Abstract
South American seasonally dry tropical forests (SDTFs) are critically endangered, with only a small proportion of their original distribution remaining. This paper presents a 12 000 year reconstruction of climate change, fire and vegetation dynamics in the Bolivian Chiquitano SDTF, based upon pollen and charcoal analysis, to examine the resilience of this ecosystem to drought and fire. Our analysis demonstrates a complex relationship between climate, fire and floristic composition over multi-millennial time scales, and reveals that moisture variability is the dominant control upon community turnover in this ecosystem. Maximum drought during the Early Holocene, consistent with regional drought reconstructions, correlates with a period of significant fire activity between 8000 and 7000 cal yr BP which resulted in a decrease in SDTF diversity. As fire activity declined but severe regional droughts persisted through the Middle Holocene, SDTFs, including Anadenanthera and Astronium, became firmly established in the Bolivian lowlands. The trend of decreasing fire activity during the last two millennia promotes the idea among forest ecologists that SDTFs are threatened by fire. Our analysis shows that the Chiquitano seasonally dry biome has been more resilient to Holocene changes in climate and fire regime than previously assumed, but raises questions over whether this resilience will continue in the future under increased temperatures and drought coupled with a higher frequency anthropogenic fire regime.
This article is part of the themed issue ‘The interaction of fire and mankind’.
Keywords: fire, Chiquitano, seasonally dry tropical forest, climate, drought, Holocene
1. Introduction
Seasonally dry tropical forests (SDTFs) comprise over 40% of the world's tropical forested ecosystems, but they are afforded little protection, and have been extensively deforested over the past several centuries. Of the remaining SDTFs, 16% occurs in South and Southeast Asia and 50% in Latin America [1]. SDTFs are limited to regions of the tropics that experience seasonal drought, occurring where annual rainfall is less than 1600 mm with a five- to six-month dry season [2]. SDTFs require fertile soils, hence they have been heavily cleared for agriculture over the past several centuries. Occurring under a similar climate, cerrado (upland) savannas differ from SDTFs in that they are structurally open, containing abundant fire-tolerant woody species and a xerophilic herbaceous ground cover. Generally, cerrado (sensu stricto) savannas occur on less fertile soils compared with SDTFs, but the boundary dynamics between SDTFs and cerrado savannas are notoriously complex and have inspired many ecological studies to disentangle the key drivers of forest–savanna ecotones (e.g. [3,4]).
Fire, whether natural or anthropogenic in origin, has long been recognized as a key control upon fine-scale SDTF–savanna boundary dynamics over short-term ecological time frames, but the relationship between fire and SDTF–savanna dynamics under high-amplitude climate change predicted over the coming century is poorly understood and a key concern for the conservation community, given the endangered status of both these ecosystems [5]. The lack of obvious plant–fire adaptations in a number of SDTF species has led many ecologists to infer that this is not a fire-adapted biome, and that it is therefore highly vulnerable to burning [6], a cause for concern in the future, as climate change is predicted to lead to increasing temperatures and drought [7], which would no doubt lead to increased risk of fire.
(a). Fire and the Bolivian Chiquitano seasonally dry tropical forest
Our study area is the Bolivian Chiquitano SDTF—the largest intact, old-growth block of SDTF in South America [1]. This semi-deciduous to deciduous forest covers most of the calcareous rolling hills of eastern Bolivia (Chiquitanía), interspersed with patches of cerrado savanna where soils are either too nutrient-poor or too thin to support forest [8]. These upland SDTF and cerrado savannas form an abrupt boundary at the Bolivia–Brazil border with the seasonally flooded savanna wetlands of the Pantanal basin, most of which lies in Brazil, to the east (figure 1). Fires occurring in the SDTF-cerrado savanna mosaics of Chiquitanía today are primarily caused by humans clearing land for agricultural purposes. The seasonally wet and dry climate, with a June–October dry season, coupled with edaphic and hydrologic controls on vegetation composition and structure, support large wildfires during the driest years, when there is abundant dry biomass [9].
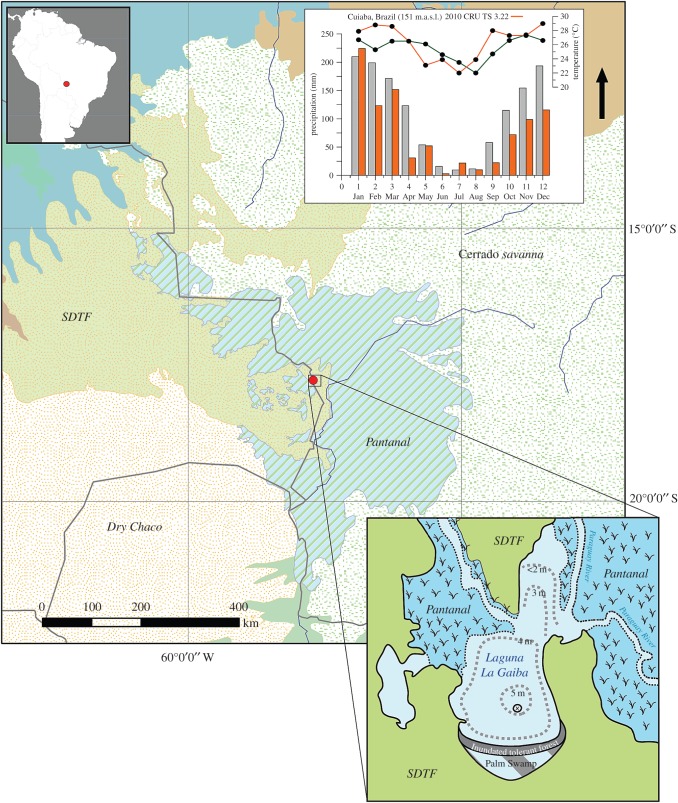
Figure 1.
Regional map of tropical South America, lower-right inset shows the location of Laguna La Gaiba (LLG) and vegetation communities around the research site, including the SDTF, cerrado savanna, Dry Chaco, inundated tolerant forest, palm swamps and the seasonally flooded Pantanal. The coring site location is marked with an ‘x’ and occurs in the deepest portion (more than 5 m) of the lake. Inset climograph from Cuiaba, Brazil, approximately 250 km northeast of Laguna La Gaiba, showing the seasonal timing and length of dry season, May to September, based on 1981–2000 climatology (grey). Red histograms and line plot show precipitation and temperature values during 2010 at LLG from gridded CRU TS 3.22 climate data.
However, a serious cause for concern among tropical ecologists and conservation biologists is that anthropogenic fires are becoming increasingly common, not only burning in savannas, but spreading into SDTF [10]. This concern is predicated on the assumption by most ecologists that, unlike savanna, SDTF is not a fire-adapted ecosystem due to the lack of fire adaptation/tolerant features of many constituent arboreal species, and, in particular, the prevalence of fire-sensitive columnar cacti (Cereus spp.) in this ecosystem [6]. However, the fact that some arboreal taxa (e.g. Astronium (Myracrodruon) urundeuva and Aspidosperma quirandy) have fire-resistant bark, raises the possibility of some degree of fire resilience within this ecosystem. The likelihood of local controlled fires escaping to become uncontrolled SDTF wildfires will only increase in the coming decades as forest flammability increases under a future warmer and drier climate [7], potentially posing a serious threat to the long-term viability of the SDTF ecosystem.
(b). Tropical climate and fire linkages
The climate of the study area—the Chiquitanía region of eastern Bolivia and the adjacent Brazilian Pantanal—experiences a highly seasonal climate whereby most of the annual precipitation falls during the austral summer (December–February; figure 1). This precipitation comes from the South American summer monsoon (SASM) and is delivered to the study area via the South American low-level jet (SALLJ) [11]. The study area has a six-month dry season and average annual precipitation between 1700 and 1000 mm, which decreases from north to south [12]. Mean annual temperature is approximately 25°C, but large changes in seasonal temperature have been recorded in the twentieth century with highs greater than 40°C during the austral summer and low temperatures below 10°C during the austral winter [10].
Modern climate–fire linkages in eastern Bolivia are influenced by both tropical Atlantic and Pacific sea surface temperatures, and moisture transport. Trade winds from the Atlantic contribute to evapotranspiration as air masses move across the Amazon basin [13]. The development of strong convection (subsidence) over Amazonia provides a link to the tropical Pacific via the east–west Walker circulation [14,15], which results in large-scale redistribution of seasonal moisture flux. The influence of climate variability on the Chiquitano SDTF is probably linked to changes in tropical Atlantic sea surface temperatures that may have a strong influence on moisture variability and fire regimes in this area [16].
Considering a more Atlantic origin for drought and fire occurrence in Chiquitanía–Pantanal may help explain changes in fire activity. Periods in the past decade when above-average convective precipitation occurs over a warm tropical North Atlantic Ocean result in subsidence to the south, over the Amazon and South Atlantic. The direct influence is a displacement of Hadley circulation and northward migration of the inter-tropical convergence zone (ITCZ), resulting in reduced precipitation across the equatorial Atlantic and, subsequently, a reduction in moisture in western and southern Amazonia [17], causing an intensified fire season [16]. For example, during the extreme drought of 2010, Chiquitanía–Pantanal experienced anomalously low precipitation and extreme fire conditions that have been linked to reduced advection of Atlantic moisture into the Amazon basin and the consequent failure of the SALLJ to deliver moisture to the study area [16] (figure 1). These recent episodes of severe drought and fire could potentially cause rapid alterations in the composition and structure of vegetation communities within the SDTF.
(c). Aims and approach
Here, we use a palaeoecological approach, based upon the analysis of pollen and charcoal from radiocarbon-dated lake sediments, to determine the long-term relationship between fire, climate and the Chiquitano SDTF over the Holocene period (the last 12 000 years). This multi-millennial time series spans intervals of time when the climate was significantly drier than present (centred on the Middle Holocene—6000 cal yr BP), thus serving as a potential analogue (albeit imperfect) of vegetation–fire–climate linkages under future increased drought predicted by most global climate models. This palaeoecological approach therefore provides the necessary long-term perspective to assess the ecological significance of SDTF responses to drought and fires observed by ecologists over recent decades.
The key aim of our study is to assess the response of the Chiquitano STDF to long-term fire activity and increased drought, and thereby test the validity of the widely held assumption that the SDTF is inherently susceptible to fire. If this hypothesis is correct, one would predict that the Bolivian Chiquitano STDF has a long-term history of low fire activity.
2. Study site and methods
The study site, Laguna La Gaiba (LLG) (−17.78°, −57.72°), is a large (> 55 km2), shallow (4–5 m deep) ‘overflow’ lake located along the course of the Paraguay River (figure 1) on the border between Bolivia and Brazil. It lies along a fault line that defines the boundary between the upland Chiquitano SDTF to the west and the seasonally flooded Pantanal wetlands to the east, which includes both savanna and treeless grasslands [18]. Seasonal rainfall over the Pantanal basin and its river catchment causes widespread floods that drain into the Paraguay River and its associated lakes. LLG is therefore closely linked hydrologically to the Pantanal basin.
This lake has yielded long-term palaeoclimate records, inferred from reconstructed lake-level changes [19–21] and pollen-based vegetation reconstructions [22,23]. These records indicate that eastern Chiquitanía and the Pantanal have undergone significant changes in hydrology, climate and biome turnover since the Last Glacial Maximum. Laguna La Gaiba is surrounded by a mosaic of plant communities, the distribution of which is dependent on local topography, edaphic conditions and hydrology. The lowest elevation areas, such as those adjacent to the northeastern boundary of LLG along the Paraguay River, support extensive flooded wetland savanna. Seasonally inundated savannas occur to the east of the main trunk of the river and support mosaics of SDTF on local areas of higher terrain, such as ancient river levees. In the immediate vicinity of LLG, the steep slopes of the Amolar Hills, rising up to 600 m above the influence of seasonal flooding, form the eastern margin of the Chiquitano SDTF [10]. Deciduousness of the SDTF increases with elevation and open-canopy scrub vegetation and cerrado savanna occur on the summit of the hills, where soils are too thin to support forest [8].
Previously published pollen abundance and richness data, together with limnological data, from LLG [21–23] are compared with a high-resolution charcoal record from the same core to enable the relationship between the Chiquitano SDTF, climate and fire, over the entire Holocene, to be explored for the first time. The LLG record extends beyond 40 000 years BP and demonstrates large-scale landscape rearrangement and catchment change in the western Pantanal at the end of the Pleistocene [21,22]. Our analysis focuses on the last 12 000 years; a period defined by landscape stability, the establishment of modern lake conditions and the spread of SDTF in eastern Chiquitanía [22,23].
The fire history reconstruction from LLG was accomplished by analysing macroscopic (more than 125 µm) sedimentary charcoal at contiguous 0.5 and 1.0 cm intervals throughout the entire sediment core [24] using the chronology of Whitney et al. [22]. Charcoal analysis for each sediment sample was completed by placing the sample in a 15 ml tube in a hot-water bath of 10% potassium hydroxide solution for 15 min and then gently washing it through a 125 µm screen. All remaining residues were examined at 36× magnification and all charcoal particles greater than 125 µm were tallied. Charcoal counts were then converted to concentration (particles cm−3) and charcoal accumulation rates (CHAR, particles cm−2 yr−1).
The CHAR record was then decomposed into two components: a low-frequency background trend and a high-frequency peaks component, referred to as fire episodes. Background charcoal (BCHAR) captures the slowly varying trends in CHAR through time as vegetation composition and structure changes, while the peaks component aids in the identification of one or more fire events [25]. At LLG, BCHAR was summarized using a locally weighted scatterplot smoothing (LOWESS) that is robust to outliers with a 300-year window width. Peak detection in the charcoal record was tested for significance using a Gaussian distribution, and only those peak values exceeding the 95th percentile were considered significant [26]. Once fire events were identified, all charcoal peaks were further screened to eliminate those peaks resulting from statistically insignificant variations or noise in CHAR [27]. Three metrics of fire activity are considered from the LLG charcoal record: CHAR or changing influx of charcoal through time; fire episode frequency, expressed as number of fires episodes or ‘peaks’ in charcoal production per 1000 years; and peak magnitude, a measure of the total amount of charcoal associated with each peak, which is probably related to the fire size, intensity and charcoal delivery to the lake.
To explore possible fire–climate–vegetation drivers at LLG through time, we obtained the relative contribution of fire and precipitation proxies in explaining temporal variation in community composition of SDTF (16 taxa), savannas (12 taxa) and riparian forests (21 taxa) by following methods similar to those proposed by Legendre et al. [28]. Because these three plant communities (SDTF, savannas and riparian forests) are distributed across distinct edaphic conditions (eutrophic, dystrophic and seasonally flooded soils, respectively), with little or no overlap in species composition, the variation partitioning analyses were performed separately for each of them. We tested the overall significance of the fire fraction (controlled for variation in moisture) and the moisture fraction (controlled for variation in fire) by applying a permutation test (999 permutations) for redundancy analysis. All proxy datasets used for the variation partitioning analyses were pre-smoothed to have the same temporal resolution, and comprise (i) macroscopic charcoal influx data (this paper), (ii) algal community change, interpreted to be controlled by precipitation-driven lake level change (first axis of principal components analysis in reference [19]), and (iii) relative pollen per cent abundance data, which have been assigned to categories reflecting each of the three main vegetation community categories around LLG [22,23] (table 1). Even though the temporal resolution applied here makes it challenging to address the role of fire and flooding on short-term ecological processes driving community turnover, recent studies have shown woody vegetation is shaped by regularly occurring floods and fires [9,29]. All variation partitioning analyses were conducted using the vegan [30] package in the R statistical environment [31].
Table 1.
Summary of the environmental proxy data used for the variation partitioning analysis, the interpretation of the proxy measurements, and sources of uncertainty in the environmental reconstructions.
| environmental variable | proxy measurement | proxy interpretation | sources of uncertainty | data references |
|---|---|---|---|---|
| fire activity | macroscopic charcoal (more than 125 µm) influx | past local fire activity is proportional to charcoal influx | errors in age model of sediment accumulation | this paper |
| vegetation | relative per cent pollen abundance | variations in pollen types reflect community change in surrounding vegetation | not all pollen types can be assigned to a single vegetation category spatial and temporal variations in relative influence of wind- versus flood-transported pollen |
Whitney et al. [22,24] |
| precipitation | Pediastrum community change | variations in proportion of algal types restricted to shallow water are controlled by lake level change; driven by regional precipitation | variation in influences of evaporation, local hydrology and precipitation on lake levels organisms respond to multiple interacting environmental variables |
Whitney & Mayle [19] |
3. Results and interpretation
Partitioning the variation explained by the environmental predictors revealed that precipitation (Pediastrum algae) and fire-related (charcoal) proxies explain 27% and 2%, respectively, of the turnover in community composition in savanna ecosystems. In addition, 2% of the turnover in savannas is a shared component between precipitation- and fire-related proxies. In the riparian forests, precipitation- and fire-related proxies explain 18% and 2%, respectively, of the turnover in community composition, while the precipitation-related proxy is the sole factor explaining turnover in SDTF composition (27%). Furthermore, 69%, 80% and 73% of the turnover in savannas, riparian forests and SDTFs, respectively, remains unexplained (figure 2).
Figure 2.
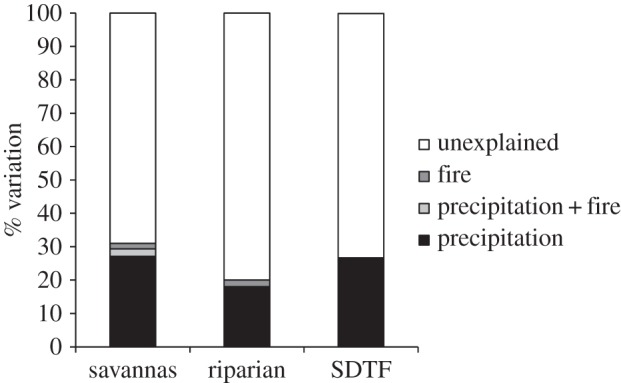
Variation partitioning by redundancy analysis to determine how much of the temporal variation in floristic composition in LLG was accounted for by the environmental variables measured (charcoal, pollen and Pediastrum proxies). Black fraction represents the precipitation component (controlled for variation in fire); light grey fraction represents the overlap between precipitation and fire components; and dark grey fraction represents the fire component (controlled for variation in precipitation). Variation partitioning among other palaeoclimate proxies (e.g. δ18O speleothem records from [32,33]) and LLG charcoal and pollen data was explored, but results were insignificant and are not shown.
Most of the turnover in community composition that we are able to explain is assigned to the precipitation-related proxy. However, the fire-related proxy or CHAR showed a weak, but still important, signature in the turnover of savannas. The clear fire adaptations of savanna plants—such as thick, corky bark; the ability to root-sprout from substantial rhizomes and protected buds—demonstrate that fire has been a key ecological force in savanna [34–36]. Because these adaptations appear to be either lacking or have received minimal research attention in present-day SDTF, ecologists have hypothesized that fire was not a strong ecological force in this biome [6]. Furthermore, even though the precipitation-related proxy explains most of the turnover in riparian communities, the fire-related proxy also showed a signature in the turnover of these communities. The ecological role of fire in SDTF, growing primarily to the west of LLG today, in the Chiquitanía region of eastern Bolivia, remains uncertain due to the paucity of suitable palaeo archives (lake and bog sediments) in this landscape. North and east of LLG are the Pantanal tropical wetlands, which are a mosaic landscape dominated by seasonally flooded savannas and riparian gallery forests, and shaped by regularly occurring floods and occasional wildfires [9,18,37].
The relative contribution of charcoal from different neighbouring ecosystems—savanna, riparian communities and SDTF—to the sediments of LLG is difficult to establish. However, because the preponderance of pollen deposited in LLG originates from upland Chiquitano SDTF to the west of LLG [22,23], it is likely that most of the charcoal entering this lake similarly originates from the Chiquitano SDTF, rather than the Pantanal wetlands to the east. Furthermore, the lack of correlation between sediment particle size and charcoal abundance supports our inference that most of the charcoal entering LLG is aeolian in origin, rather than entering the lake via erosion from the surrounding slopes or pulses of fluvial input from the Pantanal wetlands. It seems likely that, under extreme drought conditions of the Middle Holocene, fires occurring in the savanna and riparian communities of the Pantanal would have penetrated into the eastern Chiquitano SDTF.
While the environmental component (precipitation and fire) is relatively straightforward to interpret, there remains a large fraction of turnover in community composition that is unexplained (69%, 80% and 73% of the turnover in savannas, riparian forests and SDTFs, respectively) in the LLG record. There are many factors which are potentially important to determining the community composition of assemblages that we have not adequately accounted for, such as: (i) ecological drift (cf. [38]) driving stochastic rearrangements of species distribution ranges through time; (ii) biotic processes that were not measurable (e.g. competition, natural enemies); and (iii) environmental factors that were not measured and uncertainties inherent to the proxy measurements (table 1). Despite these factors, such a high proportion of unexplained variation, ranging from about 33 to 75% (e.g. [39–41]), is a common outcome in studies of floristic turnover.
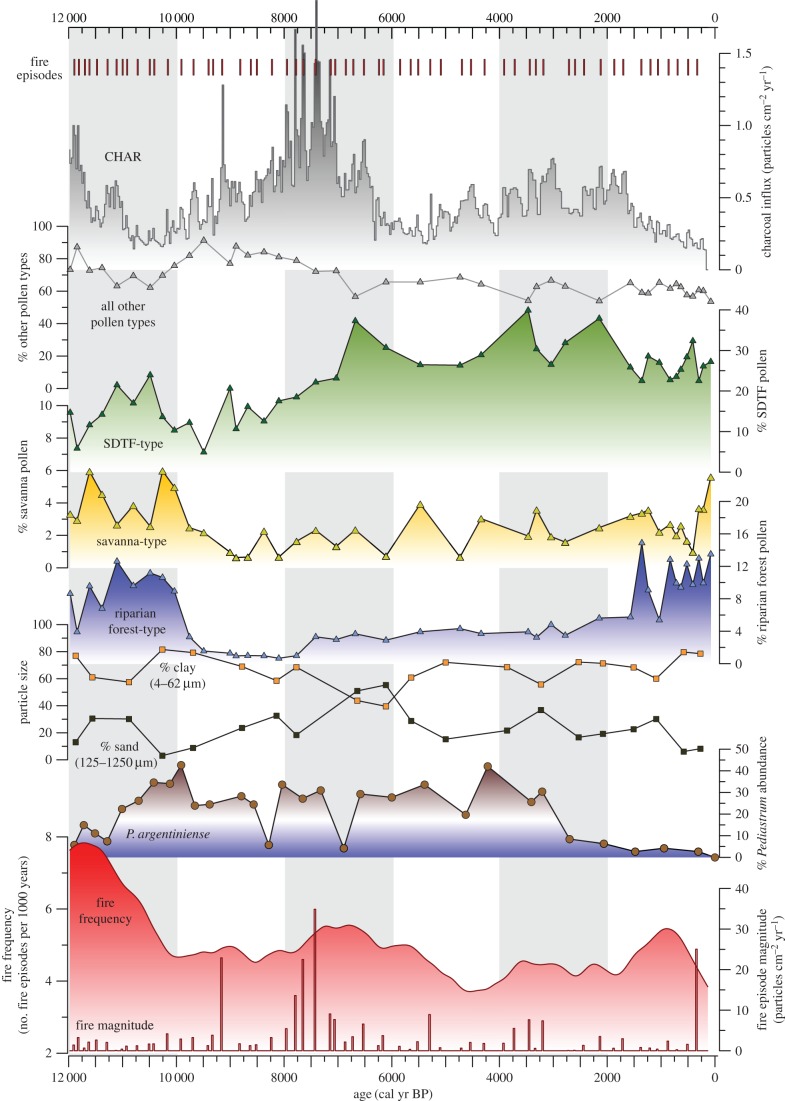
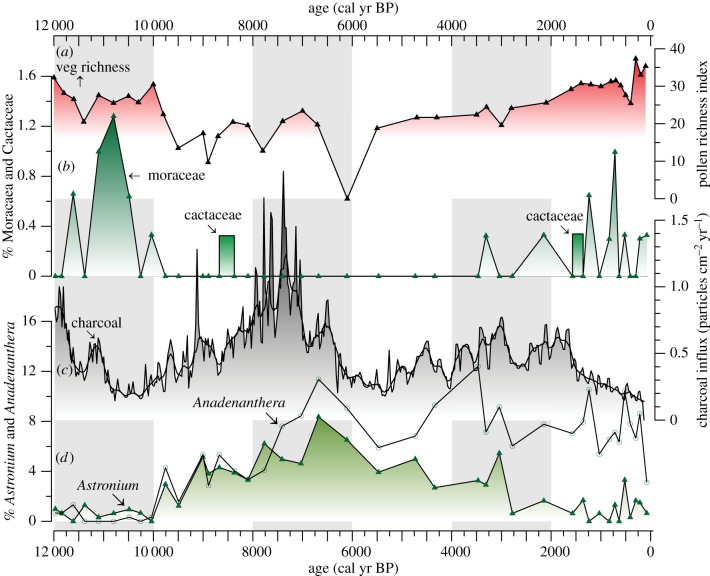
The partitioning analysis shows that drought influences SDTF community composition and our pollen data show that closed-canopy forest remained throughout the Holocene with no evidence of increased savanna-type vegetation until recently (figure 3), which would be expected if the forest was replaced by an open-structure vegetation. The period of Holocene drought, identified from high abundance of shallow water indicators (Pediastrum argentiniense algae) from 10 000 to 4400 cal yr BP [19], corresponds with compositional change shown by increases in the key dry forest taxon Astronium and a reduction in Moraceae (figure 4d,b), which is more prevalent in pollen assemblages from wetter climates [43,44]. Fire activity, or CHAR, however, does not correlate with these changes in forest composition (figure 3). Fire frequency is highest during the Holocene transition, 12 000 cal yr BP, but then low CHAR values during the Early Holocene (10 000 cal yr BP) (comparable to those of today) increase to maximum values by 7500 cal yr BP. Extreme fire activity by 7500 cal yr BP lags behind the beginning of Early Holocene drought at Botuvera Cave [32] and changes in SDTF composition at La Gaiba by approximately two millennia (figure 5b), supporting the results of our partitioning analysis that shows drought, not fire, to be the key influence on SDTF vegetation turnover. Although increasing CHAR values by 7500 cal yr BP do not significantly change the abundance of the key SDTF taxa, thus not leaving a signature in the SDTF community turnover, the increased fire pressure causes a decline in vegetation richness captured by LLG pollen richness index (figure 4a). Therefore, the combined effects of drought and fire negatively impacted plant diversity of the eastern Chiquitano SDTF as more drought-tolerant taxa (Astronium and Anadenanthera) increased in relative abundance.
Figure 3.
LLG charcoal influx (CHAR or particles cm−2 yr−1) with LOWESS showing BCHAR (black line), fire episodes (top red vertical bars) as identified by a 300 year background window and local threshold [26]. Pollen-based biome summaries [22] for SDTF-type (green), savanna-type (yellow) (which may include aquatic grasses) and riparian forest-type (blue) are presented as in Metcalfe et al. [21]. The percentage of all other pollen types not contributing to these three community types including Poaceae (averaging 46%) and Cyperaceae (averaging 16%) for the Holocene are shown in grey. Particle size analysis shows the clay (4–62 µm) and sand (125–1250 µm) fractions, Pediastrum argentiniense is shown as an indicator of shallow-water conditions [19] and fire frequency (red) is expressed as the number of fire episodes per 1000 years. Lastly, fire episode magnitude (shown at bottom as a red histogram) represents the amount of CHAR exceeding average background CHAR with each fire episode.
Figure 4.
(a) Vegetation richness, determined with rarefaction analysis of pollen data [42], (b) per cent Moraceae and Cactaceae pollen, (c) charcoal influx, and (d) per cent Anadenanthera and Astronium pollen. All pollen percentage data are based on total terrestrial pollen sum [22].
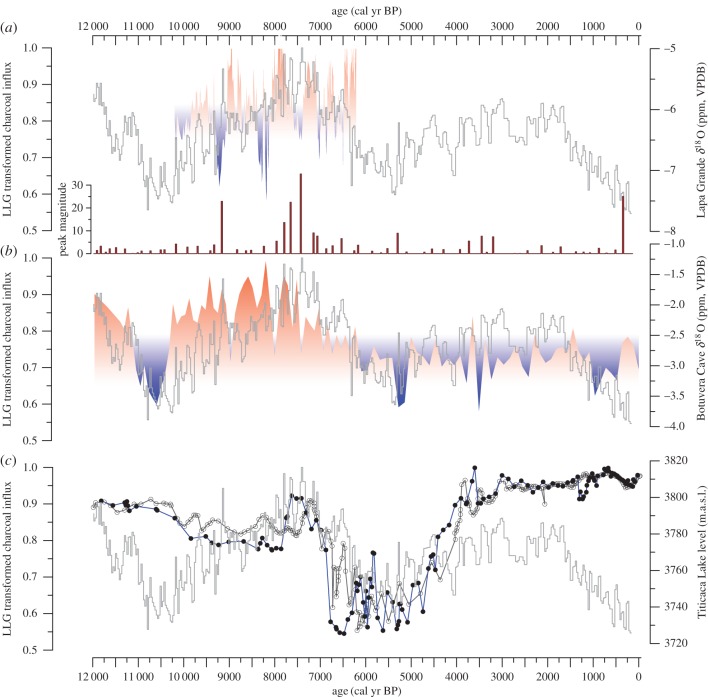
Figure 5.
LLG transformed charcoal influx using the box-cox transformation [45] is compared with three palaeo-precipitation proxies. (a) The δ18O speleothem record from Lapa Grande Cave, Brazil (–14.42º lat, –44.36º lon) [33], (b) the δ18O speleothem record from Botuvera Cave, Brazil (–27.22º lat, –49.16º lon) [32], and (c) lake level reconstruction from two sediment cores: C90 m and C150 m, from Lake Titicaca. δ13C records were used from Lake Titicaca sediments to develop transfer functions to infer past changes in lake level [46]. The charcoal influx curve is plotted in grey.
The floristic composition of the SDTF subsequently altered towards a more moisture-dependent forest community in the Late Holocene (e.g. expansion of Moraceae) as precipitation increased once more, resembling the modern forest composition by 3000 cal yr BP. This compositional change was driven by rising precipitation, beginning 4400 cal yr BP at La Gaiba [19], and corroborated by a number of lake records across the region [46,47]. Floristic changes lagged behind the reduction in fire activity by several millennia. There were no discernible variations in the pollen record concomitant with lower fire activity after 6000 cal yr BP when CHAR sharply declined (figures 3 and 4c). Again, this temporal mismatch between peak fire activity and precipitation-driven compositional changes confirms our partitioning analysis, which indicates fire is important, but not the key driver of SDTF community turnover (figure 2).
4. Discussion
(a). Influences of past fire and climate on seasonally dry tropical forest
The charcoal record from La Gaiba indicates that fire was a persistent feature in eastern Chiquitano SDTF, with periods of intensive fire activity during extended droughts. Despite the clear role of drought and fire in the development of the Chiquitano SDTF over the Holocene, there is a complex relationship at play among precipitation, floristic composition and fire at the boundary with the Pantanal. Lower precipitation, and hence reduced flooding compared with present, in the Early to Middle Holocene would have resulted in greater coverage of ignitable, dry savanna areas in the Pantanal, because the seasonal inundation of the basin and length of flood season is highly dependent on the strength of the SASM delivered to the region through the SALLJ. Palaeo-precipitation reconstructions from speleothem δ18O records approximately 1500 km southeast of LLG (downstream in the path of the SALLJ) capture the centennial-to-millennial scale droughts during the Early to Middle Holocene (figure 5a,b) that have been linked to changes in the strength of the SASM [32,33]. Modern precipitation-hydrological studies have demonstrated a strong link between annual rainfall over the Pantanal and the extent and length of flooding over the last century [12]. Furthermore, historical periods of extended drought across the Pantanal reduced seasonally inundated landscapes and vegetation types [12].
Drying across the Pantanal basin in the Early to Middle Holocene is demonstrated by the proxy data from La Gaiba [19,20], but also corroborated by several records influenced by the path of the SALLJ. These include Lake Titicaca, which progressively decreased in lake level beginning approximately 7500 cal yr BP [46], and the Lapa Grande Cave record [33], which reports several large-amplitude dry-climate anomalies, persisting for several centuries, centred at 7800 and 7400 cal yr BP (figure 5a). Similar century-long droughts occur at Botuvera Cave, located at 27°S, near the exit of the SALLJ, demonstrating the regional impact of drought conditions during the Early Holocene (figure 5b). Although stable oxygen isotope profiles differ at times in the Holocene from the LLG CHAR record, there are potential linkages among these proxies at multi-centennial and millennial scales. For example, centuries of reduced flooding in the Pantanal during extreme drought centred at 7800 cal yr BP would have limited inundation in savannas, creating optimal conditions for frequent fire ignition in these highly flammable grass-dominated systems. Our partitioning analysis supports this interpretation, as fire and drought are both shown to contribute to a considerable fraction of the variation in savanna community (figure 2).
This higher fire activity raging across the Pantanal basin during the Middle Holocene period of intense drought probably penetrated the SDTF of the eastern Chiquitano region, spreading through the undergrowth, as pollen representative of key SDTF understorey taxa, such as Clavija and Sapium, are removed from the rich diversity of taxa [23]. Interestingly, pollen of Cereus columnar cactus, one of the key SDTF taxa used to argue for the fire intolerance of SDTF [5], is present during periods of higher fire activity around 8500 and again at 1500 cal yr BP (figure 4b,c). Its presence may reflect a high degree of spatial variability and/or decreased fire frequency in the eastern Chiquitano SDTF during the Early Holocene and again in the Late Holocene. Its survival in fire-influenced SDTF may reflect its dispersal capability across the catchment, possibly favouring rocky areas with little ignitable ground cover, allowing small populations to persist (today Cereus spp. are common components of the forests around La Gaiba). Despite the inferred changes to the plant diversity and potential impacts of fire on the understorey composition as well as on the low-abundance taxa of the eastern Chiquitano SDTF during the Middle Holocene (e.g. temporary exclusion of Tabebuia and Phyllostylon), the dominant tree taxa of the dry forest (e.g. Astronium and Anadenanthera) were not replaced throughout the period of intensive fire activity (figure 4d), implying that SDTF is at least partially resistant to the fires originating in the Pantanal.
(b) Fire and conservation in seasonally dry tropical forest
Fire has been a persistent feature in the eastern Chiquitano SDTF, thus refuting our hypothesis that fire has played no role in shaping the diversity patterns in the Chiquitano SDTF (e.g. [6]). The recent decline in fire activity during the last two millennia might have fuelled the perception that fires have had a limited role in the evolution of SDTF. Critically, the floristic composition and diversity observed today in the eastern Chiquitano SDTF is a direct result of the palaeoclimate history, which in turn, had a strong influence on its history of varying fire activity. As suggested by Colinvaux [48], the high species richness across Amazonia and adjacent regions and is probably driven by the extensive land area, a wide range of habitats, as well as intermediate levels of natural disturbance, including both floods and fires.
Recent intense drought events have contributed to reductions in rainfall and soil moisture and increasing air temperatures, such as during the widespread droughts and fires of 2005 and 2010 [16,49]. Future management of fire–vegetation dynamics in the SDTF requires knowledge of natural variability on short and long time scales and consideration of how those processes link climate change to impacts on biological diversity (e.g. [50]). The maintenance of closed-canopy SDTF during periods of increased fire activity during the Early Holocene may serve as an ecological analogue for future conditions, as drought intensity and fire frequency are expected to continue increasing [7]. The Chiquitano SDTF, and neighbouring dry-forest corridor [51], however, may not be representative of all SDTF systems, as they have a very different flora and history compared to the endemic-rich, isolated SDTFs of the inter-Andean valleys. These unique and isolated SDTFs of the inter-Andean valleys have experienced an evolutionary history separate to that of lowland SDTFs, having been separated for ca 10 Myr [52]. Andean SDTFs may very probably show a sensitivity to fire that is not demonstrated by the lower-diversity Chiquitano forest biome, which have shown resilience and adaptability in occupying new regions through rapid post-glacial migration [23,53]. Regardless of whether dry forests of inter-Andean valleys would be able to maintain a closed-canopy structure in the event of high fire activity, they contain some of the highest concentrations of endemics in the world [54], and the negative impact of fires on plant richness could have a devastating effect on this biodiversity hotspot.
Our palaeoecological data show that the eastern Chiquitano SDTF withstood periods of intense droughts combined with increased fire activity during the Holocene (challenging the widely held view of susceptibility of SDTFs to fire), but the combined effects of these pressures will be exacerbated in the future, with more frequent fires of anthropogenic origin, in addition to the projected increase in drought frequency/severity and warming in the region [7]. Not only is the Chiquitano SDTF under increasing agricultural pressure, but the neighbouring Pantanal savannas have been experiencing intensifying cattle ranching activities, which include heavy use of fire and invasion of fire-tolerant grass species [10]. The Chiquitano SDTF could very probably get caught in a ‘squeeze’ from escaped fires from both systems, which alongside increasing fragmentation and drought, could cause irreversible changes to the vegetation, opening the canopy and allowing for elevated fire frequency to persist in the system. The future of SDTF ecosystems depends partly on their inherent ability to survive fire impacts.
Acknowledgements
This paper was developed through presentations and discussions emerging from the PAGES-sponsored 2013-14 La ACER workshops and the 2015 Royal Society meeting ‘The Interaction of Fire and Humankind’. We want to thank Francisco Cruz for sharing data and knowledge and two anonymous reviewers who provided many thoughtful and helpful comments greatly improving this manuscript.
Authors' contributions
M.J.P. coordinated the research and drafted the original manuscript; M.J.P. and D.M.N. performed the data analyses; M.J.P., B.S.W., F.E.M. and D.M.N. co-wrote an improved version of the manuscript and contributed equally to the editing and revisions. F.E.M., B.S.W. and E.J.d.B. contributed to fieldwork. E.J.d.B. contributed to editing and revisions; K.S.M. contributed to laboratory analysis. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Fieldwork to core Laguna La Gaiba was funded by National Geographic and The Royal Society (F.M.) and the University of Amsterdam (E.J.dB.); charcoal sample preparation and counting was funded by The University of Edinburgh (K.S.M.) and the Erasmus Programme (E.J.dB.).
References
- 1.Miles L, Newton AC, DeFries RS, Ravilous C, May I, Blyth S, Kapos V, Gordon JE. 2006. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 33, 491–505. ( 10.1111/j.1365-2699.2005.01424.x) [DOI] [Google Scholar]
- 2.Gentry AH. 1995. Diversity and floristic composition of Neotropical dry forests. In Seasonally dry tropical forests (eds Bullock SH, Mooney SA, Medina E), pp. 146–194. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Ratter JA, Pott A, Pott VJ, Cunha CN, da, Haridasan M. 1988. Observations on woody vegetation types in the Pantanal and at Corumba, Brazil. Notes R. Bot. Garden Edinb. 45, 503–525. [Google Scholar]
- 4.Bueno ML, Neves DM, Oliveira-Filho AT, Lehn CR, Ratter JA. 2013. A study in an area of transition between seasonally dry tropical forest and mesotrophic cerradão in Mato Grosso do Sul, southwestern Brazil. Edinb. J. Bot. 70, 469–486. ( 10.1017/S0960428613000164) [DOI] [Google Scholar]
- 5.Pennington RT, Lewis GP, Ratter JA. 2006. An overview of the plant diversity, biogeography and conservation of neotropical savannas and seasonally dry forests. In Neotropical savannas and seasonally dry forests: plant diversity, biogeography and conservation (eds Pennington RT, Lewis GP, Ratter JA), pp. 1–29. Boca Raton, FL: CRC Press. [Google Scholar]
- 6.Pennington RT, Lavin M, Oliveira-Filho AT. 2009. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Syst. 40, 437–457. ( 10.1146/annurev.ecolsys.110308.120327) [DOI] [Google Scholar]
- 7.IPCC. 2014. Climate Change (2014): Impacts, adaptation, and vulnerability. Part A: Global and sectoral aspects. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 8.Prance GT, Schaller GB. 1982. Preliminary study of some vegetation types of the Pantanal, Mato Grosso, Brazil. Brittonia 34, 228–251. ( 10.2307/2806383) [DOI] [Google Scholar]
- 9.de Oliveira MT, Damasceno-Júnior GA, Pott A, Paranhos Filho AC, Suarez YR, Parolin P. 2014. Regeneration of riparian forests of the Brazilian Pantanal under flood and fire influence. For. Ecol. Manag. 331, 256–263. ( 10.1016/j.foreco.2014.08.011) [DOI] [Google Scholar]
- 10.Alho CJR. 2005. The Pantanal. In The world's largest wetlands: ecology and conservation (eds Fraser LH, Keddy PA), pp. 203–264. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Zhou J, Lau K-M. 1998. Does a monsoon climate exist over South America? J. Clim. 11, 1020–1040. () [DOI] [Google Scholar]
- 12.Hamilton SK. 2002. Hydrological controls of ecological structure and function in the Pantanal wetland (Brazil). In The Ecohydrology of South American Rivers and Wetlands (ed. McClain ME.), pp. 133–158. Oxfordshire, UK: IAHS Press, Centre for Ecology and Hydrology. [Google Scholar]
- 13.Chen T-C. 1985. Global water vapor flux and maintenance during FGGE. Mon. Weather Rev. 113, 1801–1819. () [DOI] [Google Scholar]
- 14.Kousky VE, Kayano MT, Cavalcanti IFA. 1984. A review of the southern oscillation oceanic atmospheric circulation changes and related rainfall anomalies. Tellus 36A, 490–504. ( 10.1111/j.1600-0870.1984.tb00264.x) [DOI] [Google Scholar]
- 15.Walker GT. 1928. World Weather III. Mem. R. Meteorol. Soc. 2, 97–106. [Google Scholar]
- 16.Chen Y, Randerson JT, Morton DC, DeFries RS, Collatz GJ, Kasibhatla PS, Giglio L, Jin Y, Marlier ME. 2011. Forecasting fire season severity in South America using sea surface temperature anomalies. Science 334, 787–791. ( 10.1126/science.1209472) [DOI] [PubMed] [Google Scholar]
- 17.Zeng N, Yoon J-H, Marengo JA, Subramaniam A, Nobre CA, Mariotti A, Neelin JD. 2008. Causes and impacts of the 2005 Amazon drought. Environ. Res. Lett. 3, 1–9. ( 10.1088/1748-9326/3/1/014002) [DOI] [Google Scholar]
- 18.Cunha NL, Delatorre M, Rodrigues RB, Vidotto C, Gonçalves F, Dias ES, Damasceno-Júnior GA, Pott VJ, Pott A. 2012. Structure of aquatic vegetation of a large lake, western border of the Brazilian Pantanal. Braz. J. Biol. 72, 519–531. ( 10.1590/S1519-69842012000300015) [DOI] [PubMed] [Google Scholar]
- 19.Whitney BS, Mayle FE. 2012. Pediastrum species as potential indicators of lake-level change in tropical South America. J. Paleolimnol. 47, 601–615. ( 10.1007/s10933-012-9583-8) [DOI] [Google Scholar]
- 20.McGlue MM, et al. 2012. Lacustrine records of Holocene flood pulse dynamics in the upper Paraguay River watershed (Pantanal wetlands, Brazil). Quat. Res. 78, 285–294. ( 10.1016/j.yqres.2012.05.015) [DOI] [Google Scholar]
- 21.Metcalfe SE, Whitney BS, Fitzpatrick KA, Mayle FE, Loader NJ, Street-Perrott FA, Mann DG. 2014. Hydrology and climatology at Laguna La Gaiba, lowland Bolivia: complex responses to climatic forcings over the last 25 000 years. J. Quat. Sci. 29, 289–300. ( 10.1002/jqs.2702) [DOI] [Google Scholar]
- 22.Whitney BS, et al. 2011. A 45 kyr paleoclimate record from the lowland interior of tropical South America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 307, 177–192. ( 10.1016/j.palaeo.2011.05.012) [DOI] [Google Scholar]
- 23.Whitney BS, Mayle FE, Burn MJ, Guillén R, Chavez E, Pennington RT. 2014. Sensitivity of Bolivian seasonally-dry tropical forest to precipitation and temperature changes over glacial–interglacial timescales. Veg. Hist. Archaeobot. 23, 1–14. ( 10.1007/s00334-013-0395-1) [DOI] [Google Scholar]
- 24.Brown KJ, Power MJ. 2013. Charred particle analyses. In Encyclopedia of Quaternary Science, 2nd edn (ed. S Elias), pp. 716–729. Philadelphia, PA: Elsevier. [Google Scholar]
- 25.Long CJ, Whitlock C, Bartlein PJ, Millspaugh SH. 1998. A 9000-year fire history from the Oregon Coast Range, based on a high-resolution charcoal study. Can. J. For. 28, 774–787. ( 10.1139/x98-051) [DOI] [Google Scholar]
- 26.Higuera PE, Brubaker LB, Anderson PM, Hu FS, Brown TA. 2009. Vegetation mediated the impacts of postglacial climatic change on fire regimes in the south-central Brooks Range, Alaska. Ecol. Monogr. 79, 201–219. ( 10.1890/07-2019.1) [DOI] [Google Scholar]
- 27.Gavin DG, Hu FS, Lertzman K, Corbett P. 2006. Weak climatic control of stand-scale fire history during the late Holocene. Ecology 87, 1722–1732. ( 10.1890/0012-9658(2006)87%5B1722:WCCOSF%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 28.Legendre P, Borcard D, Roberts DW. 2012. Variation partitioning involving orthogonal spatial eigenfunction submodels. Ecology 93, 1234–1240. ( 10.1890/11-2028.1) [DOI] [PubMed] [Google Scholar]
- 29.Pettit NE, Naiman RJ. 2007. Fire in the riparian zone: characteristics and ecological consequences. Ecosystems 10, 673–687. ( 10.1007/s10021-007-9048-5) [DOI] [Google Scholar]
- 30.Oksanen J, et al. 2012. vegan: community ecology package. R package version 2.0-3. See http://CRAN.R-project.org/package=vegan. [Google Scholar]
- 31.R Development Core Team. 2015. R: a language and environment for statistical computing, version 3.1.0. Vienna, Austria: R Foundation for Statistical Computing. (http://www.R-project.org/) [Google Scholar]
- 32.Cruz FW, Burns SJ, Kamann I, Sharp WD, Vuille M, Cardoso AO, Ferrari JA, Dias PLS, Viana O. 2005. Insolation-driven changes in atmospheric circulation over the past 116 000 years in subtropical Brazil. Nature 434, 63–66. ( 10.1038/nature03365) [DOI] [PubMed] [Google Scholar]
- 33.Strikis NM, et al. 2011. Abrupt variation in South American monsoon rainfall during the Holocene based on a speleothem record from central-eastern Brazil. Geology 39, 1075–1078. ( 10.1130/G32098.1) [DOI] [Google Scholar]
- 34.Simon MF, Gretherc R, Queiroz LP, Skemae C, Pennington RT, Hughes CE. 2009. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc. Natl Acad. Sci. USA 106, 20 359–20 364. ( 10.1073/pnas.0903410106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann WA, Geiger EL, Gotsch SG, Rossatto DR, Silva LCR, Lau OL, Haridasan M, Franco AC. 2012. Ecological thresholds at the savanna–forest boundary: how plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 5, 759–768. ( 10.1111/j.1461-0248.2012.01789.x) [DOI] [PubMed] [Google Scholar]
- 36.Dantas VDeL, Batalha MA, Pausas JG. 2013. Fire drives functional thresholds on the savanna–forest transition. Ecology 94, 2454–2463. ( 10.1890/12-1629.1) [DOI] [PubMed] [Google Scholar]
- 37.Damasceno-Júnior GA, Semir J, Dos Santos FAM, Leitão-Filho HF. 2005. Structure, distribution of species and inundation in a riparian forest of Rio Paraguai, Pantanal, Brazil. Flora 200, 119–135. ( 10.1016/j.flora.2004.09.002) [DOI] [Google Scholar]
- 38.Hubbell SP. 2001. The unified neutral theory of biodiversity and bio geography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 39.Legendre P, Mi X, Ren H, Ma K, Yu M, Sun I, He F. 2009. Partitioning beta diversity in a subtropical broad-leaved forest of China. Ecology 90, 663–674. ( 10.1890/07-1880.1) [DOI] [PubMed] [Google Scholar]
- 40.Oliveira-Filho AT, Budke JC, Jarenkow JA, Eisenlohr PV, Neves DM. 2013. Delving into the variations in tree species composition and richness across South American subtropical Atlantic and Pampean forests. J. Plant Ecol. 6, 1–23. ( 10.1093/jpe/rtt058) [DOI] [Google Scholar]
- 41.Soininen J. 2014. A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95, 3284–3292. ( 10.1890/13-2228.1) [DOI] [Google Scholar]
- 42.Birks HJB, Line JM. 1992. The use of rarefaction analysis for estimating palynological richness from Quaternary pollen-analytical data. The Holocene 2, 1–10. ( 10.1177/095968369200200101) [DOI] [Google Scholar]
- 43.Gosling WD, Mayle FE, Tate NJ, Killeen TJ. 2005. Modern pollen-rain characteristics of tall terra firme moist evergreen forest, southern Amazonia. Quat. Res. 64, 284–297. ( 10.1016/j.yqres.2005.08.008) [DOI] [Google Scholar]
- 44.Gosling WD, Mayle FE, Tate NJ, Killeen TJ. 2009. Differentiation between Neotropical rainforest, dry forest, and savannah ecosystems by their modern pollen spectra and implications for the fossil pollen record. Rev. Palaeobot. Palynol. 153, 70–85. ( 10.1016/j.revpalbo.2008.06.007) [DOI] [Google Scholar]
- 45.Power MJ, Marlon JR, Bartlein PJ, Harrison S. 2010. Fire history and the Global Charcoal Database: a new tool for hypothesis testing and data exploration. Palaeogeogr. Palaeoclimatol. Palaeoecol. 291, 52–59. ( 10.1016/j.palaeo.2009.09.014) [DOI] [Google Scholar]
- 46.Rowe HD, Dunbar RB. 2004. Hydrologic-energy balance constraints on the Holocene lake-level history of lake Titicaca, South America. Clim. Dyn. 23, 439–454. ( 10.1007/s00382-004-0451-8) [DOI] [Google Scholar]
- 47.Baker PA, Seltzer GO, Fritz SC, Dunbar RB, Grove MJ, Tapia PM, Cross SL, Rowe HD, Broda JP. 2001. The history of South American tropical precipitation for the past 25 000 years. Science 291, 640–643. ( 10.1126/science.291.5504.640) [DOI] [PubMed] [Google Scholar]
- 48.Colinvaux P. 1987. Amazon diversity in light of the paleoecological record. Quat. Sci. Rev. 6, 93–114. ( 10.1016/0277-3791(87)90028-X) [DOI] [Google Scholar]
- 49.Marengo JA, et al. 2012. Recent developments on the South American monsoon system. Int. J. Climatol. 32, 1–21. ( 10.1002/joc.2254) [DOI] [Google Scholar]
- 50.Alho CJR, Sabino J. 2011. A conservation agenda for the Pantanal's biodiversity. Braz. J. Biol. 71, 327–335. [DOI] [PubMed] [Google Scholar]
- 51.Neves DM, Dexter KG, Pennington RT, Bueno ML, Oliveira-Filho AT. 2015. Environmental and historical controls of floristic composition across the South American Dry Diagonal. J. Biogeogr. 42, 1566–1576. ( 10.1111/jbi.12529) [DOI] [Google Scholar]
- 52.Pennington RT, Lavin M, Särkinen T, Lewis GP, Klitgaard BB, Hughes CC. 2010. Contrasting plant diversification histories within the Andean biodiversity hotspot. Proc. Natl Acad. Sci. USA 107, 13 783–13 787. ( 10.1073/pnas.1001317107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burbridge RE, Mayle FE, Killeen TJ. 2004. Fifty-thousand-year vegetation and climate history of Noel Kempff Mercado National Park, Bolivian Amazon. Quat. Res. 61, 215–230. ( 10.1016/j.yqres.2003.12.004) [DOI] [Google Scholar]
- 54.Linares-Palomino R. 2006. Phytogeography and floristics of seasonally dry forests in Peru. In Neotropical savannas and seasonally dry forests: plant diversity, biogeography and conservation (eds Pennington RT, Lewis GP, Ratter JA), pp. 257–279. Boca Raton, FL: CRC. [Google Scholar]