Abstract
Background
Mechanically offloading or shielding an incision significantly reduces scarring in both animal and first-in-human studies. Whether or not this strategy would be effective following scar revision surgery was previously unknown. In this article, the authors report that the embrace device, which uses principles of mechanomodulation, significantly improves aesthetic outcomes following scar revision surgery.
Methods
A prospective, open-label, randomized, single-center study was conducted to evaluate the appearance of scars following revision and embrace treatment. Revision surgery was performed on 12 patients, each acting as his or her own control, and outcomes were assessed at 6 months. A visual analogue scale was used to evaluate each scar, rated by four independent surgeons who were not involved in the study.
Results
Evaluation of 6-month scar images by four independent surgeons using the visual analogue scale demonstrated a highly significant improvement in scar appearance following embrace treatment (p < 0.005).
Conclusion
The embrace device represents a powerful new technology for significantly improving scar appearance following revision surgery.
CLINICAL QUESTION/LEVEL OF EVIDENCE
Therapeutic, II.
More than 200 million incisions are made in the world each year, all of which result in scarring, which is the body’s natural response to cutaneous tissue injury.1,2 Scarring creates both functional and aesthetic impairments and is associated with considerable psychosocial stress. Although there is currently no level I evidence for scar mitigation therapy, several procedures are widely used to reduce the impact of postsurgical scars. Revision surgery is the traditional treatment for improving scar appearance, generally focusing on either making the scar less noticeable or reframing the scar geometry so that it appears more like a natural anatomical crease.3,4 In the United States alone, more than 170,000 scar revision procedures were performed in 2011.5 Laser treatments have become increasingly popular in the past two decades, including carbon dioxide and pulsed dye lasers, and dermabrasion therapy is frequently used to smooth scars with irregular topology.6,7 Silicone gels, sheets, and tapes have also been used to minimize scarring postoperatively.8–10 Similarly, topical creams containing ingredients such as retinoic acid and onion extract have been used to reduce scar intensity, as have intralesional therapies containing corticosteroids or biomolecules.11,12 However, even the most scientifically grounded of these approaches, the human transforming growth factor-β3 drug Juvista (Renovo, Manchester, United Kingdom), failed to achieve efficacy in a phase III clinical trial.13
The impact of mechanical forces on fibrosis and scar formation has been known for over 100 years. Surgeons routinely strive to make incisions parallel to the Langer lines, corresponding to the orientation of native collagen fibers in the dermis, to minimize tension across the wound. Despite this intuitive knowledge of the relationship between mechanical force and scar formation, until recently, few studies had thoroughly explored the physiologic mechanisms underlying this interaction, and therapeutics aimed at reducing scarring through mechanomodulation have not been widely available.
A recent report involving both pig and human incisions treated with a tension-shielding device postoperatively showed a highly significant reduction in scarring.14 Furthermore, the biology underlying mechanomodulation to reduce fibrosis in scarring of cutaneous wounds has been rigorously studied in a transgenic mouse model.15 These basic science and clinical studies strongly support a strategy for shielding healing wounds from mechanical forces to reduce scarring.
In this article, we report clinical data using the embrace (Neodyne Biosciences, Inc., Menlo Park, Calif.) device following scar revision and potentially address the large unmet clinical need for significantly improving scar appearance following revision surgery. The embrace device creates a tension shield around the healing wound, which results in a reduced-stress environment conducive to wound healing with minimal fibrosis and scarring.14 We demonstrate the efficacy of this device through a randomized controlled trial of scar revision in 12 patients evaluated by four independent surgeons using a visual analogue scale system.
PATIENTS AND METHODS
The Investigation of a Novel Silicone Dressing to Maximize the Outcomes of Scar Revision Procedures (IMPROVE) trial was a prospective, open-label, randomized (subject as his or her own control), single-center study to evaluate the use of the Neodyne embrace device to improve the aesthetic outcome following scar revision surgery. All procedures were performed at a single outpatient surgery clinic between September of 2011 and May of 2012. This trial was approved by the Schulman Associates Institutional Review Board, Inc. (Cincinnati, Ohio).
Patients
Male and female subjects aged 18 to 65 years undergoing scar revision surgery under local anesthesia were eligible if their scars were (1) at least 12 months old, (2) linear and suitable for revision by excision and direct closure, (3) at least 2 inches in length, and (4) along a flat surface suitable for application of the embrace device and consistent medical photography. Exclusion criteria included subjects with a chronic or currently active skin disorder; subjects involved in ongoing litigation in connection with the scar to be revised; subjects with a history of collagen vascular disease; subjects diagnosed with scleroderma; subjects who currently smoke; subjects with known adverse reactions to Steri-Strip tapes (3M, St. Paul, Minn.), medical tapes, or adhesives; subjects with inability to maintain adequate care of incision; and subjects that did not qualify in the opinion of the investigators.
Device
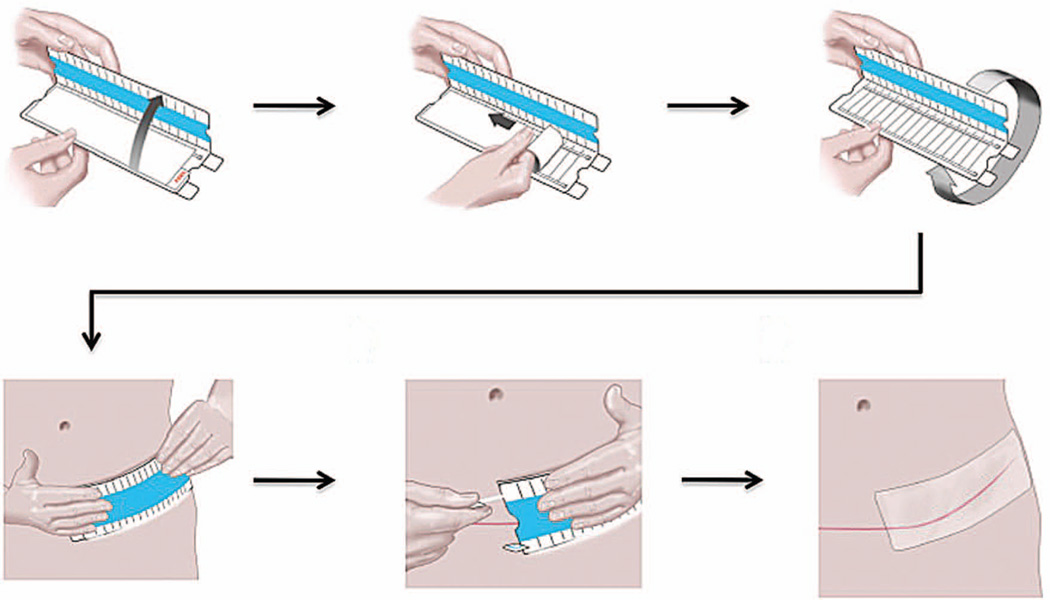
The embrace device is a 16 × 5-cm or 6 × 4-cm silicone elastomeric dressing that adheres to the skin through a pressure-sensitive silicone adhesive (Fig. 1). This device is cleared by the U.S. Food and Drug Administration for human use with a 510(k) clearance. The device is applied directly to skin following cleaning of the area. The applicator containing the device is opened completely to strain the dressing, and the device is applied directly over the center of the closed incision 1 to 4 days postoperatively. The embrace devices were provided by Neodyne Biosciences to the treating physicians throughout the study.
Fig. 1.
Application of the embrace device. The embrace device is a 16 × 5-cm or 6 × 4-cm silicone elastomeric dressing that adheres to the skin through a pressure-sensitive silicone adhesive. (Above, left) The applicator device is initially opened approximately 60 degrees, and (above, center) the protective liner is peeled away from the adhesive dressing. (Above, right) The top of the applicator is folded all the way back to prestrain the material, and (below, left) the dressing is subsequently applied directly over the center of the scar. (Below, center) Tabs above and below the applicator are pulled away to release the dressing, and (below, right) the applicator device is removed.
Treatment
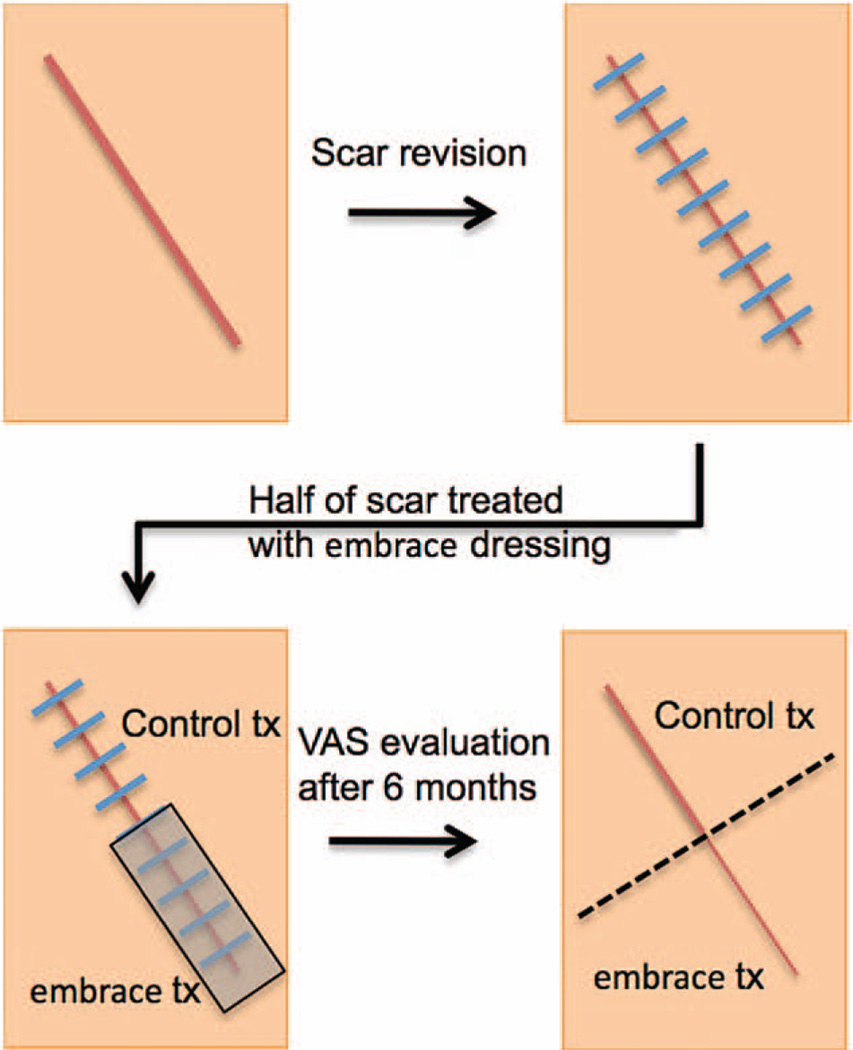
The institutional review board–approved protocol was to excise the entire scar and revise it, but only half of the revised incision was treated with the embrace device (Fig. 2). After meeting all eligibility criteria, 12 patients were enrolled. (See Figure, Supplemental Digital Content 1, which shows a Consolidated Standards of Reporting Trials (CONSORT) diagram, http://links.lww.com/PRS/A932.) Each scar was excised completely under local anesthesia and wounds were closed using suture techniques at the surgeon’s discretion (Table 1). Following the procedure, the portion of the newly closed wound to be treated with the embrace device was selected randomly by opening a sealed envelope containing instructions to assign treatment to the left/right or top/bottom portion of the incision, which were generated randomly by the study statistician before enrollment. The selected portion was treated with the embrace device throughout the full study. The embrace device was applied to approximately half of each incision by the health care provider from 1 to 4 days after the revision procedure (one subject did not receive the first dressing until 13 days after surgery because of delay in postsurgical healing). The remaining portion of the incision served as the “control” side and was treated according to the investigator’s standard of care (Table 2). Subjects returned to the investigator’s office weekly for removal of the embrace device and reapplication of a new device for up to 12 weeks/visits, with an additional visit at 6 months from the date of the revision procedure for a photographic evaluation and study exit.
Fig. 2.
Treatment algorithm. (Above) Scar revision surgery is performed under local anesthesia. (Below, left) Following the procedure, the side of each scar to be treated with the embrace device is selected randomly and the device is applied to approximately half of each incision from 1 to 4 days after the revision procedure. The remaining portion of the incision serves as the control and is treated according to the investigator’s standard of care. (Below, right) After 6 months of treatment, photographs are obtained and evaluated independently by four surgeons using the visual analogue scale (VAS) scoring system.
Table 1.
Standard Incision Closure Methods for All Incisions
| Treatment | No. of Subjects |
|---|---|
| Absorbable suture in the top dermal layer | 7 |
| Nonabsorbable suture in the top dermal layer |
1 |
| Nonabsorbable suture in the top dermal layer plus absorbable deep dermal suture |
2 |
Table 2.
Standard Postsurgical Care for Control Incisions
| Treatment | No. of Subjects |
|---|---|
| Steri-Strips alone | 6 |
| Steri-Strips plus Mederma* cream | 1 |
| No treatment | 3 |
Merz, Inc., Frankfurt am Main, Germany.
Evaluation
The sample size for this study consisted of 10 subjects with assessable data. Performance was evaluated by a visual analogue scale scar score as described below from embrace-treated and control- treated incision sites at the 6-month study endpoint.
The visual analogue scale scar scoring system was defined and validated by Duncan et al. in 2006.16 This scale consists of a 10-cm line representing scar quality, with 0 representing normal skin and 10 indicating a poor scar. The assessor places a mark along the line to represent the appearance of the scar. This mark is then translated into a score by measuring its position on the 10-cm line to one decimal place. (See Figure, Supplemental Digital Content 2, which shows the visual analogue scale, http://links.lww.com/PRS/A933.)
Prior data indicate that the standard deviation of the paired differences in visual analogue scale scores is not expected to exceed 1.46. Should the embrace dressing, on average, show an improvement of at least 1.5 points over control treatment (half that observed in a previous study), n = 10 subjects would provide 80 percent power to show that the difference is statistically significantly greater than 0.
Statistical Analysis
The visual analogue scale results are expressed as a mean ± SEM. Statistical analysis of visual analogue scale scores was carried out using a repeated measures analysis of variance procedure in R (www.r-project.org). This method makes use of each score from all reviewers, but treats them as separate measurement of the same object, rather than as independent data points. No adjustments were made for multiple hypothesis testing. A value of p < 0.05 was considered statistically significant for all comparisons.
RESULTS
Twelve subjects were initially enrolled in this study, with 10 subjects completing at least 8 weeks of dressing application and providing the final 6-month study photographs. All procedures were performed by one of three experienced plastic surgeons at a single clinic in Palo Alto, California. Seven scars were on the abdomen, two were on the breast, and one was on the neck. Patient ages ranged from 29 to 51 years, with an average age of 38.6 years, and nine of the 10 participants were women. Four patients identified themselves as Caucasian, four identified themselves as African American, one patient identified as Asian, and one patient identified as other.
Of the two patients that dropped out and did not complete the full course of treatment, one patient was lost to follow-up at 2 weeks. The second patient discontinued the study because of a wound infection on the control-treated side of the incision.
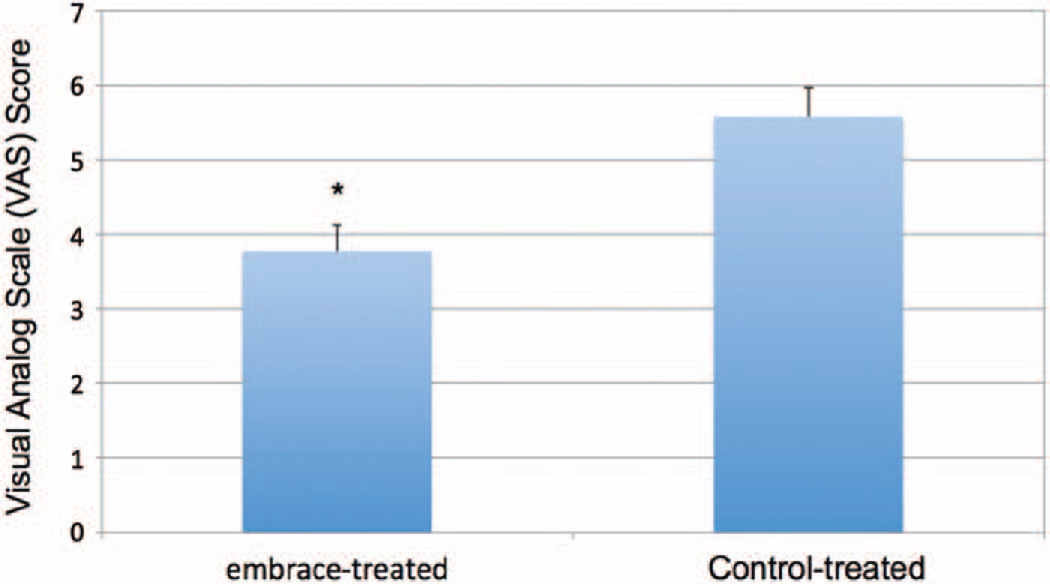
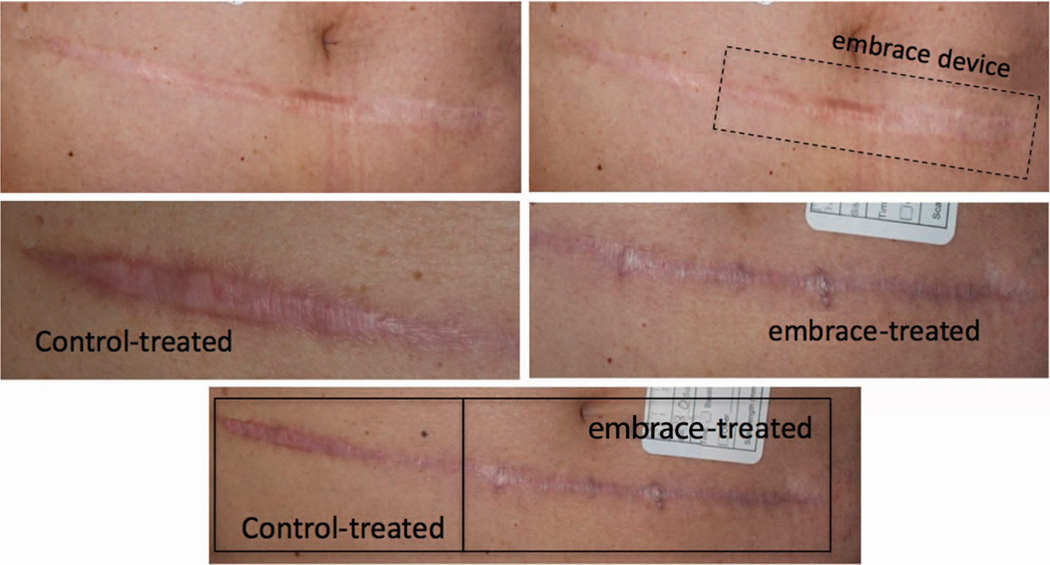
Pretreatment photographs were taken before revision surgery, and scar images were obtained at 6-month follow-up (Figs. 3 through 5). The embrace-treated and control-treated images for all 10 patients were evaluated in a blinded fashion by four experienced surgeons using the visual analogue scale at 6 months. The mean visual analogue scale score for treated scars (3.78) was significantly less than that of the control scars (5.58; p < 0.005) (Fig. 6).
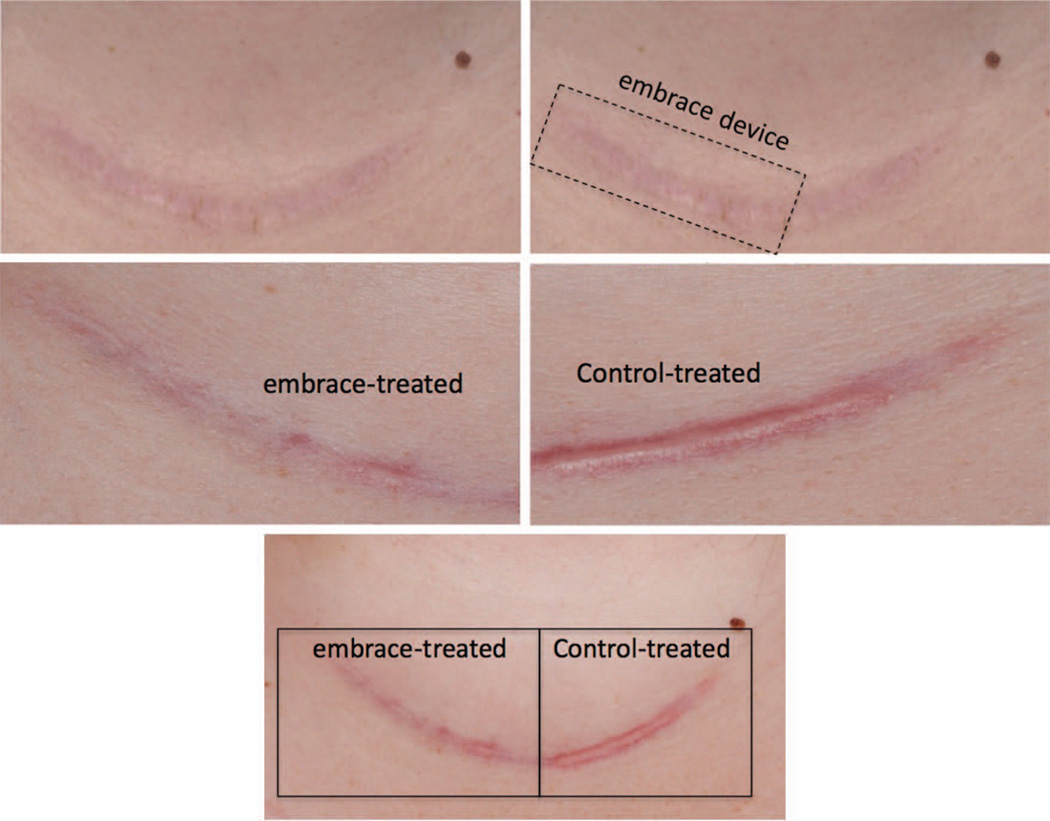
Fig. 3.
Representative photographs illustrating application of the embrace device to a neck scar following thyroidectomy. (Above, left) Preoperative photographs are obtained for each scar before revision surgery. (Above, right) Following the procedure, the side of each scar to be treated with the embrace device is randomly selected and the device is applied to approximately half of each incision, with the other side receiving the standard-of-care treatment determined by the surgeon. Six months after treatment with either the embrace device (center, left) or the investigator’s standard of care (center, right), additional photographs are taken and evaluated independently by four surgeons using the visual analogue scale scoring system. (Below) Embrace-treated (left) and control-treated (right) scar sections exhibit gross differences in appearance.
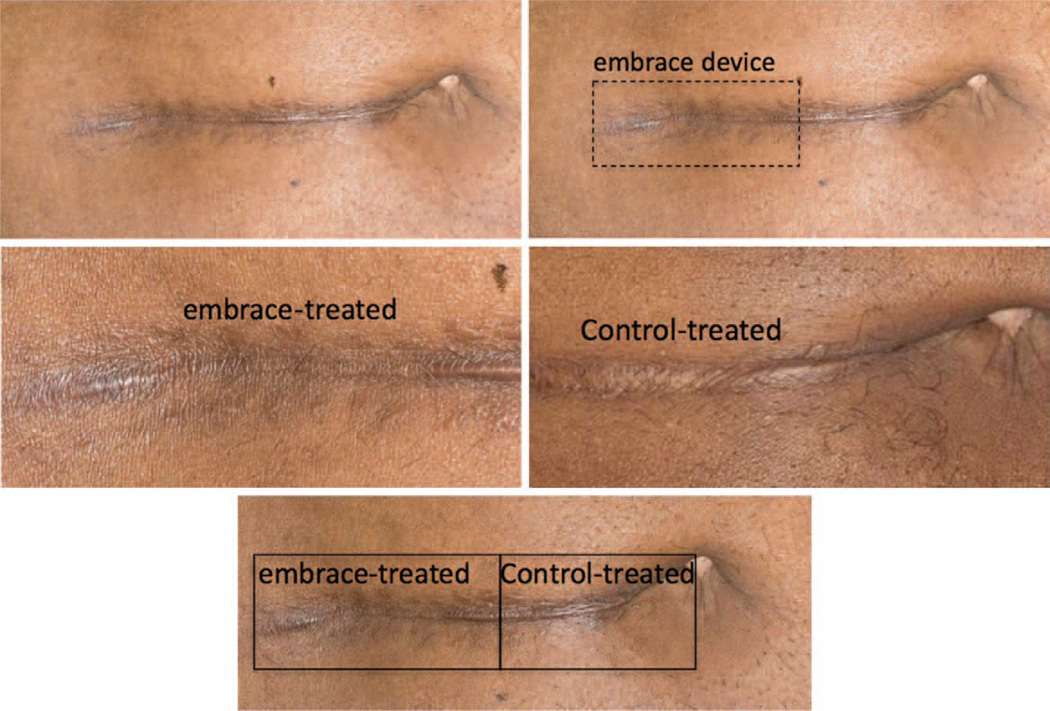
Fig. 5.
Representative photographs illustrating application of the embrace device to a laparotomy scar. (Above, left) Preoperative photographs are obtained for each scar before revision surgery. (Above, right) Following the procedure, the side of each scar to be treated with the embrace device is selected randomly and the device is applied to approximately half of each incision, with the other side receiving the standard-of-care treatment determined by the surgeon. Six months after treatment with either the embrace device (center, left) or the investigator’s standard of care (center, right), additional photographs are taken and evaluated independently by four surgeons using the visual analogue scale scoring system. (Below) Control-treated (right) and embrace-treated (left) scar sections exhibit gross differences in appearance.
Fig. 6.
Visual analogue scale comparison. Photographs of embrace-treated and control-treated scars at 6 months after revision surgery were evaluated by four independent surgeons using the visual analogue scale. Mean visual analogue scale scores for treated scars (3.78) were significantly better than those of control scars (5.58) (*p < 0.005).
In addition to the ratings by independent surgeons, we also polled each patient to determine their satisfaction with the results and asked them to evaluate three questions. Specifically, we asked them to compare the embrace-treated side with the control-treated side vis-à-vis their satisfaction with the minimization of scarring on their incision, and 100 percent of patients indicated they were either “satisfied” or “very satisfied” (Table 3). Second, we asked them to evaluate, based on the results that they observed on the embrace-treated side versus the control-treated side of their incision, how likely they were to recommend this treatment to a friend, with 90 percent of patients indicating that they were either “likely” or “very likely” to recommend it. Third, we asked patients, if they were to have another procedure that might leave a scar, how likely would they be to use the embrace treatment again, and 90 percent of patients selected “likely” or “very likely” (Table 3).
Table 3.
Subject Exit Questions
| Question |
Very Satisfied (%) |
Satisfied (%) |
Neither Satisfied nor Dissatisfied (%) |
Dissatisfied (%) |
Very Dissatisfied (%) |
| How satisfied are you with the minimization of scarring of the treated side of your incision versus the control side? |
50 | 50 | 0 | 0 | 0 |
| Question |
Very Likely (%) |
Likely (%) |
Neither Likely nor Unlikely (%) |
Unlikely (%) |
Very Unlikely (%) |
| Based on the results that you see of the treated side versus control side of your incision, how likely are you to recommend this treatment in the future? |
60 | 30 | 10 | 0 | 0 |
| If you were to have another procedure that might leave a scar, how likely would you be to use this treatment again? |
70 | 20 | 10 | 0 | 0 |
DISCUSSION
Our data report on a prospective, open-label, randomized (within-patient), single-center study evaluating the utility of the Neodyne embrace device to improve the appearance of scars following revision surgery. Because each patient acted as his or her own control, the influence of patient-specific variables was minimized. The embrace device was applied between 1 and 4 days after revision surgery, with weekly removal and reapplication of new dressings for a minimum of 8 weeks but up to 12 weeks according to each patient’s discretion.
The primary endpoint of this study was to improve the appearance of embrace-treated scars compared with control-treated scars, and this was evaluated using a visual analogue scale. For visual analogue scale evaluation, four independent surgeons (not otherwise associated with this trial) were asked to blindly evaluate the severity of each 6-month scar image on a 10-point scale.
Our data strongly support a significant improvement in scar appearance following revision and treatment with the embrace device. To our knowledge, this is the first prospective study evaluating the utility of a scar-mitigating device (embrace) to improve the appearance of scars following surgery. In this study, each subject acted as their own control. The trial design of revising the whole scar but only treating half was required by the reviewing institutional review board. Clearly, we anticipate that, going forward, clinical applications of the embrace device will entail treating the entire incision.
The visual analogue scale scoring system was chosen because it reflects what the patient sees in the overall appearance of the scar. There are numerous other scoring systems that incorporate material properties, topology, elasticity, and others, but in the end it is the visual appearance of the scar that concerns the patient. Therefore, the visual analogue scale scoring system was chosen for our analysis.
It is worthwhile mentioning that the final endpoint for this study was 6 months. This time point was chosen with an appreciation for how scars change over time. We feel that 6 months meets the criteria for the minimum acceptable time at which scar analysis should be performed, consistent with other trials.17,18 In addition, 6 months is consistent with the previous report using the embrace device in a first-in-human analysis of primary (abdominoplasty) incisions showing a significant efficacy for scar reduction.14
The highly significant differences between embrace-treated and control-treated portions of the revised incisions are supported by basic science research on mechanotransduction and fibrosis. Wong et al., in an elegant study looking at the mechanism through which mechanotransduction leads to fibrosis, showed that over 1000 genes are regulated or impacted by mechanical forces during wound repair.15 Although targeting individual genes or families of genes is an attractive strategy, the clinical data have not yielded significant results. For example, Juvista failed to meet the phase III clinical endpoint.13 The embrace device, by shielding the incision from tension brought on from mechanical forces no doubt impacts hundreds or thousands of genes and intracellular signaling pathways. Thus, this highly effective clinical result may reflect its broad impact on mechanomodulation of the healing wound.
Clearly, there are limitations to this study despite it being a randomized controlled design, as the sample size is relatively small. Despite this limitation, the highly significant difference between embrace-treated and control-treated scars, from a statistical standpoint, strongly support that the differences are real and not attributable to chance.
CONCLUSIONS
These results have important implications in clinical plastic and reconstructive surgery. It is difficult to accurately estimate how many patients worldwide are unhappy with a scar; however, the efficacy described in this randomized controlled trial suggests that the embrace device could potentially address a very large unmet need in the United States and abroad and provide a new option to both surgeons and patients in managing scars.
Supplementary Material
Fig. 4.
Representative photographs illustrating application of the embrace device to a laparotomy scar. (Above, left) Preoperative photographs are obtained for each scar prior to revision surgery. (Above, right) Following the procedure, the side of each scar to be treated with the embrace device is randomly selected and the device is applied to approximately half of each incision, with the other side receiving the standard of care treatment determined by the surgeon. Six months after treatment with either the investigator’s standard of care (center, left) or the embrace device (center, right), additional photographs are taken and evaluated independently by four surgeons using the visual analogue scale scoring system. (Below) Embrace-treated (right) and control-treated (left) scar sections exhibit gross differences in appearance.
Footnotes
This trial is registered under the name “Investigation of a Novel Silicone Dressing to Maximize the Outcomes of Scar Revision Procedures (IMPROVE),” Clinical Trials.gov identification number NCT01430130 (http://clinicaltrials.gov/show/NCT01430130).
Disclosure: Michael T. Longaker, M.D., M.B.A., and Geoffrey C. Gurtner, M.D., have equity in Neodyne Biosciences, Inc., which provided the devices used in the study and supported the clinical trial. Christy Cowley, M.P.H., Peggy McLaughlin, B.S., and Bill Beasley, B.S., are consultants to, or employed by, Neodyne Biosciences, Inc., and have equity positions in the company. Dr. Longaker analyzed the data and wrote this article while on sabbatical from Stanford University.
Supplemental digital content is available for this article. A direct URL citation appears in the text; simply type the URL address into any Web browser to access this content. A clickable link to the material is provided in the HTML text of this article on the Journal’s Web site (www.PRSJournal.com).
REFERENCES
- 1.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 2.Longaker MT. Regenerative medicine: A surgeon’s perspective. J Pediatr Surg. 2010;45:11–17. doi: 10.1016/j.jpedsurg.2009.10.004. discussion 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas JR, Somenek M. Scar revision review. Arch Facial Plast Surg. 2012;14:162–174. doi: 10.1001/archfacial.2012.223. [DOI] [PubMed] [Google Scholar]
- 4.Shockley WW. Scar revision techniques: Z-plasty, w-plasty, and geometric broken line closure. Facial Plast Surg Clin North Am. 2011;19:455–463. doi: 10.1016/j.fsc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Plastic Surgeons. [Accessed March 5, 2013];2012 Report of the 2011 Statistics National Clearinghouse of Plastic Surgery Statistics. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2011-statistics/2011_Stats_Full_Report.pdf. [Google Scholar]
- 6.Khatri KA, Mahoney DL, McCartney MJ. Laser scar revision: A review. J Cosmet Laser Ther. 2011;13:54–62. doi: 10.3109/14764172.2011.564625. [DOI] [PubMed] [Google Scholar]
- 7.Harmon CB, Zelickson BD, Roenigk RK, et al. Dermabrasive scar revision: Immunohistochemical and ultrastructural evaluation. Dermatol Surg. 1995;21:503–508. [PubMed] [Google Scholar]
- 8.Maher SF, Dorko L, Saliga S. Linear scar reduction using silicone gel sheets in individuals with normal healing. J Wound Care. 2012;21:602, 604–606, 608–612. doi: 10.12968/jowc.2012.21.12.602. [DOI] [PubMed] [Google Scholar]
- 9.Saulis AS, Mogford JH, Mustoe TA. Effect of Mederma on hypertrophic scarring in the rabbit ear model. Plast Reconstr Surg. 2002;110:177–183. doi: 10.1097/00006534-200207000-00029. discussion 184–186. [DOI] [PubMed] [Google Scholar]
- 10.Gold MH, Foster TD, Adair MA, Burlison K, Lewis T. Prevention of hypertrophic scars and keloids by the prophylactic use of topical silicone gel sheets following a surgical procedure in an office setting. Dermatol Surg. 2001;27:641–644. doi: 10.1046/j.1524-4725.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 11.Flynn TC, Coleman WP. Topical revitalization of body skin. J Eur Acad Dermatol Venereol. 2000;14:280–284. doi: 10.1046/j.1468-3083.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 12.Anthony ET, Lemonas P, Navsaria HA, Moir GC. The cost effectiveness of intralesional steroid therapy for keloids. Dermatol Surg. 2010;36:1624–1626. doi: 10.1111/j.1524-4725.2010.01696.x. [DOI] [PubMed] [Google Scholar]
- 13.Renovo. [Accessed March 7, 2013];Juvista EU Phase 3 Trial Results. Available at: http://www.renovo.com/en/news/juvista-eu-phase-3-trial-results. [Google Scholar]
- 14.Gurtner GC, Dauskardt RH, Wong VW, et al. Improving cutaneous scar formation by controlling the mechanical environment: Large animal and phase I studies. Ann Surg. 2011;254:217–225. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 15.Wong VW, Rustad KC, Akaishi S, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan JA, Bond JS, Mason T, et al. Visual analogue scale scoring and ranking: A suitable and sensitive method for assessing scar quality? Plast Reconstr Surg. 2006;118:909–918. doi: 10.1097/01.prs.0000232378.88776.b0. [DOI] [PubMed] [Google Scholar]
- 17.Bush J, Duncan JA, Bond JS, et al. Scar: Improving efficacy of avotermin administered into the wound margins of skin incisions as evaluated by a randomized, double-blind, placebo-controlled, phase II clinical trial. Plast Reconstr Surg. 2010;126:1604–1615. doi: 10.1097/PRS.0b013e3181ef8e66. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson MW, Duncan JA, Bond JS, et al. Prophylactic administration of avotermin for improvement of skin scarring: Three double-blind, placebo-controlled, phase I/II studies. Lancet. 2009;373:1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.