Abstract
Background
The objective of the present study was to determine whether single administration of the antioxidant enzyme bovine superoxide dismutase (bSOD) after radiation (RT) exposure mitigates development of pulmonary toxicity in rats.
Methods
Female F344 rats (n=60) were divided among six experimental groups: (1) RT, single dose of 21 Gy to the right hemithorax; (2) RT+5 mg/kg bSOD; (3) RT+15 mg/kg bSOD; (4) No RT; (5) sham RT+5mg/kg bSOD; and (6) sham RT+15mg/kg bSOD. A single subcutaneous injection of bSOD (5 or 15 mg/kg) was administered 24 hours postradiation. The effects of bSOD on radiation-induced lung injury were assessed by measurement of body weight, breathing frequency and histopathological changes. Immunohistochemistry was used to evaluate oxidative stress (8-OHdG+, NOX4+, nitrotyrosine+, 4HNE+ cells), macrophage activation (ED1+), and expression of profibrotic TGF-β in irradiated tissue.
Results
Radiation led to an increase in all evaluated parameters. Treatment with 15mg/kg bSOD significantly decreased levels of all evaluated parameters including tissue damage and breathing frequency starting 6 weeks post-radiation. Animals treated with 5 mg/kg bSOD trended toward a suppression of radiation-induced lung damage but did not reach statistical significance.
Conclusions
The single application of bSOD (15mg/kg) ameliorates radiation induced lung injury through suppression of ROS/RNS dependent tissue damage.
Keywords: Bovine Superoxide Dismutase, radiation induced lung injury, reactive oxygen species, fibrosis
Introduction
The probability of normal tissue complications, specifically pneumonitis/fibrosis, is a major limiting factor in achieving optimal therapeutic radiation doses for treatment of tumors in the thoracic region. In the clinical setting approximately 60 % of lung cancer patients will receive radiation therapy (RT) at some point in the course of treatment [1]. Despite improvements in radiation techniques, the incidence of lung injury after radiation therapy remains relatively high and varies between 5% and 30% [2]. At this time no U.S. Food and Drug Administration–approved therapeutic agents are available to prevent, mitigate, and/or treat lung injury caused by thoracic RT.
Although the initial ionizing event lasts only a few milliseconds, chronic production of reactive oxygen and nitrogen species (ROS and RNS, respectively) in response to RT-induced cellular stress alters the environmental milieu, leading to progressive injury. If unresolved, this injury culminates in acute pneumonitis or progressive fibrosis [3]. The production of ROS/RNS modulates gene expression of a wide array of proinflammatory and profibrogenic genes through increased expression of transcription factors such as Egr-1, NF-κβ, c-Myc, and c-Jun [4]. In addition, tissue hypoxia, which gradually develops over time as a result of ongoing inflammation and fibroobliteration of the alveoli, leads to increased vasculature dysfunction and recruitment of inflammatory mediators, further disrupting the redox balance of the tissue [5]. Activated macrophages and neutrophils are recruited to the site of hypoxia, leading to an additional increase in ROS production through oxidative burst [6]. This self-augmenting process of ROS/RNS production and inflammation leads, in turn, to delayed onset of acute pneumonitis.
Superoxide dismutases (SODs) are among the main regulators of ROS/RNS presence in the cell [7]. These metalloprotein enzymes are present in most cells and have a key role in homeostatic maintenance of intra- and extracellular ROS/RNS levels [8]. Several classes of SOD enzyme exist. They differ in the metal content of the metalloporphyrin active site within the enzyme and their location within the cell [9,10]. The role of SOD has been extensively evaluated, both in clinical and preclinical trials, in diseases correlated with ROS overproduction, including radiation-induced fibrosis of the skin and underlying tissues [11-13]. A number of investigators have shown in preclinical models that SOD overexpression or administration of SOD-like compounds can prevent and/or mitigate radiation-induced lung damage [14-18]. Kang and colleagues were the first to demonstrate that overexpression of extracellular SOD, which is primarily localized to the lung, reduces functional damage and collagen deposition in a mouse model following thoracic irradiation [15,18]. Another approach, gene therapy using inhaled or intratracheal injection of human SOD has also shown promising results [19-21] and is currently in clinical trial. SOD mimetic (e.g. Mn-porphyrin), which in addition to SOD-like catalytic activity can also neutralize a number of other free radical species, have also been shown to mitigate lung damage in both mice and rats [22-25].
Bovine SOD (bSOD; Orgotein), a member of the Cu-Zn SODs, has been studied for decades and used in various diseases from arthritis to severe respiratory distress syndrome in infants to patients receiving chemotherapy and RT [26]. We assessed the protective effects of bSOD on body weight, lung function, lung tissue damage, and extent of fibrosis caused by radiation. In addition, we investigated the mechanism of action of bSOD administration on the major pathophysiologic processes involved in radiation-induced lung injury.
Material and Methods
Animals
This study was approved by the Duke University Institutional Animal Care and Use Committee. Female Fisher 344 rats (Charles River; Wilmington, MA), 10–12 weeks of age and weighing 150–170 g, were used in this study. Animals were housed and maintained under identical conditions with food and water provided ad libitum in an institutional vivarium and according to institutional guidance. During the entire length of the study, all animals were closely monitored for pain, discomfort, and distress. Animals showing signs of distress were euthanized in accordance with institutional policies. Study animals were euthanized at a predetermined time of 20 weeks postradiation by pentobarbital overdose, and lung tissue was collected for further analysis.
Experimental design
Sixty animals (10 per group) were divided into six experimental groups (1) RT: single dose of radiation and no further treatment; (2) RT+5mg/kg: radiation and 5 mg/kg of bSOD subcutaneously 24 hours postradiation; (3) RT+15mg/kg: radiation and 15 mg/kg of bSOD subcutaneously 24 hours postradiation; (4) control: same handling procedures but with no radiation exposure or injections; (5) sham irradiation+5mg/kg: 5 mg/kg of bSOD but with no radiation; and (6) sham irradiation+15mg/kg: 15 mg/kg of bSOD but with no radiation.
Radiation and treatment
All animals receiving treatment were anesthetized by subcutaneous injection of ketamine (65 mg/kg) and xylazine (4.5 mg/kg) and placed in the prone position. A single dose of 21 Gy was delivered to the right lung using using 150-kV x-rays with a dose rate of 0.71 Gy/minute (Therapax 320; Pantak Inc., East Haven, CT). The left thorax and the rest of the body were shielded with a 12-mm lead block, a step necessary to ensure survival after exposure to the dose needed to induce late fibrotic changes. Twenty-four hours after irradiation, animals in the agent test groups were administered a subcutaneous injection of 5 or 15 mg/kg of bSOD (Oxis International; Tampa, FL). Regnault et al. conducted pharmacokinetic study in rats after oral and subcutaneous route and several doses of bSOD (0.5-20mg/kg) and showed superior bioavailability of bSOD after subcutaneous administration (6 fold higher), therefore we decided to test higher (15mg/kg) and lower (5mg/kg) doses of subcutaneous bSOD administration [27].
Follow-up and functional assessment of lung injury
The objective of this study was to determine whether administration of bSOD 24 hours after radiation exposure mitigates development of lung injury in rats. Animals were followed for 20 weeks after exposure. The overall wellbeing of the animals was evaluated by monitoring changes in body weight. Baseline measurements were conducted prior to radiation and then measured biweekly.
Breathing frequency was used as a metric for assessment of functional impairment of lungs caused by radiation. Baseline breathing frequency was measured prior to radiation and then every 2 weeks, using whole-body plethysmography (Model RM-80; Columbus Instruments, Columbus, OH). The mean values of five measurements were recorded for five animals in each group. At the end of the study (20 weeks after radiation), animals were euthanized and lung tissue was collected and fixed in 10% neutral buffered formalin for further analysis.
Histopathology and fibrosis score
The extent of lung fibrosis caused by radiation was assessed histopathologically as described in a previous study [28]. Briefly, lungs were fixed in 10% neutral buffered formalin, dissected in 5-micron thick sections, and stained with hematoxylin, eosin, and Masson’s trichrome. Slides were systematically scanned using a light microscope with a 10× objective. A scale of 0–8, with 0 representing normal lung and 8 representing severe distortion of lung structure and large tissue areas, was used by the pathologist to grade each section. The pathologist was unaware of the group assignments of the tissues.
Immunohistochemistry
We evaluated tissue levels of inflammation and oxidative stress using marker of ROS damage to DNA (8-OHdG), nitrotyrosine, 4HNE, NADPH oxidase 4 (Nox4), macrophage activation (ED1), and profibrogenic (TGF-β) as previously described [29,30]. Briefly, tissue sections were incubated overnight at 4°C with primary antibodies to macrophage marker ED1 (1:100; Serotec, Oxford, UK), 8-OHdG (8-hydroxydeoxyguanine, mouse monoclonal, 1:2000; JaICA, Shizuoka, Japan), nitrotyrosine (1:4,000; Abcam, San Diego, CA), 4HNE (1:100, JaICA, Shizuoka, Japan), Nox4 (1:400; Santa Cruz Biotechnology Inc., Santa Cruz, CA), and active TGFβ1 (1:200; Santa Cruz Biotechnology). Slides were then washed three times in phosphate-buffered saline solution for 5 minutes followed by incubation with the appropriate secondary antibody (1:200; Jackson ImmunoResearch, West Grove, PA) for 30 minutes at room temperature. Slides were then incubated with ABCElite (Vector Laboratories; Burlingame, CA) for 30 minutes at room temperature and developed using DAB working solution (Laboratory Vision; Fremont, CA). Finally, slides were counterstained with Harris hematoxylin (Fisher Scientific; Pittsburgh, PA) and mounted with cover slips. Slides were systematically scanned and 8–10 representative digital images were acquired from each slide using a 40× objective. Digital images were quantified by image analysis with Adobe Photoshop (Version 7.0; Adobe Systems, San Jose, CA) and scored by two independent observers blinded to the identity of the slides.
Statistical analysis
All results were presented as mean ± SEM. Time points for each parameter were compared against baseline controls by t tests, and all reported P values are two-sided. Statistical significance was considered with P value less than 0.05. All statistical analyses were performed using JMP software, version 6.0 (SAS Institute, Inc.; Cary, NC) as previously described [3].
Results
Body weight
In all groups exposed to radiation (RT, RT+5mg/kg, and RT+15mg/kg) a decreases in body weight was observed when compared to controls and sham-irradiated groups (5mg/kg and 15mg/kg). In irradiated animals administration of bSOD prevented weight loss. Groups RT+5mg/kg and RT+15mg/kg had significantly higher body weights in comparison with group that received only radiation starting at 8 weeks after the irradiation until the end of the study (Figure 1).
Figure 1.
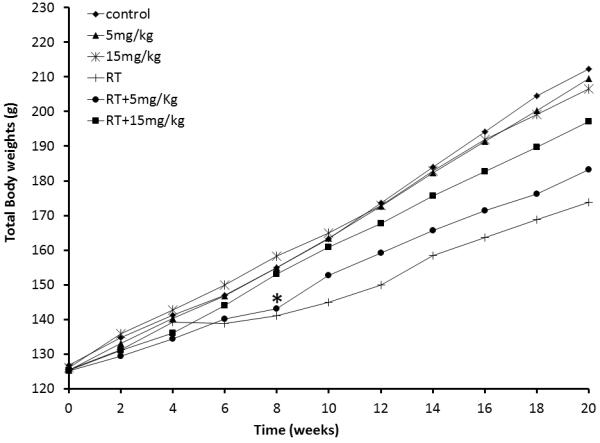
Changes in total body weight over period of 20 weeks post radiation. Starting at week 8 post radiation, groups receiving bSOD treatment (RT + 5 mg/kg and RT + 15 mg/kg) have significantly higher body weight than irradiated group (RT) (p<0.05). This difference continued to increase until the end of the experiment. The bSOD treatment alone did not have impact on the total body weight of animals. There is no difference in body weight observed in 5mg/kg and 15mg/kg groups in comparison to Control group.
Functional assessment of the lung
Radiation caused increases in breathing frequency, starting at 2 weeks after exposure in all irradiation groups (RT, RT+5mg/kg, and RT+15mg/kg). In irradiated animals treated with bSOD 24 hours after radiation there was a decrease in respiratory distress assessed by breathing frequency. Reduction in respiratory distress RT+5mg/kg and RT+15mg/kg was bSOD dose dependent: the RT+15 mg/kg dose significantly reduced breathing frequency, whereas the RT+5 mg/kg dose showed a trend in suppression of radiation-caused increase in breathing rate compared to the RT group. No negative effects on breathing frequency were observed as a result of bSOD administration in the unirradiated animals (Figure 2).
Figure 2.
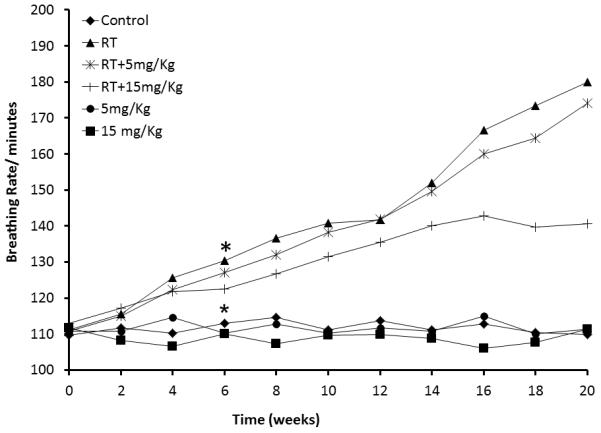
Time-related changes in breathing frequency after 21Gy irradiation to the right hemithorax. Breathing frequency increased in the first four weeks in all irradiated animals. Starting at week 6 after irradiation, breathing frequency was significantly higher in all irradiated animals (RT, RT+5mg/kg and RT+15mg/kg) when compared to nonirradiated controls (Control, 5mg/kg and 15mg/kg) (p<0.05). Importantly, breathing frequency was significantly lower in the RT + 15 mg/kg group when compared to RT and RT + 5mg/kg groups (p<0.05).
The bSOD suppresses lung damage and collagen deposition in irradiated lungs
Late effects of radiation in the exposed lung are characterized by the structural damage to the lung tissue and an increase in deposition of collagen (lung tissue fibrosis). All three radiation-exposed groups developed an increase in lung damage and collagen deposition at 20 weeks after radiation. Injection of bSOD suppressed tissue damage and collagen deposition in a dose-dependent manner. In the higher dose, RT+15mg/kg, subcutaneous injection of bSOD significantly (P < 0.05) suppressed radiation-induced tissue damage and collagen deposition scores (2.32 and 17.8, respectively) when compared with the RT-only group (6.16 and 31.6, respectively) (Figure 3, Panel A and Panel B). With the lower dose, RT+5mg/kg, there was a trend in the reduction of damage and collagen deposition; however, these differences were not statistically significant (scores, 5.2 and 24.75, respectively; P > 0.05). As expected subcutaneous injection of 5 or 15 mg/kg of bSOD was safe, with no effects on lung tissue in healthy animals (tissue damage scores of 0.16 and 0.5, respectively; collagen deposition scores of 0 and 0).
Figure 3.
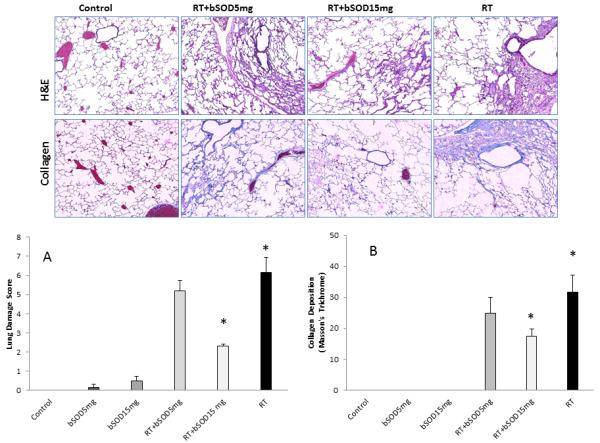
Light microscopy of hematoxylin and eosin-stained section and Masson’s trichrome- stained sections of representative lungs from control and treatment groups 20 weeks after the treatment. Panel A. Scoring results of lung tissue damage. Histopathological scoring of tissue damage showed dose dependent prevention of lung tissue damage in comparison with RT group (p<0.05). Panel B. Scoring of the amount of deposited collagen in the irradiated lungs. Collagen deposition in the irradiated lung tissue was suppressed by bSOD treatment (RT+15mg/kg vs. RT, p<0.05). Treatment with lower dose (RT+5mg/kg vs. RT) did not have significant effects despite the observed differences (p<0.05)
Postradiation application of bSOD decreases oxidative stress in irradiated lungs
In previous work we have shown that production of ROS/RNS triggers inflammation in irradiated lungs and that this is a main driving mechanism of late lung tissue damage after a single dose of radiation [3,29,31]. Therefore, we evaluated the temporal onset of oxidative stress in irradiated lungs 20 weeks after radiation exposure using immunohistochemical analysis of irradiated lung for NOX4, nitrotyrosine, 8-OHdG, and 4HNE (Figure 4 and Figure 5). As expected, radiation of 21 Gy to the right hemithorax caused a statistically significant increase in all parameters used to evaluate oxidative stress. When applied in unexposed animals, bSOD had no effect on expression of any of the analyzed markers in the 5mg/kg or 15mg/kg groups.
Figure 4.
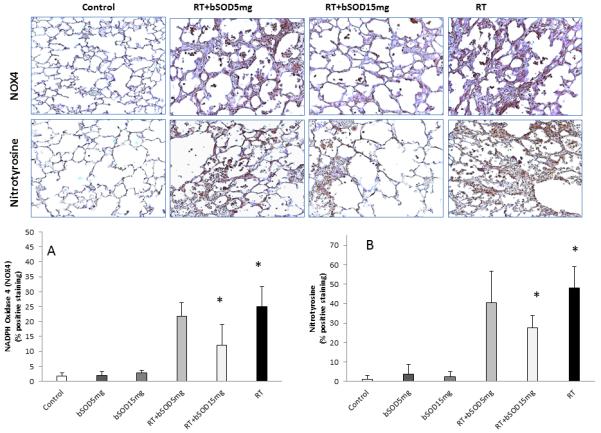
Representative immunohostochemistry images of NADPH Oxidase 4 (NOX4) and Nitrotyrosine 20 weeks after irradiation. Treatment with bSOD (15kg/mg) suppressed production of ROS/RNS, major contributors to development of delayed lung injury. Panel A. Semmi-qualitative measurement of NOX4. Irradiation caused significant increase in NOX4 presence in tissue in all irradiated groups. NOX4 expression was significantly reduced after the bSOD treatment RT+15mg/kg when compared to RT (p<0.05). RT+5mg/kg showed trend toward suppression but this difference did not reach level for statistical significance. Panel B. Semi-quantitative analysis of Nitrotyrosine, product of tyrosine nitrification by RNS and measure of RNS presence in the tissues. Nitrotyrosine expression in the lung tissue was elevated after irradiation. Our results show significantly diminished level of Nitrotyrosine in RT+15mg/kg when compared to RT (p<0.05)
Figure 5.
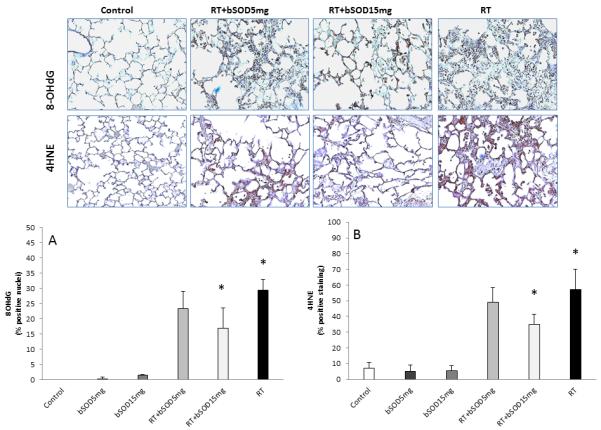
Oxidative stress (8-OHdG) and lipid peroxidation (4HNE) in irradiated lungs 20 weeks after exposure. Oxidative stress is associatedwith increased expression of 8-OHdG in irradiated lung tissue as well as lipid peroxidation measured by 4HNE. Both oxidative stress and lipid peroxidation were suppressed with bSOD treatment (RT+15mg/kg) 24h after the irradiation. Lower dose of bSOD showed trend in suppression in 8-OHdG and 4HNE but did not reach significance. Panel A: Semi-quantitative analysis of 8-OHdG. 8-OHdG-positive staining was found at significantly elevated levels after irradiation (P<0.05) and was significantly suppresed after treatment with bSOD (RT+15mg/kg vs RT, p<0.05). Panel B. Semi-quantitative analysis of 4HNE showed increase in lipid peroxidation after irradiation. Lipid peroxidation was significantly suppressed with bSOD treatment (RT+15mg/kg vs RT, p<0.05). Error bars represent ± SEM.
NOX4, a member of the NADPH oxydases family and the enzyme responsible for generation of ROS, was significantly suppressed by subcutaneous injection of bSOD when compared with the RT group (24.94% and 12%, respectively, Figure 4, Panel A). A product of tyrosine nitrification, nitrotyrosine is commonly used to measure RNS generation. In our study, 15 mg/kg of bSOD significantly suppressed RNS production after radiation. A statistically significant decrease in the percentage of nitrotyrosine+ cells was observed for the RT and RT+15mg/kg groups (47.9% and 27.4%, respectively; P < 0.05; Figure 4, Panel B). Treatment with bSOD in a dose of 15 mg/kg significantly (P < 0.05) suppressed expression of byproducts of oxygenation of DNA, 8-OHdG (29.28% vs 16.9%) (Figure 5, Panel A), and fatty acids, 4HNE (57% vs. 35%) (Figure 5, Panel B) when compared with RT alone, confirming that chronic production of ROS/RNS is an important mechanism in delayed lung tissue damage after radiation. In our experiments, we observed a trend in suppression of NOX4, nitrotyrosine, 8-OHdG, and 4HNE when animals were treated with 5 mg/kg of bSOD; however, these differences failed to reach statistical significance (P > 0.05).
Inflammation and fibrotic response are significantly suppressed by bSOD
We have previously shown that attenuation of macrophage activity and number of infiltrated cells coincides with a decrease in radiation-induced lung injury [3,32]. Here, we report that animals injected with bSOD 24 hours after radiation have a dose-dependent lower number of activated macrophages per microscopic field when compared with the RT group (RT+15mg/kg = 46.7; RT+5mg/kg = 65.35; RT = 74; RT vs. RT+15mg/kg, P < 0.05) with no effects on macrophage counts when administered in nonirradiated animals (Figure 6, Panel A).
Figure 6.
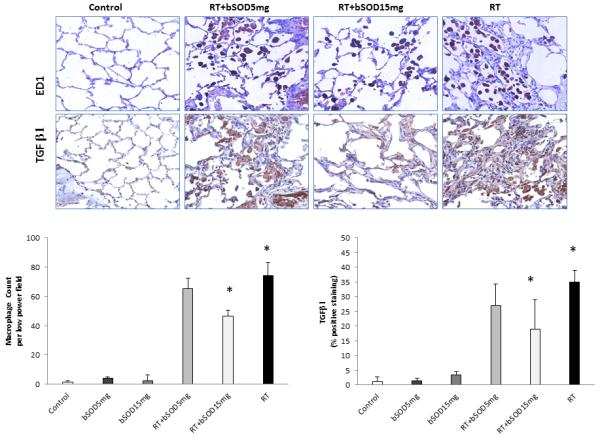
Effect of bSOD treatment on activation of lung macrophages and expression of TGF-β in lung tissue following single dose of irradiation (21Gy) to the right hemithorax. Representative immunohistological images of activated macrophages (ED1+ cells) in the volume of lung tissue and profibrinogenic TGF-β in the lungs demonstrate suppression of effects of radiation after the treatment with bSOD. Panel A. Semi-quantitative analysis of activated macrophages showed significant difference between RT+15mg/kg and RT (p<0.05). Panel B. Semi-quantitative analysis of TGF-β positive staining demonstrate significantly diminished level in RT+15mg/kg when compared to RT (p<0.05). Lower dose (RT+5mg/kg) despite the observed differences did not have statistically significant effects.
Fibrotic response in the lungs after radiation was evaluated by immunohistochemical assessment of TGF-β expression in lung tissue. TGF-β signaling is profibrotic; it increases collagen deposition in irradiated lungs and recruits fibroblasts in the irradiated tissue [33]. In animals that received no radiation, bSOD had no effect on TGF-β expression (Figure 6, Panel B). As expected, radiation induced a significant increase in TGF-β expression when compared with controls. This effect was suppressed in a dose-dependent manner by subcutaneous injection of bSOD 24 hours after exposure (Figure 6). TGF-β expression was significantly different between the RT and RT+15mg/kg groups (P < 0.05) but not between the RT and RT+5mg/kg groups.
Discussion
The risk for development of pneumonitis/fibrosis is dose limiting in radiation treatment of cancer patients with thoracic malignancies. In this proof-of-concept study, we demonstrated that subcutaneous application of bSOD ameliorates radiation-induced functional and structural lung injury. This coincided with a decrease in oxidative DNA damage, inflammation, and profibrotic TGF-β expression. In this study, we observed no adverse side effects of subcutaneous application of bSOD in healthy control animals (no radiation) receiving bSOD at doses of 5 or 15mg/kg.
This study demonstrated development of radiation-induced lung injury and functional impairment of the lungs after a single dose of 21 Gy hemithoracic irradiation. After a latent period of approximately 6 weeks during which time functional damage was not observed, symptomatic lung damage began to develop as measured by an increase in respiratory rate among animals not receiving bSOD. The results are in agreement with previous work showing chronic hypoxia and prolonged production of ROS/RNS as a driving mechanism of delayed lung tissue damage after radiation exposure [3,5,29,34]. The bSOD was able to diminish ROS/RNS production and preserve lung function. This result is in accord with immunohistochemistry showing a radiation-induced increase in 8-OhdG, nitrotyrosine, and 4HNE, with reversal of DNA oxidation and lipid peroxidation with bSOD administration. Previous studies have shown that mice overexpressing extracellular SOD [15,18] are protected against radiation-induced lung injury and that SOD-like compounds provide similar mitigation of injury in mouse or rat models of single and fractionated lung irradiation [35-37]. Interestingly, bSOD significantly affects expression of NOX4, one of the most important enzymes in ROS generation. It has been shown in vitro and in vivo that hypoxic conditions trigger NOX4 expression in an HIF-1α-dependent manner [38]. Pulmonary fibrosis, a late effect of radiation exposure, appears to be, at least in part, driven by upregulation of TGF-β, a suppressor of extracellular matrix degradation and a fibroblast activator [39,40]. Diminished ROS production after irradiation of the lungs correlates with a decrease in the expression of profibrotic TGF-β and lung fibrosis [22,23,32,35,41]. In addition, targeted suppression of TGF-β pathway inhibition at early time points with LY210976 (TGF-βR inhibitor) significantly reduced the extent of fibrosis and decreased lethality after radiation [42]. An interesting connection has recently been made with regard to NOX4 and TGF-β on the cellular level. In vitro, NOX4 has been shown to be involved in TGF-β–triggered apoptosis of vascular endothelial cells [43] and hyperproliferation of pulmonary artery smooth muscle cells [44]. The contribution of late hypercellularity to hypoxic conditions, augmenting damage in the lungs, has been well documented [5]. Hypoxic conditions trigger TGF-β production and increase extracellular concentrations of TGF-β, also activating fibroblasts in vivo [41]. Fibrinogenesis is a multifactorial process, and TGF-β signaling is one—but not the only—component. Activation of macrophages in lungs and consequent imbalance in cytokine/chemokine concentrations (i.e., increased concentrations of IL-4) in the volume of irradiated tissue significantly contribute to this late effect of radiation on lung [45]. Our results suggest that treatment with bSOD 24 hours after radiation exposure significantly reduces activation of macrophages. Present findings are in accord with previous reports that decreases in ROS/RNS production also decreased the number of ED1+ positive cells in the irradiated lung and the extent of local inflammation [23].
In the presented study, we used bSOD (Orgotein) to prevent radiation induces complications to the lungs. For the last 30 years, efficacy of bSOD has been shown in several clinical trials for treatment of acute and chronic conditions correlated with inflammation and radiation and chemotherapy [26]. It is important to highlight that several in vitro and in vivo studies evaluated SOD/SOD mimetics in models of cancer. They showed that use of SODs or SOD mimetics do not interfere with cancer response to radiation, and have beneficial effects in the healthy tissues [11,20,21,46,47]. Despite its efficacy, there are several safety concerns about its use in the humans. Orgotein is an extract of SOD enzymes from bovine liver, which raise concerns about its unwanted effects in humans due to anaphylactic shocks [48-50]. Anaphylactic reactions to the drug ultimately led to its removal from the market in the US and most European countries [26] (Spain is rare exception where Orgotein is still in clinical trials [51-53]). The underlying rationale for free radical scavenging as a therapeutic intervention to mitigate inflammatory and fibrotic disease served as the basis for development of several classes of SOD mimetics. These compounds were developed to have similar catalytic activities but to overcome the immunogenic properties of bSOD [26]. Our objective was to conduct the proof of concept mechanistic study of the bSOD application. What is important for the presented study is that a single application of the bSOD in the dose of 15mg/kg suppressed radiation induced lung injury through entire course of 20 weeks of follow up. Small molecular weight SOD mimetics are in preclinical trials with promising efficacy. However, safety and efficacy trials on large patient populations are still missing. Presented result, single application of the mitigator, would be desired feature of all the SOD mimetics currently under development, and this efficacy is yet to be achieved. One more important feature of the bSOD is that it is shown to reverse already established fibrosis [54]. Currently, no SOD mimetic can reverse already established fibrosis. The reason why SOD mimetics are not able to reverse established fibrosis may lie in the repertoire of their substrates. The SOD mimetics, aside of O2•, also modify CO3•, NO2 radicals, and likely of peroxyl radicals and alkoxyl radicals. By modifying primary oxidants, SOD mimetics modify the molecular environment in the irradiated tissue through different affected biomolecules and indirectly affect immune system in general, inflammatory pathways in particular, and secondary antioxidant pathways [55].
This proof-of-concept study demonstrated that suppression of oxidative stress by a single application of bSOD in a dose of 15 mg/kg in rats effectively protects lung function and structure after a single high dose of radiation through suppression of ROS/RNS and their debilitating effects on tissue.
Acknowledgements
This work has been supported by the National Institutes of Health Grant number RO1CA098452 and NIH-5U19-AI-067798-04
Footnotes
Conflict of interest statement:
All authors have no conflict of interest to report.
References
- [1].Tyldesley S, Boyd C, Schulze K, Walker H, Mackillop WJ. Estimating the need for radiotherapy for lung cancer: an evidence-based, epidemiologic approach. Int J Radiat Oncol Biol Phys. 2001;49(4):973–85. doi: 10.1016/s0360-3016(00)01401-2. [DOI] [PubMed] [Google Scholar]
- [2].Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, El Naqa I, Hubbs JL, Lebesque JV, Timmerman RD. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S70–6. doi: 10.1016/j.ijrobp.2009.06.091. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schaue D, Kachikwu EL, McBride WH. Cytokines in radiobiological responses: a review. Radiat Res. 2012;178(6):505–23. doi: 10.1667/RR3031.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50(4):851–5. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- [6].Ibuki Y, Goto R. Ionizing radiation-induced macrophage activation: augmentation of nitric oxide production and its significance. Cell Mol Biol (Noisy-le-grand) 2004;50 Online Pub:OL617-26. [PubMed] [Google Scholar]
- [7].Carillon J, Rouanet JM, Cristol JP, Brion R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: several routes of supplementation and proposal of an original mechanism of action. Pharm Res. 2013;30(11):2718–28. doi: 10.1007/s11095-013-1113-5. [DOI] [PubMed] [Google Scholar]
- [8].Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St Clair D, Batinic-Haberle I. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta. 2012;1822(5):794–814. doi: 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Noor R, Mittal S, Iqbal J. Superoxide dismutase--applications and relevance to human diseases. Med Sci Monit. 2002;8(9):RA210–5. [PubMed] [Google Scholar]
- [10].Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33(3):337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- [11].Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32(1):12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- [12].Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17(10):3283–90. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- [13].Lefaix JL, Delanian S, Leplat JJ, Tricaud Y, Martin M, Nimrod A, Baillet F, Daburon F. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35(2):305–12. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- [14].Breuer R, Tochner Z, Conner MW, Nimrod A, Gorecki M, Or R, Slavin S. Superoxide dismutase inhibits radiation-induced lung injury in hamsters. Lung. 1992;170(1):19–29. doi: 10.1007/BF00164752. [DOI] [PubMed] [Google Scholar]
- [15].Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57(4):1056–66. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- [16].Machtay M, Scherpereel A, Santiago J, Lee J, McDonough J, Kinniry P, Arguiri E, Shuvaev VV, Sun J, Cengel K. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother Oncol. 2006;81(2):196–205. doi: 10.1016/j.radonc.2006.09.01. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Malaker K, Das RM. Effect of superoxide dismutase on early radiation injury of lungs in the rat. Mol Cell Biochem. 1988;84(2):141–5. doi: 10.1007/BF00421048. [DOI] [PubMed] [Google Scholar]
- [18].Rabbani ZN, Anscher MS, Folz RJ, Archer E, Huang H, Chen L, Golson ML, Samulski TS, Dewhirst MW, Vujaskovic Z. Overexpression of extracellular superoxide dismutase reduces acute radiation induced lung toxicity. BMC Cancer. 2005;5:59. doi: 10.1186/1471-2407-5-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes protects the murine lung from irradiation damage. Gene Ther. 2005;12(8):685–93. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- [20].Epperly MW, Defilippi S, Sikora C, Gretton J, Kalend A, Greenberger JS. Intratracheal injection of manganese superoxide dismutase (MnSOD) plasmid/liposomes protects normal lung but not orthotopic tumors from irradiation. Gene Ther. 2000;7(12):1011–8. doi: 10.1038/sj.gt.3301207. [DOI] [PubMed] [Google Scholar]
- [21].Epperly MW, Travis EL, Sikora C, Greenberger JS. Manganese [correction of Magnesium] superoxide dismutase (MnSOD) plasmid/liposome pulmonary radioprotective gene therapy: modulation of irradiation-induced mRNA for IL-I, TNF-alpha, and TGF-beta correlates with delay of organizing alveolitis/fibrosis. Biol Blood Marrow Transplant. 1999;5(4):204–14. doi: 10.1053/bbmt.1999.v5.pm10465100. [DOI] [PubMed] [Google Scholar]
- [22].Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2008;44(6):982–9. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48(8):1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gauter-Fleckenstein B, Reboucas JS, Fleckenstein K, Tovmasyan A, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Robust rat pulmonary radioprotection by a lipophilic Mn N-alkylpyridylporphyrin, MnTnHex-2-PyP(5+) Redox Biol. 2014;2:400–10. doi: 10.1016/j.redox.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140(3):445–60. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Regnault C, Soursac M, Roch-Arveiller M, Postaire E, Hazebroucq G. Pharmacokinetics of superoxide dismutase in rats after oral administration. Biopharm Drug Dispos. 1996;17(2):165–74. doi: 10.1002/(SICI)1099-081X(199603)17:2<165::AID-BDD945>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [28].Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41(4):467–70. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Y, Zhang X, Rabbani ZN, Jackson IL, Vujaskovic Z. Oxidative stress mediates radiation lung injury by inducing apoptosis. Int J Radiat Oncol Biol Phys. 2012;83(2):740–8. doi: 10.1016/j.ijrobp.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kimura M, Rabbani ZN, Zodda AR, Yan H, Jackson IL, Polascik TJ, Donatucci CF, Moul JW, Vujaskovic Z, Koontz BF. Role of oxidative stress in a rat model of radiation-induced erectile dysfunction. J Sex Med. 2012;9(6):1535–49. doi: 10.1111/j.1743-6109.2012.02716.x. [DOI] [PubMed] [Google Scholar]
- [31].Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, Salahuddin FK, Zhang X, Clary B, Anscher MS. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173(2):165–74. doi: 10.1667/RR1816.1. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rabbani ZN, Salahuddin FK, Yarmolenko P, Batinic-Haberle I, Thrasher BA, Gauter-Fleckenstein B, Dewhirst MW, Anscher MS, Vujaskovic Z. Low molecular weight catalytic metalloporphyrin antioxidant AEOL 10150 protects lungs from fractionated radiation. Free Radic Res. 2007;41(11):1273–82. doi: 10.1080/10715760701689550. [DOI] [PubMed] [Google Scholar]
- [33].Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- [34].Jackson IL, Zhang X, Hadley C, Rabbani ZN, Zhang Y, Marks S, Vujaskovic Z. Temporal expression of hypoxia-regulated genes is associated with early changes in redox status in irradiated lung. Free Radic Biol Med. 2012;53(2):337–46. doi: 10.1016/j.freeradbiomed.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33(6):857–63. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- [36].Mahmood J, Jelveh S, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury with EUK-207 and genistein: effects in adolescent rats. Radiat Res. 2013;179(2):125–34. doi: 10.1667/RR2954.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29(18):2705–9. doi: 10.1016/j.biomaterials.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diebold I, Petry A, Hess J, Gorlach A. The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Mol Biol Cell. 2010;21(12):2087–96. doi: 10.1091/mbc.E09-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- [40].Vujaskovic Z, Groen HJ. TGF-beta, radiation-induced pulmonary injury and lung cancer. Int J Radiat Biol. 2000;76(4):511–6. doi: 10.1080/095530000138510. [DOI] [PubMed] [Google Scholar]
- [41].Jackson IL, Chen L, Batinic-Haberle I, Vujaskovic Z. Superoxide dismutase mimetic reduces hypoxia-induced O2*-, TGF-beta, and VEGF production by macrophages. Free Radic Res. 2007;41(1):8–14. doi: 10.1080/10715760600913150. [DOI] [PubMed] [Google Scholar]
- [42].Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Grone HJ, Yingling J. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18(13):3616–27. doi: 10.1158/1078-0432.CCR-11-2855. others. [DOI] [PubMed] [Google Scholar]
- [43].Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, Jiang F. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014;5:e1010. doi: 10.1038/cddis.2013.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ismail S, Sturrock A, Wu P, Cahill B, Norman K, Huecksteadt T, Sanders K, Kennedy T, Hoidal J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: the role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. Am J Physiol Lung Cell Mol Physiol. 2009;296(3):L489–99. doi: 10.1152/ajplung.90488.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Buttner C, Skupin A, Reimann T, Rieber EP, Unteregger G, Geyer P, Frank KH. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17(3):315–25. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- [46].Epperly MW, Bray JA, Krager S, Berry LM, Gooding W, Engelhardt JF, Zwacka R, Travis EL, Greenberger JS. Intratracheal injection of adenovirus containing the human MnSOD transgene protects athymic nude mice from irradiation-induced organizing alveolitis. Int J Radiat Oncol Biol Phys. 1999;43(1):169–81. doi: 10.1016/s0360-3016(98)00355-1. [DOI] [PubMed] [Google Scholar]
- [47].Hauser GJ, McIntosh JK, Travis WD, Rosenberg SA. Manipulation of oxygen radical-scavenging capacity in mice alters host sensitivity to tumor necrosis factor toxicity but does not interfere with its antitumor efficacy. Cancer Res. 1990;50(12):3503–8. [PubMed] [Google Scholar]
- [48].Nielsen OS, Overgaard J, Overgaard M, Steenholdt S, Jakobsen A, Sell A. Orgotein in radiation treatment of bladder cancer. A report on allergic reactions and lack of radioprotective effect. Acta Oncol. 1987;26(2):101–4. doi: 10.3109/02841868709091748. [DOI] [PubMed] [Google Scholar]
- [49].de Benito V, de Barrio M, de Lopez-Saez MP, Ordoqui E, Prieto-Garcia A, Sainza T, Baeza ML. Anaphylactic shock caused by impurities in orgotein preparations. Allergol Immunopathol (Madr) 2001;29(6):272–5. doi: 10.1016/s0301-0546(01)79069-6. [DOI] [PubMed] [Google Scholar]
- [50].Joral A, Boyano T, Mira J, Agud JL, Saiz F. Systemic anaphylaxis following parenteral orgotein administration. J Investig Allergol Clin Immunol. 1993;3(2):103–4. [PubMed] [Google Scholar]
- [51].Esco R, Valencia J, Coronel P, Carceller JA, Gimeno M, Bascon N. Efficacy of orgotein in prevention of late side effects of pelvic irradiation: a randomized study. Int J Radiat Oncol Biol Phys. 2004;60(4):1211–9. doi: 10.1016/j.ijrobp.2004.04.038. [DOI] [PubMed] [Google Scholar]
- [52].Escribano A, Garcia-Grande A, Montanes P, Miralles L, Garcia A. Aerosol orgotein (Ontosein) for the prevention of radiotherapy-induced adverse effects in head and neck cancer patients: a feasibility study. Neoplasma. 2002;49(3):201–8. [PubMed] [Google Scholar]
- [53].Valencia J, Velilla C, Urpegui A, Alvarez I, Llorens MA, Coronel P, Polo S, Bascon N, Esco R. The efficacy of orgotein in the treatment of acute toxicity due to radiotherapy on head and neck tumors. Tumori. 2002;88(5):385–9. doi: 10.1177/030089160208800507. [DOI] [PubMed] [Google Scholar]
- [54].Niwa Y, Somiya K, Michelson AM, Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic Res Commun. 1985;1(2):137–53. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- [55].Batinic-Haberle I, Reboucas JS, Spasojevic I. Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal. 2010;13(6):877–918. doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]


