Abstract
Context:
Neuromuscular and mechanical deficiencies are commonly studied in participants with chronic ankle instability (CAI). Few investigators have attempted to comprehensively consider sensorimotor and mechanical differences among people with CAI, copers who did not present with prolonged dysfunctions after an initial ankle sprain, and a healthy control group.
Objective:
To determine if differences exist in spinal reflex excitability and ankle laxity among participants with CAI, copers, and healthy controls.
Design:
Case-control study.
Setting:
Research laboratory.
Patients or Other Participants:
Thirty-seven participants with CAI, 30 participants categorized as copers, and 26 healthy control participants.
Main Outcome Measure(s):
We assessed spinal reflex excitability of the soleus using the Hoffmann reflex protocol. Participants' ankle laxity was measured with an instrumented ankle arthrometer. The maximum Hoffmann reflex : maximal muscle response ratio was calculated. Ankle laxity was measured as the total displacement in the anterior-posterior directions (mm) and total rotation in the inversion and eversion directions (°).
Results:
Spinal reflex excitability was diminished in participants with CAI compared with copers and control participants (P = .01). No differences were observed among any of the groups for ankle laxity.
Conclusion:
Changes in the spinal reflex excitability of the soleus that likely affect ankle stability were seen only in the CAI group, yet no mechanical differences were noted across the groups. These findings support the importance of finding effective ways to increase spinal reflex excitability for the purpose of treating neural excitability dysfunction in patients with CAI.
Key Words: neuromuscular control, sensorimotor function, joint instability, ankle sprains, soleus muscle
Key Points
Spinal reflex excitability of the soleus muscle was decreased in participants with chronic ankle instability (CAI) compared with coper and healthy control groups.
Changes in the sensorimotor system that likely affect perceived stability at the ankle joint were seen only in the CAI group, yet no mechanical differences were noted among the groups.
Because soleus spinal reflex excitability in copers more closely resembled that of control participants than those with CAI, therapeutic interventions (eg, transcutaneous electrical stimulation, joint manipulations, and reflex conditioning protocols) that can improve spinal reflex excitability dysfunction may improve the management of CAI.
Ankle sprains are among the most common lower extremity musculoskeletal injuries in sport.1 More than 28% of high school athletes sustain recurrent ankle injuries,2 a percentage that is greater than for any other injury. Additionally, up to 74% of people with an initial ankle sprain go on to experience repeated bouts of perceived giving way.3−5 Patients who report continual complications, such as repeated episodes of giving way and perceived ankle dysfunction, of the initial ankle sprain have developed chronic ankle instability (CAI).6 People with an initial ankle sprain who do not display prolonged instability and episodes of giving way are categorized as copers.7 Recently, researchers8 have focused on studying people with CAI and copers together to determine the factors that lead some individuals to return to full activity after an initial ankle sprain without any complications. Identifying differences between patients with CAI and copers will help to elucidate the factors contributing to CAI, which may help us to develop more effective intervention programs.9
Multiple negative sensorimotor outcomes in CAI populations have been shown in previous investigations,8,10−13 indicating that CAI is associated with alterations in the perception of peripheral afferent input and in the central nervous system (CNS). Previous authors have also shown that patients with CAI differ from copers in static postural control,8 dynamic stability during a single-legged landing,12 and movement patterns during functional tasks,14 supporting the association between CAI and systematic alterations in feedback and feed-forward sensorimotor control.
Although perceived instability as an estimation of functional limitations and disability is one of the most prominent characteristics of CAI, there is no consensus as to whether laxity exists in either those with CAI15 or copers.12,16 Recently, researchers16 observed a similar length change in the anterior tibiofibular ligament during the anterior drawer test and ankle inversion between participants with CAI and copers using diagnostic ultrasonography, suggesting that mechanical laxity alone may not characterize CAI. We have much to learn about the origins of the perceived and functional limitations observed in the CAI patient population, which additional assessments of alterations in the feedback and feed-forward sensorimotor functions, including spinal reflex excitability measures, may help to elucidate. Spinal reflex excitability may be decreased within the fibularis longus and soleus of the affected ankle of participants with CAI compared with the unaffected ankle.17,18 Decreased spinal reflex excitability in the affected limb of individuals with CAI suggests decreased α motor-neuron (αMN) pool availability, which plays a significant role in the muscle's motor drive.17 Decreased spinal reflex excitability has been suggested to result from deficits in peripheral sensory input after injury or supraspinal sensorimotor dysfunction (or both), which may influence presynaptic inhibition.19,20 Afferent proprioceptive deficits and reduced cutaneous sensation have been demonstrated in several studies21−24 of individuals with CAI. Bilateral decreases in corticospinal excitability have been noted within the fibularis longus of patients with CAI.11 Therefore, it appears that evaluating sensorimotor function could help to define CAI better than isolating mechanical ankle laxity, yet comparisons have not been thorough or included a group of copers. Although researchers have quantified spinal reflexive excitability in patients with CAI,11,18 few researchers have examined spinal reflex excitability in patients across the spectrum of ankle-sprain history and diversity of residual symptoms associated with CAI. Therefore, the aim of our study was to determine if differences exist in spinal reflexive excitability within the soleus, as well as in ankle laxity, among individuals with CAI, copers, and healthy controls.
METHODS
Our research was a single-blinded, case-control study involving 3 groups of individuals: those with self-reported CAI, copers, and healthy control participants. Participants were screened by an investigator, and 2 other investigators blinded to group allocation collected and analyzed the outcome measures. After screening and enrollment in the study, participants reported for 2 testing sessions. Spinal reflex excitability of the soleus and joint-laxity measures were assessed during separate sessions, in a randomly selected order. All measures were assessed on a predetermined limb. For the CAI and coper groups, the injured limb was used. If a participant had a history of bilateral ankle injury, we measured the limb with the greatest amount of self-reported instability. A randomly selected limb was used for the control group.
Participants
Ninety-three physically active participants from the university community volunteered for this study (Table 1). After we enrolled and screened participants using self-reported questionnaires, they were allocated to the CAI, coper, or control group. Thirty-seven participants were assigned to the CAI group based on recommendations from the International Ankle Consortium.15 Participants in the CAI group had to report a previous history of at least 1 ankle sprain resulting in swelling, pain, and temporary loss of function; feelings of giving way at least twice in the past 6 months; and perceived ankle instability and dysfunction during daily activities.6,15 Additionally, participants with CAI had to score ≥5 on the Ankle Instability Instrument (AII) and ≥11 on the Identification of Functional Ankle Instability (IdFAI) instrument.25,26
Table 1. .
Participant Demographics
| Characteristic |
Group |
||
| Chronic Ankle Instability (n = 37) |
Copers (n = 30) |
Controls (n = 26) |
|
| Sex, males/females | 18/19 | 13/17 | 9/17 |
| Modified physical activity? Yes/no | 11a,b/26 | 0/30 | NA |
| Mean ± SD |
|||
| Age, y | 21.95 ± 3.45 | 21.86 ± 4.32 | 21.56 ± 3.15 |
| Body mass index | 25.16 ± 3.79 | 26.21 ± 6.28 | 23.72 ± 2.84 |
| Identification of Functional Ankle Instability | 17.70 ± 3.62a,b | 4.76 ± 3.52a | 0.00 ± 0.00 |
| Ankle Instability Instrument | 5.89 ± 1.41a,b | 2.48 ± 1.21a | 0.00 ± 0.00 |
| Foot and Ankle Ability Measure—Activities of Daily Living, % | 90.29 ± 8.37a,b | 98.55 ± 2.55a | 99.96 ± 0.23 |
| Foot and Ankle Ability Measure—Sport, % | 81.89 ± 11.57a,b | 96.53 ± 5.27a | 99.55 ± 1.81 |
| Lateral ankle sprains, No. (minimum, maximum) | 2.86 ± 2.62a,b (1, 15) | 1.82 ± 1.16 | NA |
| Months since most recent lateral ankle sprain (minimum, maximum) | 40.86 ± 32.21 (4, 96) | 50.46 ± 45.64 (5, 240) | NA |
| Ankle giving-way feelings, No. (minimum, maximum) | 9.11 ± 17.71 (2, 100) | NA | NA |
Abbreviation: NA, not applicable.
Different from controls (P < .05).
Different from copers (P < .05).
Thirty participants were included in the coper group. Copers were defined as participants who had a history of ankle sprains but no reported episodes of giving way, perceived instability, or loss of function without modifying physical activity.7,12 Additionally, copers had to score <5 on the AII and <11 on the IdFAI.25,26 Twenty-six participants were included in the control group. The control-group participants were required to have no history of ankle sprain and score 0 on both the AII and IdFAI.25,26 No participants in the CAI or coper group had acutely sprained an ankle in the 3 months before testing. Participants completed the Foot and Ankle Ability Measure—Activities of Daily Living (FAAM-ADL) and Sports (FAAM-S) subscales so that we could better understand their levels of self-reported disability.27 Higher scores on the FAAM indicate less ankle disability.
Participants were asked to refrain from ingesting stimulating or depressing substances for 12 hours before testing because caffeine may affect measures of spinal reflex excitability.28 Before enrollment, the participants read and signed an informed consent form approved by the university institutional review board, which also approved the study.
Ankle-Laxity Measures
We used an instrumented ankle arthrometer (Blue Bay Medical Inc, Navarre, FL) to assess ankle-joint laxity for anterior-posterior (A-P) displacement and inversion-eversion (I-E) laxity (Figure 1), following a method previously described.29 The participant lay supine on a treatment table with a bolster under the lower leg and the upper leg secured with hook-and-loop straps around the midthigh. The arthrometer was secured to the test foot using clamps at the heel and a strap at the midfoot. A tibial pad was secured to the lower leg 5 cm above the malleolus. The testing in both directions began from a neutral ankle position.
Figure 1. .
Ankle arthrometer and participant setup.
Participants were instructed to relax and avoid contraction of ankle muscles. To test A-P displacement, the researcher applied a force of 125 N in the anterior direction, followed by the posterior direction, with total A-P displacement recorded in millimeters.29 For I-E laxity, 4000 N/mm of torque was applied first toward inversion and then eversion, with total I-E laxity measured in degrees.29 The average of 3 trials was used for analysis.
Spinal Reflex Excitability Measure
Spinal reflex excitability was assessed using previously published methods30,31 for eliciting the Hoffmann reflex (H-reflex) and muscle response (M-response). In the current study, we investigated soleus excitability because of its critical role in static balance control and ankle stabilization.32,33 In brief, participants were positioned for soleus testing with their hips flexed to 90°, knees flexed to 90°, and test ankle plantar flexed to 90°. Participants were secured to the dynamometer (System III Pro; Biodex Medical Systems, Shirley, NY) with lap and thigh straps to limit auxiliary motions of the trunk and leg. Two 10-mm pregelled Ag/AgCl electromyography electrodes (model EL503; BIOPAC Systems, Inc, Goleta, CA) were placed 1.75 mm apart over the midline of the soleus in the distal third of the lower leg30 with a ground electrode over the contralateral medial malleolus.11,30 The areas were shaved, abraded with fine sandpaper, and cleaned with isopropyl alcohol wipes before electrode placement. Electromyography signals were recorded and analyzed using AcqKnowledge software (version 4.0; BIOPAC Systems, Inc).
A 2-mm shielded disk stimulating electrode (EL254S; BIOPAC Systems, Inc) was positioned over the proximal lateral popliteal fossa to stimulate the posterior tibial nerves, and a 7-cm carbon-impregnated dispersive pad was placed over the ipsilateral quadriceps.30 The electrode was shifted to the location that elicited the largest peak-to-peak twitch response at a constant stimulus in the soleus, and this location was used for all testing trials. A constant-current 400-V maximum stimulator (DS7AH; Digitimer Ltd, Hertfordshire, England) with a rectangular current pulse of 1 millisecond was used to elicit the stimuli. AcqKnowledge software was used to visualize the signals. Stimulus intensity was increased or decreased in 2.0-mA increments with 10 seconds' rest between trials until the maximum peak-to-peak H-reflex (Hmax) was found.34 Three trials of the Hmax were then measured and recorded. To determine the maximum M-response (Mmax), the investigator increased the stimulus in increments of 10.0 mA until the peak-to-peak amplitude of the M-wave plateaued. Three Mmax trials were then measured, and the Hmax : Mmax ratio (representing Hmax normalized to Mmax) was calculated for data analysis. A greater Hmax : Mmax ratio indicates greater spinal reflex excitability of the soleus.
Statistical Analysis
For each outcome measure, we used means and standard deviations from all recorded trials for group comparisons. All statistical analyses were performed using SPSS software (version 20.0; IBM Corporation, Armonk, NY). Significance was set at P < .05 for all analyses. For laboratory outcome measures (spinal reflex excitability and mechanical joint laxity), we calculated Cohen d effect sizes using the pooled standard deviations along with 95% confidence intervals (CIs) to determine the magnitude of difference in dependent variables between groups. The strength of effect sizes was interpreted as weak (d < 0.40), moderate (d = 0.40−0.79), or strong (d ≥ 0.80).35
Demographic and Ankle-Injury Characteristics
Using separate 1-way analyses of variance, we compared demographic variables and self-reported disability among the CAI, coper, and control groups. Because of the ordinal nature, AII and IdFAI scores and other ankle-injury information were compared using independent-samples Kruskal-Wallis tests to verify group inclusion.
Spinal Reflex Excitability Variable
Using a Kolmogorov-Smirnov Z test for normality, we found that spinal reflex excitability outcome measures were not normally distributed (P < .05). Therefore, a separate independent-samples Kruskal-Wallis test was performed to compare spinal reflex excitability outcome measures among the CAI, coper, and control groups. A Mann-Whitney U test was conducted for post hoc analysis in the case of statistical significance.
Mechanical Joint Laxity Variable
According to a Kolmogorov-Smirnov Z test for normality, mechanical joint-laxity variables were normally distributed (P > .05). A separate analysis of covariance was used to examine differences among groups. The covariate was sex because the sample sizes for males and females differed among the groups, and previous researchers36 suggested that sex differences may influence mechanical joint stability. Tukey post hoc testing was conducted as needed.
RESULTS
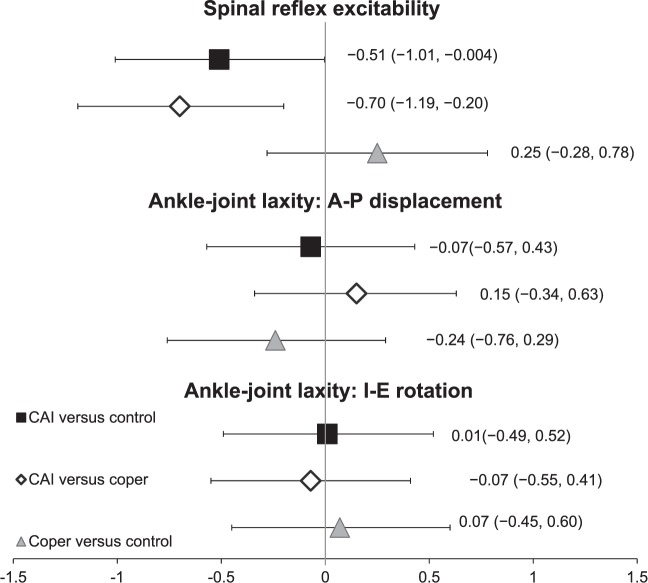
Means and standard deviations for participant demographics and self-reported questionnaire items are shown in Table 1. The groups did not differ in age, height, body mass, or physical activity. The CAI group had higher IdFAI and AII scores and lower FAAM scores than the coper and control groups, verifying the presence of the targeted condition. Means and standard deviations for all dependent variables are reported in Table 2. Effect-size calculations are provided in Figure 2.
Table 2. .
Spinal Reflex Excitability and Ankle-Joint–Laxity Measures
| Variable |
Group, Mean ± SD |
||
| Chronic Ankle Instability |
Copers |
Controls |
|
| Hmax : Mmax ratio | 0.41 ± 0.18a,b | 0.55 ± 0.22 | 0.50 ± 0.17 |
| Anterior-posterior displacement, mm | 20.84 ± 5.66 | 20.06 ± 4.71 | 21.22 ± 4.99 |
| Inversion-eversion laxity, ° | 60.20 ± 13.59 | 61.41 ± 13.55 | 59.78 ± 18.90 |
The chronic ankle instability group exhibited smaller values than the control group (P = .04).
The chronic ankle instability group exhibited smaller values than the coper group (P = .01).
Figure 2. .
Cohen d effect sizes with 95% confidence intervals for spinal reflex excitability and ankle-joint–laxity measures. Abbreviations: A-P, anterior-posterior; CAI, chronic ankle instability; I-E, inversion-eversion.
Spinal Reflex Excitability
Between-groups differences were found for soleus spinal reflex excitability (P = .02). Participants with CAI had lower Hmax : Mmax ratios than copers (P = .01) and healthy controls (P = .04) , which was supported by moderate effect sizes (CAI versus coper: d = −0.70; CAI versus control: d = −0.54) with 95% CIs that did not cross zero. Spinal reflex excitability in healthy controls was not different from that in copers (P = .42).
Ankle Laxity
No differences were noted between the groups for A-P displacement (P = .72) or I-E laxity (P = .86). All effect-size comparisons were weak with 95% CIs that crossed zero.
DISCUSSION
Our main finding was that spinal reflex excitability was decreased in participants with CAI compared with copers and healthy controls. Our findings agree with previous results17,18 that demonstrated decreased Hmax : Mmax ratios in selected ankle musculature of patients with CAI. However, spinal reflex excitability did not differ between copers and controls. This result is consistent with the results of earlier investigations that suggested mechanical ankle-joint laxity is not universal among individuals with CAI.37−39 Our current findings indicate that self-reported ankle instability may be not be associated simply with the increase in mechanical joint laxity but may be more related to sensorimotor alterations. The decrease in soleus spinal reflex excitability may negatively affect the somatosensory feedback response that is essential for activities such as postural adjustments, walking, and running.40,41 The soleus muscle has a key role in controlling the postural-sway movements of the lower limb over the base of support during standing and gait.32 Previous authors8,42−44 have consistently observed postural-control deficits and altered movement patterns during gait in individuals with CAI. Therefore, the inability to reflexively excite the soleus may alter feedback neuromuscular control around the ankle joint, leading to difficulty executing the given tasks.11
Hoffmann reflex amplitude is interpreted as the proportion of motor units activated by stimulating the afferent pathway. The mechanism leading to the lower H : M ratio associated with CAI may be attributable to both peripheral afferent input and CNS dysfunctions. Altered sensorimotor feedback control (eg, proprioceptive defect and decreased cutaneous input) may contribute to CNS alterations as evidenced by the lower H : M ratio in the soleus of the CAI group. However, potential supraspinal factors may still influence presynaptic inhibition, as descending input from higher brain centers could influence contributions from primary afferent nerves to the monosynaptic reflex response.45
It has been postulated46 that joint laxity may alter the activation of somatosensory receptors in a joint and facilitate group I nonreciprocal inhibition of the musculature crossing the joint, thereby altering neural activities within the spinal reflex pathway. We found no between-groups differences in mechanical joint-laxity measures but noted differences in spinal reflex excitability of the soleus among groups. This indicates that altered spinal reflex excitability of the soleus is likely not associated with mechanical instability of the ankle. As an exploratory analysis, we ran post hoc Spearman rank correlation analyses to evaluate the association of spinal reflex excitability of the soleus and mechanical joint-laxity measures in the CAI group. We found nonsignificant, weak correlations between the spinal reflex excitability and mechanical joint-laxity measures (total A-P displacement: rs = 0.17, P = .31; total I-E rotation: rs = 0.14, P = .41). These data indicate that perhaps a proprioceptive deficit or supraspinal mechanisms, rather than primary mechanical ankle-joint laxity, contribute to altered spinal reflex excitability of soleus in this pathologic ankle condition. We acknowledge that our experimental design did not allow us to determine which factors (altered sensory input or supraspinal alteration) induced the lower H : M ratio in participants with CAI.
Wikstrom and Brown47 suggested that a successful reorganization of the sensorimotor system after ankle sprain is the critical point when individuals develop CAI or become copers. The information from our study may support their hypothetical cascade of CAI events47 and provide insight into how copers successfully develop strategies within the sensorimotor system to manage the previous ankle injury. The small αMNs are primarily engaged in the H-reflex test.48 The soleus comprises 70% to 90% slow-twitch fibers that are mostly innervated by the small αMNs and influential in maintaining upright posture for long periods of time.33 The observed larger Hmax : Mmax ratio in copers suggests adequate recruitment of the small αMNs after an initial ankle sprain, which may help to maintain prolonged tonic contraction of the soleus. Previous investigators have shown that postural instability was associated with the ability of the CNS to control reflexive soleus muscle responses49 and that postural control was better in copers than in participants with CAI.8 It is possible that, after the ankle injury, copers restore the ability to reflexively recruit αMNs. Unfortunately, we did not collect information regarding what forms or length of rehabilitation, if any, participants completed after an initial ankle sprain, a factor that should be considered by future researchers.
Soleus spinal reflex excitability in copers more closely resembled that of control participants than of participants with CAI, which may be an important reflection of how CAI patients and copers differ. Our findings suggest that clinicians treating and rehabilitating patients with CAI should consider the manipulation of spinal reflex excitability. Because we observed a larger Hmax : Mmax ratio in copers than in participants with CAI and the self-reported feeling of ankle instability was more closely associated with decreased spinal reflex excitability of the soleus than with mechanical joint laxity, therapeutic interventions that can increase the H-reflex in the soleus could improve the management of CAI. Lower-intensity transcutaneous electrical stimulation, joint manipulations, and reflex conditioning protocols may be effective in increasing the Hmax : Mmax ratio in the soleus muscle.50−52
Although our participants with CAI demonstrated decreased spinal reflex excitability of the soleus, Klykken et al31 found an increased Hmax : Mmax ratio in the soleus after an acute ankle sprain. Differences in soleus spinal reflex excitability outcomes between that study and our investigation may be due to variations in supraspinal responses to injury in individuals with ankle injuries. Some evidence indicates that joint mechanoreceptors stimulated by joint effusion may influence the spinal reflex excitability of the lower extremity muscles, but Klykken et al31 reported weak associations between swelling and spinal reflex excitability of soleus in people with acute ankle sprains. The researchers speculated that increased spinal reflex excitability of the soleus might result from a supraspinal mechanism that is influential in modulating spinal reflex excitability aimed at positioning the injured ankle joint in a loose-packed position to increase comfort after injury. In our study, it is possible that the decreased soleus spinal reflex excitability in people with CAI may indicate a supraspinal modulation within the CNS to prevent ankle supination. Central changes in sensorimotor control have been associated with unilateral CAI,10,11,53−56 including bilateral alterations in postural control, knee kinematics, gait termination, and hip muscle-activation patterns.
A true neurophysiologic mechanism behind the differences between outcomes in spinal reflex excitability of the soleus is still uncertain, yet the current results combined with the findings of Klykken et al31 indicate that spinal reflex excitability of the soleus may be an important factor contributing to CAI. A transition point from increased to decreased soleus spinal reflex excitability that occurs within patients with CAI may not occur in copers. It is unknown how many of the participants in the Klykken et al31 study became copers, and increased spinal reflex excitability of the soleus in the injured leg could be a part of the recovery process. Authors of future prospective studies should explore the time course of the spinal reflex excitability changes after an initial lateral ankle sprain and the response to interventions during the rehabilitation process.
Although earlier researchers reported greater subtalar joint-complex laxity57 and greater elongation of the anterior talofibular ligament of CAI patients compared with healthy controls,16 we did not observe differences in mechanical joint laxity among the 3 groups and the associated effect sizes were small. Although the reason for these inconsistent findings is unclear, taken together, all cases of CAI cannot be explained only by the amount of mechanical ankle laxity.15 Our results contribute to that argument and add information to suggest that mechanical laxity may also not be a differentiating factor between CAI and coper groups. Laxity may be present in some subsets of participants with ankle instability, but caution is needed in citing this characteristic as an absolute explanatory factor for CAI. We did not measure joint hypomobility and arthrokinematic alterations, which are considered to be among the possible mechanical characteristics of CAI.58 Therefore, it remains unknown whether the presence of joint hypomobility and arthrokinematic alterations could compensate for ligamentous laxity.
One important limitation to our study was its retrospective design, making it difficult to determine if these changes were due to the condition or if the differences existed before injury. Future investigators should attempt to identify short-term and long-term spinal reflex excitability changes immediately after an ankle sprain and how these may influence the development of CAI. Recently, Hiller et al6 updated the original Hertel paradigm of CAI58 and classified CAI into 7 subgroups, including mechanical ankle instability, perceived instability, and recurrent ankle sprains. Mechanical instability was not present in the CAI group in our study; however, patients with CAI who demonstrate all 3 clinical conditions (perceived instability, recurrent ankle sprains, and mechanical instability) may have different characteristics compared with those who have perceived instability or recurrent ankle sprains (or both), copers, and healthy controls. Therefore, mechanical instability remains an important factor to be understood in the CAI paradigm, and further exploration of the mechanical and sensorimotor characteristics associated with different subgroups of participants with CAI is needed to increase our understanding of this ankle injury.
Furthermore, we did not control for the length of time since the initial lateral ankle sprain in the CAI and coper groups. It is possible that copers adopted neuromuscular strategies as more time passed after an initial ankle sprain than in participants with CAI. We did collect subjective information regarding the length of time since the most recent acute lateral ankle sprain to confirm that no participants acutely sprained an ankle in the 3 months before the study. Months since a recent lateral ankle sprain occurred did not differ between groups (Table 1). Although we know that the most recent lateral ankle sprain was not the initial ankle sprain for all CAI and coper participants, unfortunately, we did not document the time of the initial ankle sprain. Future investigation is needed to consider the length of time since initial injury and explore its effect on the characteristics of CAI and copers. Another limitation of this study was that our measurements of spinal reflex excitability were taken in the seated position. It has been suggested40,59 that comparisons between resting H-reflex data and standing H-reflex data are not compatible. Future authors should consider how to best apply these testing techniques for comparison with functional movements, which more closely relate to injurious mechanisms to the ankle.
CONCLUSIONS
Participants with CAI exhibited decreased spinal reflex excitability of the soleus compared with coper and healthy control participants; however, no differences existed between the copers and controls. Changes in the sensorimotor system that likely affect perceived stability at the ankle joint were seen only in the CAI group, yet no mechanical differences were noted among any of the groups. The reduced spinal reflex excitability of the soleus associated with CAI may be a target for clinical interventions. These findings support the importance of finding effective ways to increase spinal reflex excitability for the purpose of treating neural excitability dysfunction in patients with CAI. Further investigation is needed to determine if diminished spinal reflex excitability of the soleus predicts those who will go on to develop CAI after an ankle sprain.
ACKNOWLEDGMENTS
This study was funded by the National Athletic Trainers' Association Research & Education Foundation (Dallas, TX) through the 2013 Malacrea Master's Grant Program.
REFERENCES
- 1. Fong D, Hong Y, Chan L, Yung P, Chan K. A systematic review of ankle injury and ankle sprain in sports. Sports Med. 2007; 37 1: 73– 94. [DOI] [PubMed] [Google Scholar]
- 2. Swenson DM, Yard EE, Fields SK, Comstock RD. Patterns of recurrent injuries among US high school athletes, 2005−2008. Am J Sports Med. 2009; 37 8: 1586– 1593. [DOI] [PubMed] [Google Scholar]
- 3. Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med. 2005; 39 3: E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanen L, Docherty CL, Van Der Pol B, Simon J, Schrader J. Prevalence of chronic ankle instability in high school and division I athletes. Foot Ankle Spec. 2014; 7 1: 37– 44. [DOI] [PubMed] [Google Scholar]
- 5. Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports. 2002; 12 3: 129– 135. [DOI] [PubMed] [Google Scholar]
- 6. Hiller CE, Kilbreath SL, Refshauge KM. Chronic ankle instability: evolution of the model. J Athl Train. 2011; 46 2: 133– 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wikstrom EA, Tillman MD, Chmielewski TL, Cauraugh JH, Naugle KE, Borsa P. Discriminating between copers and people with chronic ankle instability. J Athl Train. 2012; 47 2: 136– 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wikstrom EA, Fournier KA, McKeon PO. Postural control differs between those with and without chronic ankle instability. Gait Posture. 2010; 32 1: 82– 86. [DOI] [PubMed] [Google Scholar]
- 9. Verhagen E, Bay K. Optimising ankle sprain prevention: a critical review and practical appraisal of the literature. Br J Sports Med. 2010; 44 15: 1082– 1088. [DOI] [PubMed] [Google Scholar]
- 10. Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. 2010; 38 4: 829– 834. [DOI] [PubMed] [Google Scholar]
- 11. Pietrosimone BG, Gribble PA. Chronic ankle instability and corticomotor excitability of the fibularis longus muscle. J Athl Train. 2012; 47 6: 621– 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wikstrom EA, Tillman MD, Chmielewski TL, Cauraugh JH, Naugle KE, Borsa PA. Dynamic postural control but not mechanical stability differs among those with and without chronic ankle instability. Scand J Med Sci Sports. 2010; 20 1: E137– E144. [DOI] [PubMed] [Google Scholar]
- 13. Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008; 27 3: 353– 370. [DOI] [PubMed] [Google Scholar]
- 14. Brown C, Bowser B, Simpson KJ. Movement variability during single leg jump landings in individuals with and without chronic ankle instability. Clin Biomech (Bristol, Avon). 2012; 27 1: 52– 63. [DOI] [PubMed] [Google Scholar]
- 15. Gribble PA, Delahunt E, Bleakley CM, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Athl Train. 2014; 49 1: 121– 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croy T, Saliba SA, Saliba E, Anderson MW, Hertel J. Differences in lateral ankle laxity measured via stress ultrasonography in individuals with chronic ankle instability, ankle sprain copers, and healthy individuals. J Orthop Sports Phys Ther. 2012; 42 7: 593– 600. [DOI] [PubMed] [Google Scholar]
- 17. Palmieri-Smith RM, Hopkins JT, Brown TN. Peroneal activation deficits in persons with functional ankle instability. Am J Sports Med. 2009; 37 5: 982– 988. [DOI] [PubMed] [Google Scholar]
- 18. McVey ED, Palmieri RM, Docherty CL, Zinder SM, Ingersoll CD. Arthrogenic muscle inhibition in the leg muscles of subjects exhibiting functional ankle instability. Foot Ankle Int. 2005; 26 12: 1055– 1061. [DOI] [PubMed] [Google Scholar]
- 19. Palmieri RM, Ingersoll CD, Hoffman MA, et al. Arthrogenic muscle response to a simulated ankle joint effusion. Br J Sports Med. 2004; 38 1: 26– 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000; 9 2: 135– 159. [Google Scholar]
- 21. Arnold BL, Docherty CL. Low-load eversion force sense, self-reported ankle instability, and frequency of giving way. J Athl Train. 2006; 41 3: 233– 238. [PMC free article] [PubMed] [Google Scholar]
- 22. Docherty CL, Arnold BL, Hurwitz S. Contralateral force sense deficits are related to the presence of functional ankle instability. J Orthop Res. 2006; 24 7: 1412– 1419. [DOI] [PubMed] [Google Scholar]
- 23. Hoch MC, McKeon PO, Andreatta RD. Plantar vibrotactile detection deficits in adults with chronic ankle instability. Med Sci Sports Exerc. 2012; 44 4: 666– 672. [DOI] [PubMed] [Google Scholar]
- 24. Powell MR, Powden CJ, Houston MN, Hoch MC. Plantar cutaneous sensitivity and balance in individuals with and without chronic ankle instability. Clin J Sport Med. 2014; 24 6: 490– 496. [DOI] [PubMed] [Google Scholar]
- 25. Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the ankle instability instrument. J Athl Train. 2006; 41 2: 154– 158. [PMC free article] [PubMed] [Google Scholar]
- 26. Simon J, Donahue M, Docherty C. Development of the Identification of Functional Ankle Instability (IdFAI). Foot Ankle Int. 2012; 33 9: 755– 763. [DOI] [PubMed] [Google Scholar]
- 27. Martin RL, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM). Foot Ankle Int. 2005; 26 11: 968– 983. [DOI] [PubMed] [Google Scholar]
- 28. Walton C, Kalmar J, Cafarelli E. Caffeine increases spinal excitability in humans. Muscle Nerve. 2003; 28 3: 359– 364. [DOI] [PubMed] [Google Scholar]
- 29. Hubbard TJ, Kramer LC, Denegar CR, Hertel J. Correlations among multiple measures of functional and mechanical instability in subjects with chronic ankle instability. J Athl Train. 2007; 42 3: 361– 366. [PMC free article] [PubMed] [Google Scholar]
- 30. Palmieri RM, Hoffman MA, Ingersoll CD. Intersession reliability for H-reflex measurements arising from the soleus, peroneal, and tibialis anterior musculature. Int J Neurosci. 2002; 112 7: 841– 850. [DOI] [PubMed] [Google Scholar]
- 31. Klykken LW, Pietrosimone BG, Kim KM, Ingersoll CD, Hertel J. Motor-neuron pool excitability of the lower leg muscles after acute lateral ankle sprain. J Athl Train. 2011; 46 3: 263– 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lakie M, Caplan N, Loram ID. Human balancing of an inverted pendulum with a compliant linkage: neural control by anticipatory intermittent bias. J Physiol. 2003; 551 pt 1: 357– 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tucker KJ, Tuncer M, Turker KS. A review of the H-reflex and M-wave in the human triceps surae. Hum Mov Sci. 2005; 24 5−6: 667– 688. [DOI] [PubMed] [Google Scholar]
- 34. Crone C, Nielsen J. Methodological implications of the post activation depression of the soleus H-reflex in man. Exp Brain Res. 1989; 78 1: 28– 32. [DOI] [PubMed] [Google Scholar]
- 35. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 36. Ericksen H, Gribble PA. Sex differences, hormone fluctuations, ankle stability, and dynamic postural control. J Athl Train. 2012; 47 2: 143– 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu K, Gustavsen G, Kaminski TW. Increased frequency of ankle sprain does not lead to an increase in ligament laxity. Clin J Sport Med. 2013; 23 6: 483– 487. [DOI] [PubMed] [Google Scholar]
- 38. Needle AR, Palmer JA, Kesar TM, Binder-Macleod SA, Swanik CB. Brain regulation of muscle tone in healthy and functionally unstable ankles. J Sport Rehabil. 2013; 22 3: 202– 211. [DOI] [PubMed] [Google Scholar]
- 39. Needle AR, Charles BBS, Farquhar WB, Thomas SJ, Rose WC, Kaminski TW. Muscle spindle traffic in functionally unstable ankles during ligamentous stress. J Athl Train. 2013; 48 2: 192– 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stein RB, Thompson AK. Muscle reflexes in motion: how, what, and why? Exerc Sport Sci Rev. 2006; 34 4: 145– 153. [DOI] [PubMed] [Google Scholar]
- 41. Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002; 37 1: 71– 79. [PMC free article] [PubMed] [Google Scholar]
- 42. McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord. 2008; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sefton JM, Hicks-Little CA, Hubbard TJ, et al. Sensorimotor function as a predictor of chronic ankle instability. Clin Biomech (Bristol, Avon). 2009; 24 5: 451– 458. [DOI] [PubMed] [Google Scholar]
- 44. Terada M, Bowker S, Thomas AC, et al. Alterations in stride-to-stride variability during walking in individuals with chronic ankle instability. Hum Mov Sci. 2015; 40: 154– 162. [DOI] [PubMed] [Google Scholar]
- 45. Palmieri RM, Weltman A, Edwards JE, et al. Pre-synaptic modulation of quadriceps arthrogenic muscle inhibition. Knee Surg Sports Traumatol Arthrosc. 2005; 13 5: 370– 376. [DOI] [PubMed] [Google Scholar]
- 46. Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. Semin Arthritis Rheum. 2010; 40 3: 250– 266. [DOI] [PubMed] [Google Scholar]
- 47. Wikstrom EA, Brown CN. Minimum reporting standards for copers in chronic ankle instability research. Sports Med. 2014; 44 2: 251– 268. [DOI] [PubMed] [Google Scholar]
- 48. Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Interest and limits. Neurophysiol Clin. 2000; 30 2: 67– 80. [DOI] [PubMed] [Google Scholar]
- 49. Kim KM, Hart JM, Hertel J. Influence of body position on fibularis longus and soleus Hoffmann reflexes. Gait Posture. 2013; 37 1: 138– 140. [DOI] [PubMed] [Google Scholar]
- 50. Delwaide PJ, Crenna P, Fleron MH. Cutaneous nerve stimulation and motoneuronal excitability: I, soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiatry. 1981; 44 8: 699– 707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grindstaff TL, Beazell JR, Sauer LD, Magrum EM, Ingersoll CD, Hertel J. Immediate effects of a tibiofibular joint manipulation on lower extremity H-reflex measurements in individuals with chronic ankle instability. J Electromyogr Kinesiol. 2011; 21 4: 652– 658. [DOI] [PubMed] [Google Scholar]
- 52. Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci. 2009; 29 18: 5784– 5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bullock-Saxton JE, Janda V, Bullock MI. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994; 15 6: 330– 334. [DOI] [PubMed] [Google Scholar]
- 54. Gribble PA, Robinson RH. Alterations in knee kinematics and dynamic stability associated with chronic ankle instability. J Athl Train. 2009; 44 4: 350– 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wikstrom EA, Hass CJ. Gait termination strategies differ between those with and without ankle instability. Clin Biomech (Bristol, Avon). 2012; 27 6: 619– 624. [DOI] [PubMed] [Google Scholar]
- 56. Evans T, Hertel J, Sebastianelli W. Bilateral deficits in postural control following lateral ankle sprain. Foot Ankle Int. 2004; 25 11: 833– 839. [DOI] [PubMed] [Google Scholar]
- 57. Hubbard TJ. Ligament laxity following inversion injury with and without chronic ankle instability. Foot Ankle Int. 2008; 29 3: 305– 311. [DOI] [PubMed] [Google Scholar]
- 58. Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002; 37 4: 364– 375. [PMC free article] [PubMed] [Google Scholar]
- 59. Knikou M. The H-reflex as a probe: pathways and pitfalls. J Neurosci Methods. 2008; 171 1: 1– 12. [DOI] [PubMed] [Google Scholar]