ABSTRACT
Wnt/β-catenin signaling controls intestinal stem cell (ISC) proliferation, and is aberrantly activated in colorectal cancer. Inhibitors of the ADP-ribose polymerase Tankyrase (Tnks) have become lead therapeutic candidates for Wnt-driven cancers, following the recent discovery that Tnks targets Axin, a negative regulator of Wnt signaling, for proteolysis. Initial reports indicated that Tnks is important for Wnt pathway activation in cultured human cell lines. However, the requirement for Tnks in physiological settings has been less clear, as subsequent studies in mice, fish and flies suggested that Tnks was either entirely dispensable for Wnt-dependent processes in vivo, or alternatively, had tissue-specific roles. Here, using null alleles, we demonstrate that the regulation of Axin by the highly conserved Drosophila Tnks homolog is essential for the control of ISC proliferation. Furthermore, in the adult intestine, where activity of the Wingless pathway is graded and peaks at each compartmental boundary, Tnks is dispensable for signaling in regions where pathway activity is high, but essential where pathway activity is relatively low. Finally, as observed previously for Wingless pathway components, Tnks activity in absorptive enterocytes controls the proliferation of neighboring ISCs non-autonomously by regulating JAK/STAT signaling. These findings reveal the requirement for Tnks in the control of ISC proliferation and suggest an essential role in the amplification of Wnt signaling, with relevance for development, homeostasis and cancer.
KEY WORDS: Axin, Signal Transduction, Tankyrase, Wingless, Wnt, β-catenin
Summary: In the Drosophila intestine, Tankyrase is important for Wingless signaling activation in enterocytes, thus non-autonomously regulating intestinal stem cell proliferation.
INTRODUCTION
The Wnt/β-catenin signal transduction pathway regulates intestinal stem cell (ISC) self-renewal in mammals, whereas conditional inactivation of Wnt signaling results in the loss of intestinal proliferative crypts and associated ISCs (Fevr et al., 2007; Ireland et al., 2004; Korinek et al., 1998; van Es et al., 2012). Conversely, the aberrant activation of Wnt signaling triggers development of the vast majority of colorectal carcinomas (Korinek et al., 1997; Morin et al., 1997). Despite our relatively detailed understanding of essential steps in Wnt pathway activation (Clevers and Nusse, 2012; MacDonald et al., 2009), the identification of clinically effective small-molecule inhibitors to treat Wnt-driven cancers has been challenging (Lum and Clevers, 2012). Therefore, the discovery that the ADP-ribose polymerase Tankyrase (Tnks) targets Axin, a negative regulator of Wnt signaling, for proteolysis was a breakthrough (Huang et al., 2009). Small-molecule Tnks inhibitors that disrupted Wnt signaling in cultured human cells (Chen et al., 2009; Huang et al., 2009; Lau et al., 2013; Waaler et al., 2012) and reduced the growth of colonic adenomas in mouse models (Lau et al., 2013; Waaler et al., 2012) have been identified, suggesting a promising new strategy for the treatment of Wnt-driven cancers.
However, the requirement for Tnks in physiological settings has remained less clear, because in vivo studies suggested either that Tnks was dispensable for Wnt-dependent processes, or conversely, that Tnks had tissue- or stage-specific roles. For example, in flies, genetic inactivation of Tnks resulted in no Wingless-dependent developmental phenotypes unless Axin was simultaneously overexpressed at levels high enough to abrogate Wingless signaling (Feng et al., 2014). Similarly, no defects in Wnt-dependent processes were observed after treatment of fish with Tnks inhibitors during embryonic development (Huang et al., 2009), but these inhibitors disrupted the regeneration of adult fins following injury (Chen et al., 2009; Huang et al., 2009), a process that is dependent on Wnt and several other signaling pathways (Wehner and Weidinger, 2015). Finally, functional redundancy exists between the two Tnks homologs in mice (Chiang et al., 2008) and double mutants displayed embryonic lethality, but no overt Wnt-related phenotypes (see also Discussion in Qian et al., 2011). However, a mutation in mouse Axin2 that is predicted to disrupt Tnks-dependent ADP-ribosylation paradoxically resulted in both hyperactivating and inhibiting effects on Wnt signaling in the primitive streak that were dependent on developmental stage. These opposing effects were also observed following treatment with Tnks inhibitors, suggesting complex roles in embryonic development (Qian et al., 2011). The mechanistic basis for these disparate effects of Tnks inhibition in the different in vivo models remains unknown.
In this study, we have focused on the function of Tnks in the adult Drosophila midgut, which, because of its simplicity and similarity to the vertebrate intestine, has emerged as a powerful model for studying intestinal homeostasis, regeneration and tumorigenesis (Jiang and Edgar, 2011). The activation of the Wingless pathway is graded along the length of the adult intestine, peaking at each of the boundaries between compartments and present at lower levels within compartments (Buchon et al., 2013; Tian et al., 2016). During homeostasis, Wingless signaling regulates ISC proliferation (Buchon et al., 2013; Tian et al., 2016). Here, we demonstrate that the regulation of Axin by Tnks is required for the control of adult ISC proliferation. Importantly, we find that Tnks is essential for Wingless target gene activation within regions of the gut where the Wingless pathway is activated at relatively low levels, but dispensable where Wingless pathway activity is high. Our findings suggest that, like Wingless pathway components, the role of Tnks is crucial for the non-autonomous control of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signaling in ISCs, and thereby essential to maintain intestinal homeostasis. Our findings provide the first in vivo evidence using null alleles that regulation of Axin by Tnks is essential for Wingless target gene activation under physiological conditions. The requirement for Tnks is spatially restricted within the graded range of Wnt pathway activation, suggesting that the context-dependent requirements for Tnks reflect an essential role in the amplification of signaling following Wnt stimulation.
RESULTS
Tnks is essential for control of ISC proliferation in the adult midgut
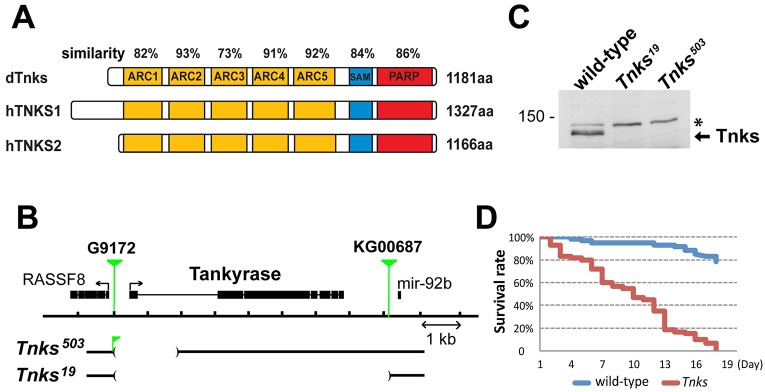
Fly genomes encode only one Tnks, which is highly conserved; the overall similarity between the Drosophila melanogaster and human TNKS1 and TNKS2 proteins is 79% (Fig. 1A). Like the human TNKS proteins, the single Drosophila Tnks contains five Ankyrin repeat clusters (ARC), a sterile α motif (SAM) and a poly(ADP-ribose) polymerase (PARP) domain (Fig. 1A) (Sbodio et al., 2002; Smith et al., 1998). ARC2, ARC4 and ARC5 in the mammalian TNKS proteins, which bind axin directly (Morrone et al., 2012), share between 91% and 93% similarity with the corresponding fly Tnks ARCs, suggesting evolutionary conservation in function.
Fig. 1.
Tnks mutants display increased mortality under reduced nutrient conditions. (A) Schematic representation of domains in Drosophila Tnks and human TNKS1 and TNKS2. ARC, Ankyrin repeat cluster; SAM, sterile alpha motif; PARP, poly-ADP-ribose polymerase catalytic domain. Percentage similarity between dTnks and hTNKS is indicated above each domain. (B) Schematic representation of the Tnks genomic region and deletions in two Drosophila Tnks null mutants, Tnks19 and Tnks503. A fragment of the P element G9172 remains in the Tnks503 mutant. (C) Tnks null mutants confirmed by immunoblotting. Immunoblots using an antibody against Tnks detect endogenous Tnks in wild-type flies, but not in Tnks null mutants. A nonspecific band serves as a control for loading (asterisk). (D) Tnks19/503 mutant flies show increased mortality compared with wild-type flies when fed with 5% sucrose solution. n=60 for each genotype.
To examine Tnks function in vivo, we isolated Drosophila Tnks mutants using either imprecise excision or hybrid element insertion (Pare et al., 2009; Parks et al., 2004) of transposons flanking the Tnks gene. We identified two alleles: Tnks503, a deletion that eliminates the Tnks transcription and translation initiation sites and Tnks19, a deletion that eliminates the Tnks gene entirely (Fig. 1B). Both alleles are null, as revealed by immunoblots of larval lysates using a Tnks antibody (Fig. 1C). Although TNKS function is essential for viability in mice, Tnks null mutant flies were viable, eclosed at a normal rate and displayed no external morphological defects under standard lab conditions, consistent with previous findings (Feng et al., 2014). However, by comparison with controls, Tnks mutant adults displayed markedly increased mortality when fed only water supplemented with sucrose (Fig. 1D).
We postulated that the high mortality in Tnks mutants might arise from digestive tract defects that compromised their ability to absorb nutrients and thus examined their guts to address this possibility. In the Drosophila midgut, the ISCs give rise to two types of daughter progenitors, the undifferentiated enteroblast (EB) and the pre-enteroendocrine (pre-EE) cell (Beehler-Evans and Micchelli, 2015; Biteau and Jasper, 2014; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006; Zeng and Hou, 2015; Zielke et al., 2014). EBs and pre-EEs differentiate into absorptive enterocytes (ECs) and secretory enteroendocrine (EE) cells, respectively. We identified midgut progenitor cells by their expression of the transcription factor escargot (esg) (Micchelli and Perrimon, 2006). In controls, progenitor cells were distributed evenly along the length of the midgut, as revealed by the esg-Gal4, UAS-GFP (esg>GFP) reporter (Fig. 2A-C). By contrast, Tnks null mutants displayed a markedly increased number of progenitor cells compared with controls, an effect that was most pronounced in the posterior midgut (Fig. 2D-F). Furthermore, multi-cell clusters of progenitor cells were detected frequently in Tnks mutants, but not in controls (Fig. 2D-F, arrows).
Fig. 2.
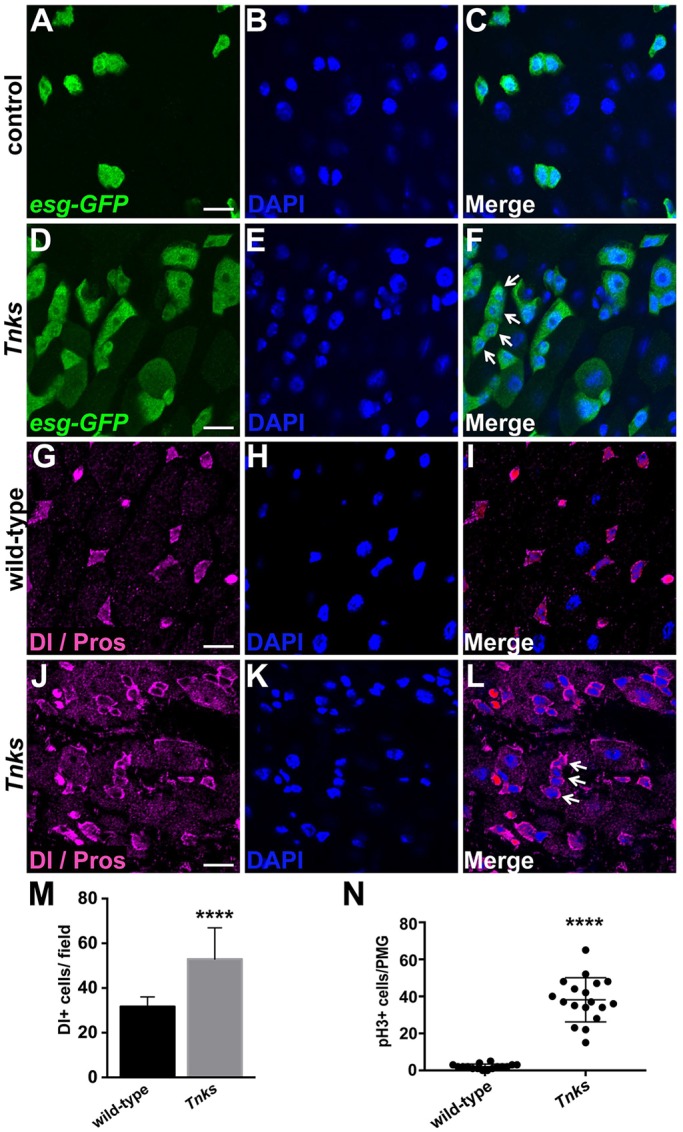
Tnks prevents overproliferation of ISCs. (A-F) Tnks mutant flies have an increased number of progenitor cells that form clusters (arrow). Midguts of 5-day-old female expressing esg-Gal4, UAS-GFP (esg>GFP) stained with anti-GFP (green) and DAPI. In control (Tnks503/+) midguts (A-C), esg>GFP+ progenitor cells are evenly spaced. (G-L) Tnks mutant flies have an increased number of ISCs that form clusters (arrow). Midguts of 5-day-old wild-type or Tnks19/503 null mutant flies stained with anti-Delta and anti-Prospero antibodies to mark ISCs and EEs, respectively. Scale bars: 10 μm. (M) Quantification of the relative number of Delta+ cells in 5-day-old wild-type (n=17) or Tnks19/503 mutant flies (n=19). (N) Quantification of pH3+ cells in posterior midgut (PMG) of 14-day-old wild-type (n=16) or Tnks19/503 mutant flies (n=18). Values are presented as means±s.d. ****P<0.0001 (t-test).
To distinguish ISCs from the other progenitor cell types, we stained wild-type or Tnks mutant midguts with the ISC-specific marker Delta (Dl) (Ohlstein and Spradling, 2007). We observed a marked increase in the number of ISCs in the midguts of Tnks mutants compared with wild-type flies (Fig. 2G-M). Notably, the increase in the number of progenitor cells in Tnks mutants became more severe with age. For example, by 14 days after eclosion, there was a large increase in the number of esg>GFP+ cells in the midgut of Tnks mutants compared with controls (Fig. S1A,C). Immunostaining with phospho-histone H3 (pH3) antibody revealed a significant increase in pH3+ cells in Tnks mutant midguts, indicating increased numbers of cells undergoing mitosis (Fig. 2N and Fig. S1B,D). Together, these findings indicate that loss of Tnks results in an aberrantly increased rate of ISC proliferation, and consequently, an increased number of progenitor cells in the adult midgut.
To determine whether Tnks is essential for regulating ISC proliferation during adulthood, we used the MARCM (mosaic analysis with a repressible cell marker) technique (Lee and Luo, 2001) to induce marked clones of either wild-type or Tnks mutant cells. We excluded transient clones by restricting our analysis to clones in the posterior midgut that contained two or more cells. When wild-type clones were induced on the day of eclosion and analyzed 4 days later, only 3.6% of the clones contained more than three cells (Fig. 3A,C). By contrast, the proportion of multi-cell clones (n>3) increased significantly following Tnks inactivation, to 22.7% (Fig. 3B,C). To address the possibility that the increased number of ISCs in Tnks mutants resulted from a block in their differentiation, we stained Tnks mutant clones for the presence of EBs, ECs and EE cells with the cell type-specific markers Suppressor of Hairless (Su(H)-lacZ), Pdm1 and Prospero, respectively (Lee et al., 2009; Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006). We found that EBs, ECs and EE cells were all present within Tnks mutant clones, indicating that Tnks is not essential for epithelial cell differentiation in the midgut (Fig. 3D-L). Therefore, Tnks is dispensable for cell differentiation, but essential for the control of ISC proliferation during homeostatic conditions in the adult gut.
Fig. 3.
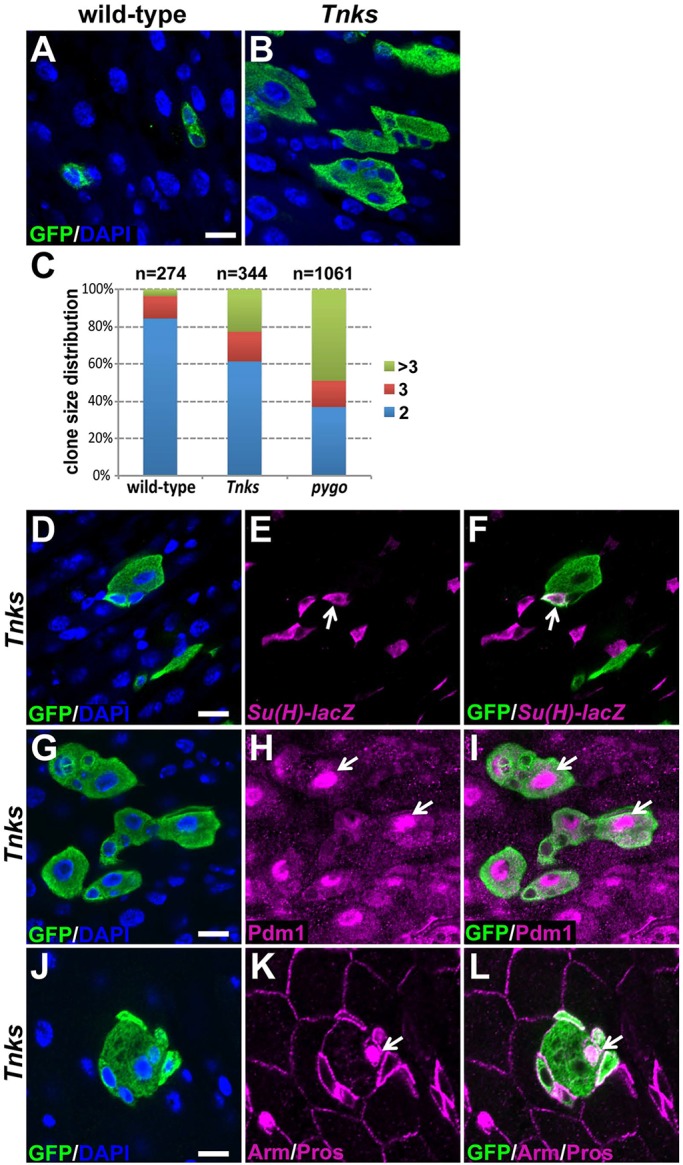
Tnks regulates ISC proliferation but not differentiation during homeostasis. MARCM clones (marked by GFP) of indicated genotypes, stained with anti-GFP (green) and DAPI. Tnks19 flies (B) have increased number of multi-cell clones compared with wild-type flies (A). (C) Quantification of clone size for wild-type, Tnks19 or pygoS123 ISC lineages in posterior midguts. Number of clones examined is indicated. (D-F) Tnks mutant ISCs can give rise to transient undifferentiated EBs (arrow). Tnks19 clones are marked by GFP (green) and EBs by Su(H)-lacZ (magenta). (G-I) Tnks mutant ISCs can differentiate into ECs (arrow). (J-L) Tnks mutant ISCs can differentiate into EE cells (arrow). Clones were induced in adults on the day of eclosion and analyzed 4 days later. Scale bars: 10 μm.
A spatially restricted requirement for Tnks within the graded activation of the Wingless pathway promotes signaling in the adult midgut
Wingless signaling was initially proposed to maintain the ISC population by preventing differentiation (Lin et al., 2008). However, more recent studies have revealed that the activation of Wingless signaling in ISCs had only mild effects on tissue self-renewal under basal homeostatic conditions, but had a major role in the response of ISCs to injury (Cordero et al., 2012; Lee et al., 2009). Recently, we reported the role of Wingless signaling in intestinal homeostasis during early adulthood (Tian et al., 2016). Our findings, based on null alleles and RNAi-mediated knockdown of multiple Wingless pathway core components, revealed that the activation of the Wingless pathway in ECs non-autonomously inhibits ISC proliferation under basal conditions during intestinal homeostasis.
Therefore, we hypothesized that defects in the regulation of ISC proliferation observed in Tnks mutants could have resulted from inhibition of Wingless signaling. To test this hypothesis, we compared the effects of inactivation of either the essential Wingless signaling transcription cofactor Pygopus (Pygo) (Kramps et al., 2002; Parker et al., 2002; Thompson et al., 2002) or Tnks under identical conditions in the adult intestine. Consistent with the results from Tnks mutant clones induced at the same stage, the percentage of multi-cell pygo null mutant clones increased significantly compared with wild-type control clones and was even greater than that observed in Tnks mutants (Fig. 3C). These findings support the hypothesis that the increased number of ISCs in Tnks mutants resulted from inactivation of Wingless signaling within intestinal compartments.
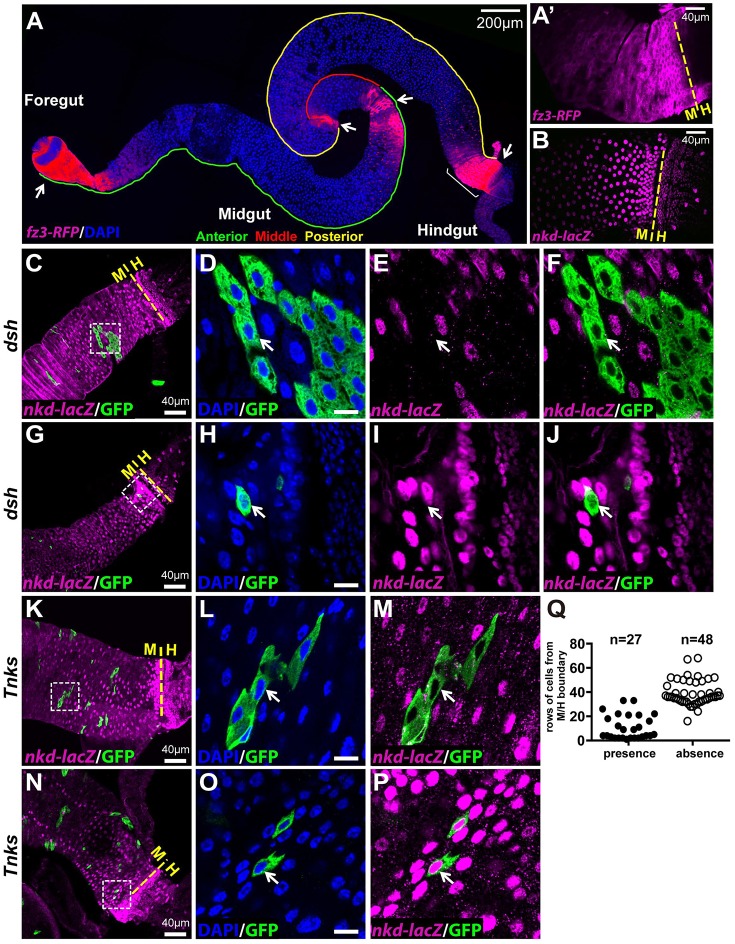
To further test the hypothesis that Tnks promotes Wingless signaling in the adult intestine, we analyzed reporters for the Wingless pathway target genes naked (nkd) and frizzled 3 (fz3) (Olson et al., 2011; Sato et al., 1999; Sivasankaran et al., 2000; Zeng et al., 2000). Previous studies revealed that the nkd-lacZ and the fz3-RFP reporters are expressed in overlapping regional gradients along the length of the adult intestine and indicated that Wingless pathway activity is highest at all the major boundaries that exist between compartments (Fig. 4A,B) (Buchon et al., 2013; Tian et al., 2016). Both reporters also revealed that Wingless pathway activity gradually diminishes to a low level with distance from the boundaries and is maintained at low levels throughout midgut compartments (Tian et al., 2016). Furthermore, both reporters are expressed in ECs, and this expression is completely lost upon inactivation of Wingless signaling (Tian et al., 2016) (Fig. 4C-J and Fig. S2E-L). Fz3-RFP is also expressed in ISCs but this expression is independent of Wingless signaling in nearly all ISCs during homeostasis (Tian et al., 2016) (Fig. S2A-D). Thus, this previous work revealed that ECs are the primary cell type in which the Wingless pathway is activated under basal conditions during homeostasis.
Fig. 4.
Tnks promotes Wingless target gene activation in regions where the pathway activity is relatively low. (A) fz3-RFP is expressed in gradients at several compartment boundaries (arrow) in midguts. (A′) Higher magnification image of the fz3-RFP gradient (magenta) near the midgut/hindgut (M/H) boundary (bracket in A) reveals that fz3-RFP is expressed in progenitor cells and ECs. (B) nkd-lacZ (magenta) also forms a gradient near the midgut/hindgut boundary, but is expressed only in ECs. (C-J) nkd-lacZ expression is dependent on Wingless signaling activation. Null mutant clones of dishevelled (dsh), an essential Wingless pathway component, are marked by GFP. nkd-lacZ (magenta) is absent in dsh mutant ECs (arrow) both away from the compartment boundary (C-F) and near the boundary (G-J). (K-P) Tnks is required for nkd-lacZ expression in regions away from the compartment boundary (K-M), but is dispensable near the boundary where Wingless pathway activity is high (N-P). Low magnification images are shown in C, G, K and N with yellow dashed line indicating the midgut/hindgut boundary. Higher magnification views of the boxed areas are shown on the right. Scale bars: 10 μm if not indicated. (Q) Analysis of nkd-lacZ expression in Tnks clones relative to the distance from the midgut/hindgut boundary. Number of clones examined is indicated.
To determine whether Tnks promotes Wingless signaling in the adult midgut, we tested whether the activation of Wingless target gene expression is dependent on Tnks. Using clonal analysis, we found that within compartments, where Wingless signaling activity is relatively low, nkd-lacZ expression was lost in Tnks mutant ECs (Fig. 4K-M). However, at the compartment boundaries, where Wingless pathway activity is high, the expression of nkd-lacZ was lost in dsh mutant cells (Fig. 4G-J), but retained in Tnks mutant cells (Fig. 4N-P). Spatial analysis of Tnks mutant clones revealed that Tnks was required for nkd-lacZ expression in ECs that were away from the midgut/hindgut boundary, but was dispensable for nkd-lacZ expression in ECs near the boundary, where Wingless pathway activity is high (Fig. 4Q). Similar results were obtained with the fz3-RFP reporter (Fig. S2E-T). These results indicated that within the intestinal epithelium, Tnks is essential for signaling in regions with relatively low pathway activation, but not required for Wingless target gene activation in regions with high levels of Wingless pathway activation.
Regulation of Axin by Tnks controls ISC proliferation
Together, these findings demonstrate that similar defects in regulation of ISC proliferation arise from inactivation of either Tnks or Wingless pathway components and provide the first evidence using null alleles that Tnks is important for the activation of Wingless target genes in a physiological context. We next tested whether this role of Tnks in promoting Wingless signaling reflects its negative regulation of Axin levels in vivo. However, the existing Axin antibodies did not allow detection of endogenous Axin in immunoblots, which was a presumed consequence of the very low Axin concentration (Tolwinski et al., 2003; Willert et al., 1999). We therefore generated a new antibody, which enabled sensitive detection of endogenous Axin; its specificity was demonstrated by the loss of Axin signal in lysates from Axin (Axn) null mutant larvae rescued to viability by a higher molecular weight Flag-Axin fusion protein (Fig. 5A). Using this antibody, we detected increased levels of Axin in lysates from Tnks mutant midguts compared with the wild type (Fig. 5B), consistent with the hypothesis that Tnks promotes Axin degradation in vivo.
Fig. 5.
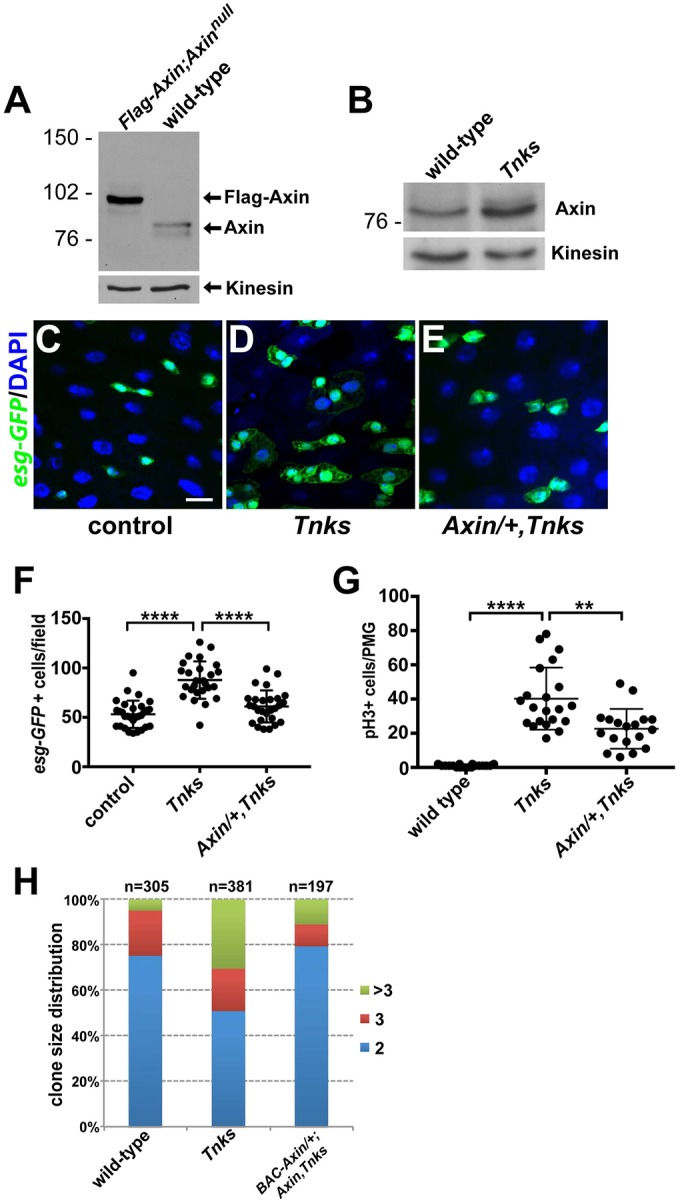
Regulation of Axin by Tnks is required for control of ISC proliferation. (A) Detection of endogenous Axin by immunoblotting. Endogenous Axin is detected only in the wild-type larvae. Axns044230 null mutant flies rescued to viability by expression of a tub>Flag-Axn transgene were used as a negative control for antibody specificity. Only the slower-migrating Flag-tagged Axin is present in these lysates, which are devoid of endogenous Axin. Kinesin was used as a loading control. (B) Axin protein levels are increased in Tnks mutants. Midgut lysates prepared from 5-day-old adult females of indicated genotypes immunoblotted with Axin antibody. (C-E) Reducing the Axn gene dosage by half restores the number of esg>GFP+ progenitor cells. 5-day-old female flies of indicated genotypes expressing esg>GFP stained with anti-GFP (green) antibody and DAPI. Scale bar: 10 μm. (F) Quantification of the relative number of esg>GFP+ ISCs and EBs cells from adult flies of indicated genotypes. Tnks503/+ flies were used as control. (G) Quantification of pH3+ cells in posterior midguts from adult flies of indicated genotypes. Each dot represents an animal and lines indicate mean±s.d. ****P<0.0001 and **P<0.01 (t-test). (H) Reducing the Axn dosage by one-half restores the number of multi-cell Tnks mutant clones. Quantification of clone size for ISC lineages of indicated genotypes. Clones were induced in adults on the day of eclosion and analyzed 4 days later. Number of clones examined is indicated.
Therefore, we sought to determine whether the overproliferation of ISCs in Tnks mutants resulted from increased Axin levels. We reasoned that if this were true, reduction of the Axn gene dosage might suppress the increased number of progenitor cells present in Tnks mutants. Indeed, the number of progenitor cells was restored to almost wild-type levels in Tnks mutants upon reduction of the Axn dosage by one-half (Fig. 5C-F). Consistently, reduction of the Axn gene dosage suppressed the proliferation of ISCs, as indicated by the reduced number of pH3+ cells (Fig. 5G). We also tested whether reducing the Axn gene dosage by half would suppress the aberrantly increased size of Tnks mutant clones. Because the Tnks and Axn genes are located on the same chromosomal arm, we performed this analysis by introduction of one copy of a BAC-Axin-V5 transgene, in which Axin is expressed from its endogenous promoter (Gerlach et al., 2014), in Tnks and Axn double-null mutant clones. We found that the size of Tnks mutant clones was decreased significantly when the Axn gene dosage was reduced (Fig. 5H). These findings further support the hypothesis that increased Axin levels resulting from loss of Tnks inhibit Wingless signaling in vivo and thereby result in ISC overproliferation.
Regulation of Wingless signaling by Tnks in ECs non-autonomously controls proliferation of neighboring ISCs
Our previous work revealed that under basal homeostatic conditions, the Wingless pathway is activated primarily in ECs and that inactivation of Wingless signaling in ECs resulted in the overproliferation of neighboring ISCs in a non-autonomous manner (Tian et al., 2016). Therefore, we sought to determine whether the loss of Tnks, like that of other Wingless pathway components, non-autonomously promotes the proliferation of neighboring ISCs. We induced either wild-type control clones or Tnks mutant clones during adulthood and identified progenitor cells with the esg-lacZ reporter. In wild-type clones, progenitor cells were distributed evenly along the midgut (Fig. 6A-C). However, in Tnks mutant clones induced at the same stage, an aberrantly increased number of wild-type progenitor cells was present in proximity to the mutant clones (Fig. 6D-F,J). Similar results were obtained when pygo mutant clones were induced in adulthood (Fig. 6G-J) (Tian et al., 2016), supporting the hypothesis that loss of Tnks results in the inactivation of Wingless signaling. By contrast, no defects were observed when Tnks mutant clones were induced during pupation and analyzed at eclosion (Fig. S3). Together, these findings raised the possibility that the increase in ISCs caused by loss of Tnks resulted from inactivation of Wingless signaling in neighboring ECs.
Fig. 6.
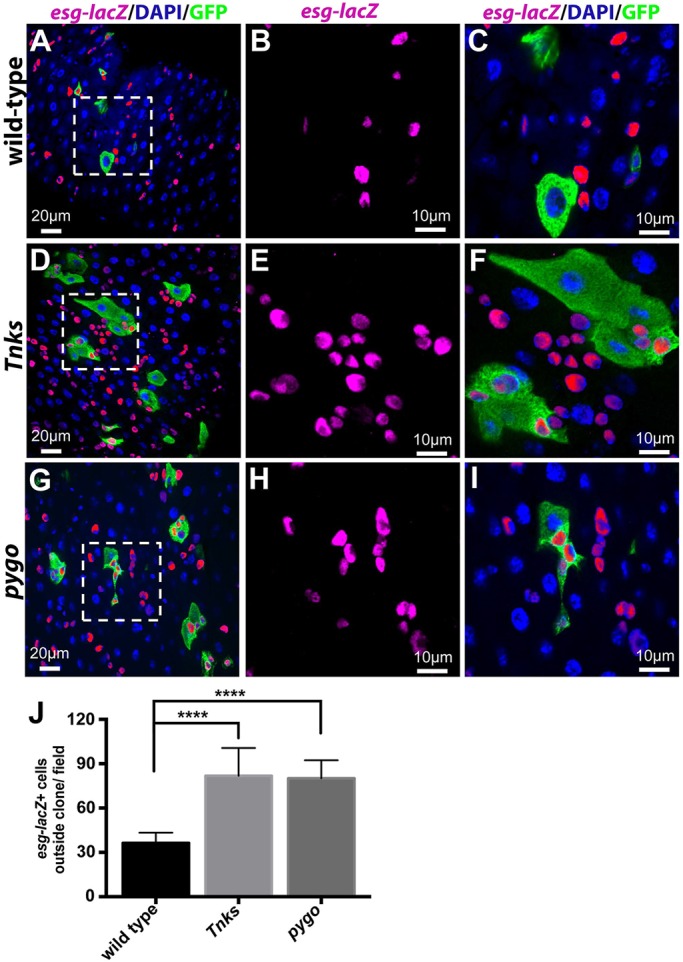
Loss of Tnks non-autonomously promotes the proliferation of neighboring ISCs. Progenitor cells (marked by esg-lacZ) in adult midguts with MARCM clones of indicated genotypes. Clones were induced in adults on the day of eclosion and examined 4 days later. Wild-type clones have no effects on the neighboring ISCs (A-C), whereas Tnks503 (D-F) or pygoS123 (G-I) null mutant clones result in the overproliferation of neighboring ISCs, as revealed by the increased number of esg-lacZ+ cells. (B,C,E,F,H,I) Higher magnification views of the boxed areas in the left panels. (J) Quantification of the relative number of progenitor cells outside the clones of indicated genotypes. n=11, 15, 17 for each genotype respectively. ****P<0.0001 (t-test). Error bars indicate s.d.
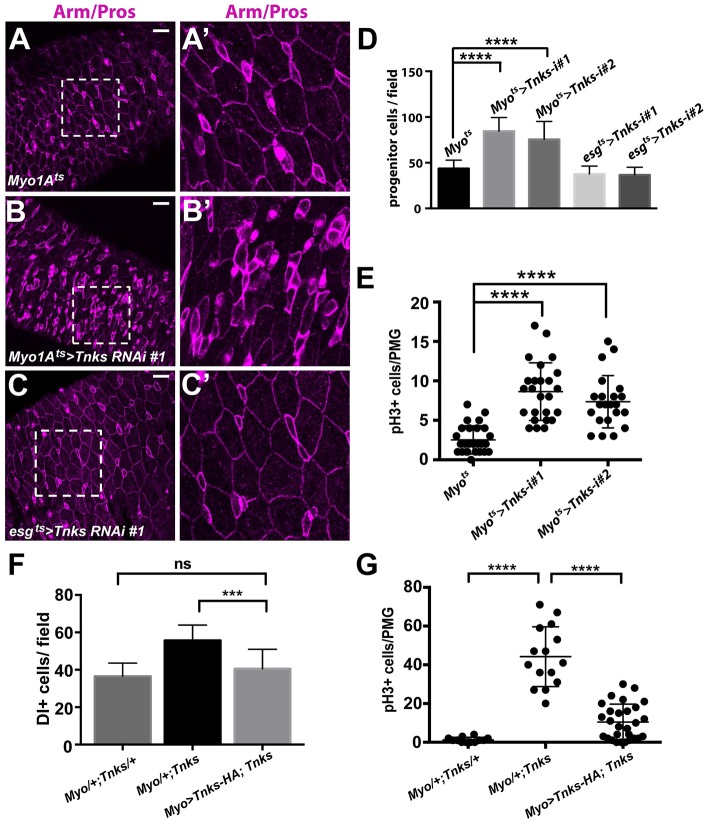
To directly test this hypothesis, we disrupted Tnks function specifically in either ECs or progenitor cells using RNAi-mediated Tnks knockdown. We used Myo1A-Gal4 or esg-Gal4 and the temperature-sensitive Gal4 repressor Gal80ts (McGuire et al., 2004) (together referred to as Myo1Ats-Gal4 or esgts-Gal4) to induce the temporal knockdown of Tnks in either ECs or progenitor cells, respectively (Jiang et al., 2009; Micchelli and Perrimon, 2006). On the day of eclosion, flies were shifted to the restrictive temperature (29°C) for 7 days and midguts were analyzed subsequently. As reported previously (Ohlstein and Spradling, 2006), ISCs and EBs were identified by their smaller size, high level of membrane-associated Armadillo/β-catenin, and lack of nuclear Prospero staining (which was used to distinguish pre-EE and EE cells from ISCs) (Fig. S4). Compared with controls (Fig. 7A,D), knockdown of Tnks in ECs resulted in an increased number of progenitor cells, which is consistent with overproliferation of ISCs (Fig. 7A-B′,D). Indeed, the number of pH3+ cells increased significantly in these animals compared with controls, further supporting the conclusion that inactivation of Tnks in ECs results in the non-autonomous overproliferation of ISCs (Fig. 7E). By contrast, no defects were observed when Tnks was knocked down in progenitor cells (Fig. 7C,D). The same findings were observed with a second, independently derived Tnks RNAi line (Feng et al., 2014), ruling out the possibility of RNAi off-target effects (Fig. 7D,E and Fig. S5A-B′). As reported previously, disruption of Wingless signaling specifically in ECs, through RNAi-mediated knockdown of essential components of the Wingless pathway, similarly leads to an increased number of ISCs, whereas their knockdown in progenitor cells has no observed effect (Fig. S5C-D′) (Tian et al., 2016).
Fig. 7.
Tnks is important in ECs to prevent ISC overproliferation. Adults expressing Tnks-RNAi #1 (B,B′) in ECs have increased number of progenitor cells compared with controls (A,A′), whereas those expressing the siRNAs in progenitor cells (C,C′) have no defects. (A′-C′) Higher magnification views of the boxed areas in A-C, respectively. Scale bars: 20 μm. (D) Quantification of the relative number of progenitor cells for flies of indicated genotype. n=13, 12, 13, 19 and 18 for each genotype, respectively. Error bars indicate s.d. ****P<0.0001 (t-test). (E) Quantification of pH3+ cells in posterior midguts from flies of indicated genotypes. Each dot represents an animal and lines indicate means±s.d. ****P<0.0001 (t-test). (F) Expression of Tnks-HA transgene in ECs suppress the increased number of ISCs in Tnks mutants. Quantification of the relative number of ISCs for flies of indicated genotype. n=14, 18 and 18 for each genotype, respectively. Error bars indicate s.d. ***P<0.001; ns, not significant (t-test). (G) Quantification of pH3+ cells in posterior midguts from 14-day-old flies of indicated genotypes. Each dot represents an animal and lines indicate means±s.d. ****P<0.0001 (t-test).
To further test the importance of Tnks function in ECs for the non-autonomous regulation of ISC proliferation, we expressed a Tnks-HA transgene in ECs using the Myo1A-Gal4 driver. Quantification of Delta (Dl)+ ISCs revealed that expressing Tnks-HA in ECs is sufficient to suppress the increased number of ISCs in Tnks mutants (Fig. 7F). As expected, the increase in pH3+ cells was also suppressed by the expression of Tnks-HA in ECs (Fig. 7G). Together, these results further supported the conclusion that Tnks loss inactivates Wingless signaling in ECs, and thereby results in the non-autonomous induction of ISC overproliferation.
Non-autonomous control of JAK/STAT signaling in ISCs by Wingless pathway activation in ECs requires Tnks
To test the possibility that loss of Tnks results in midgut apoptosis, thus causing compensatory overproliferation of ISCs, we stained Tnks mutant midguts with an antibody against the cleaved Dcp-1 caspase. The specificity of the Dcp-1 antibody was confirmed by staining the midguts of control flies expressing rpr (reaper) in ECs (Fig. S6A-D′). However, no ectopic expression of Dcp-1 was observed in Tnks mutants (Fig. S6E-F′), ruling out the presence of aberrant apoptosis.
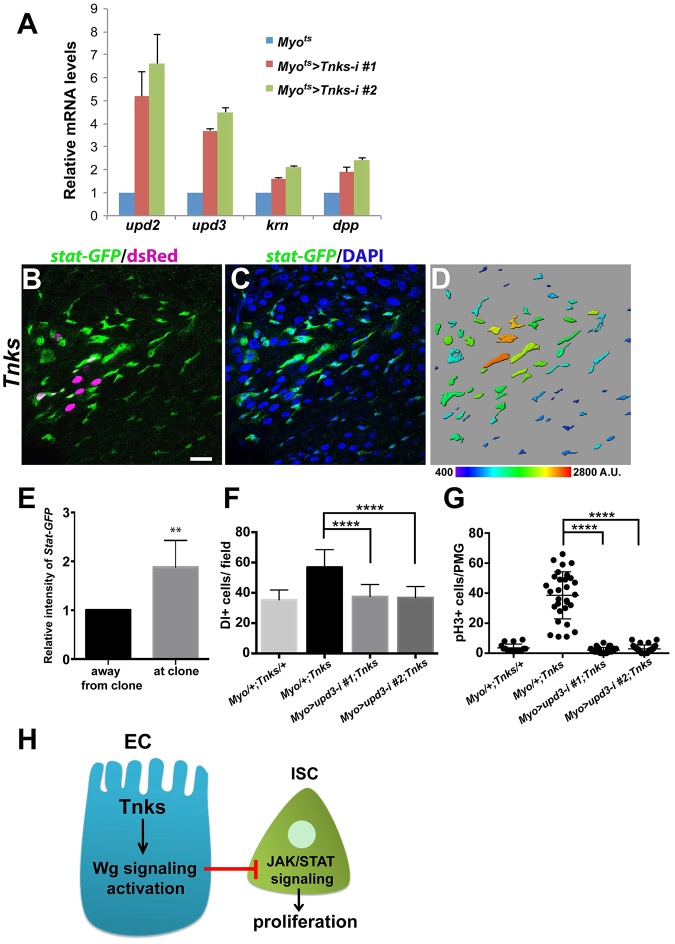
Thus, we hypothesized that inactivation of Tnks in ECs resulted in the aberrant activation of unknown signal transduction pathway(s) in neighboring ISCs, and thereby induced their overproliferation. Previous studies had revealed that several signal transduction pathways are important to control ISC proliferation, in particular the JAK/STAT, epidermal growth factor (EGF) and Decapentaplegic (Dpp)/TGF-β pathways (Biteau and Jasper, 2011; Guo et al., 2013; Jiang et al., 2011, 2009; Li et al., 2013b; Tian and Jiang, 2014; Xu et al., 2011). We therefore examined whether knockdown of Tnks in ECs resulted in increased expression of the ligands from these pathways using RT-qPCR. Indeed, we observed a 5- to 6-fold, and 3- to 4-fold increase in the levels of unpaired 2 and 3 (upd2 and upd3), respectively, two ligands of the JAK/STAT pathway that are expressed in ECs, whereas keren (krn) and dpp expression (ligands for EGF and TGF-β pathway, respectively) were increased only 1.5- to 2-fold (Fig. 8A). Similarly, previous work had revealed that RNAi-mediated knockdown of the Wingless pathway components Arrow, Pygo or Tcf (Parker et al., 2002; Thompson et al., 2002; van de Wetering et al., 1997; Wehrli et al., 2000) in ECs also resulted in a preferential increase in upd2 and upd3 expression (Tian et al., 2016). Together, these findings suggested that loss of Tnks in ECs disrupts Wingless signaling and thereby results in the aberrant activation of JAK/STAT signaling in ISCs.
Fig. 8.
Loss of Tnks non-autonomously activates JAK/STAT signaling to promote ISC proliferation. (A) Expression of Tnks-RNAi in ECs increased mRNA levels of the JAK/STAT pathway ligands upd2 and upd3. Relative mRNA levels of upd2, upd3, krn and dpp in posterior midguts of the indicated genotypes measured by RT-qPCR. Value indicates fold activation relative to controls. Myo1A/+ flies were used as control. Error bars indicate s.d. (B-D) Expression of stat-GFP in midguts with Tnks503 clones (marked by dsRed). stat-GFP (green) is ectopically expressed in progenitor cells adjacent to Tnks503 clones (magenta), compared with regions further away from the clones. (D) Color-coded representation of stat-GFP intensity. Scale bar: 20 μm. (E) Quantification of relative intensity of stat-GFP in regions away from the clones and in regions at the clones. n=5 and error bars indicate s.d. **P<0.01 (t-test). (F) Knockdown of upd3 in ECs suppresses the increased number of ISCs in Tnks mutants. Quantification of the relative number of ISCs for flies of indicated genotype. n=18, 25, 26 and 29 for each genotype, respectively. Error bars indicate s.d. ****P<0.0001 (t-test). (G) Quantification of pH3+ cells in posterior midguts from 14-day-old flies of indicated genotypes. Each dot represents an animal and lines indicate means±s.d. ****P<0.0001 (t-test). (H) A working model for the regulation of ISC proliferation by Tnks. Tnks is important for the activation of Wingless signaling in ECs. The activation of Wingless signaling inhibits activation of JAK/STAT pathway in neighboring ISCs non-autonomously, which controls their proliferation.
To test this hypothesis directly, we analyzed a stat-GFP reporter to monitor the activation of JAK/STAT signaling (Bach et al., 2007). In wild-type posterior midguts, stat-GFP is expressed primarily in ISCs. However, in guts bearing Tnks mutant clones, the level of stat-GFP expression in ISCs was markedly increased both within and near the clones (Fig. 8B-E), indicating that the JAK/STAT pathway had been aberrantly activated in a non-autonomous manner. Similarly, we recently reported that the level of stat-GFP expression was aberrantly increased in ISCs by inactivation of Wingless signaling and this effect was found both within and near the clones (Tian et al., 2016) (Fig. S7). To determine whether the ectopic expression of Upd in ECs is responsible for the overproliferation of ISCs in Tnks mutants, we knocked down upd3 in ECs using two different RNAi lines and found that knockdown of upd3 was sufficient to suppress the increased number of ISCs in Tnks mutants (Fig. 8F). Consistently, knockdown of upd3 in Tnks mutants restored the proliferation rate of ISCs to wild-type levels, as indicated by the number of pH3+ cells (Fig. 8G). Taken together, these findings demonstrated that the disruption of Wingless signaling resulting from loss of Tnks in ECs induces the non-autonomous activation of JAK/STAT signaling in neighboring ISCs, and thereby results in their aberrant proliferation (Fig. 8H).
DISCUSSION
Despite the recent focus on Tnks inhibitors as potential therapeutic agents for Wnt-driven cancers, the in vivo contexts in which Tnks is required to promote Wnt signaling have remained uncertain. Here, we addressed the function of the sole Drosophila Tnks protein in the regulation of ISC proliferation. This approach capitalized on fly genetics and circumvented the complications of functional redundancy in vertebrates. Our studies are the first to demonstrate, through analysis of null alleles under physiological conditions, that Tnks is essential for the same subset of Wingless-dependent processes that control ISC proliferation (Tian et al., 2016). Furthermore, our results reveal that the requirement for Tnks is spatially restricted to regions of relatively low Wingless pathway activation in vivo. Finally, we show that, like Wingless pathway components (Tian et al., 2016), Tnks prevents the non-autonomous activation of the JAK/STAT pathway in ISCs, and thereby prevents their aberrant proliferation. Together, our findings suggest that Tnks is essential for the Wingless-dependent control of ISC proliferation and thus for the maintenance of intestinal homeostasis, and elucidate the underlying mechanism.
This work builds on the recent discovery that the activation of the Wingless pathway is graded along the length of the adult intestine, peaking at distinct compartment boundaries, but also present at low levels throughout intestinal compartments (Buchon et al., 2013; Tian et al., 2016). We find that Tnks is not required unconditionally for signaling throughout the entire length of these gradients of Wingless pathway activity. Indeed, Tnks is essential for Wingless target gene activation and the control of ISC proliferation within compartments, where the levels of Wingless pathway activation are relatively low, but is dispensable for target gene activation at compartment boundaries, where Wingless pathway activity peaks. On the basis of these findings, we postulate that the spatially restricted requirement for Tnks reflects its role in the amplification of signaling. At low levels of Wingless pathway activation, the nuclear level of β-catenin might be near the threshold required for target gene activation and the increased Axin levels resulting from Tnks inactivation would lower β-catenin levels below this critical threshold. By contrast, at high Wingless concentration, β-catenin levels would surpass the threshold necessary for target gene activation, even following loss of Tnks. This spatially restricted requirement for Tnks might explain disparities in recent in vivo studies regarding the requirement for Tnks in Wnt-directed physiological processes (Feng et al., 2014; Huang et al., 2009). Furthermore, our studies might have relevance for Tnks function in Wnt gradients in vertebrates, such as in the graded activation of the Wnt pathway along mammalian intestinal crypts. Moreover, the efficacy of Tnks inhibitors could reflect an essential function for Tnks in the amplification of pathway activity in Wnt-driven cancers.
Depletion of Tnks has no obvious effects on wing development or the expression of Wingless target genes in larval wing discs unless Axin levels are raised above the endogenous level (Feng et al., 2014; Wang et al., 2016). The threshold at which Axin levels inhibit Wingless signaling in wing discs is between 3- and 9-fold above the endogenous Axin level (Wang et al., 2016) and more than 3-fold in embryos (Yang et al., 2016). However, the phenotypes resulting from loss of Tnks in the midgut resemble those resulting from disruption of the Wingless pathway and are rescued by reducing the Axn gene dosage. This finding suggests Tnks is crucial to maintain Axin levels below a physiological threshold and thus to ensure proper activation of Wingless signaling in midgut, and suggest that the threshold at which Axin levels disrupt signaling is lower in the adult intestine compared with other tissues. Alternatively, other mechanisms may promote Axin degradation to compensate for Tnks loss in other tissues or developmental stages, whereas such compensatory mechanisms might not exist in the adult intestine. As intestinal homeostasis is disrupted in Tnks mutants, the physiological functions of the midgut, primarily in digestion and absorption of nutrients, are likely to be impaired. Under standard lab conditions, Tnks mutant flies exhibit no obvious survival disadvantage when compared with wild-type flies. However, with limited nutrient supply, the integrity of the midgut becomes vital for maintaining organismal fitness.
Previous studies indicated that Wingless pathway activation is required autonomously for long-term ISC self-renewal and for ISC proliferation upon tissue damage (Cordero et al., 2012; Lin et al., 2008). In addition, Wingless pathway activation in ECs controls ISC proliferation non-autonomously, and is thereby crucial to maintain intestinal homeostasis (Tian et al., 2016). Here, we have found that like Wingless pathway components, Tnks is essential for this process; Tnks inactivation disrupts the expression of Wingless target genes in ECs, leading to the hyperactivation of the JAK/STAT pathway and thereby the overproliferation of nearby ISCs. As observed for other Wingless pathway components, inactivation of Tnks in mutant clones results in aberrantly increased proliferation of neighboring ISCs. In addition, like other Wingless components, knockdown of Tnks specifically in ECs, but not in progenitors, results in ISC overproliferation, further supporting this non-autonomous mechanism. These findings suggest that to ensure proper proliferation of ISCs during homeostasis, the Wingless pathway status in ECs must be tightly regulated in a process that requires Tnks.
As the intestine serves as a crucial barrier against pathogens and toxins, the maintenance of intestinal integrity is essential for survival. Many conserved signal transduction pathways regulate ISC behavior following injury, including the EGF, JAK/STAT, Wingless/Wnt, TGF-β/Dpp and insulin pathways (Biteau and Jasper, 2011; Choi et al., 2011; Cordero et al., 2012; Guo et al., 2013; Jiang et al., 2011, 2009; Lee et al., 2009; Li et al., 2013b; O'Brien et al., 2011; Tian and Jiang, 2014; Xu et al., 2011). The interplay between these different signaling pathways ensures homeostatic renewal as well as rapid regenerative responses to injury. We provide evidence that loss of Tnks in ECs upregulates expression of upd2 and upd3 and thereby induces the aberrant activation of JAK/STAT signaling in ISCs, placing Wingless signaling upstream of the JAK/STAT pathway in the non-autonomous regulation of ISC proliferation. Therefore, this Tnks-dependent mechanism ensures that JAK/STAT signaling is largely repressed in ISCs under basal conditions, but is poised for rapid activation during the regenerative response to intestinal injury.
MATERIALS AND METHODS
Flies and genetics
The Tnks503 and Tnks19 mutants were isolated as described in supplementary Materials and Methods. Other stocks used were: esg-Gal4, UAS-GFP (Micchelli and Perrimon, 2006), esgK606 (Micchelli and Perrimon, 2006), Su(H)GBE-lacZ (Micchelli and Perrimon, 2006), fz3-RFP (Olson et al., 2011), nkd-lacZ (Zeng et al., 2000), 10× stat-GFP (Bach et al., 2007), hs-flp, UAS-mCD8:GFP; tub-Gal4, FRT82B, tub-Gal80 (Li et al., 2013a), hs-flp, tub-gal4, UAS-dsRed; FRT82B, tub-gal80 (Guo et al., 2013), hs-flp, tub-Gal80, FRT19A; tub-Gal4, UAS-mCD8:GFP (Choi et al., 2011), FRT82B (BDSC), 82B pygoS123 (Thompson et al., 2002), dshV26 FRT19A (Klingensmith et al., 1994; Wehrli and Tomlinson, 1998), tub>Flag-Axin; Axins044230 (Peterson-Nedry et al., 2008), Axins044230 (Hamada et al., 1999), BAC Axin-V5 integrated at the VK30 site (PBac{y[+]-attP-9A}VK00030) (Gerlach et al., 2014), Myo1A-Gal4 (Karpowicz et al., 2013), Myo1A-Gal4, tub-Gal80ts, UAS-GFP (Jiang et al., 2009), esg-Gal4, tub-Gal80ts, UAS-GFP (Micchelli and Perrimon, 2006), UAS-Tnks RNAi#1 (VDRC#106238), UAS-Tnks RNAi#2 (Tnks-RNAi-2) (Feng et al., 2014), UAS-dsh RNAi (VDRC#31306), UAS-upd3 RNAi#1 (VDRC#106869), UAS-upd3 RNAi#2 (VDRC#27136), UAS-rpr3-5/TM3. Canton S flies were used as controls, and all crosses were performed at 25°C unless otherwise indicated. Only adult female flies were analyzed in this study.
Clonal analysis and RNAi experiments
Mitotic clones were generated using the MARCM system (Lee and Luo, 2001). Adult flies were subjected to a 30 min heat shock in a 37°C water bath on the day of eclosion. dsh mutant clones were induced by two 2 h heat shocks, with at least 3 h between consecutive heat shocks. After heat shock, flies were maintained at 25°C for 4-6 days before analysis. For analysis of fz3-RFP expression (Fig. 4 and Fig. S2), clones were induced in 3rd instar larvae by a single 2 h heat shock and examined 1-2 days after eclosion, as fz3-RFP expression becomes less homogenous with age. For quantification of clone size, only clones in the posterior midgut were included in the analysis (Fig. 3C and Fig. 5G). For RNAi experiments, crosses were maintained at 22°C and progeny of desired genotypes were collected on the day of eclosion and kept at 29°C for 7 or 10 days before dissection.
Antibodies
Antibodies against Axin and Tnks were generated as described in supplementary Materials and Methods. Both antibodies were used at 1:1000 for immunoblots. Other primary antibodies used were chicken anti-GFP (Abcam, ab13970, 1:10,000), mouse anti-Arm (DSHB, 1:20), mouse anti-Prospero (DSHB, 1:100), mouse anti-Delta (DSHB, 1:100), rabbit anti-dsRed (Clontech, 632496, 1:1000), mouse anti-β-gal (Promega, Z3788, 1:5000), rabbit anti-β-gal (MP Biomedicals, 559762, 1:5000), rabbit anti-Pdm1 (Lee et al., 2009) (gift from Dr Xiaohang Yang, Institute of Molecular and Cell Biology, Singapore, 1:200), rabbit anti-phospho-histone H3 (Ser10) (Millipore, 06-570, 1:1000), rabbit anti-cleaved Dcp-1 (Cell Signaling, 9578S 1:100), rabbit anti-Kinesin Heavy Chain (Cytoskeleton, AKIN01-A, 1:10,000 for immunoblots). Secondary antibodies used for immunostaining were goat or donkey Alexa Fluor 488 or 555 conjugates (Invitrogen, 1:400) and goat Cy5 conjugates (Jackson ImmunoResearch, 1:200). Secondary antibodies used for immunoblots were goat anti-guinea pig HRP-conjugates (Jackson ImmunoResearch, 1:5000) or goat anti-rabbit HRP-conjugates (Bio-Rad, 1:10,000).
Immunostaining and immunoblots
For immunostaining, adult intestines were dissected in PBS, fixed in 4% paraformaldehyde in PBS for 45 min. For Delta staining, intestines were fixed in 8% formaldehyde, 200 mM sodium cacodylate, 100 mM sucrose, 40 mM potassium acetate, 10 mM sodium acetate and 10 mM EGTA for 20 min at room temperature (O'Brien et al., 2011). Tissues were then washed in PBS with 0.1% Triton X-100, followed by incubation in PBS with 0.1% Tween-20 and 10% BSA for 1 h at room temperature. Incubation with primary antibodies was performed at 4°C overnight in PBS with 0.5% Triton X-100. Incubation with secondary antibodies was for 2 h at room temperature. Specimens were mounted in Prolong Gold (Invitrogen). Fluorescent images were obtained on a Nikon A1RSi confocal microscope and processed using Adobe Photoshop software.
For midgut lysates used in immunoblots, midguts from 5-day-old adults were dissected in cold PBS, and then treated with 1× Trypsin in EDTA (Corning Life Sciences) for 2 h at room temperature. Tissues were washed with PBS and homogenized in 4× Laemmli loading buffer. Lysates were incubated for 5 min at 100°C before SDS-PAGE analysis.
RT-qPCR
Adult females were shifted to 29°C after eclosion for 7 days before analysis. Total RNA was extracted from 35 posterior midguts and cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen). cDNA was analyzed in triplicate using the StepOnePlus Real-Time PCR system (Applied Biosystems). Expression of the target genes was measured relative to that of rpl32 (rp49). Experiments were performed three times with independent biological samples and representative results are shown in Fig. 8A. Primers used are listed in supplementary Materials and Methods.
Life-span assay
For the life-span assay, 5-day-old wild-type or Tnks19/503 mutant flies were placed in empty vials containing a 4×2.5 cm piece of chromatography paper. As a feeding medium, 400 μl of 5% sucrose solution was applied to the paper. Flies were transferred to new vials with fresh sucrose solution each day. Sixty flies of each genotype were used in this assay and 15 flies were placed in each vial. Experiments were performed at 29°C.
Quantification and statistics
For ISC quantification, flies were stained with anti-Delta and anti-Prospero antibodies. Images of the R5a region (Buchon et al., 2013) were obtained with a 60× objective and the total number of Dl+ cells in a field of 0.023 mm2 were counted. Progenitor cells were identified by esg>GFP (Fig. 5F), esg-lacZ (Fig. 6J) or by their smaller size, strong membrane-associated Arm and absence of nuclear Prospero (Fig. 7D). For quantification, the total number of progenitor cells (Fig. 5F and Fig. 7D), or progenitor cells outside indicated clones (Fig. 6J), was counted in a field of 0.032 mm2 within R5a. In Tnks mutants, in which some ECs were also marked by esg>GFP, only progenitor cells were counted. For quantification of pH3+ cells, the total number of pH3+ cells in the posterior midgut of the indicated genotypes was counted. For quantification of stat-GFP immunostaining intensity, each stat-GFP+ cell was identified using Imaris software (Bitplane). The mean intensity in cells within a field (40 μm×40 μm) surrounding a Tnks mutant clone or an equal field at least 50 μm away from the clone was measured. The relative intensity was calculated and shown in Fig. 8E. All t-tests were performed using Prism (GraphPad).
Acknowledgements
We thank Benjamin Ohlstein, Julia Cordero, Craig Micchelli, Ramanuj DasGupta, Norbert Perrimon, Claude Desplan, Konrad Basler, Matthew Scott, Yanrui Jiang, Jean-Paul Vincent, Xinhua Lin, Gary Struhl, Bruno Lemaitre, Xiaohang Yang and Yu Cai for kind gifts of flies and reagents. We also thank the Bloomington Stock Center and the Vienna Drosophila Research Center for fly stocks, the Drosophila Genomics Resource Center and Developmental Studies Hybridroma Bank for reagents. We thank Lucy Erin O'Brien, Rongwen Xi, Julia Cordero and Bruno Lemaitre for providing protocols and advice, Victoria Marlar, Arvonn Tully, and Ann Lavanway for technical support, and Girish Deshpande and Claudio Pikielny for thoughtful comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Z.W., A.T., H.B. and Y.A. conceived the study. Z.W., A.T., H.B., O.T.-B., E.Y. and Y.A. designed and performed the experiments. H.N. provided reagents. Z.W. and Y.A. wrote the manuscript.
Funding
This work was funded by grants from the Office of Extramural Research, National Institutes of Health [RO1CA105038 to Y.A., P40OD018537 to the BDSC], the Emerald Foundation (to Y.A.), the Norris Cotton Cancer Center (to Y.A.) and the National Science Foundation [DBI-1039423 for the purchase of a Nikon A1RSi confocal microscope]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.127647/-/DC1
References
- Bach E. A., Ekas L. A., Ayala-Camargo A., Flaherty M. S., Lee H., Perrimon N. and Baeg G.-H. (2007). GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7, 323-331. 10.1016/j.modgep.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Beehler-Evans R. and Micchelli C. A. (2015). Generation of enteroendocrine cell diversity in midgut stem cell lineages. Development 142, 654-664. 10.1242/dev.114959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B. and Jasper H. (2011). EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045-1055. 10.1242/dev.056671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B. and Jasper H. (2014). Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 7, 1867-1875. 10.1016/j.celrep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Osman D., David F. P. A., Yu Fang H., Boquete J.-P., Deplancke B. and Lemaitre B. (2013). Morphological and molecular characterization of adult midgut compartmentalization in Drosophila. Cell Rep. 3, 1725-1738. 10.1016/j.celrep.2013.04.001 [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M. E., Tang W., Lu J., Ma Z., Fan C.-W., Wei S., Hao W., Kilgore J., Williams N. S. et al. (2009). Small molecule–mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100-107. 10.1038/nchembio.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y. J., Hsiao S. J., Yver D., Cushman S. W., Tessarollo L., Smith S. and Hodes R. J. (2008). Tankyrase 1 and tankyrase 2 are essential but redundant for mouse embryonic development. PLoS ONE 3, e2639 10.1371/journal.pone.0002639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N. H., Lucchetta E. and Ohlstein B. (2011). Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc. Natl. Acad. Sci. USA 108, 18702-18707. 10.1073/pnas.1109348108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. and Nusse R. (2012). Wnt/beta-catenin signaling and disease. Cell 149, 1192-1205. 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Stefanatos R. K., Scopelliti A., Vidal M. and Sansom O. J. (2012). Inducible progenitor-derived Wingless regulates adult midgut regeneration in Drosophila. EMBO J. 31, 3901-3917. 10.1038/emboj.2012.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Li X., Ray L., Song H., Qu J., Lin S. and Lin X. (2014). The Drosophila tankyrase regulates Wg signaling depending on the concentration of Daxin. Cell. Signal. 26, 1717-1724. 10.1016/j.cellsig.2014.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevr T., Robine S., Louvard D. and Huelsken J. (2007). Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol. Cell. Biol. 27, 7551-7559. 10.1128/MCB.01034-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. P., Emmink B. L., Nojima H., Kranenburg O. and Maurice M. M. (2014). Wnt signalling induces accumulation of phosphorylated beta-catenin in two distinct cytosolic complexes. Open Biol. 4, 140120 10.1098/rsob.140120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Driver I. and Ohlstein B. (2013). Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J. Cell Biol. 201, 945-961. 10.1083/jcb.201302049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada F., Tomoyasu Y., Takatsu Y., Nakamura M., Nagai S.-i., Suzuki A., Fujita F., Shibuya H., Toyoshima K., Ueno N. et al. (1999). Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283, 1739-1742. 10.1126/science.283.5408.1739 [DOI] [PubMed] [Google Scholar]
- Huang S.-M. A., Mishina Y. M., Liu S., Cheung A., Stegmeier F., Michaud G. A., Charlat O., Wiellette E., Zhang Y., Wiessner S. et al. (2009). Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614-620. 10.1038/nature08356 [DOI] [PubMed] [Google Scholar]
- Ireland H., Kemp R., Houghton C., Howard L., Clarke A. R., Sansom O. J. and Winton D. J. (2004). Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology 126, 1236-1246. 10.1053/j.gastro.2004.03.020 [DOI] [PubMed] [Google Scholar]
- Jiang H. and Edgar B. A. (2011). Intestinal stem cells in the adult Drosophila midgut. Exp. Cell Res. 317, 2780-2788. 10.1016/j.yexcr.2011.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., McEwen D. G. and Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343-1355. 10.1016/j.cell.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Grenley M. O., Bravo M.-J., Blumhagen R. Z. and Edgar B. A. (2011). EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84-95. 10.1016/j.stem.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P., Zhang Y., Hogenesch J. B., Emery P. and Perrimon N. (2013). The circadian clock gates the intestinal stem cell regenerative state. Cell Rep. 3, 996-1004. 10.1016/j.celrep.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith J., Nusse R. and Perrimon N. (1994). The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 8, 118-130. 10.1101/gad.8.1.118 [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B. and Clevers H. (1997). Constitutive transcriptional activation by a beta -catenin-Tcf complex in APC-/- colon carcinoma. Science 275, 1784-1787. 10.1126/science.275.5307.1784 [DOI] [PubMed] [Google Scholar]
- Korinek V., Barker N., Moerer P., van Donselaar E., Huls G., Peters P. J. and Clevers H. (1998). Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379-383. 10.1038/1270 [DOI] [PubMed] [Google Scholar]
- Kramps T., Peter O., Brunner E., Nellen D., Froesch B., Chatterjee S., Murone M., Züllig S. and Basler K. (2002). Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109, 47-60. 10.1016/S0092-8674(02)00679-7 [DOI] [PubMed] [Google Scholar]
- Lau T., Chan E., Callow M., Waaler J., Boggs J., Blake R. A., Magnuson S., Sambrone A., Schutten M., Firestein R. et al. (2013). A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 73, 3132-3144. 10.1158/0008-5472.CAN-12-4562 [DOI] [PubMed] [Google Scholar]
- Lee T. and Luo L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251-254. 10.1016/S0166-2236(00)01791-4 [DOI] [PubMed] [Google Scholar]
- Lee W.-C., Beebe K., Sudmeier L. and Micchelli C. A. (2009). Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development 136, 2255-2264. 10.1242/dev.035196 [DOI] [PubMed] [Google Scholar]
- Li X., Erclik T., Bertet C., Chen Z., Voutev R., Venkatesh S., Morante J., Celik A. and Desplan C. (2013a). Temporal patterning of Drosophila medulla neuroblasts controls neural fates. Nature 498, 456-462. 10.1038/nature12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhang Y., Han L., Shi L. and Lin X. (2013b). Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev. Cell 24, 133-143. 10.1016/j.devcel.2012.12.010 [DOI] [PubMed] [Google Scholar]
- Lin G., Xu N. and Xi R. (2008). Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119-1123. 10.1038/nature07329 [DOI] [PubMed] [Google Scholar]
- Lum L. and Clevers H. (2012). Cell biology. The unusual case of Porcupine. Science 337, 922-923. 10.1126/science.1228179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K. and He X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9-26. 10.1016/j.devcel.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S. E., Mao Z. and Davis R. L. (2004). Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 10.1126/stke.2202004pl6 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A. and Perrimon N. (2006). Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475-479. 10.1038/nature04371 [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B. and Kinzler K. W. (1997). Activation of beta -catenin-Tcf signaling in colon cancer by mutations in beta -catenin or APC. Science 275, 1787-1790. 10.1126/science.275.5307.1787 [DOI] [PubMed] [Google Scholar]
- Morrone S., Cheng Z., Moon R. T., Cong F. and Xu W. (2012). Crystal structure of a Tankyrase-Axin complex and its implications for Axin turnover and Tankyrase substrate recruitment. Proc. Natl. Acad. Sci. USA 109, 1500-1505. 10.1073/pnas.1116618109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L. E., Soliman S. S., Li X. and Bilder D. (2011). Altered modes of stem cell division drive adaptive intestinal growth. Cell 147, 603-614. 10.1016/j.cell.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B. and Spradling A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470-474. 10.1038/nature04333 [DOI] [PubMed] [Google Scholar]
- Ohlstein B. and Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988-992. 10.1126/science.1136606 [DOI] [PubMed] [Google Scholar]
- Olson E. R., Pancratov R., Chatterjee S. S., Changkakoty B., Pervaiz Z. and DasGupta R. (2011). Yan, an ETS-domain transcription factor, negatively modulates the Wingless pathway in the Drosophila eye. EMBO Rep. 12, 1047-1054. 10.1038/embor.2011.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare A. C., Dean D. M. and Ewer J. (2009). Construction and characterization of deletions with defined end points in Drosophila using P elements in trans. Genetics 181, 53-63. 10.1534/genetics.108.094193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D. S., Jemison J. and Cadigan K. M. (2002). Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129, 2565-2576. [DOI] [PubMed] [Google Scholar]
- Parks A. L., Cook K. R., Belvin M., Dompe N. A., Fawcett R., Huppert K., Tan L. R., Winter C. G., Bogart K. P., Deal J. E. et al. (2004). Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36, 288-292. 10.1038/ng1312 [DOI] [PubMed] [Google Scholar]
- Peterson-Nedry W., Erdeniz N., Kremer S., Yu J., Baig-Lewis S. and Wehrli M. (2008). Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev. Biol. 320, 226-241. 10.1016/j.ydbio.2008.05.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Mahaffey J. P., Alcorn H. L. and Anderson K. V. (2011). Tissue-specific roles of Axin2 in the inhibition and activation of Wnt signaling in the mouse embryo. Proc. Natl. Acad. Sci. USA 108, 8692-8697. 10.1073/pnas.1100328108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Kojima T., Ui-Tei K., Miyata Y. and Saigo K. (1999). Dfrizzled-3, a new Drosophila Wnt receptor, acting as an attenuator of Wingless signaling in wingless hypomorphic mutants. Development 126, 4421-4430. [DOI] [PubMed] [Google Scholar]
- Sbodio J. I., Lodish H. F. and Chi N.-W. (2002). Tankyrase-2 oligomerizes with tankyrase-1 and binds to both TRF1 (telomere-repeat-binding factor 1) and IRAP (insulin-responsive aminopeptidase). Biochem. J. 361, 451-459. 10.1042/bj3610451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R., Calleja M., Morata G. and Basler K. (2000). The Wingless target gene Dfz3 encodes a new member of the Drosophila Frizzled family. Mech. Dev. 91, 427-431. 10.1016/S0925-4773(99)00313-5 [DOI] [PubMed] [Google Scholar]
- Smith S., Giriat I., Schmitt A. and de Lange T. (1998). Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 282, 1484-1487. 10.1126/science.282.5393.1484 [DOI] [PubMed] [Google Scholar]
- Thompson B., Townsley F., Rosin-Arbesfeld R., Musisi H. and Bienz M. (2002). A new nuclear component of the Wnt signalling pathway. Nat. Cell Biol. 4, 367-373. 10.1038/ncb786 [DOI] [PubMed] [Google Scholar]
- Tian A. and Jiang J. (2014). Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. eLife 3, e01857 10.7554/eLife.01857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian A., Benchabane H., Wang Z. and Ahmed Y. (2016). Regulation of stem cell proliferation and cell fate specification by wingless/Wnt signaling gradients enriched at adult intestinal compartment boundaries. PLoS Genet. 12, e1005822 10.1371/journal.pgen.1005822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski N. S., Wehrli M., Rives A., Erdeniz N., DiNardo S. and Wieschaus E. (2003). Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev. Cell 4, 407-418. 10.1016/S1534-5807(03)00063-7 [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Cavallo R., Dooijes D., van Beest M., van Es J., Loureiro J., Ypma A., Hursh D., Jones T., Bejsovec A. et al. (1997). Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789-799. 10.1016/S0092-8674(00)81925-X [DOI] [PubMed] [Google Scholar]
- van Es J. H., Haegebarth A., Kujala P., Itzkovitz S., Koo B.-K., Boj S. F., Korving J., van den Born M., van Oudenaarden A., Robine S. et al. (2012). A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell. Biol. 32, 1918-1927. 10.1128/MCB.06288-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaler J., Machon O., Tumova L., Dinh H., Korinek V., Wilson S. R., Paulsen J. E., Pedersen N. M., Eide T. J., Machonova O. et al. (2012). A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 72, 2822-2832. 10.1158/0008-5472.CAN-11-3336 [DOI] [PubMed] [Google Scholar]
- Wang Z., Tacchelly-Benites O., Yang E., Thorne C. A., Nojima H., Lee E. and Ahmed Y. (2016). Wnt/Wingless pathway activation is promoted by a critical threshold of Axin maintained by the tumor suppressor APC and the ADP-ribose polymerase Tankyrase. Genetics. 203, 1-13. 10.1534/genetics.115.183244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner D. and Weidinger G. (2015). Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 31, 336-343. 10.1016/j.tig.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Wehrli M. and Tomlinson A. (1998). Independent regulation of anterior/posterior and equatorial/polar polarity in the Drosophila eye; evidence for the involvement of Wnt signaling in the equatorial/polar axis. Development 125, 1421-1432. [DOI] [PubMed] [Google Scholar]
- Wehrli M., Dougan S. T., Caldwell K., O'Keefe L., Schwartz S., Vaizel-Ohayon D., Schejter E., Tomlinson A. and DiNardo S. (2000). arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407, 527-530. 10.1038/35035110 [DOI] [PubMed] [Google Scholar]
- Willert K., Shibamoto S. and Nusse R. (1999). Wnt-induced dephosphorylation of axin releases beta -catenin from the axin complex. Genes Dev. 13, 1768-1773. 10.1101/gad.13.14.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N., Wang S. Q., Tan D., Gao Y., Lin G. and Xi R. (2011). EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev. Biol. 354, 31-43. 10.1016/j.ydbio.2011.03.018 [DOI] [PubMed] [Google Scholar]
- Yang E., Tacchelly-Benites O., Wang Z., Randall M. P., Tian A., Benchabane H., Freemantle S., Pikielny C., Tolwinski N. S., Lee E. et al. (2016). Wnt pathway activation by ADP-ribosylation. Nat. Commun. 7, 11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X. and Hou S. X. (2015). Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 142, 644-653. 10.1242/dev.113357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Wharton K. A. Jr., Mack J. A., Wang K., Gadbaw M., Suyama K., Klein P. S. and Scott M. P. (2000). naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403, 789-795. 10.1038/35001615 [DOI] [PubMed] [Google Scholar]
- Zielke N., Korzelius J., van Straaten M., Bender K., Schuhknecht G. F. P., Dutta D., Xiang J. and Edgar B. A. (2014). Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell Rep. 7, 588-598. 10.1016/j.celrep.2014.03.020 [DOI] [PubMed] [Google Scholar]