Abstract
In the past decade, substantial evidence supports the paradigm that stem cells exert their reparative and regenerative effects, in large part, through the release of biologically active molecules acting in a paracrine fashion on resident cells. The data suggest the existence of a tissue microenvironment where stem cell factors influence cell survival, inflammation, angiogenesis, repair and regeneration in a temporal and spatial manner.
Keywords: Stem cell, myocardial infarction, heart disease, regeneration and therapy
Development of the Paracrine Hypothesis
Stem cell therapy for tissue repair and regeneration holds great therapeutic potential1. The ability of stem cells to develop into various cell types, and the ease with which they can be expanded in culture, has led to a great deal of interest in their use as therapeutic agents to treat a wide range of diseases. Various embryonic and adult stem cells, isolated from a variety of different tissues including brain, heart, kidney, and bone marrow, have been assessed for their therapeutic potential (Table 1)1,2. Of these, adult stem cells from the bone marrow have been studied widely in clinical trials. Indeed, bone marrow derived mesenchymal stem cells (MSCs) have been used to treat a very large and diverse set of diseases; including myocardial infarction, Parkinson’s disease, Crohn’s disease, cancer, amongst others3–7. Currently over 100 clinical trials using MSCs are active in the United States alone (from ClinicalTrials.org) and the results of these preliminary studies have been encouraging.
Table 1.
Stem cells used in regenerative medicine and for which paracrine effects have been shown.
| Tissue | Cell |
|---|---|
| Bone marrow | mesenchymal stem cells (MSCs) |
| hematopoietic stem cells | |
| bone marrow-derived mononuclear cells | |
| Unfractionated bone marrow cells | |
| Heart | cardiac progenitor/stem cells |
| Adipose | mesenchymal stem cells |
| Skeletal muscle | myoblasts |
| Circulation | Endothelial progenitor cells |
It has been shown that injection of adult stem cells (Table 1) into the injured heart has beneficial effects. Originally, it was believed that these stem cells engrafted into the damaged tissue and differentiated into cardiomyocytes, vascular or other cells8, 9. In vitro, MSCs treated with 5-azacytidine were shown to differentiate into cardiac-like muscle cells10. Moreover, hematopoietic (hematopoietic lineage negative c-Kit positive) stem cells were reported to regenerate infarcted myocardium by differentiating into cardiomyocytes9. A number of other groups also demonstrated that MSCs possess the ability to differentiate into cardiomyocytes. Indeed, studies have demonstrated that engrafted MSCs in vivo can improve cardiac function and remodeling11–13.
However, it has been shown that following injection adult stem cells suffered from poor survivability14. Moreover, it has not been possible to reproduce the earlier studies which showed that bone marrow derived stem cells differentiate into cardiac cells. Utilizing a GFP mouse, Balsam et al could not find any evidence that bone marrow derived hematopoietic lineage negative c-Kit positive cells differentiated into cardiomyocytes when injected into infarcted myocardium. Instead, these cells adopted a typical hematopoietic fate15. Moreover, using genetic tracing techniques Murry et al were unable to identify differentiation of hematopoietic stem cells into cardiomyocytes in any of their 145 transplants into normal and injured adult mouse hearts16. These findings, and others, have called into question the plasticity of bone marrow derived stem cells and their direct role in tissue regeneration. Fusion with recipient cells within the tissue was also proposed to be a mechanism by which injected adult stem cells exerted their beneficial effects17 however again the frequency of this event was found to be relatively low18, 19.
Based on the above studies, it is now clear that although engraftment can result in improved cardiac function11–13, the small number of adult stem cells engrafted cannot directly generate sufficient cardiomyocytes to account for the therapeutic benefits observed. How can one explain the apparent tissue reparative and regenerative effects of these cells? Recent evidence suggests the importance of the paracrine mechanism of stem cell action. Our laboratory was among the first to report that the administration of conditioned medium from adult stem cells was sufficient to recapitulate the beneficial effects of the cells in vitro and in vivo20. This observation and similar reports from other laboratories have led to the proposal that adult stem cells exert their therapeutic benefits via the release of biologically active proteins, or paracrine factors, acting on resident cells. Indeed, there is now a large body of evidence supporting the hypothesis that paracrine factors are essential for the reparative effects of adult stem cells following delivery into the injured heart. Adult stem cells secrete a wide variety of growth factors and chemokines that can promote cardiac repair. Elevated levels of proteins such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and insulin-like growth factor 1 (IGF1) are found in the heart following injection of adult stem cells21, 22.
In this review we will discuss the mechanisms through which paracrine factors released by stem cells promote cardiac repair and regeneration. We will propose the following novel concepts: (1) paracrine factors released by stem cells influence adjacent and distant cells differentially by their concentration gradients and thus creating a tissue microenvironment, (2) paracrine factors are often pleiotropic in nature and act on multiple mechanisms and different cell types, and (3) paracrine factors can influence in temporal and spatial manners the post- myocardial repair and regenerative responses.
Effects of the Paracrine factors
It has been demonstrated that paracrine factors promote cardiac regeneration through a number of mechanisms including cardiomyocyte proliferation, cytoprotection, differentiation of resident stem cells, neovascularization, and by limiting inflammatory and pro-fibrotic processes. Below is a review of these paracrine actions.
Survival/Cytoprotection
Adult stem cells in an ischemic environment promote cardiomyocyte survival via the paracrine release of cytoprotective molecules. We have shown that cell culture medium conditioned by hypoxic MSCs reduces rat cardiomyocyte apoptosis and necrosis when exposed to conditions that promote cell death20.Over-expression of the pro-survival protein Akt1 greatly enhances the cytoprotective capabilities of MSCs. To further validate the cytoprotective potential of MSCs, we studied the effect of the conditioned media in vivo using a rat model of coronary occlusion. We showed that administration of culture media from Akt MSC reduced infarct size and restored cardiac function in the rodent model of MI23. Our findings have been replicated by others in a large animal model.24. Taken together these studies validate that MSCs promote cardiomyocyte survival via paracrine factors and that Akt is crucial for this process23. We are not alone in reporting these paracrine effects of bone marrow derived adult stem cells25–27. Takahashi et al showed that rat bone marrow mononuclear cells release proteins such as VEGF, PDGF, IGF-1 and IL-1b, some of which were significantly enhanced by hypoxia. The conditioned media of bone marrow mononuclear cells strongly inhibited cardiomyocyte apoptosis and preserved their contractile capacity25. Moreover, Uemura et al demonstrated that bone marrow stromal cells, which showed up-regulation of Akt following brief anoxia, prevented cardiomyocyte apoptosis in a co-culture model. This study went on to show that bone marrow stromal cells markedly inhibited LV remodeling following myocardial infarction26.
In the course of our research we have identified a number of novel paracrine factors. Secreted frizzled related protein 2 (Sfrp2) showed the highest fold difference in expression between Akt1- and un-modified MSCs. When delivered to hypoxic cardiomyocytes Sfrp2 inhibited caspase-3 activity and prevented apoptosis28. On the basis that Sfrp proteins are Wnt antagonists we analyzed Wnt expression in hypoxic cardiomyocytes; identifying Wnt3a as a potential candidate. Cardiomyocyte apoptosis in response to hypoxia-reoxygenation was significantly augmented by Wnt3a acting via β-catenin. Sfrp2 was found to bind directly to Wnt3a and significantly attenuated Wnt3a-induced caspase activity in a dose dependent fashion29. We also identified C3orf58 as a novel paracrine factor secreted from MSCs. By virtue of how the gene was regulated in MSCs we named C3orf58 as HASF for Hypoxic induced Akt regulated Stem cell Factor. HASF, a relatively novel ~49kDa protein with no recognizable domains apart from a signal peptide, has been previously associated with human familial autism30. A single dose of purified HASF protein injected into the heart immediately following myocardial infarction prevented the loss of cardiac function associated with this type of injury. Analysis of the heart tissue showed that HASF reduced the number of TUNEL positive nuclei as well as inhibiting caspase activation and mitochondrial pore opening. The cytoprotective effects of HASF were lost in mice lacking PKCε31.
Immunomodulation/Inflammation
Adult stem cells when injected into myocardium dampen the inflammatory state associated with injury by down-regulating expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and MCP-132. These effects have a paracrine component; conditioned media prepared from cultured MSCs was found to inhibit damage to isolated adult rat cardiomyocytes in response to MCP-132. In contrast to MSCs, EPCs actively secrete pro-inflammatory cytokines such as MCP-1. Moreover, these cells can be stimulated to produce the procoagulant protein tissue factor by lipopolysaccharide suggesting under certain conditions EPCs could promote thrombosis33, 34.
MSCs possess immune-modulatory properties that affect a broad range of cells involved in the immune response. MSCs inhibit T-cell proliferation and cytotoxity; rendering the T-cells unresponsive35. Paracrine factors released by MSCs, as well as direct interaction between the two cell types36, are believed to be important. Paracrine factors released by MSCs, such as TGFβ, HGF, nitric oxide, indoleamine 2,3-dioxygenase, and prostaglandin-E2 (PGE2), inhibit T-cell function37. Under certain conditions MSCs release T-cell activators such as IL-6, IL-1 and RANTES37. IL-6 may also be important for the effect of MSCs upon B-cells and further underscores the spatial aspect of the paracrine hypothesis which we will discuss below. Depending upon the strength of the stimulus MSCs either promote or inhibit IgG production by B-cells38. MSCs also prevent dendritic cell maturation and function via the release of IL-6 and PGE239, 40. The latter molecule is also required for the inhibitory effect of MSCs on Natural Killer (NK) cell proliferation, cytokine production, and cytotoxicity41. Finally, MSCs also secrete interleukin 1 receptor antagonist which inhibits the release of the pro-inflammatory cytokine TNFα from activated macrophages42.
Macrophages can promote angiogenesis and tissue healing through a number of secreted molecules43–45. Following transplantation of MSCs into infarcted tissue large numbers of macrophages collect at the sites of injection despite an overall reduction in the population of these cells in the heart46. Various reports have indicated that MSCs switch macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype both in vitro and in vivo47–49, potentially through secreted factors such as IGF-150 and IL-1051. These studies suggest that MSCs contribute to the overall recovery of cardiac function following myocardial infarction in part via their effects on macrophages. Indeed the regenerative and reparative effects on transplanted MSCs are reduced following transient depletion of macrophages52.
Cardiomyocyte proliferation
Following cardiac injury cardiomyocytes are lost in significant numbers. Lower vertebrates such as the zebrafish possess mechanisms to replace these cardiomyocytes53. Specifically, the remaining cardiomyocytes de-differentiate, re-enter the cell-cycle and proliferate. These mechanisms are either absent or inactive in the adult mammalian heart. Indeed, cardiomyocyte proliferation is known to be relatively infrequent54–57. Finding strategies which increase cardiomyocyte proliferation is an important endeavor.
Recent research has uncovered a number of MSC paracrine factors that mediate cardiomyocyte proliferation. These paracrine mediators of cardiomyocyte proliferation tend to be either growth factors or extracellular matrix proteins.
Fibroblast growth factor 2 (FGF2) promotes cardiomyocyte proliferation in vitro via PKCε, a process also potentially involving connexin-43 phosphorylation58. Similarly, again in vitro, platelet-derived growth factor (PDGF) increases the proliferation of cardiomyocytes via Akt activation, inactivation of GSK-3beta and the subsequent down-regulation of the cyclin-dependent kinase inhibitor p2759. Neuregulin-1 (NRG1), a member of the EGF family, stimulates DNA synthesis in both neonatal and adult cardiomyocytes through its specific receptor ErbB460, 61. Mononucleated cardiomyocytes were found to be capable of karyokinesis, whereas binucleated cells were not61. Pertinently, injection of NRG1 into adult mice promoted regeneration following myocardial infarction61. This molecule is now undergoing small scale clinical trials as a therapeutic agent to treat congestive heart failure62. Though tempting to speculate de-differentiation as the underlying process by which NRG1 promotes cardiomyocyte proliferation Bersell et al could not find any evidence of sarcomere disassembly for example61. Despite this finding it is possible that pro-proliferative paracrine factors promote cardiomyocyte proliferation via de-differentiation, and this process underpins the robust regeneration found in the neonatal mouse heart57, 63–65. It is particularly notable that growth factors such as FGF, PDGF and NRG1 mediate cardiomyocyte proliferation through PI3K. Under hypoxic conditions Adipose derived stromal cells secrete the pro-inflammatory cytokine IL-6; and curiously this protein has also been linked with augmentation of cardiomyocyte proliferation via Stat3 and ERK1/266.
Fibronectin and collagen, acting via their receptor integrin-β1, are pro-proliferative67. Periostin is an ECM protein secreted by adipose-derived MSCs68, and has been reported to promote cardiomyocyte proliferation via integrin (alphaV, beta1, beta3 and beta5) mediated activation of PI3K69. In a myocardial infarction model periostin induced cardiomyocytes to re-enter the cell-cycle and this was associated with improvements in cardiac function69. Interestingly, activation of PI3K was sufficient to recapitulate the effects of periostin. Other researchers found that periostin had no effect on cardiomyocyte proliferation70. Periostin also appears to be associated with increased myocardial fibrosis71, 72 though it should be noted that delivery of the protein into the pericardial space improved cardiac function following myocardial infarction71.
Our novel paracrine factor, HASF was found to increase DNA synthesis in cultured rat neonatal ventricular cardiomyocytes by 60%, a level of stimulation comparable in intensity to FGF. Importantly, evidence of cytokinesis was observed in a murine model73. The proliferative effects of HASF were found to be mediated by PI3K and the cell-cycle regulator cyclin-dependent kinase 7 (CDK7)73. We are currently investigating in more detail the molecular pathways by which HASF promotes cardiomyocyte proliferation. Utilizing yeast two-hybrid and co-immunoprecipitation assays we identified a direct interaction between the Insulin-like Growth Factor-1 Receptor (IGF1R) and HASF74. Subsequent studies with pharmacological inhibitors and siRNA mediated knockdown showed that the beneficial effects of HASF are mediated by the IGF1R74. HASF is a much larger molecule than IGF-1, and indeed many of the commonly investigated growth factors, and may activate different pathways. Indeed, whereas IGF-1 promotes cardiac hypertrophy75, HASF does not73. This finding itself highlights the potential clinical benefits of the HASF protein.
Cardiac remodeling
Cardiac injury with significant cell loss and functional imparment leads to cardiac remodeling which is mediated by a significant change in the ECM including fibrosis, cardiomyocyte hypertrophy and changes in ventricular dimension and function. Paracrine factors released by adult stem cells can alter the ECM and prevent post-infarction remodeling. In a number of animal models MSCs decrease fibrosis in a number of tissues including the heart22, lung76, liver77 and kidney78. Stem cells, such as MSCs, express a number of proteins that regulate the extracellular matrix such as metalloproteinases (MMPs), serine proteases, and serine protease inhibitors, suggesting that transplanted MSCs can inhibit fibrosis through a paracrine action79. MSC transplantation has been shown to inhibit post-MI increases in the expression of collagens-1 and –III as well as the tissue inhibitor of metalloproteinase (TIMP)-180. Conditioned media prepared from MSCs strongly inhibits cardiac fibroblast proliferation and inhibits the production of collagen-I and –III from these cells81. Growth differentiation factor-11 (GDF11), a circulating protein that was revealed as a rejuvenating factor for the aging heart by parabiosis experiments, actively prevents fibrosis following myocardial injury82.
We have demonstrated that Sfrp2 prevents fibrosis. Injected into infarcted rat myocardium 2 days after injury Sfrp2 inhibited MI-induced collagen type-I deposition as well as left ventricular fibrosis. Activity of Bmp1, a key enzyme involved in the regulation of collagen biosynthesis and maturation, was repressed by a high concentration of Sfrp283. Despite our findings there are a number of reports which have ascribed a pro-fibrotic role for Sfrp2. Kobayashi et al found that fibrosis was reduced in Sfrp2-null mice following myocardial infarction84. Similarly, Mastri et al reduced fibrosis and improved cardiac function following the intraperitoneal delivery of a Sfrp2 neutralizing antibody into cardiomyopathic hamsters85. Why is there a discrepancy between the studies? Sfrp proteins have biphasic effects depending upon their concentration; with Wnt86 and BMP185 signaling being augmented or inhibited at low or high concentrations respectively. Indeed, Mastri et al were unable to identify Sfrp2 in the hamster heart whereas we used a large dose of the protein. As mentioned in a recent commentary a more detailed analysis of the concentration dependence of the effects of Sfrp2 is needed87. The biphasic effects of Sfrp proteins highlight the importance of a spatial component to the paracrine hypothesis. Considering that MSCs secrete Sfrp2, cells in close proximity to the paracrine source will be exposed to high concentrations of Sfrp2. Thus, in the microenvironment formed by the injected adult stem cells one would expect Sfrp2 to behave in anti-fibrotic fashion.
Metabolism and Contractility
Injury alters cardiac metabolism with a switch from the typical fatty acid oxidation to glucose uptake, and a shift to lactate production88. Moreover, in the infarct border zone the phosphocreatine-to-ATP ratio increases89. These changes influence infarct size and remodeling.
Injection of MSCs into the hearts of pigs following MI partially prevented the metabolic changes in the heart associated with injury. Due to the low engraftment of the injected cells it was proposed that the MSCs were thwarting metabolic changes via paracrine factors90. This has also been observed in a rat model of MI. Here, Akt overexpression significantly increased the ability of MSCs to inhibit changes in metabolism; sparing phosphocreatine stores and limiting glucose uptake91.
There is evidence that the administration of adult stem cells promotes cardiac contractility. Indeed, we witnessed a large increase in spontaneous contractility of adult rat ventricular cardiomyocytes exposed to conditioned media from hypoxic Akt1-MSCs23. The strong and synchronized contraction suggested that the conditioned media contained inotropic factors that had a positive effect on cardiomyocyte contractility. Similarly, Takahashi et al found that conditioned media from bone marrow mononuclear cells maintained fractional shortening and maximal rate of re-lengthening of adult rat ventricular cardiomyocytes in culture25. Conditioned media was more effective in preserving contractility if the bone marrow mononuclear cells were exposed to hypoxic conditions. Both of these studies suggest that the release of inotropic paracrine factors is increased by hypoxia. The identity of these inotropic paracrine factors is currently unknown; however IGF-1, a growth factor released by MSCs, can promote cardiomyocyte contractility in vitro92.
Neovascularization
Another important effect of adult stem cells in the ischemic myocardium is neovascularization. For example, injection of bone marrow mononuclear cells into ischemic myocardium resulted in increased regional blood flow and capillary density93. Moreover, the administration of MSCs following permanent occlusion increases capillary density94, 95. Only a very small number of these stem cells engraft and differentiate into vascular structures15, 96.
The molecular pathways that control angiogenesis are well characterized and involve proteins such as VEGF, bFGF, HGF, and angiopoietin, amongst others. These molecules are also secreted by bone marrow derived stem cells suggesting that exogenously delivered adult stem cells promote vessel formation via the paracrine release of known pro-angiogenic factors95, 97, 98. Support of this paradigm has come from a number of studies. Tse et al compared a number of different types of bone marrow derived cells for their ability to improve cardiac function in a swine model of chronic ischemia. Bone marrow mononuclear cells were the most effective, and the authors ascribed the increased capillary density arising from the injections of these cells to the paracrine release of VEGF and angiopoietin-299. Similarly, conditioned media from bone marrow mononuclear cells increases vessel density in a rat model of acute MI25. The Epstein laboratory found that injection of MSCs into the adductor muscle following distal femoral artery injection improved distal limb perfusion and increased the number of mid-thigh conductance vessels. The injected MSCs were not observed to incorporate into collaterals indicating that the effects they observed were paracrine in nature95. Using a murine hind-limb ischemia model they also observed that conditioned media from MSCs enhanced collateral flow recovery and remodeling; improving limb function98. Conditioned media from these MSCs enhanced endothelial and smooth muscle cell proliferation in vitro. VEGF is an important pro-angiogenic paracrine factor as ablation of this gene significantly inhibits the ability of MSCs to promote functional recovery in the injured heart100. However antibodies targeting VEGF and FGF only partially attenuated the effect of the conditioned media98; indicating that MSCs release other pro-angiogenic proteins besides these two growth factors.
Endothelial progenitor cells (EPCs) also promote angiogenesis via paracrine mechanisms. Conditioned media derived from EPCs promotes angiogenesis in ischemic myocardium101. VEGF and stromal derived factor 1 (SDF-1) are among the responsible bioactive molecules. Both proteins are secreted by EPCs and promote endothelial cell migration as well as capillary formation via differentiation independent mechanisms102.
Resident stem cell activation
The heart contains a number of resident stem cells which are defined by a number of markers including c-Kit and Sca-1. The most heavily researched resident stem cell in the heart is the c-Kit cardiac progenitor cell (CPC). These CPCs are believed to be capable of promoting regeneration via mobilization into injured tissue and differentiation into mature cardiac cells. Intracoronary infusion of autologous c-Kit+ CPCs improves ventricular systolic function and reduces infarct size in patients with heart failure after myocardial infarction103, 104. These c-Kit+ cells are also beneficial in rodent models; improving cardiac function when injected into infarcted myocardium. However, in all of these studies it was apparent that donor c-Kit+ cell differentiation into mature cardiac cells, such as cardiomyocytes, was too low to account for the functional benefits. The authors concluded that paracrine effects must be responsible for the regenerative effects of the injected c-Kit+ cardiac stem cells105, 106. The nature of these paracrine factors remains to be identified.
Recent data suggests that paracrine factors released by adult stem cells significantly augment the ability of resident c-Kit+ cardiac stem cells to differentiate into cardiomyocytes. Conditioned medium derived from cultured MSCs promotes cardiac progenitor cell (CPC) proliferation and differentiation. The growth factor IGF-1, a paracrine factor released by MSCs, promotes resident stem mobilization107 and commitment to the cardiac lineage108,109.
In the course of our research we identified Abi3bp as a putative paracrine factor released by MSCs. There is little known about Abi3bp except for roles in neural cell differentiation and a number of anti-tumorigenic properties. We found that Abi3bp formed extensive extracellular matrix deposits when secreted by MSCs110. Abi3bp had dramatic effects on MSC differentiation. When we assessed the differentiation of MSCs prepared from Abi3bp knockout mice we found that osteogenesis was completely ablated with chrondogenesis and adipogenesis severely impaired. In addition, Abi3bp inhibited MSC proliferation110. Considering the close relationship between MSCs and CPCs we hypothesized that Abi3bp would have similar affects upon CPC proliferation and differentiation. Indeed Abi3bp promoted c-Kit+ CPC differentiation, whilst proliferation was inhibited, both in vitro and in vivo111. Integrin-β1 was found to be crucial for the effect of Abi3bp on c-Kit+ CPCs. Genetic ablation of Abi3bp was associated with adverse recovery following MI111. This is likely due to the effects on CPC differentiation as cardiomyocyte proliferation was unaffected by the loss of Abi3bp expression111.
Extracellular vesicles & Exosomes
Recent evidence suggests that the paracrine functions of MSCs, and other cell-types, are potentially mediated by extracellular vesicles (EVs). There are a number of EV subtypes, such as exosomes and microvesicles. Exosomes, the most numerous subtype, are released upon fusion of a multivesicular body with the plasma membrane whereas microvesicles are released directly from the cell membrane112. EVs were originally thought to be a mechanism by which cells disposed of waste materials. Many lines of research now point towards EVs as important mediators of cell–cell communication, immunomodulation, proliferation, cell-senescence, and differentiation by transferring various bio-active cargoes such as proteins, lipids, mRNAs, as well as miRNAs, from one cell to another112–114.
Exosomes derived from stem cells such as MSCs have been shown to protect against injury and promote regeneration in a number of models. In vitro, cardiomyocyte protection from cell death by MSCs is partially mediated by the transfer of miRNA-221 contained within EVs, reducing caspase activity in the target cells115. Similarly, exosomes derived from bone marrow CD34+ cells and cardiac progenitor cells promote angiogenesis in cultured endothelial cells116, 117. In vivo, fractionation studies indicated that only the fraction of MSC conditioned media containing products of greater than 1000 kDa (100–220 nm), the typical size of exosomes, provided protection in a mouse model of myocardial ischemia and reperfusion injury118. The same group later purified exosomes from MSCs and found that they reduced infarct size post myocardial injury119. The protective effect of exosomes are not limited to the heart, indeed EVs derived from MSCs protect the kidney from ischemia-reperfusion injury120–122.
Considering that EVs can deliver multiple bio-active molecules at the same time they hold much promise as therapeutic agents to deliver paracrine factors in vivo. To this end a number of researchers are actively investigating novel approaches to EV delivery such as incorporating tags to aid in their isolation, over-expression of key cargoes, and designing synthetic EV structures to aid scalability for clinical use123–125.
Emerging concepts in Paracrine mechanisms: temporal and spatial pleiotropic actions and the creation of microenvironment
Effects of paracrine factors after myocardial injury are dynamic, multifaceted and multi-phased. The healing process following a myocardial injury is a complex sequence of time dependent events involving cell death (apoptosis and necrosis), inflammation, fibroblast proliferation, collagen deposition, neovascularization, cardiac remodeling and, in a limited manner, cardiac regeneration. In this section of the review, we propose the following new concepts that: (1) paracrine factors released by stem cells influence adjacent and distant cells differentially by their concentration gradients and thus creating a tissue microenvironment, (2) paracrine factors have pleiotropic actions on different cells and multiple mechanisms, and (3) paracrine factors can exert temporal and spatial effects on cardiac repair and regenerative events.
Release of paracrine factors produces concentration gradients and creates unique tissue microenvironment
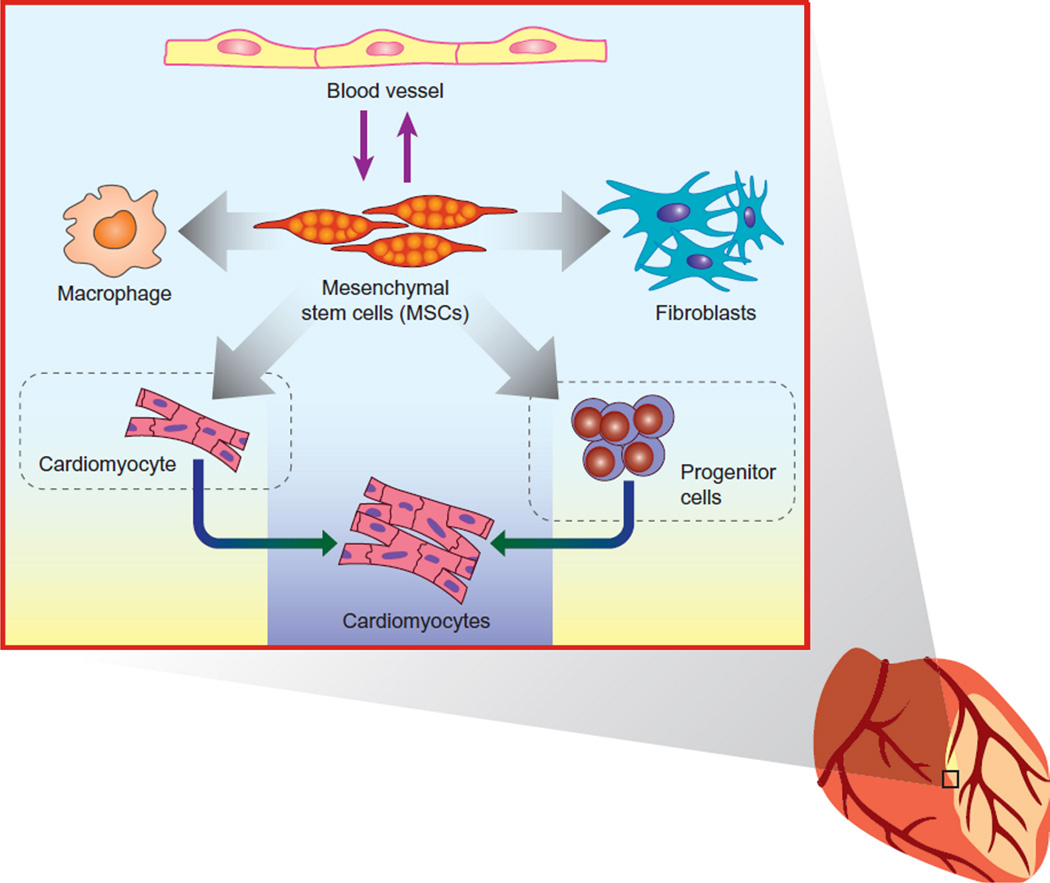
Once the stem cells are established in the injured myocardium via endogenous mobilization or exogenous administration, they release paracrine factors that will form concentration gradients. The concentration gradient of the factors will influence adjacent and distant cells differentially and thus creating a unique microenvironment within the cardiac tissue. These concentration gradients have the potential to impact cardiac repair and regeneration in a number of ways previously unconsidered. The concentration of the paracrine factor, or in other words the spatial proximity to the stem cell, may directly influence the response of resident cells to the secreted protein (Figure 1). Though currently undefined in the context of paracrine factors acting in the heart there are other settings where concentration gradients of secreted proteins have been shown to dictate cell behavior. For example spatial proximity to an IL-2 source affects the magnitude and direction of the T-cell response126. Moreover, it is well established that paracrine concentration gradients are critical for the normal development of the embryo127,128. Proteins such as EGF, FGF, Wingless/Wnt, and BMP generate concentration gradients that provide spatial information to generate distinct cell types in a specific three-dimensional pattern128. In the developing embryo these paracrine factors have a specific range that is dependent on their diffusion capacity and interaction with proteoglycans128. Our own data and that of others regarding the role of Sfrp2 upon cardiac fibrosis also suggests that paracrine factor concentration gradients can have a dramatic effect on cell behavior. We have found that administrating a high concentration of Sfrp2 prevents fibrosis following MI83. In contrast, genetic deletion of Sfrp284 and Sfrp2 neutralizing antibodies85 reduce fibrosis, suggesting that Sfrp2 is pro-fibrotic. This can be explained by the biphasic properties of Sfrp proteins which augment or inhibit Wnt86/ BMP185 signaling at low or high concentrations respectively.
Figure 1.
Paracrine factors affect different cell types and create a microenvironment that is influenced by concentration gradients, with temporal and spatial summation of cellular responses. (This figure is taken from149 with permission)
Concentration gradients provide a conduit to attract cells to a specific site. In the context of myocardial infarction proteins released from dying cells release chemoattractants which mediate the infiltration of pro-inflammatory immune cells into the injured tissue129. Modifying MSCs to express proteins such as CCR1130 allows MSCs to follow the same chemotactic gradients as pro-inflammatory immune cells. This is particularly pertinent as MSCs release a myriad of paracrine factors that inhibit the function of pro-inflammatory immune cells. By analogy, it is tempting to speculate that concentration gradients of paracrine factors released by injected stem cells act as a chemoattractant for therapeutically beneficial cells. One possibility would be resident progenitor cells as they migrate in vitro in response to IGF-1131; a paracrine factor released by MSCs.
Adapting concepts from immunology and neuron function132, 133 we also suggest that stem cell derived paracrine factor concentration gradients affect cell behavior via temporal and spatial summation. Signaling is typically a transient event, for example when bound to ligand receptor tyrosine kinases are internalized and degraded134. In contrast, the regenerative processes mediated by stem cell paracrine factors are very prolonged. How transient signaling events initiated by paracrine factors are accumulated over time and integrated into a prolonged biological response is thus a pivotal question. This question has been addressed in non-cardiac settings. For example, temporal and spatial summation, where signaling events are built up incrementally, have been proposed to explain the dichotomy between T-cell commitment to cytokine production and proliferation which requires sustained signaling and the rapid loss of a signal from an activated T-cell receptor132. Considering that each event that follows myocardial infarction is prolonged, and that for each of these events several stem cell paracrine factors will have a direct influence, temporal and spatial summation has been under-appreciated in the paracrine model.
Once the adult stem cells are in their microenvironment the secretory profile of the cell is also likely to change with time. Indeed, autocrine/paracrine signals from embryonic stem cells, which are necessary for their self-renewal and differentiation, are temporal in nature135.
Paracrine Factors are Pleiotropic
What has become very apparent to us during the course of our research is that paracrine factors released by stem cells are pleiotropic. This pleiotropic ability of stem cell derived paracrine factors allows them to influence cardiac repair and regeneration at multiple points following myocardial injury (Figure 2).
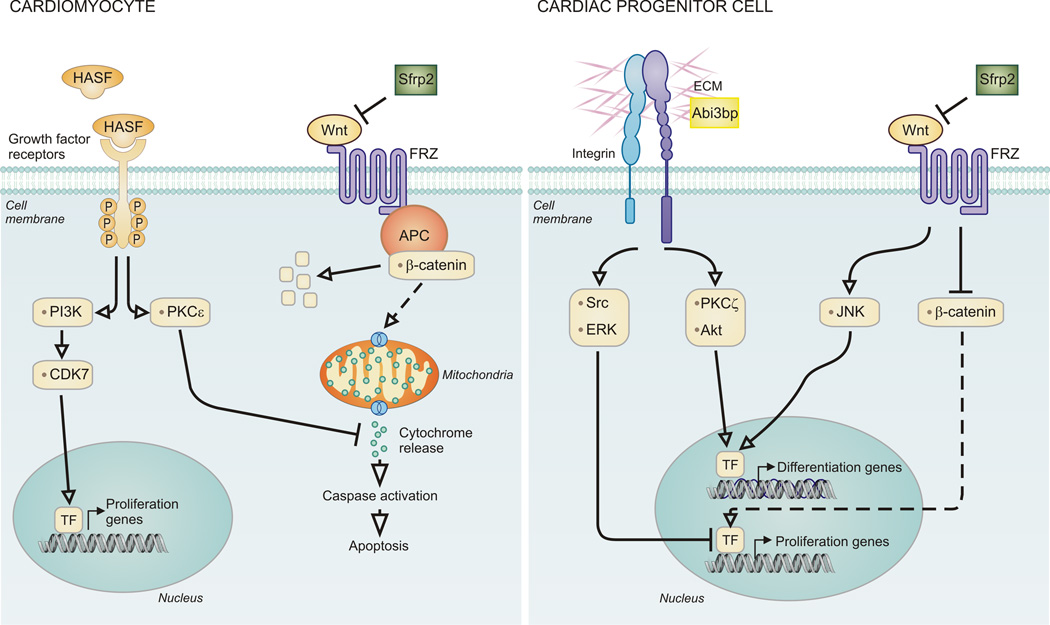
Figure 2. Paracrine factors are pleiotropic.
For illustration, we show the cellular effects of 2 selective paracrine factors on the cardiomyocyte. Left: HASF and Sfrp2 inhibit cardiomyocyte apoptosis through divergent pathways. HASF, following binding to a growth factor receptor, inhibits cytochrome release from mitochondria via PKCe. In contrast Sfrp2 inhibits Wnt activation of Frizzled Receptors. This induces b-catenin degradation via the APC complex. Right: Abi3bp and Sfrp2 promote cardiac progenitor cell differentiation as well as inhibiting proliferation. Abi3bp activates integrin-b1. Src and ERK activation work together to inhibit proliferation. PKCz and Akt activation switch on cardiac genes. Sfrp2 sequesters Wnt, preventing activation of Frizzled Receptors. This promotes JNK activation and cardiac gene expression. Inhibition of b-catenin blocks the proliferation pathway in these cells.
As mentioned above we have identified Sfrp2 and HASF as two cytoprotective paracrine factors (Figure 2). These two paracrine factors utilize two different mechanisms. Sfrp2 promotes the protection of cardiomyocytes by binding to the pro-apoptotic protein Wnt3a29. In contrast, HASF prevents cardiomyocyte cell-death through PKCε31. As noted in a recent editorial HASF has a number of novel features as a cytoprotective factor136. Over-expression of PKCε promotes cardiac hypertrophy137, whereas that of HASF does not73. Moreover, pharmacological inhibition of PKCε did not affect HASF mediated activation of Akt and it is curious feature of HASF that high levels of Akt activity are not important for the cytoprotective effects of this paracrine factor136. Certainly “protection of the heart without promoting ventricular hypertrophy or dysfunction is a unique and important feature of HASF”136. As described earlier our recent research has identified that the IGF1R mediates the beneficial effects of HASF74. This finding, which implies that HASF is a novel member of the IGF family of growth factors, suggests that HASF may have additional roles beyond those currently discovered, for example effects on metabolism. Both HASF and Sfrp2 have effects on the injured heart beyond simply protecting against cell death. HASF promotes cardiomyocyte proliferation both in vitro and in vivo. Importantly, evidence of cytokinesis was observed in a murine model73. HASF appears to utilize common growth-factor receptor tyrosine kinase pathways, though there are significant differences. Whereas IGF-1 promotes cardiac hypertrophy75, HASF does not73. As stated earlier, in addition to promoting cytoprotection, we have found that Sfrp2 prevents fibrosis by inhibiting Bmp183. Moreover we have recently shown that Sfrp2 inhibits Sca-1 cardiac progenitor cell (CPC) proliferation and primes the cells for differentiation138. This switch from proliferation to differentiation occurred via Sfrp2 binding to Wnt6, which inhibited canonical Wnt signaling and activated non-canonical Wnt/Planar Cell Polarity signaling through JNK138.
We originally identified the protein Abi3bp as an autocrine regulator of MSC biology110. This molecule is also pleiotropic. Abi3bp was found to strongly promote resident c-Kit+ cardiac progenitor differentiation both in vitro and in vivo111. Abi3bp belongs to the proteoglycan family of extracellular matrix proteins. These proteoglycans modify the fibrillar structure of the extracellular matrix which has significant effects upon cell adhesion, migration, and proliferation139. Moreover, proteoglycans regulate the activities of secreted proteins. For example, the heparan sulfate chains of proteoglycans bind to fibroblast growth factors, enabling the growth factor to cross-link and activate their cell-surface receptors139. It is possible therefore that Abi3bp, by virtue of being a proteoglycan, not only modifies the extracellular matrix of the scar in a fashion that promotes repair and regeneration but also regulates the activities of other paracrine factors released by MSCs.
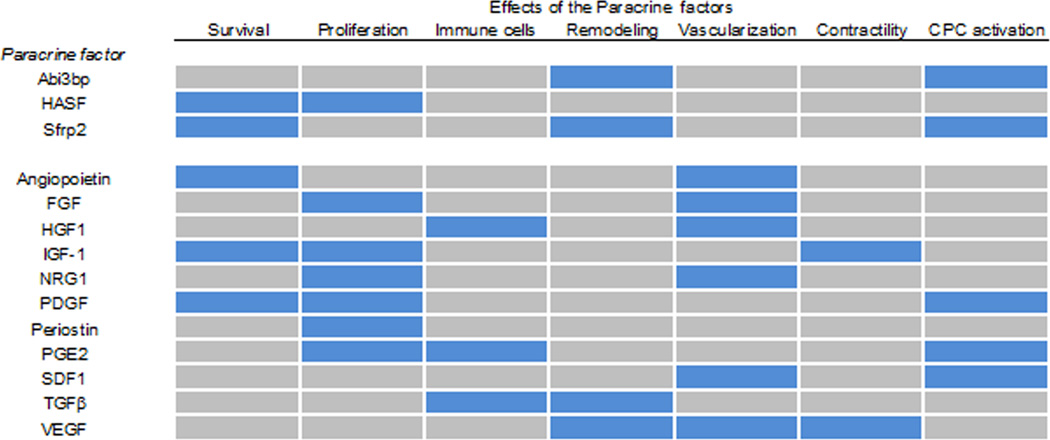
Many other paracrine factors also possess pleiotropic properties. These include IGF-1, FGF, PDGF, and VEGF (Figure 3). Given the fact that response to cardiac injury involves complex, dynamic and time dependent events, paracrine factors can influence myocardial pathobiology a multifaceted temporal manner on different cell types and via different mechanisms as discussed below.
Figure 3.
Paracrine factors described in this review are listed with the effects they have upon the heart post-myocardial infarction. Blue represents an effect, grey no effect.
Paracrine Factors Promote Repair and Regeneration at Multiple Time-Points
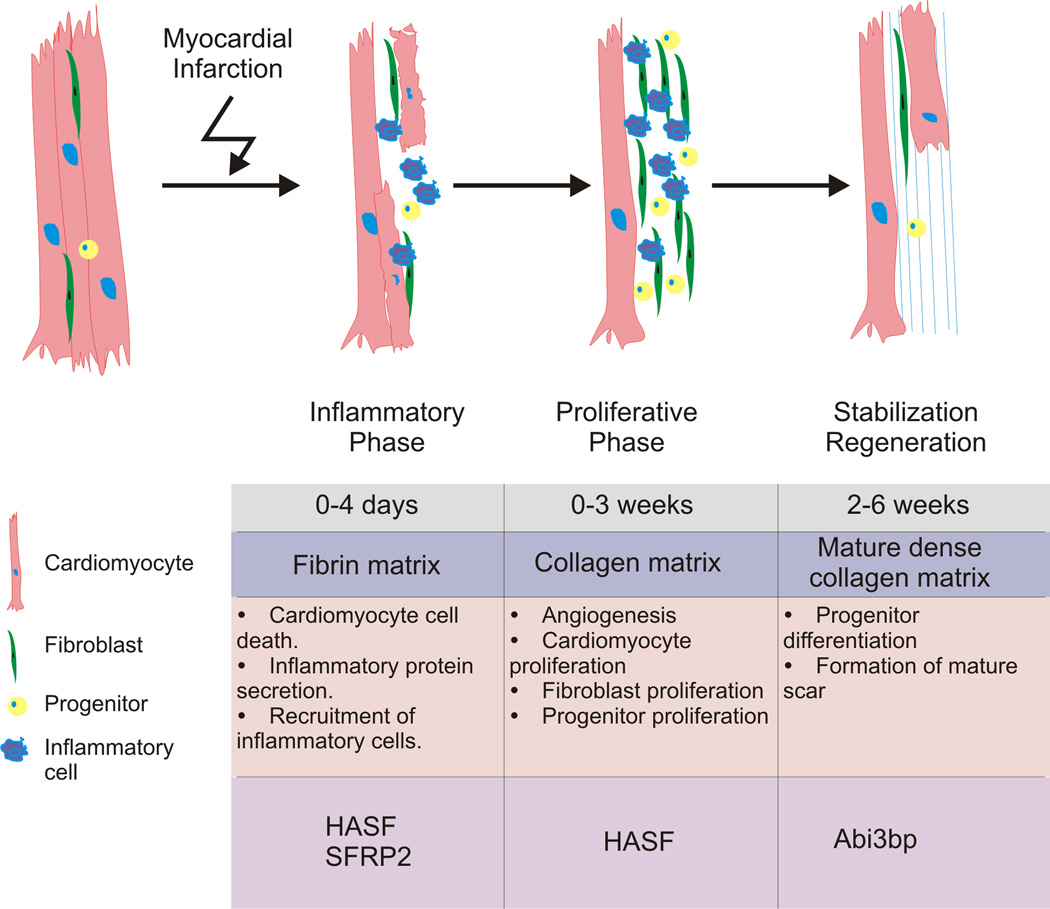
As we have described above paracrine factors released by MSCs affect all of the events that occur following myocardial infarction (Figure 4). Using our own research as an example HASF and Sfrp2 prevent cardiomyocyte cell-death, the first event that occurs following a myocardial infarction. Both Sfrp2 and Abi3bp promote resident progenitor cells to differentiate, an event which begins ~1 week after myocardial infarction. Finally HASF promotes the remaining cardiomyocytes to proliferate. Considering the pleiotropic actions of paracrine factors (Table 2), they can influence the post-myocardial injury sequence of responses such as inflammation, fibrosis, neovascularization, remodeling, and cardiomyocyte proliferation. This hypothesis is contingent on the existence of stem cells or other secretory cells albeit at low levels throughout the cardiac repair and regenerative process. Indeed, this is supported by the evidence of stem cells presence in low levels weeks after MI140, 141.
Figure 4.
Paracrine factors affect different temporal events after myocardial injury influencing different stages of the reparative and regenerative processes.
One question one can ask is, does the secretory profile of MSCs change in a positive fashion in response to the temporal sequence of events that occur following a myocardial infarction?
The inflammatory response is robustly active immediately following myocardial infarction. Increased expression of the cytokine interleukin-6 (IL-6) is one of the hallmarks of this inflammatory response. Intriguingly, IL-6 modifies MSC paracrine function by stimulating these cells to secrete VEGF142 which as discussed earlier promotes new blood vessel formation in the myocardium. Other pro-inflammatory cytokines released by the dying myocardium have similar effects to IL-6. TNF-a, IL-1 and IFN-g have all been shown to stimulate MSC secretion of a number of growth factors such as epidermal growth factor (EGF), VEGF, hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF1) and angiopoietin. These growth factors go on to regenerate the myocardium through the formation of new capillaries, cardiomyocyte proliferation and resident progenitor cell differentiation143. Taking these findings one step further, pre-activating MSCs with pro-inflammatory cytokines prior to their delivery into the heart may have therapeutic applications by stimulating MSC paracrine effects as has recently been shown in a radiation-induced intestinal injury model144.
In a similar vein, the extracellular remodeling which occurs post-myocardial infarction has been shown to modulate the secretory profile of MSCs. The remodeled matrix was found to promote the secretion of a number of proangiogenic, anti-fibrotic, and immunomodulatory paracrine factors from MSCs, most notably HGF and SDF-1145.
Studies in brain injury models suggest that MSCs are not passive players but actively sense their environment to affect repair. Administration of MSCs at different time-points following brain ischemia injury had markedly different effects, stimulating cell proliferation when injected three days after injury and stimulating axonal remodeling when injected 10 days after ischemia. Pathway-focused PCR array analysis revealed that that a number of genes encoding secreted factors were differentially regulated in the MSCs injected at the two-time points. This led the authors to conclude that the MSCs were actively sensing the microenvironment and changing their secretory profile according to the needs of the milieu, adapting to the specific signals provided by the injured brain146, 147.
Conclusions and Future Directions in Paracrine Factor Research
In summary, the paracrine hypothesis is a natural extension of the traditional concept of the stem cell niche to include the role of factors released by stem cells upon their microenvironment influencing the tissue’s response to injury. As mentioned above, paracrine factors create a specific microenvironment, impacting the biology of cells within that niche. Understanding the temporal and spatial components underlying the regenerative properties of paracrine factors in the injured heart will clarify the complex process of repair and regeneration.
It is apparent from a large number of studies that transplanted adult stem cells fail to integrate and differentiate into mature cardiac cells. Moreover, there is an extensive loss of the cells following transplantation. So why not simply inject more adult stem cells? This is neither practical nor desirable. Some of these adult stem cells are particularly rare, for example c-Kit+ CPCs. Though these cells can in certain circumstances be amplified ex vivo the amount of time taken to get a sufficient quantity is considerable; too long to be clinically useful. It should also be borne in mind that cardiac injury tends to occur in mid to late life. Increasing age significantly impairs the ability of stem cells to renew and differentiate97, 148; potentially limiting an individualized treatment strategy using the patient’s own cells. Potentially allogenic cells from young or, notwithstanding ethical concerns, fetal donors could be utilized. However, immunosuppression would then be necessary to prevent cell rejection.
We, and others, have shown that the release of paracrine factors mediate the majority of the effects of transplanted adult stem cells. Utilizing that knowledge many researchers, including ourselves, have genetically engineered adult stem cells to augment the paracrine effect or increase cell survival and engraftment1. For example, our modifications of MSCs include overexpression of Akt120, to augment the paracrine effect of these cells as well promoting their survival,
It is important to note that in the field of cell therapy consistent and reproducible results among laboratories is a major issue. There are initiatives being pursued to standardize procedures and nomenclature. However, especially for the rare adult stem cells, growth conditions and the number of passages will give rise to a different population with each isolate. This is less than ideal for a standardized therapeutic modality. Substituting the paracrine factors for the adult stem cells is thus a sensible approach. The treatment strategy can be defined and reproducible; furthermore any difficult issues involve the use of cells are avoided.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This work was supported by National Heart, Lung, and Blood Institute grants RO1 HL81744, HL72010, and HL73219 (to V.J.D.); and the Edna and Fred L. Mandel Jr. Foundation (to V.J.D., and M.M.). M.M. was also supported by an American Heart Association National Scientist Development Award (10SDG4280011).
NON-STANDARD ABBREVIATIONS AND ACRONYMS
- bFGF
basic Fibroblast growth factor
- CPC
Cardiac progenitor cell
- GFP
Green Fluorescent Protein
- HASF
Hypoxic induced Akt regulated Stem cell Factor
- HGF
Hepatocyte growth factor
- IGF1
Insulin-like growth factor
- MI
Myocardial infarction
- MSC
Mesenchymal stem cell
- Sfrp2
Secreted frizzled related protein 2 (Sfrp2)
- VEGF
Vascular endothelial growth factor
Footnotes
DISCLOSURES
None of the authors have any real or apparent conflict(s) of interest to disclose.
References
- 1.Hodgkinson CP, Gomez JA, Mirotsou M, Dzau VJ. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Human gene therapy. 2010;21:1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teo AK, Vallier L. Emerging use of stem cells in regenerative medicine. Biochem J. 2010;428:11–23. doi: 10.1042/BJ20100102. [DOI] [PubMed] [Google Scholar]
- 3.Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6:139–148. doi: 10.1038/nrendo.2009.274. [DOI] [PubMed] [Google Scholar]
- 4.Loebinger MR, Bilton D, Wilson R. Upper airway 2: Bronchiectasis, cystic fibrosis and sinusitis. Thorax. 2009;64:1096–1101. doi: 10.1136/thx.2008.112870. [DOI] [PubMed] [Google Scholar]
- 5.Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010;6:211–218. doi: 10.1586/eci.09.86. [DOI] [PubMed] [Google Scholar]
- 6.Kumar AH, Caplice NM. Clinical potential of adult vascular progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:1080–1087. doi: 10.1161/ATVBAHA.109.198895. [DOI] [PubMed] [Google Scholar]
- 7.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 9.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 10.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 11.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation research. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Liang D, Gao X, Zhao C, Qin X, Xu Y, Su T, Sun D, Li W, Wang H, Liu B, Cao F. Selective inhibition of inositol hexakisphosphate kinases (IP6Ks) enhances mesenchymal stem cell engraftment and improves therapeutic efficacy for myocardial infarction. Basic Res Cardiol. 2014;109:417. doi: 10.1007/s00395-014-0417-x. [DOI] [PubMed] [Google Scholar]
- 13.Mathieu E, Lamirault G, Toquet C, Lhommet P, Rederstorff E, Sourice S, Biteau K, Hulin P, Forest V, Weiss P, Guicheux J, Lemarchand P. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PloS one. 2012;7:e51991. doi: 10.1371/journal.pone.0051991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 16.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 17.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 18.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circulation research. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 19.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature medicine. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 21.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. The Journal of clinical investigation. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 23.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 24.Lim SY, Kim YS, Ahn Y, Jeong MH, Hong MH, Joo SY, Nam KI, Cho JG, Kang PM, Park JC. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovascular research. 2006;70:530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. American journal of physiology Heart and circulatory physiology. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 26.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circulation research. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 27.Xu M, Uemura R, Dai Y, Wang Y, Pasha Z, Ashraf M. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. Journal of molecular and cellular cardiology. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Deb A, Zhang Z, Pachori A, He W, Guo J, Pratt R, Dzau VJ. Secreted frizzled related protein 2 protects cells from apoptosis by blocking the effect of canonical Wnt3a. Journal of molecular and cellular cardiology. 2009;46:370–377. doi: 10.1016/j.yjmcc.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J, Guo J, Beigi F, Hodgkinson CP, Facundo HT, Zhang Z, Espinoza-Derout J, Zhou X, Pratt RE, Mirotsou M, Dzau VJ. HASF is a stem cell paracrine factor that activates PKC epsilon mediated cytoprotection. Journal of molecular and cellular cardiology. 2014;66:157–164. doi: 10.1016/j.yjmcc.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Ingram DA, Murphy MP, Saadatzadeh MR, Mead LE, Prater DN, Rehman J. Release of proinflammatory mediators and expression of proinflammatory adhesion molecules by endothelial progenitor cells. Am J Physiol Heart Circ Physiol. 2009;296:H1675–H1682. doi: 10.1152/ajpheart.00665.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Stefano R, Barsotti MC, Armani C, Santoni T, Lorenzet R, Balbarini A, Celi A. Human peripheral blood endothelial progenitor cells synthesize and express functionally active tissue factor. Thromb Res. 2009;123:925–930. doi: 10.1016/j.thromres.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. Journal of biomedical science. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 37.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regenerative medicine. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scandinavian journal of immunology. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 39.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, Cantos C, Jorgensen C, Noel D. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an interleukin-6-dependent mechanism. Stem cells. 2007;25:2025–2032. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 40.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 41.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 42.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yano T, Miura T, Whittaker P, Miki T, Sakamoto J, Nakamura Y, Ichikawa Y, Ikeda Y, Kobayashi H, Ohori K, Shimamoto K. Macrophage colony-stimulating factor treatment after myocardial infarction attenuates left ventricular dysfunction by accelerating infarct repair. J Am Coll Cardiol. 2006;47:626–634. doi: 10.1016/j.jacc.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Troidl C, Mollmann H, Nef H, Masseli F, Voss S, Szardien S, Willmer M, Rolf A, Rixe J, Troidl K, Kostin S, Hamm C, Elsasser A. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J Cell Mol Med. 2009;13:3485–3496. doi: 10.1111/j.1582-4934.2009.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, Leor J. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116:I38–I45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 47.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental hematology. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 50.Santini MP, Tsao L, Monassier L, Theodoropoulos C, Carter J, Lara-Pezzi E, Slonimsky E, Salimova E, Delafontaine P, Song YH, Bergmann M, Freund C, Suzuki K, Rosenthal N. Enhancing repair of the mammalian heart. Circulation research. 2007;100:1732–1740. doi: 10.1161/CIRCRESAHA.107.148791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, Dimmeler S. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circulation research. 2008;103:203–211. doi: 10.1161/CIRCRESAHA.108.178475. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, Leor J. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–1901. doi: 10.1016/j.jacc.2013.07.057. [DOI] [PubMed] [Google Scholar]
- 53.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 54.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. The American journal of physiology. 1997;272:H220–H226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 57.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kardami E, Banerji S, Doble BW, Dang X, Fandrich RR, Jin Y, Cattini PA. PKC-dependent phosphorylation may regulate the ability of connexin43 to inhibit DNA synthesis. Cell communication & adhesion. 2003;10:293–297. doi: 10.1080/cac.10.4-6.293.297. [DOI] [PubMed] [Google Scholar]
- 59.Hinrichsen R, Haunso S, Busk PK. Different regulation of p27 and Akt during cardiomyocyte proliferation and hypertrophy. Growth factors. 2007;25:132–140. doi: 10.1080/08977190701549835. [DOI] [PubMed] [Google Scholar]
- 60.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. The Journal of biological chemistry. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 61.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 62.Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. The New England journal of medicine. 2012;367:2150–2153. doi: 10.1056/NEJMcibr1203156. [DOI] [PubMed] [Google Scholar]
- 63.Hodgkinson CP, Dzau VJ. Conserved microRNA program as key to mammalian cardiac regeneration: insights from zebrafish. Circulation research. 2015;116:1109–1111. doi: 10.1161/CIRCRESAHA.115.305852. [DOI] [PubMed] [Google Scholar]
- 64.Haubner BJ, Adamowicz-Brice M, Khadayate S, Tiefenthaler V, Metzler B, Aitman T, Penninger JM. Complete cardiac regeneration in a mouse model of myocardial infarction. Aging (Albany NY) 2012;4:966–977. doi: 10.18632/aging.100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DI, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Przybyt E, Krenning G, Brinker MG, Harmsen MC. Adipose stromal cells primed with hypoxia and inflammation enhance cardiomyocyte proliferation rate in vitro through STAT3 and Erk1/2. Journal of translational medicine. 2013;11:39. doi: 10.1186/1479-5876-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Developmental cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heo SC, Lee KO, Shin SH, Kwon YW, Kim YM, Lee CH, Kim YD, Lee MK, Yoon MS, Kim JH. Periostin mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth in a xenograft lung adenocarcinoma model. Biochimica et biophysica acta. 2011;1813:2061–2070. doi: 10.1016/j.bbamcr.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature medicine. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 70.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circulation research. 2009;104:e1–e7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ladage D, Yaniz-Galende E, Rapti K, Ishikawa K, Tilemann L, Shapiro S, Takewa Y, Muller-Ehmsen J, Schwarz M, Garcia MJ, Sanz J, Hajjar RJ, Kawase Y. Stimulating myocardial regeneration with periostin Peptide in large mammals improves function post-myocardial infarction but increases myocardial fibrosis. PloS one. 2013;8:e59656. doi: 10.1371/journal.pone.0059656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao S, Wu H, Xia W, Chen X, Zhu S, Zhang S, Shao Y, Ma W, Yang D, Zhang J. Periostin expression is upregulated and associated with myocardial fibrosis in human failing hearts. Journal of cardiology. 2014;63:373–378. doi: 10.1016/j.jjcc.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Beigi F, Schmeckpeper J, Pow-Anpongkul P, Payne JA, Zhang L, Zhang Z, Huang J, Mirotsou M, Dzau VJ. C3orf58, a novel paracrine protein, stimulates cardiomyocyte cell-cycle progression through the PI3K-AKT-CDK7 pathway. Circulation research. 2013;113:372–380. doi: 10.1161/CIRCRESAHA.113.301075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bareja A, Hodgkinson C, Payne A, Pratt R, VJ D. Abstract 15731: Hypoxia and Akt Induced Stem Cell Factor Exerts Cardioprotective Effects via Specific Binding to the Insulin-Like Growth Factor-1 Receptor. Circulation. 2014;130:A15731. [Google Scholar]
- 75.Duerr RL, Huang S, Miraliakbar HR, Clark R, Chien KR, Ross J., Jr Insulin-like growth factor-1 enhances ventricular hypertrophy and function during the onset of experimental cardiac failure. The Journal of clinical investigation. 1995;95:619–627. doi: 10.1172/JCI117706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. Journal of hepatology. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 78.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlondorff D, Anders HJ. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney international. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 79.Ohnishi S, Yasuda T, Kitamura S, Nagaya N. Effect of hypoxia on gene expression of bone marrow-derived mesenchymal stem cells and mononuclear cells. Stem cells. 2007;25:1166–1177. doi: 10.1634/stemcells.2006-0347. [DOI] [PubMed] [Google Scholar]
- 80.Xu X, Xu Z, Xu Y, Cui G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coronary artery disease. 2005;16:245–255. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 81.Ohnishi S, Sumiyoshi H, Kitamura S, Nagaya N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS letters. 2007;581:3961–3966. doi: 10.1016/j.febslet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 82.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall'Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim MJ, Serwold T, Wagers AJ, Lee RT. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He W, Zhang L, Ni A, Zhang Z, Mirotsou M, Mao L, Pratt RE, Dzau VJ. Exogenously administered secreted frizzled related protein 2 (Sfrp2) reduces fibrosis and improves cardiac function in a rat model of myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21110–21115. doi: 10.1073/pnas.1004708107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, Yamada C, Liu TC, Huang G, Basson CT, Kispert A, Greenspan DS, Sato TN. Secreted Frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nature cell biology. 2009;11:46–55. doi: 10.1038/ncb1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mastri M, Shah Z, Hsieh K, Wang X, Wooldridge B, Martin S, Suzuki G, Lee T. Secreted Frizzled-related protein 2 as a target in antifibrotic therapeutic intervention. American journal of physiology Cell physiology. 2014;306:C531–C539. doi: 10.1152/ajpcell.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uren A, Reichsman F, Anest V, Taylor WG, Muraiso K, Bottaro DP, Cumberledge S, Rubin JS. Secreted frizzled-related protein-1 binds directly to Wingless and is a biphasic modulator of Wnt signaling. The Journal of biological chemistry. 2000;275:4374–4382. doi: 10.1074/jbc.275.6.4374. [DOI] [PubMed] [Google Scholar]
- 87.Ostrom RS. A new molecular target for blunting organ fibrosis. Focus on "Secreted Frizzled-related protein 2 as a target in antifibrotic therapeutic intervention". American journal of physiology Cell physiology. 2014;306:C527–C528. doi: 10.1152/ajpcell.00020.2014. [DOI] [PubMed] [Google Scholar]
- 88.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 89.Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, Swingen C, Zhang G, Feygin J, Ochiai K, Bransford TL, From AH, Bache RJ, Zhang J. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol. 2006;291:H648–H657. doi: 10.1152/ajpheart.01387.2005. [DOI] [PubMed] [Google Scholar]
- 90.Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol. 2007;293:H1772–H1780. doi: 10.1152/ajpheart.00242.2007. [DOI] [PubMed] [Google Scholar]
- 91.Gnecchi M, He H, Melo LG, Noiseaux N, Morello F, de Boer RA, Zhang L, Pratt RE, Dzau VJ, Ingwall JS. Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells. 2009;27:971–979. doi: 10.1002/stem.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freestone NS, Ribaric S, Mason WT. The effect of insulin-like growth factor-1 on adult rat cardiac contractility. Molecular and cellular biochemistry. 1996;163–164:223–229. doi: 10.1007/BF00408662. [DOI] [PubMed] [Google Scholar]
- 93.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 94.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 95.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 96.Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 97.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 98.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circulation research. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 99.Tse HF, Siu CW, Zhu SG, Songyan L, Zhang QY, Lai WH, Kwong YL, Nicholls J, Lau CP. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. European journal of heart failure. 2007;9:747–753. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Markel TA, Wang Y, Herrmann JL, Crisostomo PR, Wang M, Novotny NM, Herring CM, Tan J, Lahm T, Meldrum DR. VEGF is critical for stem cell-mediated cardioprotection and a crucial paracrine factor for defining the age threshold in adult and neonatal stem cell function. Am J Physiol Heart Circ Physiol. 2008;295:H2308–H2314. doi: 10.1152/ajpheart.00565.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–S64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PloS one. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: interactions during muscle regeneration. Trends in immunology. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Engels MC, Rajarajan K, Feistritzer R, Sharma A, Nielsen UB, Schalij MJ, de Vries AA, Pijnappels DA, Wu SM. Insulin-like growth factor promotes cardiac lineage induction in vitro by selective expansion of early mesoderm. Stem cells. 2014;32:1493–1502. doi: 10.1002/stem.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakanishi C, Yamagishi M, Yamahara K, Hagino I, Mori H, Sawa Y, Yagihara T, Kitamura S, Nagaya N. Activation of cardiac progenitor cells through paracrine effects of mesenchymal stem cells. Biochemical and biophysical research communications. 2008;374:11–16. doi: 10.1016/j.bbrc.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 110.Hodgkinson CP, Naidoo V, Patti KG, Gomez JA, Schmeckpeper J, Zhang Z, Davis B, Pratt RE, Mirotsou M, Dzau VJ. Abi3bp is a multifunctional autocrine/paracrine factor that regulates mesenchymal stem cell biology. Stem cells. 2013;31:1669–1682. doi: 10.1002/stem.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodgkinson CP, Gomez JA, Payne AJ, Zhang L, Wang X, Dal-Pra S, Pratt RE, Dzau VJ. Abi3bp regulates cardiac progenitor cell proliferation and differentiation. Circulation research. 2014;115:1007–1016. doi: 10.1161/CIRCRESAHA.115.304216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Molecular therapy : the journal of the American Society of Gene Therapy. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. doi: 10.3389/fimmu.2014.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 115.Yu B, Gong M, Wang Y, Millard RW, Pasha Z, Yang Y, Ashraf M, Xu M. Cardiomyocyte protection by GATA-4 gene engineered mesenchymal stem cells is partially mediated by translocation of miR-221 in microvesicles. PloS one. 2013;8:e73304. doi: 10.1371/journal.pone.0073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circulation research. 2011;109:724–728. doi: 10.1161/CIRCRESAHA.111.253286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 120.Bruno S, Grange C, Collino F, Deregibus MC, Cantaluppi V, Biancone L, Tetta C, Camussi G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PloS one. 2012;7:e33115. doi: 10.1371/journal.pone.0033115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Reis LA, Borges FT, Simoes MJ, Borges AA, Sinigaglia-Coimbra R, Schor N. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PloS one. 2012;7:e44092. doi: 10.1371/journal.pone.0044092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, Tetta C, Camussi G. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27aoverexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep. 2013;8:1876–1882. doi: 10.3892/mmr.2013.1738. [DOI] [PubMed] [Google Scholar]
- 124.van der Meel R, Fens MH, Vader P, van Solinge WW, Eniola-Adefeso O, Schiffelers RM. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 125.van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 2013;65:1284–1298. doi: 10.1016/j.addr.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 126.Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. The Journal of biological chemistry. 2011;286:34883–34892. doi: 10.1074/jbc.M111.276329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Itoh N, Ohta H. Fgf10: a paracrine-signaling molecule in development, disease, and regenerative medicine. Current molecular medicine. 2014;14:504–509. doi: 10.2174/1566524014666140414204829. [DOI] [PubMed] [Google Scholar]
- 128.Perrimon N, Pitsouli C, Shilo BZ. Signaling mechanisms controlling cell fate and embryonic patterning. Cold Spring Harbor perspectives in biology. 2012;4:a005975. doi: 10.1101/cshperspect.a005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. European journal of clinical investigation. 2013;43:986–995. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circulation research. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rachmilewitz J, Lanzavecchia A. A temporal and spatial summation model for T-cell activation: signal integration and antigen decoding. Trends in immunology. 2002;23:592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
- 133.Magee JC. Dendritic integration of excitatory synaptic input. Nature reviews Neuroscience. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- 134.Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harbor perspectives in biology. 2013;5:a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Przybyla L, Voldman J. Probing embryonic stem cell autocrine and paracrine signaling using microfluidics. Annual review of analytical chemistry. 2012;5:293–315. doi: 10.1146/annurev-anchem-062011-143122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miura T. HASF, a PKC-epsilon activator with novel features for cardiomyocyte protection. Journal of molecular and cellular cardiology. 2014;69:1–3. doi: 10.1016/j.yjmcc.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 137.Ferreira JC, Brum PC, Mochly-Rosen D. betaIIPKC and epsilonPKC isozymes as potential pharmacological targets in cardiac hypertrophy and heart failure. Journal of molecular and cellular cardiology. 2011;51:479–484. doi: 10.1016/j.yjmcc.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmeckpeper J, Verma A, Yin L, Beigi F, Zhang L, Payne A, Zhang Z, Pratt RE, Dzau VJ, Mirotsou M. Inhibition of Wnt6 by Sfrp2 regulates adult cardiac progenitor cell differentiation by differential modulation of Wnt pathways. Journal of molecular and cellular cardiology. 2015;85:215–225. doi: 10.1016/j.yjmcc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mizumoto S, Yamada S, Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol. 2015;34:35–42. doi: 10.1016/j.sbi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 140.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. Journal of molecular and cellular cardiology. 2006;41:876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 141.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 142.Herrmann JL, Weil BR, Abarbanell AM, Wang Y, Poynter JA, Manukyan MC, Meldrum DR. IL-6 and TGF-alpha costimulate mesenchymal stem cell vascular endothelial growth factor production by ERK-, JNK-, and PI3K-mediated mechanisms. Shock. 2011;35:512–516. doi: 10.1097/SHK.0b013e31820b2fb9. [DOI] [PubMed] [Google Scholar]
- 143.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. Pre-activation of mesenchymal stem cells with TNF-alpha, IL-1beta and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. doi: 10.1038/srep08718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Sullivan KE, Quinn KP, Tang KM, Georgakoudi I, Black LD., 3rd Extracellular matrix remodeling following myocardial infarction influences the therapeutic potential of mesenchymal stem cells. Stem Cell Res Ther. 2014;5:14. doi: 10.1186/scrt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Velthoven CT, Kavelaars A, Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res. 2012;71:474–481. doi: 10.1038/pr.2011.64. [DOI] [PubMed] [Google Scholar]
- 147.van Velthoven CT, Kavelaars A, van Bel F, Heijnen CJ. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J Neurosci. 2010;30:9603–9611. doi: 10.1523/JNEUROSCI.1835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogorek B, Isobe K, Wybieralska E, Borghetti G, Pesapane A, Sorrentino A, Mangano E, Cappetta D, Mangiaracina C, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi AM, Kajstura J, Hosoda T, Rota M, Anversa P, Leri A. c-Kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circulation research. 2014;114:41–55. doi: 10.1161/CIRCRESAHA.114.302500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hodgkinson C, Gomez J, Bareja A, Dzau V. Role of Paracrine Mechanisms. In: Perin E, Miller L, Taylor D, Willerson J, editors. Stem Cell and Gene Therapy for Cardiovascular Disease. Elsevier; 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.