Abstract
Metabolic profiling has great potential to help the diagnosis and prognosis of cancer patients. Fanconi Anemia (FA) tumor suppressor signaling has been instrumental in understanding human-tumorigenesis. However, this instrumental understanding has never been demonstrated at the metabolic level. Here we show that impaired FA signaling can lead cells to exhibit metabolic signatures of tumorigenesis. This is consistent with our original studies of the roles of FA signaling in suppressing non-FA tumorigenesis at functional and genetic levels. Using ultra-performance liquid chromatography-mass spectroscopy and gas chromatography-mass spectrometry, we characterized metabolic alterations in bladder cancer cells carrying an intact or impaired FA pathway. The latter was obtained by ectopically expressing FAVL (FAVL-high), which we previously found to be capable of inactivating FA signaling. 18 metabolites, end products of cell proliferation and/or apoptosis, were significantly different between FAVL-high and -low cells. Methionine, phenylalanine and threonine, resulting from a tumorigenic process, were substantially increased in FAVL-high cells. With this study, we achieved genomic, functional, and metabolomic characterization on roles of FA signaling in the development of human cancer. Furthermore, this study provides novel insights into how to translate FA basic research into strategies for producing effective biomarkers in human cancer diagnosis and prognosis.
Keywords: Metabolomics, Bladder cancer, FA pathway, FAVL, Cell proliferation
INTRODUCTION
Metabolomics is the study of the small molecule composition (metabolites <1,000 Da) present within in bio-fluids, tissue samples, and cell lines. Genomics (genomic variance), transcriptomics (gene expression), proteomics (protein abundance) and metabolomics (metabolite concentrations) are examples of several “omics” levels by which biological systems are studied in both healthy and diseased states. These complement each other and provide readouts that are closest to the clinical endpoint.1 Following prostate cancer, bladder cancer (BC) is the second most commonly diagnosed malignancy of the urinary system. It is a major cause of mortality around the world with approximately 150,000 deaths annually. However, within the US alone it is estimated that BC will account for approximately 16,000 deaths of the 74,000 new cases in 2015.2 In recent years, numerous urine-based bladder tumor markers (UBBTMs) have been evaluated for the diagnosis of BC. Most notably, bladder tumor antigen (BTA), urinary nuclear matrix protein 22 (NMP22), ImmunoCyt, fibrin degradation product (FDP), and UroVysion assays have all achieved FDA approval for diagnostic purposes.3 These protocols are, however, limited by their poor sensitivity, invasiveness, and cost. Unfortunately, delayed diagnosis, in turn is associated with an increased risk of death from BC, even for patients within the low-risk threshold of the disease. Therefore, an accurate, highly sensitive, specific and low cost diagnostic test to identify patients early on with BC is desperately needed.
Fanconi anemia (FA), a genetic disease that affects approximately 1-3 of 500,000 newborns, is characterized by a broad spectrum of congenital abnormalities such as short stature, abnormalities of the skin, arms, head, eyes, and kidneys, bone marrow failure, a predisposition to hematological and solid malignancies, and the presence of spontaneous and induced chromosomal breakages. Hallmarks of FA include: chromosomal fragility and a hypersensitivity to drugs that induce DNA inter or/and intra strand cross-links (ICLs). Until now, 19 FA genes have been identified (FANC-A, -B, -C, -D1, -D2, -E, -F, -G, -I, -J, -L, -M, -N, -O, -P, -Q, -R, -S and -T).4-6 These 19 FA genes encode for proteins involved in a common DNA repair pathway (FA-BRCA), which is responsible for repair of DNA ICLs.7 Therefore, the absence or dysfunction of any one of these proteins can drastically reduce DNA repair efficiency. When this process is impaired, an accumulation of chromosomal breakage occurs, leading to genomic instability, aging and ultimately cancer.8 We discovered the novel alternative splice variant of FANCL, named FAVL, through which we demonstrated the essential role of an intact FA-BRCA signaling pathway in suppressing the development of non-FA human cancer. We reported that at a functional level, FAVL impairment in the FA pathway contributes to BC development,9,10 also at a genetic level amongst the general population, we documented that the mutated FA pathway resulting from any one mutated FA gene is significantly associated with the development of human BC.11 These studies, for the first time, hold a profound implication in directly advancing our understanding of the BC development, achieved through contributions of functional and genetic evidence that the FA pathway performs tumor suppressor functions in cancer patients without FA, which extends to the exciting field of metabolomics in this study.
The application of metabolic profiling provides unique information, which can reveal molecular mechanisms when used in conjunction with genome-wide analysis such as gene expression.12 In cancer cells, the Warburg effect describes the occurrence of metabolic reprogramming,13 which is characterized by an increase in glucose uptake, and flux into anabolic pathways to promote cell growth.14 Currently, metabolomics has demonstrated its practicality by the identification of BC biomarkers in urine,15,16 plasma and tissue samples.17,18 Additionally, metabolic studies in cell culture are highly valuable19 as they can identify functional biomarkers that represent cellular processes,20,21 and/or provide insights into the individuality of various cancer cell lines.22,23 These are essential for a comprehensive understanding of cancer cell biology and to complement clinical studies.19 In this study, we validated our novel findings that the tumorigenecity of the FAVL impaired FA pathway contributes to BC development at the metabolic level.
METHODS
Cell culture and chemicals
Human urinary bladder transitional cell carcinoma cell line T24 (HTB-4) was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cell lines used were prepared as previously described.9 HTB-4 cells were used to compare and accurately assess the effects of FAVL. HTB-4 cells with ectopically expressed FAVL were termed FAVL-high cells; and empty vector-transfected controls cells accordingly were termed FAVL-low cells. For the experiments, cells were trypsinized, one million cells were counted and washed with 1X PBS; cell pellets were frozen at −80°C. Three replicates were prepared for each cell types. Ethanol, pyridine, methoxyamine hydrochloride, C8–C30 fatty acid methyl esters (FAMEs), and ammonium acetate were purchased from Sigma Aldrich (St. Louis, MO). LC-MS Optima grade methanol and acetonitrile, formic acid, N-Methyl-N-trimethylsilyltrifluoroacetamide (MSTFA) with 1% TMCS, and hexane were obtained from Fisher Sci. (Fair Lawn, NJ). The ultra-pure water was produced by Millipore Advantage A10 system with a LC-MS Polisher filter (Billerica, MA).
Sample preparation for metabolic profiling
The cell line samples were prepared as previously described with modifications.24-26 Appropriate weight of homogenizer beads and 50 μL of cold-water were added to the cell line samples for the first-step of extraction. Each aliquot of 270-μL mixture of ethanol: chloroform (3:1 = v/v) was added to the extracts for the second-step extraction. The sample extracts were centrifuged at 4°C and 14,500 rpm for 20 min. The supernatant was used for targeted metabolic profiling of lipids with UPLC-MS/MS, and for untargeted metabolic profiling with GC-TOFMS.
Lipid profiling
Each aliquot of 20 μL of the supernatant was added to a 96-well Biocrates Kit plate (Biocrates Life Sciences, Austria) for lipid quantitation. After samples were dried under nitrogen, each 300 μL of extraction solvent (5 mM ammonium acetate in methanol) was added and the kit plate was gently shaken at room temperature for 30 min. The sample extracts were filtered through the 0.45 μm membrane of the kit plate and each aliquot of 20 μL of sample was diluted with 380 μL of methanol with 5 mM ammonium acetate for flow injection analysis (FIA) analysis of lipids.
Untargeted metabolomics profiling with GC-TOFMS
Each aliquot of 250-μL above supernatant was dried with a freeze dryer. The dried samples were derivatized by methoxyamine hydrochloride in pyridine and subsequently by MSTFA. Retention indices C8–C30 fatty acid methyl esters (FAMEs) were added for retention time correction.
Metabolite measurements
Lipid analysis with UPLC-MS/MS26
An Acquity ultra performance liquid chromatography coupled to a Xevo TQ-S mass spectrometer (UPLC-MS/MS, Waters Corp., Milford, MA) was used for targeted metabolite analysis of 140 lipids in cell line samples. Each 10 μL of sample was directly injected into the mass spectrometer with elution solvent (methanol with 5 mM ammonium acetate) at a varied flow rate from 30 to 200 μL/min within 3 min.
Untargeted metabolomic profiling with GC-TOFMS25
Each 1-μl sample was analyzed on an Agilent 7890A gas chromatography coupled to a Leco Pegasus time of flight mass spectrometer (Leco Corp., St Joseph, MI) for global metabolite profiling. The analytes were introduced with a splitless mode to achieve maximum sensitivity and separated on an Rtx-5 MS capillary column (30 m × 0.25 mm I.D., 0.25 μm) (Restek, Bellefonte, PA). The column temperature was initially set to 80 °C for 2 min, increased to 300 °C for 12 min, and maintained at 300 °C for 5 min. The solvent delay was set to 4.4 min. The front inlet temperature, transferline temperature, and source temperature were set to 260 °C, 270 °C, and 220 °C, respectively. The mass spectrometer was operated on a full scan mode from 50 to 500 at an acquisition rate of 20 spectra/sec.
Statistical data analysis
The raw LC-MS/MS data files were processed with Target Lynx Application Manager (Waters Corp., Milford, MA) to extract peak area and retention time of each metabolite. The raw GC-TOFMS data files were processed with Chroma TOF software (Leco Corp., St Joseph, MI) to extract peak signal and retention time for each metabolite. The detected metabolites were annotated with our internal standard library using an automated mass spectral data processing (AMSDP) software package.27 PCA was applied to visualize the overall difference between the low and high FAVL cells along with SIMCA-P 12.0.1 (Umetrics, Umeå, Sweden). Nonparametric statistical analysis i.e., Mann Whitney U test was used for searching the significantly different metabolites between the groups with a critical p value of 0.05 and 0.01.
Molecular pathway and network analysis in Ingenuity Pathway Analysis (IPA)
To systematically understand the metabolites in FAVL elevated cells compare to control cells, we uploaded the metabolite lists (with HMDB IDs) and the change folds of the differentially expressed metabolites onto an IPA server (http://3crapps.cc.hawaii.edu/IPA/). Canonical pathways and chemical-protein interaction networks were generated based on the knowledge sorted in the Ingenuity Pathway Knowledge base. A ratio of the number of metabolites that map to the canonical pathway divided by the total number of molecules that map to the pathway was displayed. Fisher's exact test was used to calculate the p value to determine the probability that the association between the metabolites and the canonical pathway was explained by chance alone. The network score was based on the hypergeometric distribution and was calculated with the right-tailed Fisher's exact test. The higher a score was, the more relevant the eligible submitted molecules were to the network.
RESULT
The FA pathway is frequently mutated during the development of BC
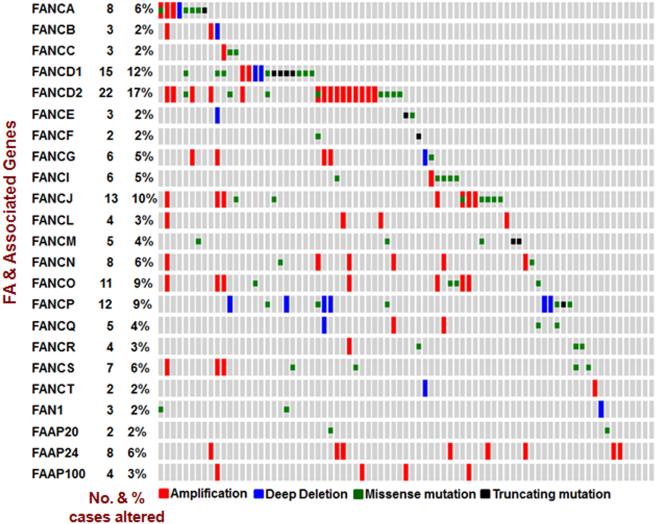
New tools and recent technologies have stimulated an in-depth understanding to the nature of cancer research especially in cellular response. As a result, the translation of the gained knowledge into methods of diagnosing and treating cancers has improved. The multi-component FA signaling pathway is expending, whereby the learned knowledge was suddenly off date and could not effectively help develop methods aiding clinical practice as expected. We were the first to report that the mutated FA pathway occurs in a variety of human cancers at a considerable rate. However, those rates are changing when we learned that additional players are also critical members for constituting the pathway. It has come to our realization that we need to update those upon which we emphasize the importance of the FA tumor suppressor signaling pathway in the suppression of human tumor development. We herein analyzed 23 key players in the FA pathway (FANCA/B/C/D1/D2/E/F/G/I/J/L/M/N/O/P/Q/R/S/T and FAN1, FAAP20/24/100), composed of 19 FA genes known and 4 functional partners. As we analyzed for 16 FA genes previously, we cross-referenced the available clinical information provided by the Cancer Genome Atlas (TCGA) via c-BioPortal 28,29. We found that, at the genetic level, the frequency of the mutated FA pathway is about 59% among 127 bladder cancer cases analyzed. As shown in Figure 1, the 23 players in the FA pathway do not act all normally among the majority (75/127) of bladder cancer cases tested.
Figure 1. Key players in the FA pathway are highly mutated in BC development.
23 genes (FANCA/B/C/D1/D2/E/F/G/I/J/L/M/N/O/P/Q/R/S/T and FAN1, FAAP20/24/100), encoding key players of the FA pathway, were analyzed by using online data sets of 127 BC tissue sample and data-mining tools (the c-BioPortal for Cancer Genomics and the data sets from the TCGA research Network).
Metabolic profiling is significantly differentiated between BC cells harboring an intact and impaired FA pathway
Metabolomics is certainly complementary to other “omics”, such as genomics and proteomics. It is important to recognize that the profiling taken from gene and/or protein expression only provides a partial characterization of the pathophysiological status. This can be due to the fact that a single gene can have a functional role in a number of cellular processes 18. The changes in the metabolome, however, can more accurately reflect the ultimate status of all cellular processes occurred. To date, the relationship between the mutated FA pathway and human cancer in a metabolic-wide manner has yet to be elucidated.
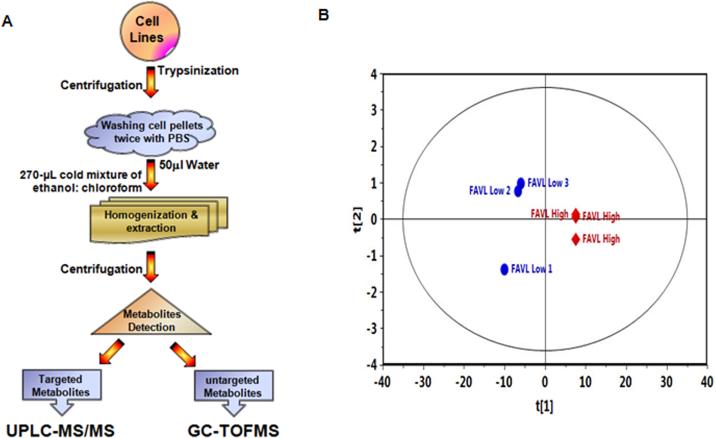
Our previous studies strongly suggest that FAVL is often highly expressed in a variety of human cancers, including lung, prostate and BCs.9,10 At a functional level, FAVL appears to be a tumor promotion factor, which may function throughout all phases of tumor development within the bladder by deactivating FA signaling. Therefore, it has become a useful tool to study how an intact pathway suppresses human tumorigenesis. Using targeted (UPLC-MS/MS) and untargeted (GC-TOFMS) metabolomics analysis we determined the metabolite alterations within a set of human urinary bladder transitional cell carcinoma cells (HTB-4). The HTB4 cells consisted of either ectopically expressing FAVL (FAVL-high cells) or the corresponding empty vector (FAVL-low cells). As shown in Table 1, the dataset resulted in 206 individual metabolites, consisting of 141 metabolites measured by LC-MS platform and 65 metabolites measured by GC-MS. Principal component analysis (PCA) revealed clear separation between FAVL-low and high cells. This included a tight clustering of biological triplicates (Figure 2), which confirmed their strong metabolic diversity. To provide a broader insight into the data set, we applied the Mann Whitney U test to analyze all 206 metabolites, searching for differences in metabolites between groups, with respect to the critical p value (0.05 and 0.01). We found that, of the 206 metabolites (Figure 3 & Table 1), 80 were significantly altered in FAVL-high cells compared to FAVL-low ones (Table 2).
Table 1.
Targeted and Untargeted Metabolic Profile
| Platform | GC-MS | LC-MS | Total |
|---|---|---|---|
| Measured Metabolites | 65 | 141 | 206 |
| Differential Metabolites (p≤ 0.05 with t-test) | 16 | 56 | 72 |
| Differential Metabolites (p≤ 0.01 with t-test) | 2 | 6 | 8 |
Figure 2. Overall metabolic profiling of BC cells carrying an intact or impaired FA pathway.
A. Schematic workflow for both targeted (UPLC-MS/MS) and untargeted (GC-TOFMS) analyses of FAVL-high and low cells (carrying impaired and intact FA signaling respectively). The results are normally represented as metabolic area ratios corrected by international standards. B. OPLS-DA score plots of metabolic profiles derived from the data of GC–TOFMS among FAVL-high and low samples.
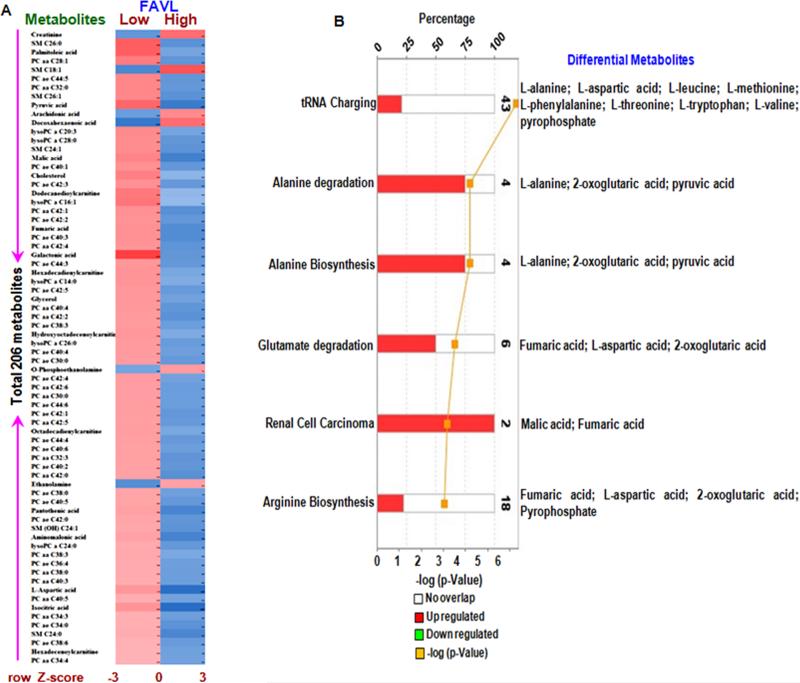
Figure 3. Metabolic profiles for BC cells carrying an impaired FA pathway compared to cells carrying an intact FA pathway.
A. The heatmap for the intensity variations of the total 206 metabolites identified with GC-TOFMS and UPLC-MS/MS together. B. Most significantly altered canonical pathways and its metabolites revealed by IPA analysis.
Table 2.
Differentially Expressed GC-MS Platform Metabolites between low and high FAVL Expressed Cells
| Metabolic group/ metabolites | p-value | Ratio (vector/ FAVL) |
|---|---|---|
| Differential Metabolites (p≤ 0.05 with t-test) | ||
| Pyruvic acid | 1.02 × 10−2 | 1.72 |
| Arachidonic acid | 1.21 × 10−2 | 0.64 |
| Docosahexaenoic acid | 1.28 × 10−2 | 0.72 |
| Malic acid | 1.47 × 10−2 | 1.88 |
| Cholesterol | 1.51 × 10−2 | 1.17 |
| Fumaric acid | 1.80 × 10−2 | 1.88 |
| Galactonic acid | 1.94 × 10−2 | 5.72 |
| Glycerol | 2.16 × 10−2 | 1.49 |
| O-Phosphoethanolamine | 2.57 × 10−2 | 0.29 |
| Octadecadienylcarnitine | 2.74 × 10−2 | 1.81 |
| Ethanolamine | 3.15 × 10−2 | 0.45 |
| Pantothenic acid | 3.45 × 10−2 | 1.91 |
| Aminomalonic acid | 3.73 × 10−2 | 1.73 |
| L-Aspartic acid | 4.26 × 10−2 | 1.56 |
| Isocitric acid | 4.32 × 10−2 | 1.92 |
| Hexadecenoylcarnitine | 4.88 × 10−2 | 1.77 |
|
Differential Metabolites (p≤ 0.01 with t-test) | ||
| Creatinine | 5.07 × 10−2 | 0.79 |
| Palmitoleic acid | 3.45 × 10−2 | 1.56 |
Cellular processes are occurring differently between bladder cancer cells carrying an intact and an impaired FA pathway (FAVL-low and -high cells, respectively)
To investigate the metabolic roles of FAVL in BC, we performed molecular pathway and network analysis using Ingenuity Pathway Analysis (IPA). Following data processing, a heat map of the metabolites was constructed to visualize the changes in metabolites within FAVL-high cells compared to FAVL-low cells (Figure 3A). We found that 18 metabolites (pyruvic acid, arachidonic acid, docosahexaenoic acid, malic acid, cholesterol, fumaric acid, galactonic acid, glycerol, o-phosphoethanolamine, octadecadienylcarnitine, ethanolamine, pantothenic acid, aminomalonic acid, L-aspartic acid, isocitric acid, hexadecenoylcarnitine, creatinine and palmitoleic acid) (Table 3) were significantly elevated in FAVL-high cells out of 80 identified differential metabolites (Table 1). Among those, 11 metabolites were involved in fatty acid metabolism, 5 metabolites were involved in carbohydrate metabolism and 2 metabolites were involved in amino acid metabolism (Table 2). IPA revealed that tRNA charging (9/43), alanine degradation (3/4), alanine biosynthesis (3/4), glutamate degradation (3/6), and arginine biosynthesis (2/4) (Figure 3B and Table 3) were the 5 most significantly altered pathways in FAVL-high cells compared to FAVL-low cells, all of which are typically active during tumorigenesis.
Table 3.
Summary of Metabolomics Analysis
| Canonical Pathways | p-value | Overlap |
|---|---|---|
| tRNA Charging | 5.20 × 10−7 | 20.9 % (9/43) |
| Alanine Degradation III | 6.23 × 10−5 | 75.0 % (3/4) |
| Alanine Biosynthesis II | 6.23 × 10−5 | 75.0 % (3/4) |
| Glutamate Degradation II | 3.01 × 10−4 | 50.0 % (3/6) |
| Renal Cell Carcinoma Signaling | 6.50 × 10−4 | 100.0 % (2/2) |
| Diseases and Bio Functions | p-value | Metabolites |
|---|---|---|
| Cancer | 4.81 × 10−2 - 1.09 × 10−7 | 13 |
| Gastrointestinal Disease | 4.81 × 10−2 - 1.09 × 10−7 | 14 |
| Hepatic System Disease | 4.81 × 10−2 - 1.09 × 10−7 | 9 |
| Organismal Injury and Abnormalities | 4.95 × 10−2 - 1.09 × 10−7 | 18 |
| Metabolic Disease | 4.81 × 10−2 - 2.33 × 10−4 | 11 |
| Molecular and Cellular Functions | p-value | Metabolites |
|---|---|---|
| Cellular Growth and Proliferation | 4.81 × 10−2 - 7.00 × 10−6 | 10 |
| Small Molecule Biochemistry | 4.95 × 10−2 - 3.91 × 10−6 | 21 |
| Cellular Function and Maintenance | 4.95 × 10−2 - 2.71 × 10−6 | 16 |
| Amino Acid Metabolism | 4.81 × 10−2 - 3.91 × 10−6 | 12 |
| Carbohydrate Metabolism | 4.81 × 10−2 - 2.71 × 10−5 | 11 |
| Toxicology Functions | ||
|---|---|---|
| Clinical Chemistry and Hematology | p-value | Metabolites |
| Increased Levels of Albumin | 4.36 × 10−6 - 4.36 × 10−6 | 5 |
| Increased Levels of LDH | 4.21 × 10−1 - 9.40 × 10−2 | 3 |
| Increased Levels of Alkaline Phosphatase | 4.81 × 10−2 - 4.81 × 10−2 | 1 |
| Increased Levels of Blood Urea Nitrogen | 9.40 × 10−2 - 9.40 × 10−2 | 1 |
| Increased Levels of ALT | 1.16 × 10−1 - 1.16 × 10−1 | 1 |
| Nephrotoxicity | p-value | Metabolites |
|---|---|---|
| Renal Damage | 4.81 × 10−2 - 2.56 × 10−3 | 4 |
| Kidney Failure | 4.36 × 10−1 - 4.95 × 10−2 | 3 |
| Renal Degeneration | 2.44 × 10−2 - 2.44 × 10−2 | 1 |
| Renal Tubule Injury | 4.81 × 10−2 - 4.81 × 10−2 | 1 |
| Nephrosis | 9.40 × 10−2 - 9.40 × 10−2 | 1 |
| Top molecules | Fold change | |
|---|---|---|
| D-mannose | ⇑ 3.205 | |
| acetyl-L-carnitine | ⇑ 2.524 | |
| D-pantothenic acid | ⇑ 1.913 | |
| fumaric acid | ⇑ 1.883 | |
| malic acid | ⇑ 1.881 | |
| pyruvic acid | ⇑ 1.717 | |
| L-aspartic acid | ⇑ 1.559 | |
| glycerol | ⇑ 1.485 | |
| L-tryptophan | ⇑ 1.445 | |
| L-methionine | ⇑ 1.404 | |
Biological functional pathways are altered significantly in FAVL-high BC cells compared to FAVL-low ones
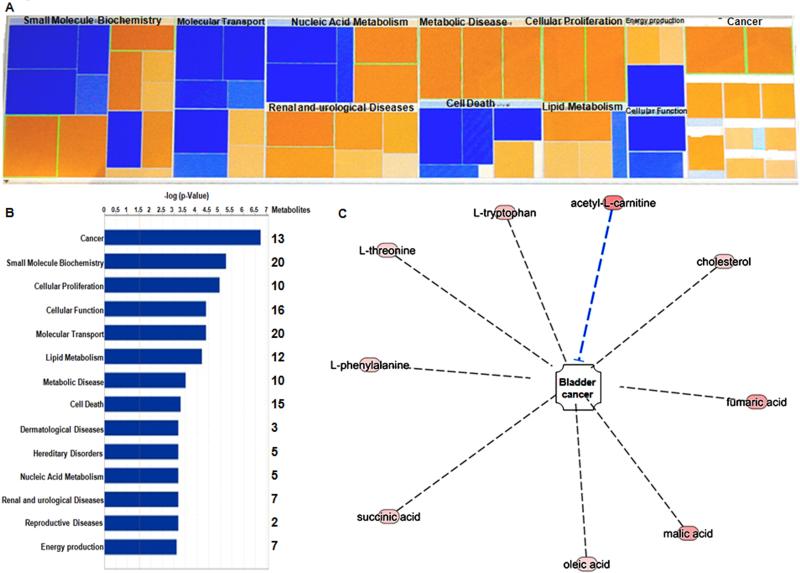
To ensure robustness, we normalized metabolic data via a cell count, as described previously 22. Additionally, we excluded normalization against protein content, as it has been previously reported to introduced a high degree of error into the data set 30. All 206 metabolites identified by GC-MS and LC-MS platform were uploaded to the IPA website for functional analysis. The metabolic functions (canonical pathways, diseases and bio functions, molecular and cellular functions, toxicology functions and top molecules involved in these metabolic profiles) revealed by IPA analysis (Figure 4A) are summarized in Table 3. The most significantly altered bio functions were observed to be cancer (p= 1.83X10−7 - 2.59X10−2; 13 metabolites), small molecule biochemistry (p= 5.69X10−6 - 4.87X10−2; 20 metabolites), cellular proliferation (p= 1.08X10−5 - 2.57X10−2; 10 metabolites), cellular function (p= 4.14X10−4 – 4.34X10−2; 16 metabolites) and molecular transport (p= 4.04X10−4 – 4.87X10−2; 20 metabolites) (Figure 4B). The 5 most significant molecular and cellular functions were cellular growth and proliferation (10 metabolites), small molecule biochemistry (21 metabolites), cellular function and maintenance (16 metabolites), amino acid metabolism (12 metabolites) and carbohydrate metabolism (11 metabolites) (Table 3). We also determined the most up-regulated molecules (D-mannose, acetyl-L-carnitine, D-pantothenic acid, fumaric acid, malic acid, pyruvic acid, L-aspartic acid, glycerol, L-tryptophan and L-methionine) are involved in the molecular remodeling of metabolic functions during the progression of BC.
Figure 4. Integrative molecular modeling of metabolic functions in BC progression.
A. Heatmap of molecular and cellular functions associated with metabolite markers revealed by IPA analysis. Color coding displays z-scored data: blue indicates lower levels and orange indicates higher levels of metabolite intensity. B. Top networks of metabolite markers are associated with Molecular and Cellular functions. C. 8 metabolites associated with the activation of BC were identified via IPA analysis (L-tryptophan, L-threonine, L-phenylalanine, succinic acid, oleic acid, malic acid, fumaric acid and cholesterol), also acetyl L-carnitine was found to inhibit BC progression.
The activities of cell proliferation and cell death are significantly altered in FAVL-high BC cells compared to FAVL-low BC cells
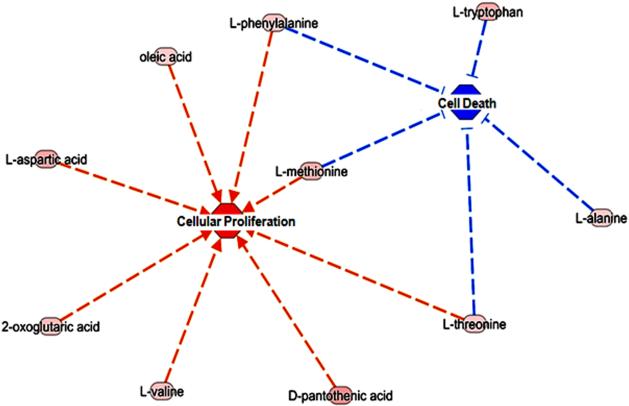
Previously in the xenograft tumor model we reported that FAVL elevation increased in vivo growth potential robustly. These findings suggest that FAVL is an unrecognized cause of BC development, which may heavily contribute to BC development and/or progression. Here, we first sought to confirm our findings at the metabolite level. To validate the role of FAVL in BC, we performed metabolic pathway analysis. This was based on the metabolites that were significantly altered in FAVL-high cells. As shown in Figure 4C, 8 metabolites associated with the activation of BC development were identified via IPA analysis (L-tryptophan, L-threonine, L-phenylalanine, succinic acid, oleic acid, malic acid, fumaric acid and cholesterol). Interestingly, acetyl L-carnitine was also increased, which is a resultant metabolite of BC progression. This suggests that tumorigenesis is a net outcome of two simultaneously occurring processes of promotion and inhibition. In terms of TCA, its intermediates, including succinic acid, malic acid and fumaric acid were observed to be higher in FAVL-high cells compared to FAVL-low cells. In addition, the levels of L-tryptophan, L-threonine and L-phenylalanine (involved in the entry into TCA31) are increased in FAVL-high cells compared to FAVL-low ones, each of which can lead to an elevated level of lactic acids in TCA. This could be attributed to the “Warburg effect”, by which in cancer cells, glucose is often converted into lactic acid.31 To further validate FAVL as a promoter of cell proliferation and an inhibitor of cell death at the metabolic level, we found 8 elevated metabolites in FAVL-high cells (2-oxoglutaric acid, D-pantothenic acid, L-aspartic acid, L-methionine, L-phenylalanine, L-threonine, L-valine and oleic acid) were associated with a promoted cellular proliferation (p= 4.81X10−2 - 7.00X10−6) as well as 5 metabolites (L-alanine, L-methionine, L-phenylalanine, L-tryptophan and L-threonine) are products of a reduced activity in cell death (p= 2.59X10−2 - 5.17X10−4) (Figure 5 and Table 3). Amongst the amino acid intermediates, methionine, phenylalanine and threonine were involved in both processes of promoted cell proliferation and the mitigated cell death. Overall, our data suggest that FAVL elevation is an important tumor promotion factor contributing to the formation of BC at the metabolic level.
Figure 5. Metabolites in BC cells carrying an impaired FA pathway are involved in both cell proliferation & cell death.
Most significantly differential network between cellular proliferation and cell death. 8 metabolites (2-oxoglutaric acid, D-pantothenic acid, L-aspartic acid, L-methionine, L-phenylalanine, L-threonine, L-valine and oleic acid), resulting from cellular proliferation are significantly altered between BC cells with or without an intact FA pathway. 5 metabolites (L-alanine, L-methionine, L-phenylalanine, L-tryptophan and L-threonine) which are the end-products of the reduced activity in cell death. Amongst the amino acid intermediates, methionine, phenylalanine and threonine were involved in both activities of promoted cell proliferation and inhibited cell death (Red line means activation and blue line means inhibition).
DISCUSSION
Metabolomics is a relatively new area of study and the latest addition to the “omics” family of genomics, transcriptomics, and proteomics. The central dogma of molecular biology describes the flow of biological information in a system, from DNA to RNA to protein to metabolites.32 Our previous studies reported that, at both the functional and genetic level, impairment to the FA pathway contributes to the development of BC.10,11 The studies presented herein demonstrate for the first time that the FAVL induced impairment of the FA pathway contributes to BC at the metabolic level. In this study, we systematically profiled metabolites in FAVL-low and -high cells using GC-TOFMS and UPLC-MS/MS. PCA was used to establish a clear separation of metabolites between FAVL-low and high cells. These differences clearly demonstrated the tumorigenic potential initiated by FAVL impairment to the FA pathway.
Currently, through the use of modern metabolomic tools, overall metabolite compositions can be determined in different types of samples. In comparison to healthy cells, the metabolism of cancer cells is generally characterized by the elevated catabolic conversion of glucose into lactate (high-energy demand of strongly proliferating cancer cells), known as the Warburg effect. Other characteristics include the preferential metabolism of specific amino acids, such as valine, isoleucine, and glutamine.33 We observed 18 metabolites, which were significantly altered between FAVL-low and high cells. These metabolites were mainly involved in fatty acid, carbohydrate and amino acid metabolism. Fatty acid beta-oxidation and cell membrane synthesis are known to be dysregulated within cancerous cells.34 Whereby, lipids are involved in many tumor associated processes including cell dislodgement, invasion, migration, and proliferation.35 In addition, the precursors for amino acids, nucleotides, and lipids that are needed for proliferating tumor cells are generated via glucose metabolism through glycolysis. Glycolysis creates a continuous pool of pyruvate that can be converted into acetyl CoA by the pyruvate dehydrogenase complex. Following this, acetyl CoA can be used for de novo fatty acid synthesis.36 Furthermore, increased levels of fatty acids have been observed early in cancer progression and during carcinogenesis.37 Another source of energy is derived from Glutamine, an abundant amino acid, via glutaminolysis. This pathway is utilized by tumor cells when glycolysis alone is not sufficient for energy production. Additionally, the constituents released following the degradation of glutamine are then available to interact in anabolic processes. Glucose and glutamine can both be metabolized to nucleic acids, amino acids and lipids that are required for cell proliferation.38 Besides the increased synthesis of amino acids, nucleotides and lipids, which are essential in tumor proliferation, other metabolites have been reported as tumor biomarkers in the literature. In a hypoxic environment such as BC, Alanine is produced through the transamination of pyruvate.39 Glycine, an essential precursor for purine synthesis is decreased following hypoxia-inducible factor 1 (HIF-1) signaling in BC.40 As shown in Figure 5, the 8 metabolites resulting from the increased cellular proliferation; 2-oxoglutaric acid, D-pantothenic acid, L-aspartic acid, L-methionine, L-phenylalanine, L-threonine, L-valine and oleic acid were elevated in FAVL-high cells in comparison to FAVL-low cells. In addition, 5 metabolites; L-alanine, L-methionine, L-phenylalanine, L-tryptophan and L-threonine, which are the end products of inhibiting cell death were also elevated in FAVL-high cells compared to FAVL-low cells. These striking differences demonstrate a tumor promotion potential initiated from FAVL impairment to the FA pathway.
Increased glucose metabolism has been hypothesized to drive the bioenergetic needs of cancer cells. However, it is clear that highly proliferative cancer cells require additional supplies of biosynthetic precursors, which are unable to be met by glucose metabolism. Thus there is the requirement of anaplerosis via glutaminolysis and/or pyruvate carboxylation and other sources such as fatty acids.41,42 IPA on these metabolites revealed molecular and cellular functions that 10 metabolites were associated with cellular growth and proliferation, 21 with small molecule biochemistry, 16 with cellular function and maintenance, 12 with amino acid metabolism and 11 with carbohydrate metabolism summarized in Table 3.
The studies presented herein validate the tumorigenic potential of FAVL via metabolite signatures of potent proliferative activity that promotes the development of human BC. These data provide further evidence that FAVL impairment to the FA pathway contributes to the development of BC, perhaps both primary and recurrent BC at the metabolic level. With this addition, we achieved genomic, functional, to metabolomic characterization on the roles of FA signaling in the development of human cancer. Further, this study provides novel insights into how to translate FA basic research into strategies to development effective biomarkers for human cancer diagnosis and prognosis.
ACKNOWLEDGEMENTS
The work is supported partly by R01CA188251 & R01CA136532 to PF. This study is also partly supported by the Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, and University of Hawaii Cancer Center, Honolulu, HI.
REFERENCES
- 1.Patel S, Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J Pharm Biomed Anal. 2015;107:63–74. doi: 10.1016/j.jpba.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nature reviews. Cancer. 2015;15(1):25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 3.Mitra AP, Cote RJ. Molecular screening for bladder cancer: progress and potential. Nat Rev Urol. 2010;7(1):11–20. doi: 10.1038/nrurol.2009.236. [DOI] [PubMed] [Google Scholar]
- 4.Sawyer SL, Tian L, Kahkonen M, Schwartzentruber J, Kircher M, University of Washington Centre for Mendelian, G.; Consortium, F. C. Majewski J, Dyment DA, Innes AM, et al. Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov. 2015;5(2):135–142. doi: 10.1158/2159-8290.CD-14-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouffard F, Plourde K, Belanger S, Ouellette G, Labrie Y, Durocher F. Analysis of a FANCE Splice Isoform in Regard to DNA Repair. J Mol Biol. 2015;427(19):3056–3073. doi: 10.1016/j.jmb.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Bogliolo M, Surralles J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Current opinion in genetics & development. 2015;33:32–40. doi: 10.1016/j.gde.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Kottemann MC, Smogorzewska A. Fanconi anaemia and the repair of Watson and Crick DNA crosslinks. Nature. 2013;493(7432):356–363. doi: 10.1038/nature11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neveling K, Endt D, Hoehn H, Schindler D. Genotype-phenotype correlations in Fanconi anemia. Mutat Res. 2009;668(1-2):73–91. doi: 10.1016/j.mrfmmm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Zhao D, Park HK, Wang H, Dyer RB, Liu W, Klee GG, McNiven MA, Tindall DJ, Molina JR. FAVL elevation in human tumors disrupts Fanconi anemia pathway signaling and promotes genomic instability and tumor growth. The Journal of clinical investigation. 2010;120(5):1524–1534. doi: 10.1172/JCI40908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panneerselvam J, Park HK, Zhang J, Dudimah FD, Zhang P, Wang H, Fei P. FAVL impairment of the Fanconi anemia pathway promotes the development of human bladder cancer. Cell cycle. 2012;11(15):2947–2955. doi: 10.4161/cc.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen Y, Lee YH, Panneerselvam J, Zhang J, Loo LW, Fei P. Mutated Fanconi anemia pathway in non-Fanconi anemia cancers. Oncotarget. 2015;6(24):20396–20403. doi: 10.18632/oncotarget.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 14.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z, Lin L, Gao Y, Chen Y, Yan X, Xing J, Hang W. Bladder cancer determination via two urinary metabolites: a biomarker pattern approach. Mol Cell Proteomics. 2011;10(10):M111 007922. doi: 10.1074/mcp.M111.007922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renwick AG, Thakrar A, Lawrie CA, George CF. Microbial amino acid metabolites and bladder cancer: no evidence of promoting activity in man. Hum Toxicol. 1988;7(3):267–272. doi: 10.1177/096032718800700307. [DOI] [PubMed] [Google Scholar]
- 17.Liu S, Zhang L, Sun Q, Wang F, Xi S, Sun G. The distribution in tissues and urine of arsenic metabolites after subchronic exposure to dimethylarsinic acid (DMAV) in rats. Biol Trace Elem Res. 2015;164(2):219–225. doi: 10.1007/s12011-014-0208-0. [DOI] [PubMed] [Google Scholar]
- 18.Chan EC, Pasikanti KK, Hong Y, Ho PC, Mahendran R, Raman Nee Mani L, Chiong E, Esuvaranathan K. Metabonomic profiling of bladder cancer. J Proteome Res. 2015;14(2):587–602. doi: 10.1021/pr500966h. [DOI] [PubMed] [Google Scholar]
- 19.Cuperlovic-Culf M, Barnett DA, Culf AS, Chute I. Cell culture metabolomics: applications and future directions. Drug Discov Today. 2010;15(15-16):610–621. doi: 10.1016/j.drudis.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Bohm A, Halama A, Meile T, Zdichavsky M, Lehmann R, Weigert C, Fritsche A, Stefan N, Konigsrainer A, Haring HU. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS One. 2014;9(4):e93148. doi: 10.1371/journal.pone.0093148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halama A, Riesen N, Moller G, Hrabe de Angelis M, Adamski J. Identification of biomarkers for apoptosis in cancer cell lines using metabolomics: tools for individualized medicine. J Intern Med. 2013;274(5):425–439. doi: 10.1111/joim.12117. [DOI] [PubMed] [Google Scholar]
- 22.Halama A, Moller G, Adamski J. Metabolic signatures in apoptotic human cancer cell lines. OMICS. 2011;15(5):325–335. doi: 10.1089/omi.2010.0121. [DOI] [PubMed] [Google Scholar]
- 23.Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4(7):551–561. doi: 10.1038/nrc1390. [DOI] [PubMed] [Google Scholar]
- 24.Ni Y, Xie G, Jia W. Metabonomics of human colorectal cancer: new approaches for early diagnosis and biomarker discovery. Journal of proteome research. 2014;13(9):3857–3870. doi: 10.1021/pr500443c. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Cai G, Zhou B, Li D, Zhao A, Xie G, Li H, Cai S, Xie D, Huang C. A distinct metabolic signature of human colorectal cancer with prognostic potential. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(8):2136–2146. doi: 10.1158/1078-0432.CCR-13-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu Y, Zhou B, Su M, Baxter S, Zheng X, Zhao X, Yen Y, Jia W. Mass spectrometry-based quantitative metabolomics revealed a distinct lipid profile in breast cancer patients. International journal of molecular sciences. 2013;14(4):8047–8061. doi: 10.3390/ijms14048047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni Y, Qiu Y, Jiang W, Suttlemyre K, Su M, Zhang W, Jia W, Du X. ADAP-GC 2.0: deconvolution of coeluting metabolites from GC/TOF-MS data for metabolomics studies. Analytical chemistry. 2012;84(15):6619–6629. doi: 10.1021/ac300898h. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6(269):pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva LP, Lorenzi PL, Purwaha P, Yong V, Hawke DH, Weinstein JN. Measurement of DNA concentration as a normalization strategy for metabolomic data from adherent cell lines. Analytical chemistry. 2013;85(20):9536–9542. doi: 10.1021/ac401559v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan AW, Gill RS, Schiller D, Sawyer MB. Potential role of metabolomics in diagnosis and surveillance of gastric cancer. World journal of gastroenterology : WJG. 2014;20(36):12874–12882. doi: 10.3748/wjg.v20.i36.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halama A, Guerrouahen BS, Pasquier J, Diboun I, Karoly ED, Suhre K, Rafii A. Metabolic signatures differentiate ovarian from colon cancer cell lines. Journal of translational medicine. 2015;13:223–234. doi: 10.1186/s12967-015-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Enriquez S, Hernandez-Esquivel L, Marin-Hernandez A, El Hafidi M, Gallardo-Perez JC, Hernandez-Resendiz I, Rodriguez-Zavala JS, Pacheco-Velazquez SC, Moreno-Sanchez R. Mitochondrial free fatty acid beta-oxidation supports oxidative phosphorylation and proliferation in cancer cells. The international journal of biochemistry & cell biology. 2015;65:209–221. doi: 10.1016/j.biocel.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Fernandis AZ, Wenk MR. Lipid-based biomarkers for cancer. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2009;877(26):2830–2835. doi: 10.1016/j.jchromb.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Rui L. Energy metabolism in the liver. Compr Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel W, Fang HB, Vafai SB, Vazquez F, Puigserver P, Boros L. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71(21):6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz-Pinedo C, El Mjiyad N, Ricci JE. Cancer metabolism: current perspectives and future directions. Cell Death Dis. 2012;3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conde VR, Oliveira PF, Nunes AR, Rocha CS, Ramalhosa E, Pereira JA, Alves MG, Silva BM. The progression from a lower to a higher invasive stage of bladder cancer is associated with severe alterations in glucose and pyruvate metabolism. Exp Cell Res. 2015;335(1):91–98. doi: 10.1016/j.yexcr.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Guin S, Pollard C, Ru Y, Ritterson Lew C, Duex JE, Dancik G, Owens C, Spencer A, Knight S, Holemon H. Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease. J Natl Cancer Inst. 2014;106(5) doi: 10.1093/jnci/dju062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeBerardinis RJ. Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med. 2008;10(11):767–777. doi: 10.1097/GIM.0b013e31818b0d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang QY, Wan YP, Wang JL, Shen WR, Chen ZQ, Zhao M. [Comparison of different definitions on metabolic syndrome in obese children]. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30(12):1297–1301. [PubMed] [Google Scholar]