Abstract
Background
The Japan Guidelines of Lung Cancer Therapy recommend epidermal growth factor receptor-tyrosine kinase inhibitors as a first-line therapy for advanced/recurrent non-small cell lung cancer patients with epidermal growth factor receptor mutation. Although survival periods in recent reports of epidermal growth factor receptor-tyrosine kinase inhibitor treatment have been getting longer, the reasons why are unclear. We investigated the survival, prognostic factors and real-world treatment of non-small cell lung cancer patients with epidermal growth factor receptor mutation in clinical practice.
Methods
Non-small cell lung cancer patients (n = 1660) who started first-line treatment from January 2008 to December 2012 were enrolled. Patients were diagnosed with epidermal growth factor receptor mutation-positive advanced/recurrent non-small cell lung cancer by histology or cytology samples. The primary objective was to estimate overall survival. The secondary objectives were to determine prognostic factors, real-world treatment patterns and efficacy of gefitinib treatment. We calculated the treatment exposure rate for each treatment category using the following formula: exposure rate = person-years for the treatment category/total person-years × 100.
Results
The median overall survival was 30.8 months. Sex, age, histology, epidermal growth factor receptor mutation type, clinical stage and performance status affected overall survival. The exposure rates for all epidermal growth factor receptor-tyrosine kinase inhibitors, gefitinib and platinum-doublet chemotherapy were 62.1, 46.4 and 8.5% respectively. Overall 56.1% of patients were administered gefitinib as first-line therapy, and 39.0% were treated with ≥2 epidermal growth factor receptor-tyrosine kinase inhibitor regimens. The median progression-free survival in the first-line gefitinib group was 11.4 months. Factors affecting prognosis were sex, histology, clinical stage and performance status.
Conclusion
Epidermal growth factor receptor-tyrosine kinase inhibitors, especially gefitinib, are major components of the treatment regimens for epidermal growth factor receptor mutation-positive non-small cell lung cancer. Switching and re-challenging with epidermal growth factor receptor-tyrosine kinase inhibitors were also practiced in Japan.
Keywords: EGFR tyrosine kinase inhibitor, gefitinib, non-small cell lung cancer, mutation
Introduction
The Japan Guidelines of Lung Cancer Therapy currently recommend epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) as a first-line therapy for EGFR mutation-positive advanced/recurrent non-small cell lung cancer (NSCLC) (1). Previously, platinum-based doublet chemotherapy was the standard of care, but it had a poor prognosis with a median overall survival (OS) of 8–10 months and the 1-year survival rate was 30–35%. After the approval of gefitinib, a selective EGFR-TKI targeting mutated EGFR, in Japan in July 2002, Takano et al. reported that the survival period was extended in patients with EGFR mutation-positive NSCLC (2). A number of recent studies have reported the extended median OS of patients with EGFR mutation-positive NSCLC treated with EGFR-TKIs: the 2013 NEJ002 study by Inoue et al. reported a median OS of 27.7 months with gefitinib (3); the 2014 WJOG3405 study by Yoshioka et al. reported a median OS of 34.8 months with gefitinib (4) and the 2014 JO22903 study by Katakami et al. reported a median OS of 36.3 months with erlotinib (5). A recent report by Kato et al. reported a median OS of 46.9 months with afatinib and 35.8 months with chemotherapy in a Japanese subset of the Lux-Lung3 study (6). Although these studies indicate that EGFR-TKIs extended the median OS of EGFR mutation-positive NSCLC patients, the impact of EGFR-TKIs in actual clinical use is unclear. Moreover, these recent clinical studies suggesting increased OS of NSCLC patients since the introduction of several EGFR-TKIs have only shown the prolongation of progression-free survival (PFS), but not OS. With this in mind, what treatments contributed to the longer OS reported in prior clinical trials? Considering the greater efficacy and large number of treatment options currently available owing to the contributed development of anti-cancer drugs, we can predict that there is a ‘best treatment regimen’ which accounts for the improvement in OS. However, no evidence for ‘best treatment regimen’ has been reported to date. Therefore, we undertook a retrospective study to determine what kind of treatments EGFR mutation-positive NSCLC patients receive in real-world clinical practice in Japan to identify which clinical factors might contribute to the longer OS duration in EGFR-TKI-treated patients.
Patients and methods
Study design and participants
This study was a multicentre, observational, retrospective study of advanced/recurrent NSCLC patients with EGFR mutation. Patients were enrolled at 17 centres in Japan. Once the patient was registered in this study, clinical information regarding each patient was retrospectively extracted from the medical chart and entered into a patient database.
Eligible patients were diagnosed with EGFR mutation-positive advanced/recurrent NSCLC by histology or cytology samples and were initiated with first-line treatment between January 2008 and December 2012. Patients treated with unauthorized drugs as of 31st December 2014 were excluded from the study.
This study was approved by the Ethics Review Board or Institutional Review Board at each participating site. The study was performed in accordance with the Declaration of Helsinki and was categorized as a study without human samples as defined by the Japanese guidelines presented by the Ministry of Health, Labour and Welfare: ‘Ethical guidelines for epidemiologic research, dated 17 June 2002’, ‘Ethical guidelines for clinical research, dated 30 July 2003’ and ‘Ethical guidelines for medical research involving human subjects, dated 22 December 2014’.
Data extraction
We extracted these following clinical data from the patients' medical charts.
Patient characteristics:
Date of NSCLC diagnosis, sex, age at the time of diagnosis, histological diagnosis, clinical staging at initial diagnosis, distant metastasis organ, Eastern Cooperative Oncology Group (ECOG) performance status (PS), smoking history, smoking exposure and type of EGFR mutation.
Survival status at the end of December 2014, date of death or date of last follow-up.
NSCLC treatments:
Treatment medication, date of the first dose, date of the last dose, PS at the start of each treatment line, best response for each treatment line, reason for discontinuation, maintenance therapy, radiotherapy, region of radiotherapy, brain metastasis and date of progressive disease [PD; defined according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 (7) or clinical PD].
Outcomes
The aim of this study was to investigate the real-world treatment regimens and survival of patients with EGFR mutation-positive advanced/recurrent NSCLC. The primary objective was to estimate OS of the patients. The secondary objectives were to determine prognostic factors, real-world treatment regimens and efficacy of gefitinib treatment.
Statistical analysis
Survival curves were drawn according to the Kaplan–Meier method. We analysed relationships between survival time and baseline factors in univariate analyses. Baseline factors were the year of diagnosis, the year treatment commenced, sex, age, histology, EGFR mutation type, clinical stage, PS, smoking history and first-line treatment category. Cox regression analysis was carried out with multiple factors. The efficacy of gefitinib as first-line treatment was also determined by Cox regression analysis.
We created a ‘treatment map’ indicating each treatment period and treatment time by color-coding each treatment category. Five categories of treatment were used: gefitinib ± α, other EGFR-TKI ± α, platinum-doublet chemotherapy ± bevacizumab (BV), other chemotherapy ± BV and other (including crizotinib, alectinib). ‘α’ meant chemotherapy or BV or both. We calculated the treatment exposure rate for each treatment category using the following formula:
We finalised this statistical analysis plan before closing the database to reduce bias that might occur because it was a retrospective study. This study is registered with ClinicalTrials.gov (NCT02475720).
Results
Patient characteristics
Overall, 1660 patients were included in the study, but 3 patients did not fulfil the eligibility criteria; therefore, 1657 patients were analysed. Of these patients, 380 were alive, 965 were dead and 312 were lost to follow-up at the end of December 2014. At the start of first-line treatment, 929 patients (56.1%) were treated with gefitinib and 728 patients (43.9%) were treated with other regimens (gefitinib + α: n = 33; other EGFR-TKI ± α: n = 98; platinum-doublet chemotherapy ± BV: n = 509; other chemotherapy ± BV: n = 86 and other treatments: n = 2).
The patient characteristics are shown in Table 1. The median number of treatment regimens was 2. Overall, 64.8% of patients were female and the median age was 67.0 years. In total, 95.2% patients had adenocarcinoma, 66.7% patients had stage IV disease, 43.8% had only one metastatic organ, 21.4% had two metastatic organs and 17.4% had ≥3 metastatic organs. With regard to EGFR mutation, 50.1% had deletion 19 (Del19) and 41.5% had L858R mutation. Other EGFR mutation types were G719X (2.4%), L861Q (1.4%), exon 21 point mutations except L858R and L861Q (2.9%), etc.
Table 1.
Patient characteristics
| All patients (%) | First-line gefitinib (%) | |
|---|---|---|
| Total number of patients | 1657 (100.0) | 929 (100) |
| Number of treatment regimens, median (range) | 2.0 (1–16) | 2.0 (1–16) |
| Year treatment commenced | ||
| 2008 | 194 (11.7) | 81 (8.7) |
| 2009 | 298 (18.0) | 170 (18.3) |
| 2010 | 361 (21.8) | 184 (19.8) |
| 2011 | 413 (24.9) | 234 (25.2) |
| 2012 | 391 (23.6) | 260 (28.0) |
| Sex | ||
| Male | 584 (35.2) | 270 (29.1) |
| Female | 1073 (64.8) | 659 (70.9) |
| Age, years median (range) | 67.0 (27–97) | 69.0 (27–97) |
| Histology | ||
| Ad | 1577 (95.2) | 898 (96.7) |
| Sq | 48 (2.9) | 19 (2.0) |
| Large | 5 (0.3) | 2 (0.2) |
| AdSq | 9 (0.5) | 5 (0.5) |
| Other | 18 (1.1) | 5 (0.5) |
| EGFR mutation type | ||
| Del 19 | 814 (49.1) | 467 (50.3) |
| L858R | 667 (40.3) | 383 (41.2) |
| Other | 146 (8.8) | 60 (6.5) |
| Del19 + L858R | 7 (0.4) | 5 (0.5) |
| Del19 + T790M | 4 (0.2) | 2 (0.2) |
| Del19 + other | 5 (0.3) | 3 (0.3) |
| L858R + T790M | 5 (0.3) | 3 (0.3) |
| L858R + other | 9 (0.5) | 6 (0.6) |
| Clinical stage | ||
| IIIB | 125 (7.5) | 44 (4.7) |
| IV | 1105 (66.7) | 600 (64.6) |
| Recurrence | 427 (25.8) | 285 (30.7) |
| Number of metastatic organs | ||
| 0 | 289 (17.4) | 154 (16.6) |
| 1 | 725 (43.8) | 394 (42.4) |
| 2 | 354 (21.4) | 214 (23.0) |
| ≥3 | 289 (17.4) | 167 (18.0) |
| Performance status | ||
| 0 | 654 (39.5) | 334 (36.0) |
| 1 | 681 (41.1) | 369 (39.7) |
| 2 | 117 (7.1) | 85 (9.1) |
| 3 | 81 (4.9) | 70 (7.5) |
| 4 | 12 (0.7) | 11 (1.2) |
| Unknown | 112 (6.8) | 60 (6.5) |
| Smokings | ||
| Smoker | 646 (39.0) | 306 (32.9) |
| Non-smoker | 981 (59.2) | 604 (65.0) |
| Unknown | 30 (1.8) | 19 (2.0) |
Ad, adenocarcinoma; Sq, squamous cell carcinoma; large, large cell carcinoma; AdSq, adenosquamous cell carcinoma.
Gefitinib was administered as the first-line treatment in 81/194 patients who started treatment in 2008 and increased to 260/391 patients in 2012. The median age of the first-line gefitinib group was 69.0 years and was significantly older than that in the other therapy group (66.0 years; P < 0.001, Wilcoxon test). The percentage of patients with a PS of ≥2 was 17.8% in the first-line gefitinib group, which was significantly greater than that in the other therapy group (6.0%; P< 0.001, Wilcoxon test).
Real-world treatments
In the top 10 treatment sequences, 420 patients were treated with gefitinib ± α alone (Supplementary data, Fig. S1). In total, 526 patients were treated only with EGFR-TKIs (including gefitinib) without platinum-doublet chemotherapy and 223 patients experienced switching from gefitinib to another EGFR-TKI in these top 10 treatment sequences.
Only 3.3% of all patients did not receive EGFR-TKI treatment and 49.8% patients were not administered platinum-doublet chemotherapy (Supplementary data, Table S1). Overall, 39.0% of patients received ≥2 EGFR-TKI regimens.
We show the ‘treatment map’ in 1594 patients whose duration of the first-line treatment was confirmed (Supplementary data, Fig. S2). We calculated the ‘exposure rate’ for each category by determining the person-years for each treatment category as a percentage of the number of person-years for all patients combined. The exposure rate was 62.1% for EGFR-TKI (46.4% for gefitinib ± α), 8.5% for platinum doublet ± BV, 8.0% for other chemotherapy ± BV and 21.4% for the untreated period (data not shown).
OS of all patients
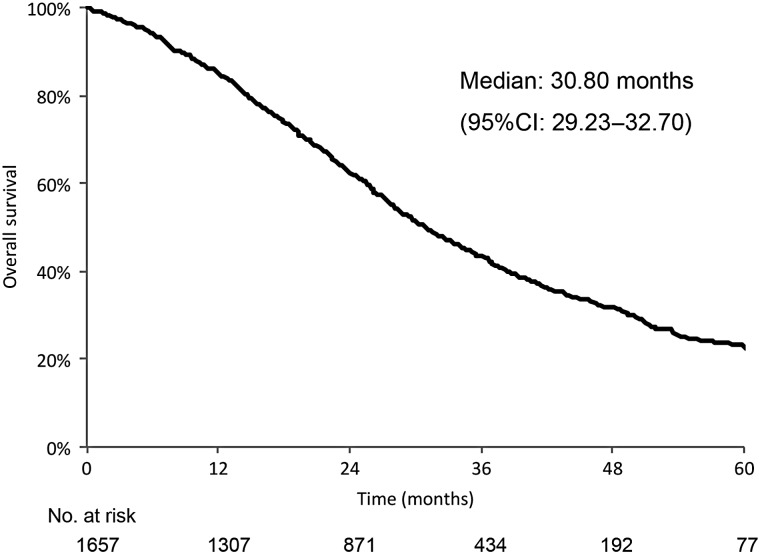
The OS curve for all patients is shown in Fig. 1. The median OS was 30.8 months. In univariate analyses, we found that OS was influenced by the following variables: year of diagnosis, sex, age, histology, EGFR mutation type, clinical stage, PS and first-line treatment category. The year treatment commenced and smoking history were not associated with OS in univariate analysis (data not shown). Cox regression analysis revealed that sex, age, histology, EGFR mutation type, clinical stage and PS were independently associated with OS (Table 2).
Figure 1.
Overall survival of all patients. CI, confidence interval.
Table 2.
Cox regression of overall survival
| N | Multivariate Step down procedure |
||
|---|---|---|---|
| HR | 95% CI | ||
| Sex | |||
| Male | 584 | Ref | – |
| Female | 1073 | 0.819 | (0.716, 0.936) |
| Age, years | |||
| <60 | 343 | Ref | – |
| 60–69 | 580 | 0.971 | (0.813, 1.161) |
| 70–79 | 521 | 1.097 | (0.915, 1.315) |
| ≥80 | 209 | 1.568 | (1.247, 1.972) |
| Unknown | 4 | 0.716 | (0.100, 5.146) |
| Histology | |||
| Ad | 1577 | Ref | – |
| Other | 80 | 2.000 | (1.512, 2.646) |
| EGFR mutation type | |||
| Del19 only | 814 | Ref | – |
| L858 only | 667 | 1.093 | (0.952, 1.254) |
| Del19 + L858R | 7 | 1.636 | (0.518, 5.159) |
| Other | 169 | 1.381 | (1.123, 1.698) |
| Clinical stage | |||
| IIIB | 125 | Ref | – |
| IV | 1105 | 1.984 | (1.535, 2.565) |
| Recurrent | 427 | 1.017 | (0.765, 1.351) |
| Performance status | |||
| 0 | 654 | Ref | – |
| 1 | 681 | 1.514 | (1.308, 1.752) |
| 2 | 117 | 2.982 | (2.326, 3.822) |
| 3 | 81 | 4.198 | (3.169, 5.561) |
| 4 | 12 | 3.179 | (1.491, 6.778) |
| Unknown | 112 | 1.020 | (0.765, 1.360) |
HR, hazard ratio; CI, confidential interval; Ad, adenocarcinoma.
Survival of patients on first-line gefitinib therapy
The PFS curve of patients on first-line gefitinib therapy is shown in Supplementary data, Fig. S3. The median PFS was 11.4 months (95% CI: 10.40–12.30, Supplementary data, Fig. S3). In univariate analyses, we found that PFS was influenced by the following factors: year of diagnosis, sex, histology, clinical stage and PS. The year treatment commenced, age and EGFR mutation type were not associated with PFS in univariate analysis (data not shown). Cox regression analysis showed that sex, histology, clinical stage and PS were independently associated with PFS (Table 3).
Table 3.
Cox regression of progression-free survival after first-line gefitinib therapy
| N | Multivariate Step down procedure |
||
|---|---|---|---|
| HR | 95% CI | ||
| Sex | |||
| Male | 269 | Ref | – |
| Female | 654 | 0.812 | (0.697, 0.946) |
| Histology | |||
| Ad | 893 | Ref | – |
| Other | 30 | 1.858 | (1.266, 2.725) |
| Clinical stage | |||
| IIIB | 44 | Ref | – |
| IV | 595 | 1.747 | (1.227, 2.486) |
| Recurrent | 284 | 0.961 | (0.664, 1.391) |
| Performance status | |||
| 0 | 334 | Ref | – |
| 1 | 365 | 1.441 | (1.221, 1.701) |
| 2 | 85 | 2.125 | (1.640, 2.754) |
| 3 | 69 | 2.388 | (1.789, 3.187) |
| 4 | 11 | 1.769 | (0.898, 3.484) |
| Unknown | 59 | 0.928 | (0.680, 1.268) |
HR, hazard ratio; CI, confidential interval; Ad, adenocarcinoma.
The median OS of patients on first-line gefitinib therapy was 28.5 months (95% CI: 26.40–31.03, Supplementary data, Fig. S3). Sex, age, histology, clinical stage and PS were independently associated with OS in Cox regression analysis (data not shown).
Discussion
This study was retrospective, and therefore allowed us to assemble a large data-set of patients with EGFR mutation-positive NSCLC in the real world without patient selection bias. Therefore, we consider the results of this study to reflect the ‘real world setting’.
The median OS was 30.8 months in this real-world cohort of EGFR mutation-positive NSCLC patients (Fig. 1), and the median OS after first-line gefitinib was 28.5 months. This is reasonable when comparing data from previous trials in which the median OS after first-line EGFR-TKI ranged from 27.7 to 46.9 months (3–6). Factors that affected OS were sex, age, histology, clinical stage and PS confirmed the findings of previous studies. Although the median OS after first-line gefitinib was shorter than the median OS for all patients combined, these included patients with poor PS, as discussed later. The median PFS after first-line gefitinib was 11.4 months and was similar to values of 9.2–10.8 months in previous studies (8,9). Factors affecting PFS in patients from previous studies also included sex, histology, clinical stage and PS. This confirms the effect of EGFR-TKI treatment for the elderly because age did not affect PFS.
The exposure rate of EGFR-TKIs was 62.1% (46.4% for gefitinib) which is similar to the value of 65.3% (46.9% for gefitinib) in a study of 335 patients treated between 2002 and 2010 (10). Exposure rate of platinum-doublet chemotherapy was showed only actual exposure because platinum-doublet chemotherapy is generally administered until four to six cycles or unacceptable toxicity and then most patients have drug holidays. On the other hand, EGFR-TKIs could be continued even after PD or worsening PS and could be administered several times by switching or re-challenge. Therefore, we could not directly compare exposure rates between EGFR-TKIs and platinum-doublet chemotherapy. This is a limitation of the present study. Our current findings indicated that more than half of the patients were treated with gefitinib and 64.0% were treated with an EGFR-TKI as first-line therapy. Platinum-doublet chemotherapy was administered to 30.7% of patients as first-line therapy (data not shown). There may be the background that first-line EGFR-TKI treatment was recommended in 2010 by the Clinical Guidelines for Lung Cancer when we see that the proportion of patients given an EGFR-TKI as a first-line treatment increased between 2008 and 2012 (Table 1). In a previous meta-analysis, first-line EGFR-TKI showed a significantly greater PFS compared with first-line platinum-doublet chemotherapy (11). In addition, according to the patient characteristics, an EGFR-TKI was more commonly used as a first-line treatment in patients with poor PS. In the PS 2, 3 or 4 patients, 72.6, 86.4 and 91.7% received gefitinib as their first-line treatment. Therefore, this suggests that EGFR-TKI is an irreplaceable therapeutic tool in these patients.
However, of note, it was common to switch between EGFR-TKIs (Supplementary data, Fig. S1). Furthermore, 39.0% of patients had multiple courses of EGFR-TKI therapy (Supplementary data, Table S1). This suggests that switching between EGFR-TKIs and re-challenge with EGFR-TKIs are common practice. Switching between EGFR-TKIs (12) and re-challenge with EGFR-TKIs (13–15) were previously reported to be effective treatment options. We will initiate a study to analyse the treatment sequence to investigate whether switching of and re-challenge with EGFR-TKIs influence the survival of patients.
The administration of EGFR-TKIs beyond disease progression is generally considered another treatment strategy that might affect prognosis. Of note, some patients may have been administered an EGFR-TKI beyond disease progression in this study. It was reported that the continuation of EGFR-TKI beyond RECIST PD was also useful for patients who initially responded to gefitinib (16–18). When disease progresses during EGFR-TKI treatment, it is thought that the tumors developed resistance to EGFR-TKIs. Numerous studies on the mechanisms underlying resistance to EGFR-TKIs have been reported, including secondary mutations in EGFR T790M (19,20), MET amplification (21,22), PTEN down regulation (23,24), CRKL amplification (25), increased expression of HGF (26), FAS-NF-κB pathway activation (27), epithelial–mesenchymal transition (28) and phenotypic conversion to small cell lung cancer (28,29).We hope that in the near future, new agents that uniquely target each mechanism will be approved to prolong OS.
In conclusion, this study provided real-world evidence for the survival and treatment of patients with EGFR mutation-positive advanced/recurrent NSCLC. EGFR-TKIs comprised a major part of the treatment strategy, especially for patients with poor PS in the real world. Switching of and re-challenging with EGFR-TKIs were also performed in Japan. Switching and re-challenging might further extend the survival of patients with EGFR mutation-positive advanced/recurrent NSCLC. We are planning additional analysis regarding the treatment sequence in these patients to optimize EGFR-TKI treatment.
Funding
This study was sponsored by AstraZeneca. Funding to pay the Open Access publication charges for this article was provided by AstraZeneca.
Supplementary data
Supplementary data are available at http://www.jjco.oxfordjournals.org.
Conflict of interest statement
Akira Inoue received consulting fees from AstraZeneca, Chugai Pharmaceutical, and Boehringer Ingelheim. Satoshi Morita and Fumio Imamura received consulting fees from AstraZeneca. Takashi Seto received consulting fees from Astellas Pharma, AstraZeneca, Bayer Yakuhin, Chugai Pharmaceutical Co., Daiichi Sankyo Co., Eisai Co., Eli Lilly Japan, Fuji Pharma Co., Hisamitsu Pharmaceutical Co., Kyowa Hakko Kirin Co., MSD, Nippon Boehringer Ingelheim Co., Novartis Pharma, Ono Pharmaceutical Co., Pfizer Japan, Sanofi, Sumitomo Dainippon Pharma Co., Taiho Pharmaceutical Co., Takeda Pharmaceutical Co., and Yakult Honsha Co. Isamu Okamoto received honoraria from AstraZeneca K.K. Kazuhiko Nakagawa received honoraria from Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., AstraZeneca K.K., Pfizer Japan Inc., and Nippon Boehringer Ingelheim Co., Ltd. Satoshi Muto is an employee of AstraZeneca K.K. Masahiro Fukuoka declares advisory affiliations with Ono Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., and Astellas, and received consulting fees from Chugai Pharmaceutical Co., Daiichi Sankyo, Eisai Co., Ltd., and Kyowa Hakko Kirin Co., Ltd. Kazushi Yoshida and Nobuyuki Yamamoto have no conflicts of interest to declare.
Supplementary Material
Acknowledgements
The authors thank all the participants of this study and H. Oda at Medical TOUKEI Corporation for performing data analyses.
References
- 1.The Japan Lung Cancer Society. Japan Guidelines of Lung Cancer Therapy. 2014 ed Japan: Kanehara Shuppan; 2014. [Google Scholar]
- 2.Takano T, Fukui T, Ohe Y et al. EGFR mutations predict survival benefit from gefitinib in patients with advanced lung adenocarcinoma: a historical comparison of patients treated before and after gefitinib approval in Japan. J Clin Oncol 2008;26:5589–95. [DOI] [PubMed] [Google Scholar]
- 3.Inoue A, Kobayashi K, Maemondo M et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin–paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54–9. [DOI] [PubMed] [Google Scholar]
- 4.Yoshioka H, Mitsudomi T, Morita S et al. Final overall survival results of WJTOG 3405, a randomized phase 3 trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer (NSCLC) harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2014;32:8117. [Google Scholar]
- 5.Katakami N, Nishio M, Gotoh K et al. Phase II trial to evaluate Erlotinib as a 1st line treatment to NSCLC with EGFR mutation: overall survival of JO22903. Haigan 2014;54:349. [Google Scholar]
- 6.Kato T, Yoshioka H, Okamoto I et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci 2015;106:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Can 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 8.Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–8. [DOI] [PubMed] [Google Scholar]
- 9.Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–8. [DOI] [PubMed] [Google Scholar]
- 10.Nishino K, Imamura F, Morita S et al. A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer 2013;82:299–304. [DOI] [PubMed] [Google Scholar]
- 11.Lee CK, Wu YL, Ding PN et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol 2015;33:1958–65. [DOI] [PubMed] [Google Scholar]
- 12.Tang C, Gao H, Li X et al. Different treatment orders achieved similar clinical results: a retrospective study for retreatment of epidermal growth factor receptor tyrosine kinase inhibitors in 120 patients with non-small-cell lung cancer. J Cancer Res Clin Oncol 2014;140:427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomizawa Y, Fujita Y, Tamura A et al. Effect of gefitinib re-challenge to initial gefitinib responder with non-small cell lung cancer followed by chemotherapy. Lung Cancer 2010;68:269–72. [DOI] [PubMed] [Google Scholar]
- 14.Oh IJ, Ban HJ, Kim KS, Kim YC. Retreatment of gefitinib in patients with non-small-cell lung cancer who previously controlled to gefitinib: a single-arm, open-label, phase II study. Lung Cancer 2012;77:121–7. [DOI] [PubMed] [Google Scholar]
- 15.Xia GH, Zeng Y, Fang Y et al. Effect of EGFR-TKI retreatment following chemotherapy for advanced non-small cell lung cancer patients who underwent EGFR-TKI. Cancer Biol Med 2014;11:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishie K, Kawaguchi T, Tamiya A et al. Epidermal growth factor receptor tyrosine kinase inhibitors beyond progressive disease: a retrospective analysis for Japanese patients with activating EGFR mutations. J Thorac Oncol 2012;7:1722–7. [DOI] [PubMed] [Google Scholar]
- 17.Asami K, Okuma T, Hirashima T et al. Continued treatment with gefitinib beyond progressive disease benefits patients with activating EGFR mutations. Lung Cancer 2013;79:276–82. [DOI] [PubMed] [Google Scholar]
- 18.Yang JJ, Chen HJ, Yan HH et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33–9. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Boggon TJ, Dayaram T et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med 2005;352:786–92. [DOI] [PubMed] [Google Scholar]
- 20.Pao W, Miller VA, Politi KA et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelman JA, Zejnullahu K, Mitsudomi T et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039–43. [DOI] [PubMed] [Google Scholar]
- 22.Bean J, Brennan C, Shih JY et al. MET amplification occurs with or without T790 M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA 2007;104:20932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamasaki F, Johansen MJ, Zhang D et al. Acquired resistance to erlotinib in A-431 epidermoid cancer cells requires down-regulation of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res 2007;67:5779–88. [DOI] [PubMed] [Google Scholar]
- 24.Kokubo Y, Gemma A, Noro R et al. Reduction of PTEN protein and loss of epidermal growth factor receptor gene mutation in lung cancer with natural resistance to gefitinib (IRESSA). Br J Cancer 2005;92:1711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung HW, Du J, Boehm JS et al. Amplification of CRKL induces transformation and epidermal growth factor receptor inhibitor resistance in human non-small cell lung cancers. Cancer Discov 2011;1:608–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yano S, Yamada T, Takeuchi S et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol 2011;6:2011–7. [DOI] [PubMed] [Google Scholar]
- 27.Bivona TG, Hieronymus H, Parker J et al. FAS and NF-κB signalling modulate dependence of lung cancers on mutant EGFR. Nature 2011;471:523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sequist LV, Waltman BA, Dias-Santagata D et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suda K, Mizuuchi H, Maehara Y, Mitsudomi T. Acquired resistance mechanisms to tyrosine kinase inhibitors in lung cancer with activating epidermal growth factor receptor mutation—diversity, ductility, and destiny. Cancer Metastasis Rev 2012;31:807–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.