BSLE patients measuring levels of anti-type VII collagen autoantibodies throughout their disease course for an extended time frame would be helpful in determining whether they could be reliable disease markers.
Acknowledgments
We are indebted to Rose Cannon for her administrative support in the preparation of this manuscript.
Funding/Support: This study was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR061441. The content is solely the responsibility of the authors and does not necessarily represent the official views of the University of Texas Southwestern Medical Center at Dallas and its affiliated academic and health care centers, the National Center for Research Resources, and the National Institutes of Health.
| Role of the Sponsors: | Funding/Sponsor was involved? | |
| Design and conduct of the study | Yes _____ | No __X___ |
| Collection, management, analysis, and interpretation of data | Yes _____ | No __X___ |
| Preparation, review, or approval of the manuscript | Yes _____ | No __X___ |
| Decision to submit the manuscript for publication | Yes _____ | No __X___ |
Abbreviations
- BSLE
Bullous Systemic Lupus Erythematosus
- DLE
Discoid Lupus Erythematosus
- EBA
Epidermolysis Bullosa Acquisita
- ELISA
Enzyme-Linked Immunosorbent Assay
- NC
Noncollagenous domain
- SLE
Systemic Lupus Erythematosus
- UTSW
University of Texas Southwestern
Footnotes
Author Contributions: Mr. Grabell and Dr. Chong had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Grabell, Yancey, and Chong
Analysis and interpretation of data: Grabell, Matthews, Yancey, and Chong
Drafting of the manuscript: Grabell and Chong
Critical revision of the manuscript for important intellectual content: Grabell, Matthews, Yancey, and Chong
Statistical analysis: Grabell and Chong
Obtained funding: Yancey and Chong
Administrative, technical, or material support: Matthews, Yancey, and Chong
Study supervision: Chong
Financial disclosure for relationships relevant to this manuscript: There are no conflicts of interest relevant to this manuscript. Financial Disclosure for all other relationships: Dr. Chong is an investigator for Daavlin Corporation. Dr. Yancey has served in the advisory boards for Stiefel/GlaxoSmithKline and Mary Kay, Inc.
REFERENCES
- 1.Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatologic clinics. 2011 Oct;29(4):649–653. doi: 10.1016/j.det.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Chan LS, Lapiere JC, Chen M, et al. Bullous systemic lupus erythematosus with autoantibodies recognizing multiple skin basement membrane components, bullous pemphigoid antigen 1, laminin-5, laminin-6, and type VII collagen. Arch. Dermatol. 1999 May;135(5):569–573. doi: 10.1001/archderm.135.5.569. [DOI] [PubMed] [Google Scholar]
- 3.Gammon WR, Murrell DF, Jenison MW, et al. Autoantibodies to type VII collagen recognize epitopes in a fibronectin-like region of the noncollagenous (NC1) domain. J. Invest. Dermatol. 1993 May;100(5):618–622. doi: 10.1111/1523-1747.ep12472291. [DOI] [PubMed] [Google Scholar]
- 4.Morris NP, Keene DR, Glanville RW, Bentz H, Burgeson RE. The tissue form of type VII collagen is an antiparallel dimer. The Journal of biological chemistry. 1986 Apr 25;261(12):5638–5644. [PubMed] [Google Scholar]
- 5.Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003 Oct 16;349(16):1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 6.Parente MG, Chung LC, Ryynanen J, et al. Human type VII collagen: cDNA cloning and chromosomal mapping of the gene. Proceedings of the National Academy of Sciences of the United States of America. 1991 Aug 15;88(16):6931–6935. doi: 10.1073/pnas.88.16.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lapiere JC, Woodley DT, Parente MG, et al. Epitope mapping of type VII collagen. Identification of discrete peptide sequences recognized by sera from patients with acquired epidermolysis bullosa. The Journal of clinical investigation. 1993 Oct;92(4):1831–1839. doi: 10.1172/JCI116774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sitaru C, Mihai S, Otto C, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J. Clin. Invest. 2005 Apr;115(4):870–878. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodley DT, Ram R, Doostan A, et al. Induction of epidermolysis bullosa acquisita in mice by passive transfer of autoantibodies from patients. J. Invest. Dermatol. 2006 Jun;126(6):1323–1330. doi: 10.1038/sj.jid.5700254. [DOI] [PubMed] [Google Scholar]
- 10.Arbuckle MR, James JA, Kohlhase KF, Rubertone MV, Dennis GJ, Harley JB. Development of anti-dsDNA autoantibodies prior to clinical diagnosis of systemic lupus erythematosus. Scand J Immunol. 2001 Jul-Aug;54(1–2):211–219. doi: 10.1046/j.1365-3083.2001.00959.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee LA. Cutaneous lupus in infancy and childhood. Lupus. 2010 Aug;19(9):1112–1117. doi: 10.1177/0961203310370347. [DOI] [PubMed] [Google Scholar]
- 12.Provost TT, Talal N, Harley JB, Reichlin M, Alexander E. The relationship between anti-Ro (SS-A) antibody-positive Sjogren's syndrome and anti-Ro (SS-A) antibody-positive lupus erythematosus. Arch. Dermatol. 1988 Jan;124(1):63–71. [PubMed] [Google Scholar]
- 13.Ishikawa O, Zaw KK, Miyachi Y, Hashimoto T, Tanaka T. The presence of anti-basement membrane zone antibodies in the sera of patients with non-bullous lupus erythematosus. The British journal of dermatology. 1997 Feb;136(2):222–226. [PubMed] [Google Scholar]
- 14.Fujii K, Fujimoto W, Ueda M, Makino E, Arata J. Detection of anti-type VII collagen antibody in Sjogren's syndrome/lupus erythematosus overlap syndrome with transient bullous systemic lupus erythematosus. Br. J. Dermatol. 1998 Aug;139(2):302–306. doi: 10.1046/j.1365-2133.1998.02372.x. [DOI] [PubMed] [Google Scholar]
- 15.Noe MH, Chen M, Woodley DT, Fairley JA. Familial epidermolysis bullosa acquisita. Dermatol. Online J. 2008;14(12):2. [PubMed] [Google Scholar]