Abstract
This Communication describes a chemically responsive polymer film that is capable of detecting low levels of a specific applied molecular signal (thiol) and subsequently initiating a self-propagating reaction within the material that converts the nonfluorescent film into a globally fluorescent material. We illustrate that the intensity of the resulting fluorescent material is independent of the quantity of the applied thiol, whereas the rate to reach the maximum level of signal is directly proportional to the quantity of the signal. In contrast, a control film, which lacks functionality for mediating the self-propagating reaction, provides a maximum change in fluorescence that is directly proportional to the quantity of the applied thiol. This level of nonamplified signal is 78% lower in intensity (when initiated with 100 µM of applied thiol) than is achieved when the material contains functionality that supports the self-powered, self-propagating amplification reaction.
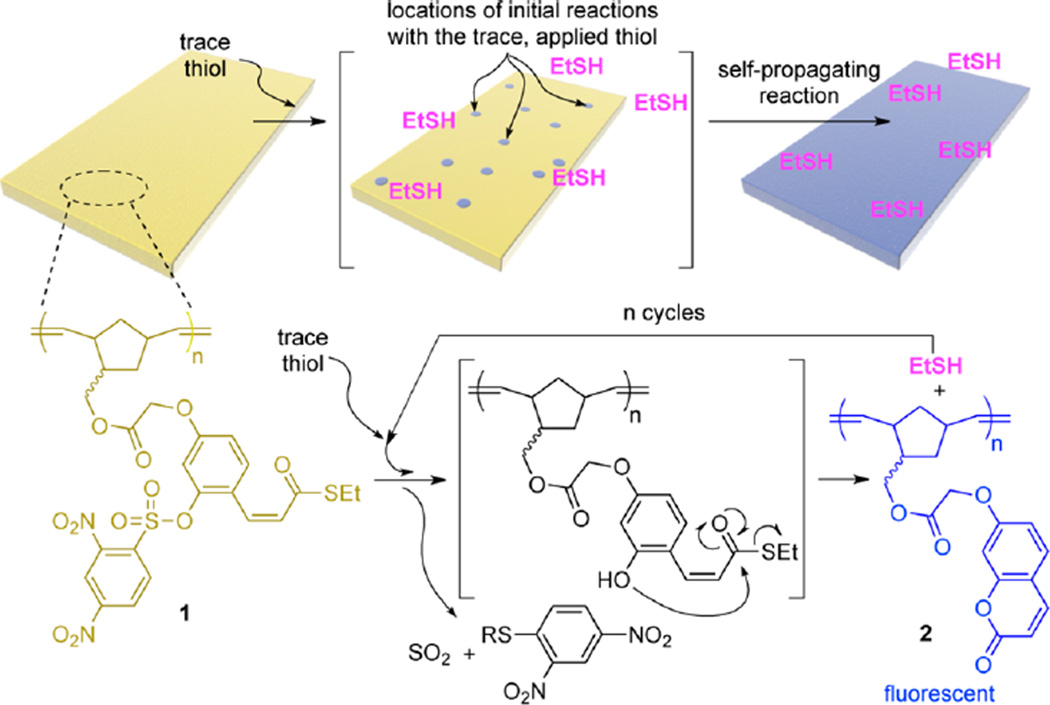
The ability of a self-propagating reaction to impart an autonomous, amplified, macroscopic response to a material (without assistance from reagents supplied in solution) is exceedingly rare in synthetic materials, but is common in living systems (e.g., Venus flytraps, touch-me-nots, or sea cucumbers).1 Few reagents, reactions, and polymers are able to achieve biological-like amplified responses in materials;2 thus, the vast majority of stimuli-responsive materials are designed to change macroscopically when large quantities of signal is applied.3 As a step toward overcoming this limitation, herein we describe a self-propagating reaction that occurs in a polymeric material (Figure 1), thus offering a means for amplifying a molecular detection event into a macroscopic change in a material. The response arising from the self-propagating reaction is analogous to living systems in that the material changes equally regardless of whether the material is exposed to trace levels of the specific applied signal or stoichiometric quantities.
Figure 1.
Illustration of a polymeric film that transforms completely from nonfluorescent to fluorescent when exposed to trace levels of added thiol. The added thiol initiates a self-propagating reaction within the film that amplifies the fluorescent output. The bottom half of the figure depicts the specific polymer (1) and self-propagating reaction that provides the global change from nonfluorescent to fluorescent across the entire material.
In brief, this Communication provides two key advances: (i) A novel design for a small molecule signal amplification reagent (which we ultimately convert into a monomer). This single reagent enables a self-propagating amplification reaction while simultaneously minimizing background reaction, thus enabling substantial signal amplification in aqueous media. (ii) We then demonstrate the use of the reagent to create a polymeric material that changes its macroscopic properties (optical properties in this case) autonomously in response to local and even fleeting stimuli. Such materials are highly sought after, yet are largely unrealized. Possible applications of such materials could include new types of reconfigurable camouflage, self-healing materials, reconfigurable biomedical materials, debondable adhesives, and materials that change porosity and/or wetting properties to prevent exposure of underlying objects, among a variety of other possible applications. This Communication offers one path forward for realizing this long-term potential, with a focus on changing the optical properties of the material.
Polymer 1 is key to our design: each repeating unit in 1 contains the functionality needed to support the self-propagating reaction. This functionality includes a 2,4-dinitrobenzenesulfonyl (Nosyl) protected phenol, which cleaves selectively in response to added thiol.4 Removal of the Nosyl group allows the liberated phenol to cyclize into the thioester, releasing one equivalent of ethanethiol while simultaneously forming an equivalent of fluorescent, polymerbound 7-alkoxycoumarin (2) (Figure 1). The released ethanethiol is free to react with another Nosyl group on a neighboring repeating unit in the polymeric material, thus continuing the self-propagating reaction and, consequently, amplifying the quantity of fluorescent 2. Since each reaction between ethanethiol and the Nosyl group in 1 simultaneously consumes and produces one equivalent of thiol, the net concentration of thiol remains constant throughout the self-propagating reaction. In other words, a single detection event between a material made from 1 and an applied thiol should lead to conversion of the entire material into 2.
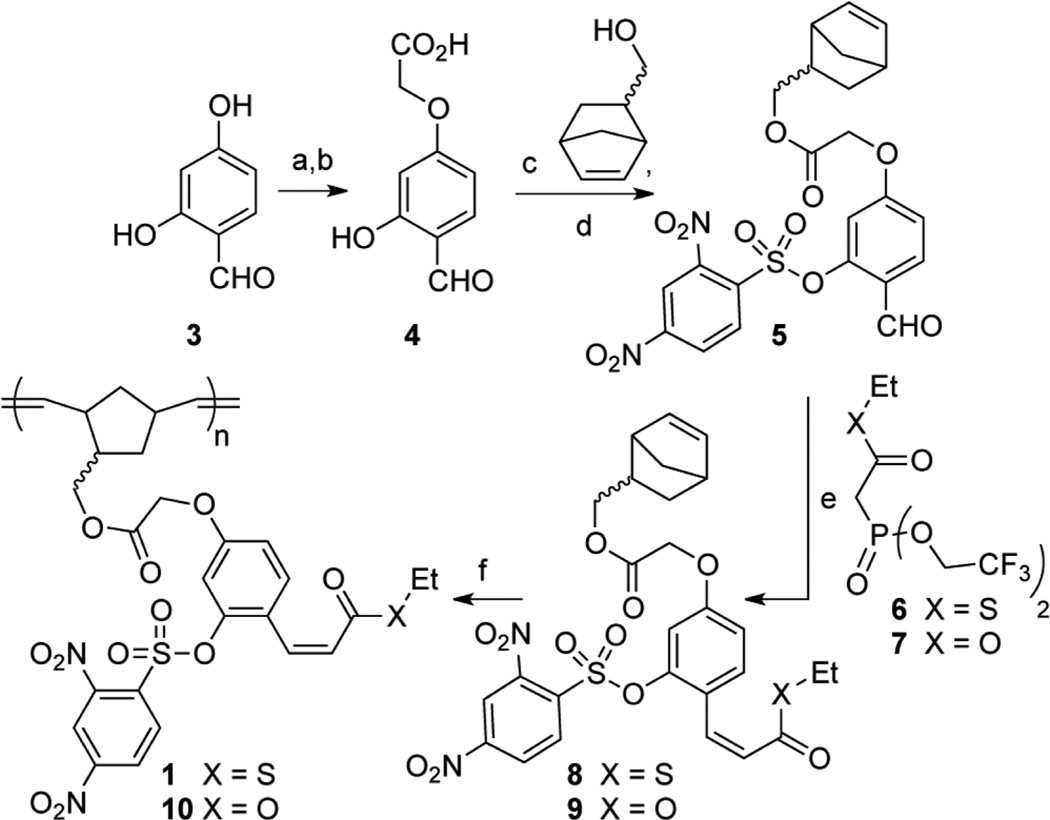
To test this design, we prepared polymer 1 in six linear steps using the route depicted in Scheme 1. We also used a similar sequence to prepare control polymer 10, which differs from 1 only by replacement of the ethyl thioester (1) with an ethyl ester (10) (Scheme 1). Control polymer 10 is capable of consuming one equivalent of added thiol and producing one equivalent of fluorescent product 2, but not of releasing thiol to continue propagating the reaction. Thus, 10 provides a benchmark for a stoichiometric response between the plastic and a specific quantity of an applied thiol.
Scheme 1. Synthesis of Polymer 1 and Control Polymer 10a.
aReagents and conditions: (a) BrCH2CO2tBu, NaHCO3, 70 °C, DMF (42%); (b) TFA, CH2Cl2; (c) HBTU, DIEA, DMAP, MeCN (42% over 2 steps); (d) DNBS-Cl, DIEA, CH2Cl2, −78 to 20 °C (87%); (e) K2CO3, 18-crown-6, THF, −20 °C (66% for 8 and 43% for 9); (f) (i) Grubb’s second gen. cat., CH2Cl2, 20 °C; (ii) ethyl vinyl ether (81% for 1 and 82% for 10).
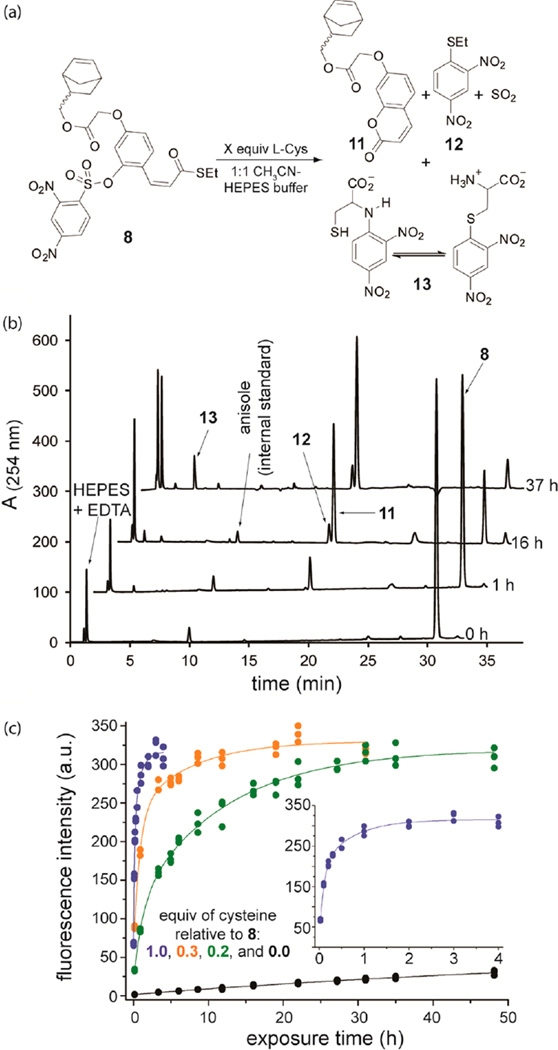
Before evaluating whether 1 is capable of supporting a self-propagating reaction in the context of a macroscopic plastic, we first tested monomer 8 since it is readily soluble and the products of its reaction with added thiol are easily analyzed using liquid chromatography coupled to a mass spectrometer (LCMS). Treatment of 8 (2 mM) in 1:1 MeCN–buffered water (pH 7.4) with 0.1 equiv of l-cysteine (a model thiol) yielded the expected 7-alkoxycoumarin product (11, Figures 2a,b), ethanethioether 12, and l-cysteine adduct of the cleaved Nosyl group (13) (Figures 2a,b). Production of ethanethioether 12, in particular, is consistent with a self-propagating reaction, as is the observation that nearly all of 8 is consumed during the experiment despite the 10-fold excess of 8 relative to the added thiol.
Figure 2.
Demonstration that monomer 8 is capable of supporting the self-propagating reaction. (a) The reaction conditions and products when 8 is exposed to thiol (cysteine in this example). (b) LCMS chromatograms that reveal the temporal change in concentration of reactants and products when 8 (2 mM) in 1:1 MeCN–buffered water (pH 7.4) is exposed to 0.1 equiv of l-cysteine at 23 °C. (c) Kinetics (obtained from fluorescence measurements) that support the self-propagating reaction in (a). These results demonstrate that even trace levels of added thiol elicit a maximum fluorescence response over time. The graph includes all data corresponding to three replicates per equivalent of added cysteine. The inset clarifies the response for 1 equiv of cysteine. The λex for coumarin 11 is 320 nm, while the λem is 380 nm. The lines are simulated data for the reaction A + B → C + A (see the text for details) superimposed on the experimental data points.
Similar experiments using 8 and different initial concentrations of added l-cysteine were analyzed using fluorescence spectroscopy to quantify the intensity of fluorescence from 11 and hence the relative quantity of 11 produced during the self-propagating reaction (Figure 2c). These experiments revealed that the same level of fluorescent signal is obtained regardless of whether 8 is treated with 1 equiv or 0.2 equiv of added thiol (Tables S1 and S3), and that only the time required to reach this maximum level of signal varies between reactions. In the absence of added thiol, minimal background fluorescence was observed over the duration of the experiment, which is a highly desirable feature for an amplification reaction.5
Moreover, the experimental fluorescence data is consistent with the self-propagating reaction A + B → C + A, where A = thiol, B = reagent 8, and C = coumarin 11. Simulations of this reaction (eq 1) superimpose with the experimental data, as illustrated in Figure 2c.
| (1) |
In this equation, Imeasured is the normalized fluorescence intensity, [A]0 and [B]0 represent the concentrations of A and B (respectively) at the outset of the reaction, k is the rate constant for the reaction, and t is exposure time.6
We next tested whether this self-propagating reaction would be effective in the context of a solid-state material made from polymer 1. Since we planned to use the same 1:1 MeCN–buffered water solvent system as used for 8, we first evaluated whether solid plastics made from 1 would dissolve in the solvent. The goal of this experiment was to use a solvent that did not dissolve solid 1 to ensure that subsequent experiments involved measurements of the self-propagating reaction at the interface of solid 1 and an applied signal in an aqueous solution. No noticeable change in size of a cup-shaped object made from 1 (Figure S1) was observed when the object was immersed in 1:1 MeCN–buffered water (pH 7.4) for 45 h, thus confirming the suitability of this solvent for the solid-state experiments.7
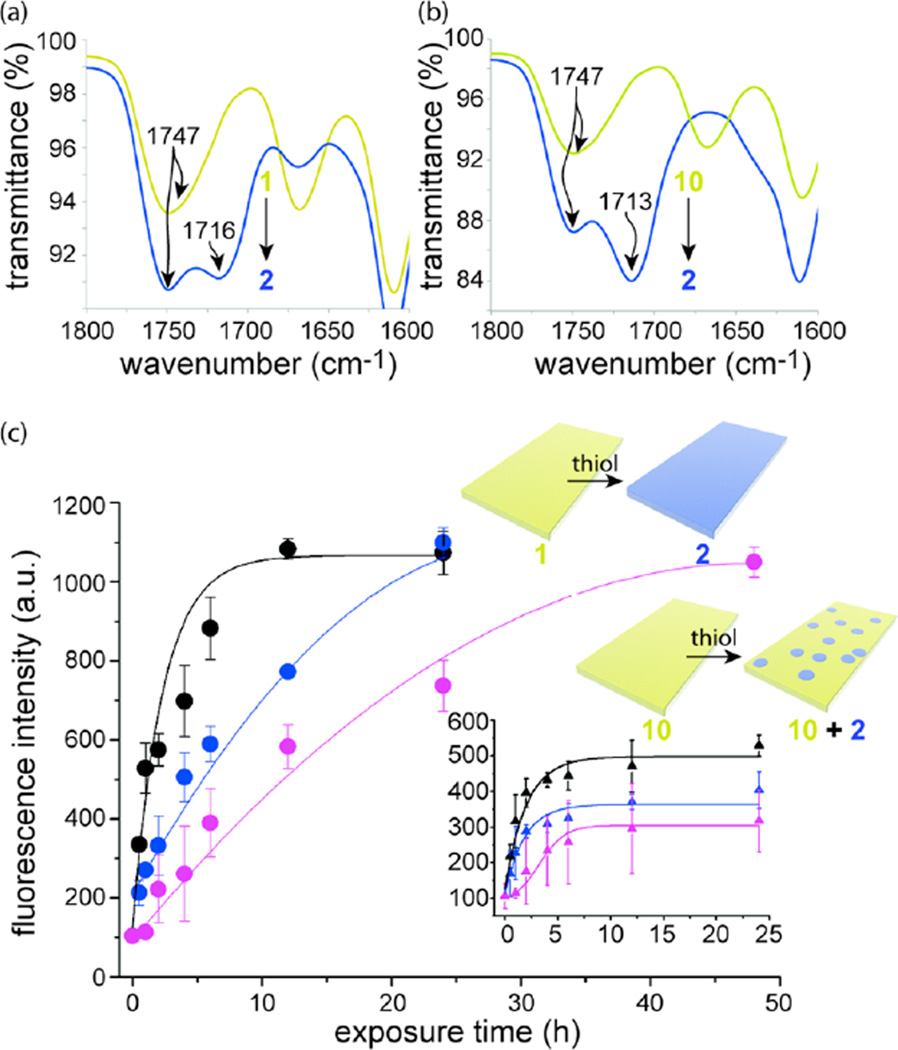
Since the plastic made from 1 was not affected dramatically by the aqueous solution, we solvent-cast films of the polymer as well as control polymer 10 on glass slides. Separate films were exposed for 3 h to 1:1 MeCN–buffered water containing l-cysteine. After rinsing and drying the films, we used infrared spectroscopy (IR) to evaluate whether the esters (in films of 10) and thioesters (in films of 1) had transformed into lactones. Indeed, when solutions containing l-cysteine were exposed to the films, the single carbonyl IR signature of the starting polymer films (e.g., ~1750 cm−1) converted into two peaks (~1750 and ~1720 cm−1) (Figures 3a,b). In the absence of l-cysteine, no change in IR signature was observed for either polymer. These combined results confirm that cleavage of the Nosyl group, and subsequent cyclization to the lactone of 7-alkoxycoumarin indeed occurs when the solid films are wet with solutions containing thiols.
Figure 3.
Self-propagating reactions in films. (a,b) Change in IR signature in the carbonyl region when a film of 1 (a) or 10 (b) is exposed to 10 mM thiol (l-cysteine) in 1:1 MeCN–buffered water (pH 7.4) for 3 h. (c) Kinetics (obtained from fluorescence measurements) when 0.1 mM (pink data), 1 mM (blue data), and 10 mM (black data) solutions of l-cysteine were exposed to films of polymers 1 and 10 at 23 °C. The circular data points correspond to films made from 1; the triangular data in the inset graph correspond to films made from 10 (the axis labels are the same as the larger graph). The data represent the averages of three measurements, and the error bars reveal the standard deviations from these averages. In the context of solid state films, the λex = 320 nm; λem = 430 nm. The lines are provided to visually differentiate the data sets.
To test the effects of self-propagation on global changes in the optical properties of a material made from 1, we used the technique of spin coating to create 0.5 cm-wide × 1 cm-long × 7.2 ± 1.9 nm-thick films of polymer 1 and 10 (6.7 ± 0.9 nm-thick) on strips of polypropylene. Individual films were immersed at 23 °C in 0.2 mL of 1:1 MeCN–buffered water (pH 7.4) containing different initial concentrations of added thiol (l-cysteine). At specified time points, samples were removed from solution and dried. Time-dependent measurements of fluorescence (λex = 320 nm; λem = 430 nm) for these dry films revealed the expected relationship (for a self-propagating reaction) between the quantity of added thiol, and the reaction time required for the film to reach a maximum (and equal) level of fluorescence signal (Figure 3c). We also observed minimal background reaction in the absence of l-cysteine (the background signal increased only by 10% after 72 h of exposing a film of 1 to the MeCN–buffered water; Table S8).
In contrast, but also expected, the kinetics for solid-state experiments using films of 10 were consistent with 1:1 responses between signal (added thiol) and the readout (fluorescence) (Figure 3c, inset). Films made from 10 reached a maximum fluorescence signal faster than films made from 1, which is a result that is consistent with self-propagating reactions continuing at the solid–liquid interface between films of 1 (but not 10) and the surrounding solution until all accessible Nosyl groups react and convert 1 into 2. Thus, these solid-state studies confirm the ability of thiols to propagate a macroscopic change in the optical properties of a plastic when the plastic encounters a low dose of an applied signal at the interface of the plastic and an aqueous solution.
These findings support the concept of using self-propagating amplification reactions to create materials with the ability to autonomously and completely transform themselves in response to trace levels of specific applied signals. When viewed together with two recent demonstrations of autoinductive reactions in materials (one in a solid-state film8 and the other dispersed in a hydrogel9), it is becoming increasingly apparent that self-propagating reactions can be used to impart a dramatic and preplanned change to the global properties of materials. These advances, the most critical of which is the signal-induced self-propagating reaction, are key for enabling a new type of biomimetic, amplified response in synthetic materials.
Future expansions of this type of stimuli-responsive material may benefit from (i) use of polymer backbones other than poly(norbornene) and (ii) recent discoveries of autoinductive10 (including dendritic chain reaction11), autocatalytic,12 and catalytic signal amplification reactions13 in small molecules. Inclusion of polymer-bound detection reagents (that release thiols in response to another analyte, such as an enzyme10,11) into a material along with polymer 1 will substantially expand the scope of the materials as well.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health: R01GM105686. We thank Gregory G. Lewis for preparing the sample holder for fluorescence measurements.
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Materials, procedures, tables of data, and figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Fratzl P, Barth FG. Nature. 2009;462:442–448. doi: 10.1038/nature08603. [DOI] [PubMed] [Google Scholar]; (b) Forterre Y, Skotheim JM, Dumais J, Mahadevan L. Nature. 2005;433:421–425. doi: 10.1038/nature03185. [DOI] [PubMed] [Google Scholar]
- 2.(a) Grinthal A, Aizenberg J. Chem. Soc. Rev. 2013;42:7072–7085. doi: 10.1039/c3cs60045a. [DOI] [PubMed] [Google Scholar]; (b) Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Science. 2008;319:1370–1374. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]; (c) DiLauro AM, Lewis GG, Phillips ST. Angew. Chem. 2015;127:6298–6303. doi: 10.1002/anie.201501320. [DOI] [PubMed] [Google Scholar]
- 3.(a) Zhai L. Chem. Soc. Rev. 2013;42:7148–7160. doi: 10.1039/c3cs60023h. [DOI] [PubMed] [Google Scholar]; (b) Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, Tsukruk VV, Urban M, Winnik F, Zauscher S, Luzinov I, Minko S. Nat. Mater. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]; (c) Liu F, Urban MW. Prog. Polym. Sci. 2010;35:3–23. [Google Scholar]; (d) Theato P, Sumerlin BS, O’Reilly RK, Epps TH., III Chem. Soc. Rev. 2013;42:7055–7056. doi: 10.1039/c3cs90057f. [DOI] [PubMed] [Google Scholar]; (e) Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S. Chem. Soc. Rev. 2013;42:7421–7435. doi: 10.1039/c3cs60094g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Molla MR, Rangadurai P, Pavan GM, Thayumanavan S. Nanoscale. 2015;7:3817–3837. doi: 10.1039/c4nr04563g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Li X, Qian S, He Q, Yang B, Li J, Hu Y. Org. Biomol. Chem. 2010;8:3627–3630. doi: 10.1039/c004344c. [DOI] [PubMed] [Google Scholar]; (b) Wang S-P, Deng W-J, Sun D, Yan M, Zheng H, Xu J-G. Org. Biomol. Chem. 2009;7:4017–4020. doi: 10.1039/b909760k. [DOI] [PubMed] [Google Scholar]
- 5.Over the course of the 50 h exposure time in Figure 2c, the level of background thiol remained exceedingly low, which would result in a slow reaction between reagent 8 and thiol. Presumably the quantity of thiol at ~50 h is sufficiently high to initiate a slow amplification reaction. However, we did not continue the experiment further to explore this amplification reaction since all test samples were complete.
- 6.A derivation of eq 1 is provided in the Supporting Information.
- 7.Softening of the cup-shaped objects at their edges is consistent with minor swelling of the plastic via absorption of solvent at the surface of the object. This minor degree of softening indicates that the solvent wets solids made from 1, which allows thiols in solution to interact with the solid surface.
- 8.Kim H, Baker MS, Phillips ST. Chem. Sci. 2015;6:3388–3392. doi: 10.1039/c5sc00701a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshii T, Onogi S, Shigemitsu H, Hamachi I. J. Am. Chem. Soc. 2015;137:3360–3365. doi: 10.1021/ja5131534. [DOI] [PubMed] [Google Scholar]
- 10.(a) Mohapatra H, Schmid KM, Phillips ST. Chem. Commun. 2012;48:3018–3020. doi: 10.1039/c2cc17566e. [DOI] [PubMed] [Google Scholar]; (b) Baker MS, Phillips ST. J. Am. Chem. Soc. 2011;133:5170–5173. doi: 10.1021/ja108347d. [DOI] [PubMed] [Google Scholar]; (c) Yeung K, Schmid KM, Phillips ST. Chem. Commun. 2013;49:394–396. doi: 10.1039/c2cc36861g. [DOI] [PubMed] [Google Scholar]
- 11.(a) Avital-Shmilovici M, Shabat D. Soft Matter. 2010;6:1073–1080. [Google Scholar]; (b) Sella E, Shabat D. J. Am. Chem. Soc. 2009;131:9934–9936. doi: 10.1021/ja903032t. [DOI] [PubMed] [Google Scholar]; (c) Sella E, Weinstain R, Erez R, Burns NZ, Baran PS, Shabat D. Chem. Commun. 2010;46:6575–6577. doi: 10.1039/c0cc02195d. [DOI] [PubMed] [Google Scholar]; (d) Karton-Lifshin N, Shabat D. New J. Chem. 2012;36:386–393. [Google Scholar]; (e) Perry-Feigenbaum R, Sella E, Shabat D. Chem. - Eur. J. 2011;17:12123–12128. doi: 10.1002/chem.201101796. [DOI] [PubMed] [Google Scholar]
- 12.Mohapatra H, Phillips ST. Anal. Chem. 2012;84:8927–8931. doi: 10.1021/ac302582h. [DOI] [PubMed] [Google Scholar]
- 13.Goggins S, Marsh BJ, Lubben AT, Frost CG. Chem. Sci. 2015;6:4978–4985. doi: 10.1039/c5sc01588j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.