Abstract
Neurobiological research in genetically tractable organisms relies heavily on robust assays for behavioral phenotypes. The simple body plan of the nematode Caenorhabditis elegans makes it particularly amenable to the use of automated microscopy and image analysis to describe behavioral patterns quantitatively. This protocol provides an approach for obtaining uniform illumination during worm tracking. Good lighting can be more of an art than a science. Once the system is set up, it will be necessary to play with it, testing the results after each adjustment to ensure that the analysis software is able to clearly identify the worm and its boundaries. Although the protocol was developed for use in a single-worm tracker, it addresses factors important for the generation of reproducible, standardized images in all systems.
Materials
It is essential that you consult the appropriate Material Safety Data Sheets and your institution's Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Equipment
Imaging setup
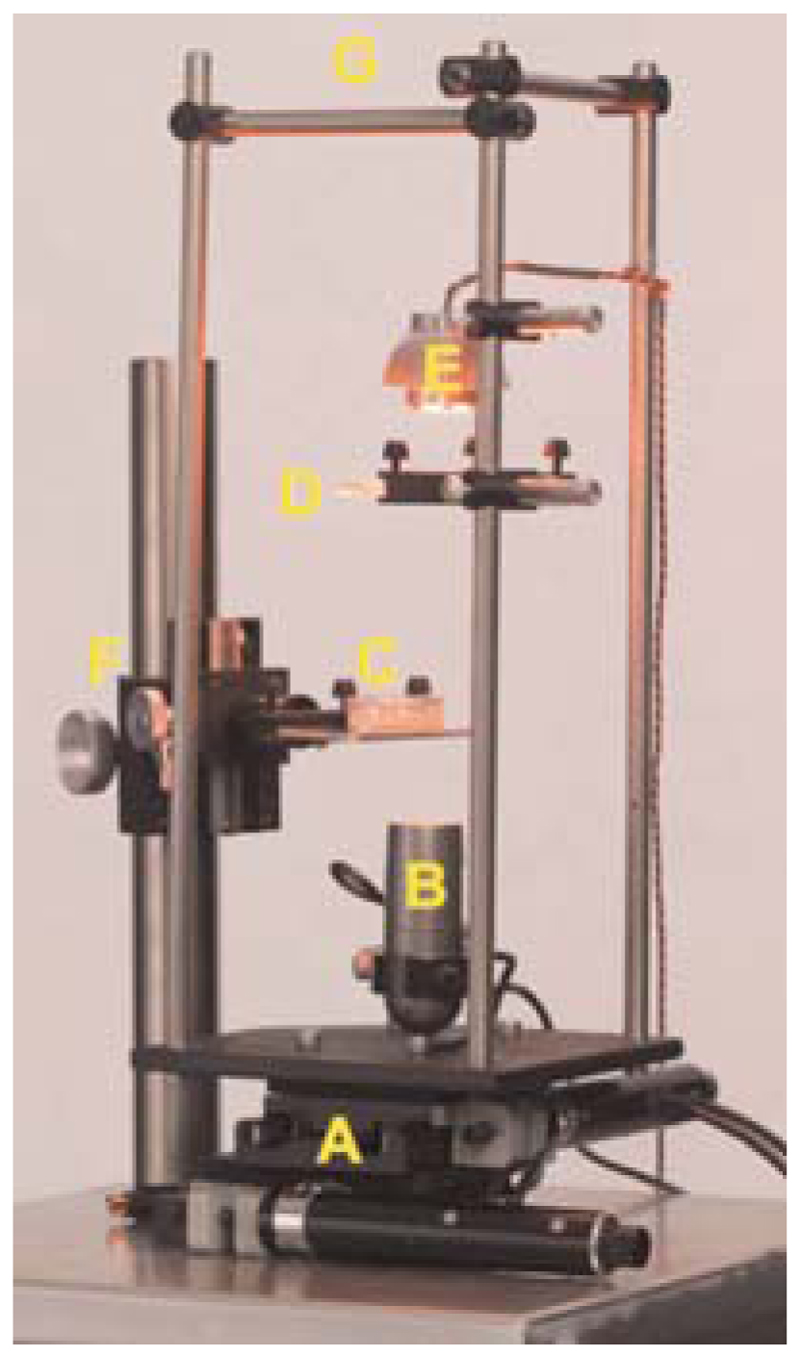
The hardware setup, including stage, camera, and light source, is shown in Figure 1. Illumination components (including light source, camera, filters) are discussed in the Method.
Figure 1.
Hardware: (A) The stage, composed of x- and y-axis actuators and linear translation stages; (B) the camera; (C) the sample, a worm on an agar Petri dish; (D) a diffuser above a collimating Fresnel lens, to provide uniform illumination; (E) a high-intensity red light-emitting diode (LED) for illumination; (F) course and fine focusing knobs to focus the sample in view of the camera; (G) a rigid cage to center the illumination above the camera and eliminate vibrations caused by moving the stage.
Method
-
1.
Choose the appropriate wavelength(s) (e.g., use the red spectrum to avoid stimulating lite-1 receptor, blue for the converse; cameleon, GCaMP, and various neuronal reporters have published constraints for their excitation and emission spectra; and channelrhodopsins and halorhodopsins have published constraints for their excitation spectra).
-
2.
Obtain your light source. Bright, single light-emitting diodes (LEDs) and arrays of LEDs are available at a variety of wavelengths, and are inexpensive. Alternatively, a selection of bulbs (e.g., halogen, mercury, xenon) exist that have high-intensity emission much of the way from ultraviolet, across visible light, and into the infrared spectrum. If you need an intense, tight spectrum unavailable through LEDs or filtered bulbs, lasers provide a great, albeit expensive, option.
-
3.
Your lighting must remain uniform despite camera movement. If your camera and light source move independently from each other, several companies make light boxes that achieve uniform light over large areas. Unfortunately, inexpensive light boxes can be weak; therefore, one needs to check that their illumination is sufficient to provide the desired video frame rates.
-
4.
Purchase appropriate filter sets to achieve tight spectral bands when necessary. Multiband excitation and emission will likely require one or more dichroic mirrors, in addition to your filter set, to differentiate between emission bands.
-
5.
Your light source intensity significantly affects the camera exposure time. Test the two in conjunction to achieve fast image captures free from motion blur.
-
6.
Good contrast means that the worm edges are significantly distinguished (much darker or much lighter) from the background. This includes the head and tail, which are often difficult to delineate from their background, as well as worm tracks, which can often be as dark as the worm itself, leading to confusion between body and background. Therefore, consider the following factors to achieve good contrast.
-
i.
The distance of the light source from the worm: Greater distances yield more directional lighting, improving edge contrast at the expense of intensity.
-
ii.
Using a tight filter grating (e.g., a laptop privacy filter) to restrict angled light: Similar to distance, the grating leads to more directional light and, therefore, better contrast; but, once again, this is at the expense of intensity.
-
iii.
Using a diffusing filter (e.g., ground or colored glass) to achieve uniform lighting within your camera’s field of view: Vignetting, caused by nonuniform lighting, can lead to image edges that are as dark as or darker than worm edges and, therefore, to confusion between the worm and its background.
-
iv.
Using a collimating lens to achieve uniform, directional light: As the previous factors show, this greatly increases worm edge contrast.
-
v.
Maintaining an unobstructed view of the worm: Among other suggestions, this can be achieved by resting the worm plate on a transparent platform and flipping it such that the worm faces the video camera directly as opposed to being filmed through the agar; note that worms on agar appear behaviorally unaffected when crawling upside down.
-
i.
Related information
A discussion of the principles of single-worm tracking, along with a protocol, can be found in Preparation of Samples for Single-Worm Tracking (Yemini et al. 2011a). A discussion of various methods for recording many worms simultaneously, including a protocol, can be found in Tracking Movement Behavior of Multiple Worms on Food (Yemini et al. 2011b).
References
- Yemini E, Kerr RA, Schafer WR. Preparation of samples for single-worm tracking. Cold Spring Harb Protoc. 2011a doi: 10.1101/pdb.prot066993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemini E, Kerr RA, Schafer WR. Tracking movement behavior of multiple worms on food. Cold Spring Harb Protoc. 2011b doi: 10.1101/pdb.prot067025. [DOI] [PMC free article] [PubMed] [Google Scholar]