Abstract
Chronic rhinosinusitis (CRS) is a broad clinical syndrome that is characterized by prolonged mucosal inflammation of the nose and paranasal sinuses, and is typically divided into two subtypes based on the presence or absence of nasal polyps. The etiology and pathogenesis of both forms remain areas of active research. Over the last 15 years, a number of hypotheses have been proposed to explain all or part of the clinical CRS spectrum. These hypotheses reflect the concept that CRS results from a dysfunctional interplay between individual host characteristics and factors exogenous to the host. Six broad theories on CRS etiology and pathogenesis are discussed as follows: (1) the “fungal hypothesis,” (2) the “superantigen hypothesis,” (3) the “biofilm hypothesis,” and (4) the “microbiome hypothesis,” all of which emphasize key environmental factors, and (5) the “eicosanoid hypothesis” and (6) the “immune barrier hypothesis,” which describe specific host factors. These theories are reviewed, and the evidence supporting them is critically appraised.
Keywords: Chronic rhinosinusitis, Sinusitis, Nasal polyposis, Pathogenesis, Pathophysiology, Etiology
Introduction
Chronic rhinosinusitis (CRS) is a clinical syndrome defined by persistent symptomatic inflammation of the mucosa of the nasal cavities and paranasal sinuses, with a prevalence estimated to be approximately 10 % of the population in the Western world [1••, 2, 3]. The syndrome is defined by the subjective presence of at least two of the following symptoms for a minimum of 12 weeks: nasal obstruction, nasal discharge, facial pain, or olfactory dysfunction. Due to symptomatic overlap with other common conditions, objective confirmation of sinonasal mucosal inflammation is required using nasal endoscopy or diagnostic imaging [1••]. This definition of CRS is purposely broad, encompassing a spectrum of clinical variants, inflammatory profiles, histologic features, and associated co-morbidities. While this definition intentionally avoids causation, a small fraction of CRS cases occur in association with known genetic disorders [e.g., Kartagener's syndrome or cystic fibrosis (CF)], autoimmune disorders (e.g., Wegener's granulomatosis), or immunodeficiencies (e.g., human immunodeficiency virus infection). Cases of CRS that occur in these settings are local manifestations of a systemic disease and will typically exhibit a somewhat more specific histology and clinical course. The overwhelming majority of CRS cases are idiopathic however, with classification systems commonly dividing the disorder into two phenotypes based on nasal endoscopy: chronic rhinosinusitis without nasal polyps (CRSsNP) and chronic rhinosinusitis with nasal polyps (CRSwNP). Although quite simplistic, the separation remains useful in many clinical settings, since the vast majority of nasal polyps in Western countries are eosinophilic and steroid responsive, which helps to guide therapy [1••].
Historically, CRSsNP was considered the result of an incompletely treated or unresolved bacterial infection, while CRSwNP was regarded as a noninfectious disorder linked to atopy [1••]. Research over the last 20 to 30 years has indicated that the etiology and pathogenesis for both forms are far more complex and overlapping [1••, 4–7]. To be precise, the terms etiology and pathogenesis are often used interchangeably, but etiology more commonly refers to a primal cause, while pathogenesis describes the cellular or molecular pathways leading from the etiology to the clinical phenotype. The development of a detailed classification system for CRS based on molecular pathways or endotypes is a goal, but this awaits a much more detailed understanding of the inflammatory disease processes and the correlation of proposed endotypes with clinical course and response to therapy [8•]. Strict definition of endotypes at this juncture could limit, rather than enable, CRS research by reducing the ability of investigators to recruit patient groups of ample size. At present for CRS, several broad hypotheses have been proposed that emphasize one or more host or environmental factors that have been associated with CRS etiology. While the relative importance of each factor remains a matter of debate, there is an emerging consensus on two points: (1) specific factors likely vary in importance in individual patients and (2) CRS is an antegrade process in that the mucosal inflammation is most commonly triggered by exogenous agents inhaled through the nose. Overall, this leads to the concept that CRS is most inclusively described as a dysfunctional interaction that occurs at the site of interface between the host and the environment—the sinonasal mucosa [1••, 4–7].
Environmental factors implicated in CRS include allergens, toxins, and microbial agents, while host factors center on defects of the immune system. Nasal allergens have been found to induce pathophysiologic changes within the paranasal sinuses that are somewhat similar in kind, if not degree, to the responses seen in CRS [9, 10]. However, inflammatory profiles associated with atopy include skin test responsiveness and elevated immunoglobulin (Ig) E, and this is not universal in CRS patients [11, 12]. In addition, the severity of CRS, as measured by both clinical and radiographic parameters, has not been found to demonstrate significant correlations with atopic status [13, 14]. Overall, most investigators do not view allergic rhinitis as a causative factor in CRS but rather as a superimposed problem that contributes to a variable but relatively mild degree to the inflammation seen in CRS [1••]. Environmental toxins are intriguing albeit largely theoretical causes of CRS, as evidence is weak. Some data exist for tobacco smoke as an etio-logic factor, but it is not clear how this could account for the type 2 cytokine profile seen in severe forms of CRS (see below) [1••]. The most prominent environmental factors in CRS are believed to be microbial, including both fungi and bacteria. Among host factors implicated in CRS are defects in the mechanical, innate, and adaptive components of the immune system. Overall, six broad hypotheses on CRS etiology and pathogenesis have thus far been proposed, each describing a central role for one or more aspects of these factors: (1) the “fungal hypothesis,” (2) the “superantigen hypothesis,” (3) the “biofilm hypothesis,” and (4) the “microbiome hypothesis,” all of which emphasize key environmental factors, and (5) the “eicosanoid hypothesis” and (6) the “immune barrier hypothesis,” which focus on host factors.
Fungal Hypothesis of CRS
Investigators from the Mayo Clinic were the first to hypothesize that all or nearly all cases of CRS were driven by host responses to common airborne fungal elements [15, 16]. Using specialized detection techniques, fungal microorganisms were identified in the sinonasal cavities of essentially 100 % of both CRS and healthy control patients [17]. These novel findings, later confirmed by other investigators, were combined with in vitro studies demonstrating a hyperreactivity of peripheral blood mononuclear cells (PBMCs) to Alternaria antigens [18]. This response was obtained in CRS patients but was absent in controls and thus was proposed to reflect an immunologic sensitization to fungal antigens independent of atopy. It was hypothesized to occur in both polypoid and non-polypoid CRS, varying only in disease intensity [19]. Later studies demonstrated that nasal mucus from patients with CRS triggered eosinophil migration [20] and that a 60-kDa component of Alternaria elicited eosinophil degranulation via protease-activated receptors (PARs) [21]. In summary, Alternaria and possibly other fungi were proposed to serve a dual role: first, inhaled and processed fungal proteins were presented to sensitized T cells triggering a cytokine response that activated and attracted eosinophils to the mucosal surface. Second, these eosinophils then targeted the fungi as an aberrant host defense response, with degranulation and collateral tissue damage mediating the symptoms of CRS. Activated eosinophils, obtained from CRS patients, were shown on videos attacking fungi, providing persuasive visual evidence to support this hypothesis.
Despite widespread initial interest in this theory involving Alternaria as a primary etiologic agent in CRS, the fungal hypothesis as originally proposed has been largely abandoned for multiple reasons. First, eosinophils are not generally considered important cell types in the defense against fungi, and the videos of eosinophils attacking fungi were felt to reflect non-specific eosinophil “activation” as opposed to any immunologic specificity for the fungi in vivo. Secondly, two separate attempts to replicate the sensitization of PBMCs to fungal antigens in CRS patients failed, indicating that the original findings were clearly not universal [22, 23]. Third and most importantly, double-blind, placebo-controlled trials involving antifungal agents failed to show any evidence of efficacy in modifying the disease process of CRS [24, 25]. Follow-up studies on the mucus of CRS patients treated with topical amphotericin also failed to show effects on any relevant CRS-related cytokine or chemokine [26]. While fungi are no longer viewed as primary etiologic agents for the development of CRS, an increased burden of fungal colonization is still regarded as an important disease modifier. Fungi contain intrinsic proteases that can induce cytokines via activation of PAR receptors on numerous cell types, possibly driving T helper type (Th) 2 responses [25, 27–30]. Fungal extracts can inhibit JAK-STAT1 signaling in epithelium, an effect that may inhibit Th1 and promote Th2 responses [31]. Fungi also likely play a key role in classic allergic fungal sinusitis or AFS [32]. Lastly, fungal cell walls contain chitin, which has been shown to induce a Th2 response in some human and animal models, although any role in CRS remains unclear [33–35]. Currently, most investigators suspect that fungi likely play a significant role in a subpopulation of CRS patients.
Bacteria-Based Hypotheses of CRS
In addition to fungi, bacteria also colonize the sinonasal tract of both healthy and CRS patients, but the microbiome of this area of the body remains relatively poorly characterized, with only a few studies thus far utilizing sensitive 16s molecular techniques [1••, 36, 37•]. Historical data, however, using mostly culture-based techniques, have long suggested the importance of bacteria, most prominently Staphylococcus aureus, in CRS [38, 39]. In addition to surface colonization, Staphylococcus is also capable of residing within the epithelial cells and macrophages of CRS patients [40–42]. Under normal circumstances, bacteria, including Staphylococcus, elicit a Th17 inflammatory host defense response, and one of the most problematic issues confounding a bacterial hypothesis for CRS etiology is therefore the difficulty in explaining the Th2 response seen in the tissues of the most recalcitrant patients [1••]. Nevertheless, three bacterial-based hypotheses have been proposed as follows: (1) the superantigen hypothesis, (2) the biofilm hypothesis, and (3) the microbiome hypothesis.
Superantigen Hypothesis
The superantigen hypothesis proposes that superantigenic (SAGs) exotoxins produced by the Staphylococcus bacteria amplify local eosinophilic responses via an array of mechanisms, thereby fostering polyp formation [43, 44]. In support of this theory, studies from CRSwNP have demonstrated the presence of S. aureus in a high percentage of polyp patients [45, 46]. Staphylococcal superantigens have also been detected in polyp homogenates, but not in control or CRSsNP tissues [47]. These toxins act by triggering a massive and uncontrolled immunologic response activating as many as 30 % of the T cell population in affected individuals, compared to the 0.001 % that is activated in a normal antigen-specific immune response [48]. They bind to the T cell receptor outside the antigen-binding groove, as well as the human leukocyte antigen (HLA) class II histocompatibility complex of antigen-presenting cells. By this mechanism, superantigens bypass the normal steps of antigen recognition and promote polyclonal T lymphocyte proliferation and massive cytokine release, which in the case of typical nasal polyps, has a strong Th2 component. Many other cell types are affected, including B cells, resulting in a local polyclonal IgE response in nasal polyps [43, 47, 49–56].
The T and B cell responses seen in polyp tissues are considered immunologic “footprints” of SAG effects, contrasting with the lack of evidence for any Alternaria-specific effects [1••]. These footprints are demonstrated in only about half of the studied CRSwNP cases, however. Furthermore, significant portions of controls, CRSsNP patients, and CF patients are colonized with Staphylococcus, but none of the tissues from these groups demonstrate any SAG effects [7]. Consequently, it is not clear if SAGs are causative per se, but they may only accentuate the inflammatory response already present in the tissues. In eosinophilic polyps, a SAG effect would therefore greatly enhance the intensity of a Th2-skewed response that had already been established, creating a more clinically severe phenotype. As a consequence, Staphylococcus superantigens are generally seen as disease modifiers for the development of nasal polyposis, rather than discrete etiologic agents.
Biofilm Hypothesis
Bacterial biofilms have been implicated as important features of the endogenous sinonasal bacteria in both CRSsNP and CRSwNP with detection rates of 42–75 % in patients undergoing sinus surgery [57–61]. Biofilms are highly organized, complex structures composed of communities of bacteria encased within a protective extracellular matrix. This external matrix, composed of polysaccharides, nucleic acids, and proteins, provides a mechanism for bacteria to reduce their metabolic rates in conditions that are less than optimal for growth, protecting them from both host defenses and conventional antibiotics. Prior studies have shown that in the sinonasal cavities, bacterial biofilms contribute to a 10- to 1000-fold decrease in the susceptibility to antibiotics when compared with that of free-floating, planktonic bacteria of the same species. Bacteria within these biofilms, however, retain the capacity to release planktonic bacteria and possibly exotoxins, which may be responsible for stimulating the inflammatory responses in CRS. Multiple bacterial species, including S. aureus, Pseudomonas aeruginosa, Streptoccoccus pneumonia, Haemophilus influenza, and Moraxella catarrhalis, are known to produce biofilms, but S. aureus biofilms are most commonly associated with recalcitrant CRS. Moreover, unlike other bacteria, S. aureus has the theoretical capability through SAG release or possibly other means to enhance the robust Th2 adaptive immune response seen in most cases of severe CRS [62].
A biofilm hypothesis for CRS etiology has not been fully articulated, and there is no direct evidence to suggest that bacterial biofilms play a role in the initial establishment of CRS [63]. Furthermore, it is likely that while the density and type may vary, biofilms are a common bacterial property present in both healthy and diseased states. In theory, chance elements could permit the establishment of pathogenic biofilms or more extensive biofilms on the mucosal surface, whereupon these structures might aid in the perpetuation of the prolonged sinonasal mucosal inflammation seen in resistant CRS. Absent superantigen, however, biofilms have no accepted mechanism whereby they can facilitate or even enhance the type 2 cytokine pattern exhibited in most severe forms of CRS. Consequently, based on current data, any biofilm hypothesis would default to the superantigen hypothesis from the standpoint of CRS pathogenesis [1••].
Microbiome Hypothesis
The sinonasal microbiome has not been extensively studied using more sensitive culture-independent 16s molecular techniques. The limited studies comparing normal controls and CRS patients indicate similar overall bacterial burden but marked differences in relative abundance and diversity [36, 46, 64, 65]. In other better-studied organ systems, most notably the gastrointestinal tract and skin, evidence suggests that externally induced changes in the microbiome may mediate chronic inflammation via the secondary proliferation of pathogenic flora that would normally be suppressed by commensals [66]. These commensal microrganisms act in part by secreting antimicrobial proteins and producing lipid byproducts that help maintain homeostasis by depressing pathogen growth [67, 68••]. This has been interpreted to suggest that restoration of the microbiome via probiotics or inoculation with a sample of healthy bacteria may be associated with resolution of inflammation [69]. A preliminary study in CRS has suggested that antibiotics or perhaps virally induced changes in the sinonasal microbiome would similarly permit the emergence of pathogenic organisms mediating CRS [65]. This microbiome hypothesis of CRS will require a more extensive study, but even if validated to some extent, it will likely need to invoke other elements to account for the Th2 inflammation seen in CRS. It is also likely that the microbiome varies within the anatomic sites of the nose and sinuses, precluding simple inoculation methods of treatment [70•]. Lastly, it should also be noted that probiotics and microbial transplants have been successful primarily in pseudomembraneous enterocolitis, an antibiotic-induced disorder with an underlying normal immune system, potentially very much unlike CRS. These treatments have been far less effective in the management of inflammatory bowel disease, a complex genetic and environmentally driven disorder more analogous to CRS [6, 71].
Host-Related Hypotheses of CRS
The mucosal immune system possesses the inherent capability to protect the host from injury induced by environmental agents, and defects in this system could theoretically account for the chronic inflammation characteristic of CRS. Under normal circumstances, multiple integrated components act in a coordinated fashion to provide effective immune defense against foreign material, including microbial agents, at the mucosal interface. First in contact with the outside world is the physical or mechanical barrier, consisting of airway mucus, the mucociliary escalator, and tight junctional complexes between epithelial cells, all acting to limit stimulation of the immune system by foreign material. Backing up this mechanical barrier is the innate immune system, which in part consists of endogenous antimicrobials secreted either constitutively or inducibly by various host cell types into the nasal mucus. Nevertheless, if the foreign pathogenic stimulus is sufficiently strong, an adaptive immune response with highly specific T and B proliferation will be initiated. In overview, the nature, strength, and duration of the immune response will be matched to the exogenous stimulus. In CRS, the inflammatory response becomes chronic, suggesting sustained stimulation of the immune system or an excessive, inappropriate response. Thus far, two broad host-related hypotheses have been suggested: the eicosanoid hypothesis and the immune barrier hypothesis.
Eicosanoid Hypothesis
Eicosanoids are signaling molecules secreted by a wide variety of cell types with immunologic and inflammatory properties generated by metabolism of arachidonic acid. Defects in the eicosanoid pathway, which have long been closely associated with aspirin intolerance, have more recently been implicated as a contributing cause to CRSwNP in general [7, 72]. Increased synthesis of pro-inflammatory leukotrienes and decreased synthesis of anti-inflammatory prostaglandins have been identified in both aspirin-tolerant and aspirin-sensitive polyps, suggesting a broader role in chronic Th2 sinonasal disease. In addition, some data suggests that eicosanoids may modulate the effects of Staphylococcal superantigens as well [73, 74]. While intriguing, the amount of evidence is still relatively scant; hence, the eicosanoid hypothesis has been implied, rather than more formally articulated. Furthermore, the relatively modest clinical efficacy of leukotriene inhibitors in the management of nasal polyposis has dampened widespread interest in this pathway as a major factor in the development of CRS.
Immune Barrier Hypothesis
The immune barrier hypothesis proposes that defects in the physical barrier and the innate immune response predispose to the development of CRS when challenged by relatively common microbial agents [5, 6]. This emphasis on events at the mucosal interface stands in contrast to most prior work, which emphasized downstream cellular infiltration and most importantly, a Th2-skewed adaptive response. Upstream, the physical barrier consists of airway mucus that traps foreign matter and intercellular tight junctions between respiratory epithelial cells, forming a semi-permeable barrier limiting access across the mucosa. The first evidence for upstream defects leading to CRS came from CF patients, who have an established abnormal mucociliary flow and a very high incidence of CRS, both with and without nasal polyps [75]. Even carriers of the CF mutation without the clinical disease have a significantly higher prevalence of CRSsNP, emphasizing the importance of mucocilairy flow to nasal and sinus homeostasis [76]. Later studies indicated that defective mucociliary clearance was present more broadly in CRS, increasing transit time and, secondarily, exposure to foreign material [77, 78]. Subsequent studies have suggested a weakened mechanical barrier as well, with diminished tight junctional proteins and increased susceptibility to exogenous protease degradation [79–81]. Functional studies demonstrated that the barrier was porous in CRSwNP, permitting increased access of foreign material across the epithelium [82, 83]. Recently, elevated levels of Oncostatin M, which is a cytokine that can cause the disassembly of tight junctions, have been reported in affected tissues, and may be hypothesized to play a role in barrier defects in CRS [84]. Overall, these data suggest that mucociliary dysfunction is present in both forms of CRS, but a porous barrier is more closely linked to CRSwNP.
The innate immune system functions as the next level of defense after the mechanical barrier. One prominent component consists of innate antimicrobials that are secreted by epithelial cells, glands, and innate effector cells. A partial list includes the following: defensins, lysozyme, S100s, cathelicidins, lactoferrin, pentraxin-3, complement, PLUNC, and the collectins [1••]. The distribution of these proteins varies at distinct anatomic subsites, and there is considerable regional variation in expression within the nose and sinuses [85]. Presumably, this regional variation in host defense molecules is a reflection of regional differences in the nasal microbiome or vice versa [70•]. Tonic levels of antimicrobial proteins are secreted at the basal state, increasing upon the stimulation of pattern recognition receptors (PRRs) present on most cell types, including epithelial cells. In CRS, the expression of some antimicrobials is increased, and this is presumably the appropriate response to exogenous stimulation. Others, including lactoferrin, PLUNC, S100s, and possibly more, are decreased in CRS [86–89]. Lactoferrin and the S100s are decreased in both forms of CRS, while PLUNC is decreased only in nasal polyposis. PLUNC may be particularly relevant to CRS, since it has anti-biofilm properties and is secreted in high levels at the uncinate process. The S100 family is important for differentiation, wound healing, and barrier re-constitution, in addition to its antimicrobial effects. Overall, the presence of these presumed immune defects in CRS supports the hypothesis that primary abnormalities in host defense molecules contribute to an abnormal microbiome, increased pathogen access, and the inducement of a sustained compensatory adaptive response.
PRR signaling, including the Toll receptor family, is important in the secretion of the aforementioned innate antimicrobials for host defense. A decreased number or perhaps diminished function of some PRRs may account for the data observed in CRS patients. For the most part, results are inconsistent in regard to changes in PRR expression or signaling in CRS patients (for review, see Fokkens et al. [1••]). Recent data, however, has indicated that bitter taste receptors (T2R), which are present in the nose and normally not associated with host defense, may be defective in some subtypes of CRSsNP patients [90]. Physiologically, these receptors respond to bacterial products inducing increased ciliary beat frequency and the release of nitric oxide (NO), defensins, and possibly other innate antimicrobials. Variations in the T2R genes have been associated with diminished signaling properties, which may predispose to CRSsNP. Another potentially relevant pathway involves the cytokine IL-22, the IL-22r, and the transcription factor STAT3, which are important in the broad regulation of mucosal immunity, including host defense and post-injury regeneration [91–94]. In CRS, diminished IL-22r has been demonstrated in CRSwNP [95] and diminished STAT3 activity is present in both forms of CRS when compared to controls [96]. Although suggestive, further studies will be necessary to validate the clinical significance of these observations.
The immune barrier hypothesis proposes that mechanical and innate immune barrier defects are broadly present in CRS, including CRSwNP and CRSsNP, resulting in an abnormal microbiome, increased exposure to foreign material, and excessive compensatory innate cellular and adaptive immune responses. This hypothesis, however, does not account for the variation in the type of adaptive inflammation seen in the various phenotypes of CRS. As discussed above, CRS is currently divided into two groups based on the presence or absence of nasal polyps but more comprehensive classification systems have been considered based on cellular infiltrates, cytokine expression, or tissue remodeling patterns. Polyps in Caucasians are most commonly characterized by high levels of tissue eosinophils, neutrophils, mast cells, and B cells; a type 2 biased cytokine profile; and a remodeling pattern consisting of decreased collagen, increased fibrin, and increased tissue edema. In contrast, CRSwNP in Asian populations exhibits a more variable pattern of tissue infiltration and cytokine expression, with more neutrophils and fewer eosinophils, but demonstrates a somewhat similar remodeling pattern. Likewise, CF polyps exhibit neutrophilic infiltration with a Th17 cytokine bias. CRSsNP variants in Caucasian and Asian populations are less well characterized, but both typically demonstrate increased collagen (for review, see Fokken et al. [1]). As a result, attempts have been made to classify CRS based on differences in the adaptive response, such as the pattern of T helper cells within the tissue [97•]. In regard to the immune barrier hypothesis, these collective observations suggest that besides deficits in the mechanical barrier or innate antimicrobials, additional variations in the host immune response are likely necessary to explain the full range of the CRS syndrome. In short, while a defective immune barrier may predispose to the development of CRS, additional defects likely exist in the communication with innate effector responses or the transition to the adaptive response. Variations in this second component may therefore grossly account for the separation of CRSsNP and CRSwNP, as well as the other patterns described above.
Nasal polyps in Caucasians are by far the most thoroughly studied form of CRS, 80 % of which display the cellular infiltration and cytokine patterns described above. The molecular mechanism driving this process is not completely clear, but the epithelial cytokines TSLP and IL-33 have been implicated [98••, 99]. These cytokines have the capacity to directly activate innate lymphocyte populations (ILC2s), which then release substantial amounts of type 2 cytokines, including IL-5 and IL-13 [100•]. In addition, IL-33 and TSLP directly bind dendritic cells, helping to skew the adaptive response in a Th2 direction [101, 102]. Nasal polyps also exhibit extensive B cell infiltrates with local IgA, IgE, and IgG production, while some CRS patients, furthermore, express autoreactive antibodies through the regulation of the protein BAFF [103–105]. The presence of these antibodies in proximity to innate effector cells that possess Fc receptors may lead to degranulation and the observed tissue damage and remodeling changes seen in nasal polyposis [106, 107•]. Evidence for specific cytokines or signaling pathways that may address the other phenotypes of CRS is thus far relatively scant.
The immune barrier hypothesis is the broadest and most inclusive hypothesis for CRS etiology and pathogenesis thus far proposed, as it can account for, rather than conflict with, the most prominent data supporting the other theories [see Fig. 1]. It remains unclear whether any barrier defects are due to primary genetic variation in the host, epigenetic changes induced by environmental insults, or both. It should be kept in mind, however, that epidemiological studies indicate, for the most part, that CRS patients exhibit chronic inflammation in the nose, sinuses, and often the lower respiratory tract [108, 109]. This makes it more likely that the host genes mediating CRS pathogenesis will primarily be those that govern the immunobiology of the respiratory mucosa, as opposed to the systemic immune response.
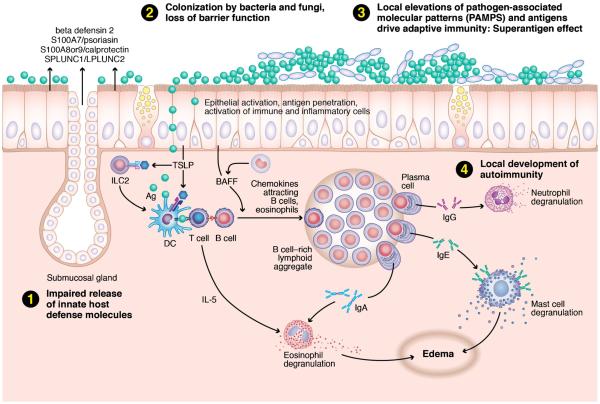
Fig. 1.
The “immune barrier hypothesis” posits that the chronic inflammation associated with CRS is linked to a defective mechanical and innate immune barrier. This deficit leads to a compensatory innate effector cell recruitment and activation, coupled with a robust adaptive immune response. The nature of this compensatory response likely varies among CRS phenotypes. Describes the proposed pathway for a typical case of Caucasian nasal polyposis. Reduced host defense molecules (1) lead to an abnormal microbiome (2), likely in biofilm format. Impaired mucociliary flow and a porous mechanical barrier lead to increased access of PAMPS stimulating a compensatory response (3). In Caucasian polypoid CRS, the cytokines BAFF and TSLP have been implicated in the observed innate and adaptive response. TSLP acts on type 2 innate lymphocytes (ILC2s) to release the type 2 cytokines IL-5 and IL-13. TSLP also acts on dendritic cells, fostering Th2 lymphocyte differentiation with an amplified type 2 cytokine response. Superantigens, if present, will accentuate the T cell response expressed in the tissue; in this case, the response is skewed to Th2. BAFF drives a strong local B cell immunoglobulin response, including autoreactive antibodies in severe cases (4). Collectively, this results in the recruitment and activation of innate effector cells that possess Fc receptors for the locally produced immunoglobulins. In the presence of large amounts of antigen that penetrate the weakened barrier, this should lead to widespread effector cell degranulation and tissue damage
Conclusions
The etiology and pathogenesis of CRS remain an active area of research. Fungi and bacteria have been implicated as important environmental factors contributing to chronic mucosal inflammation and have thus formed the basis of the fungal hypothesis, the superantigen hypothesis, the biofilm hypothesis, and the microbiome hypothesis. Increasing evidence, however, shows that these exogenous agents are unable to explain the full gamut of clinical variants, inflammatory profiles, and histologic patterns of CRS. Fungi and bacteria are thus generally viewed as disease modifiers of CRS, which accentuate, rather than cause, a dysfunctional immune response in CRS hosts. The eicosanoid hypothesis and the immune barrier hypothesis, in contrast, highlight the contribution of host variables in CRS pathogenesis. The immune barrier hypothesis specifically emphasizes the likely role of genetics and epigenetics in the development of functional deficits at the level of the sinonasal epithelium. These defects ultimately result in a dysfunctional inflammatory response when triggered by external stimuli, with some supporting data provided by the environmentally based hypotheses. In summary, while there is strong evidence supporting an important role for both host and environmental factors in CRS pathophysiology, we have a very incomplete understanding of the molecular pathways that lead to the tissue manifestations and clinical symptomatology that define CRS. Much work is thus necessary at the clinical level to define distinct phenotypes of disease beyond with and without polyps and then to link those phenotypes with molecular pathways of disease.
Acknowledgments
Robert Schleimer reports grants from NIH and personal fees from Intersect ENT, GlaxoSmithKline, Allakos, Aurasense, Merck, BioMarck, and Sanofi. In addition, Dr. Schleimer has a patent on Siglec-8 and Siglec-8 ligand-related patents licensed to Allakos.
Research Funding The study was supported by the Chronic Rhinosinusitis Integrative Studies Program (CRISP) U19-AI106683.
Footnotes
Compliance with Ethics Guidelines Conflict of Interest Kent Lam and Robert C. Kern declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;3:1–298. p preceding table of contents. [PubMed] [Google Scholar]; This is an updated and comprehensive reference document created by international collaboration that serves as the standard guideline for definitions, classifications, and diagnostic criteria for acute and chronic rhinosinusitis.
- 2.Pleis JR, Lucas JW, Ward BW. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat. 2009;10:1–157. [PubMed] [Google Scholar]
- 3.Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–8. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee S, Lane AP. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr Infect Dis Rep. 2011;13:159–68. doi: 10.1007/s11908-011-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18:21–6. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22:549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Crombruggen K, Zhang N, Gevaert P, et al. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–32. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 8•.Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131:1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]; An international consensus report, this recent statement highlights the continued research endeavors to define CRS variants according to both clinical characteristics as phenotypes and also pathophysiologic mechanisms as endotypes.
- 9.Baroody FM, Mucha SM, Detineo M, Naclerio RM. Nasal challenge with allergen leads to maxillary sinus inflammation. J Allergy Clin Immunol. 2008;121:1126–32. doi: 10.1016/j.jaci.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Baroody FM, Mucha SM, de Tineo M, Naclerio RM. Evidence of maxillary sinus inflammation in seasonal allergic rhinitis. Otolaryngol Head Neck Surg : Off J Am Acad Otolaryngol Head Neck Surg. 2012;146:880–6. doi: 10.1177/0194599811435972. [DOI] [PubMed] [Google Scholar]
- 11.Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clinical Immunol. 2013;131:1075–1083. 1083, e1071–1077. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz DelCastillo F, Jurado-Ramos A, Fernandez-Conde BL, et al. Allergenic profile of nasal polyposis. J Investig Allergol Clin Immunol. 2009;19:110–6. [PubMed] [Google Scholar]
- 13.Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23:145–8. doi: 10.2500/ajra.2009.23.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson S, Douglas R, Wormald PJ. The relationship between atopy and chronic rhinosinusitis. Am J Rhinol. 2006;20:625–8. doi: 10.2500/ajr.2006.20.2907. [DOI] [PubMed] [Google Scholar]
- 15.Sasama J, Sherris DA, Shin SH, et al. New paradigm for the roles of fungi and eosinophils in chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2005;13:2–8. doi: 10.1097/00020840-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Davis LJ, Kita H. Pathogenesis of chronic rhinosinusitis: role of airborne fungi and bacteria. Immunol Allergy Clin N Am. 2004;24:59–73. doi: 10.1016/S0889-8561(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 17.Ponikau JU, Sherris DA, Kern EB, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999;74:877–84. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 18.Braun H, Buzina W, Freudenschuss K, et al. `Eosinophilic fungal rhinosinusitis': a common disorder in Europe? Laryngoscope. 2003;113:264–9. doi: 10.1097/00005537-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Shin SH, Ponikau JU, Sherris DA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–75. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Wei JL, Kita H, Sherris DA, et al. The chemotactic behavior of eosinophils in patients with chronic rhinosinusitis. Laryngoscope. 2003;113:303–6. doi: 10.1097/00005537-200302000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Inoue Y, Matsuwaki Y, Shin SH, et al. Nonpathogenic, environmental fungi induce activation and degranulation of human eosinophils. J Immunol. 2005;175:5439–47. doi: 10.4049/jimmunol.175.8.5439. [DOI] [PubMed] [Google Scholar]
- 22.Douglas R, Bruhn M, Tan LW, et al. Response of peripheral blood lymphocytes to fungal extracts and staphylococcal superantigen B in chronic rhinosinusitis. Laryngoscope. 2007;117:411–4. doi: 10.1097/MLG.0b013e31802c0707. [DOI] [PubMed] [Google Scholar]
- 23.Orlandi RR, Marple BF, Georgelas A, et al. Immunologic response to fungus is not universally associated with rhinosinusitis. Otolaryngol–Head Neck Surg : Off J Am Acad Otolaryngol-Head Neck Surg. 2009;141:750–756. e751–752. doi: 10.1016/j.otohns.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Ebbens FA, Scadding GK, Badia L, et al. Amphotericin B nasal lavages: not a solution for patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2006;118:1149–56. doi: 10.1016/j.jaci.2006.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Ebbens FA, Fokkens WJ. The mold conundrum in chronic rhinosinusitis: where do we stand today? Curr Allergy Asthma Rep. 2008;8:93–101. doi: 10.1007/s11882-008-0018-6. [DOI] [PubMed] [Google Scholar]
- 26.Ebbens FA, Georgalas C, Luiten S, et al. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: a multicenter randomized controlled study. Laryngoscope. 2009;119:401–8. doi: 10.1002/lary.20064. [DOI] [PubMed] [Google Scholar]
- 27.Shin SH, Lee SH, Jeong HS, Kita H. The effect of nasal polyp epithelial cells on eosinophil activation. Laryngoscope. 2003;113:1374–7. doi: 10.1097/00005537-200308000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Shin SH, Lee YH, Jeon CH. Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Otolaryngol. 2006;126:1286–94. doi: 10.1080/00016480500395179. [DOI] [PubMed] [Google Scholar]
- 29.Rudack C, Steinhoff M, Mooren F, et al. PAR-2 activation regulates IL-8 and GRO-alpha synthesis by NF-kappaB, but not RANTES, IL-6, eotaxin or TARC expression in nasal epithelium. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2007;37:1009–22. doi: 10.1111/j.1365-2222.2007.02686.x. [DOI] [PubMed] [Google Scholar]
- 30.Matsuwaki Y, Wada K, White T, et al. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. Int Arch Allergy Immunol. 2012;158(Suppl 1):19–29. doi: 10.1159/000337756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhushan B, Homma T, Norton JE, et al. Suppression of epithelial STAT1 activation by extracts of Aspergillus fumigatus. Am J Respir Cell Mol Biol. 2014 doi: 10.1165/rcmb.2014-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan MW. Allergic fungal rhinosinusitis. Otolaryngol Clin N Am. 2011;44:697–710. doi: 10.1016/j.otc.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–82. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 35.Ramanathan M, Jr, Lee WK, Lane AP. Increased expression of acidic mammalian chitinase in chronic rhinosinusitis with nasal polyps. Am J Rhinol. 2006;20:330–5. doi: 10.2500/ajr.2006.20.2869. [DOI] [PubMed] [Google Scholar]
- 36.Feazel LM, Frank DN, Ramakrishnan VR. Update on bacterial detection methods in chronic rhinosinusitis: implications for clinicians and research scientists. Int Forum Allergy Rhinol. 2011;1:451–9. doi: 10.1002/alr.20071. [DOI] [PubMed] [Google Scholar]
- 37•.Feazel LM, Robertson CE, Ramakrishnan VR, Frank DN. Microbiome complexity and Staphylococcus aureus in chronic rhinosinusitis. Laryngoscope. 2012;122:467–72. doi: 10.1002/lary.22398. [DOI] [PMC free article] [PubMed] [Google Scholar]; This cross-sectional observational study demonstrates the utility of DNA pyrosequencing in complementing culture analysis in better understanding the biodiversity of the intranasal microbiota.
- 38.Brook I. The role of bacteria in chronic rhinosinusitis. Otolaryngol Clin N Am. 2005;38:1171–92. doi: 10.1016/j.otc.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Larson DA, Han JK. Microbiology of sinusitis: does allergy or endoscopic sinus surgery affect the microbiologic flora? Curr Opin Otolaryngol Head Neck Surg. 2011;19:199–203. doi: 10.1097/MOO.0b013e328344f67a. [DOI] [PubMed] [Google Scholar]
- 40.Corriveau MN, Zhang N, Holtappels G, et al. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23:461–5. doi: 10.2500/ajra.2009.23.3367. [DOI] [PubMed] [Google Scholar]
- 41.Sachse F, Becker K, von Eiff C, et al. Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy. 2010;65:1430–7. doi: 10.1111/j.1398-9995.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 42.Tan NC, Foreman A, Jardeleza C, et al. Intracellular Staphylococcus aureus: the Trojan horse of recalcitrant chronic rhinosinusitis? Int Forum Allergy Rhinol. 2013;3:261–6. doi: 10.1002/alr.21154. [DOI] [PubMed] [Google Scholar]
- 43.Bachert C, Gevaert P, Holtappels G, et al. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- 44.Bachert C, Zhang N, Patou J, et al. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–8. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 45.Van Zele T, Gevaert P, Watelet JB, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Ramakrishnan VR, Feazel LM, Abrass LJ, Frank DN. Prevalence and abundance of Staphylococcus aureus in the middle meatus of patients with chronic rhinosinusitis, nasal polyps, and asthma. Int Forum Allergy Rhinol. 2013;3:267–71. doi: 10.1002/alr.21101. [DOI] [PubMed] [Google Scholar]
- 47.Seiberling KA, Conley DB, Tripathi A, et al. Superantigens and chronic rhinosinusitis: detection of staphylococcal exotoxins in nasal polyps. Laryngoscope. 2005;115:1580–5. doi: 10.1097/01.mlg.0000168111.11802.9c. [DOI] [PubMed] [Google Scholar]
- 48.Seiberling KA, Grammer L, Kern RC. Chronic rhinosinusitis and superantigens. Otolaryngol Clin N Am. 2005;38:1215–36. doi: 10.1016/j.otc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein JM, Ballow M, Schlievert PM, et al. A superantigen hypothesis for the pathogenesis of chronic hyperplastic sinusitis with massive nasal polyposis. Am J Rhinol. 2003;17:321–6. [PubMed] [Google Scholar]
- 50.Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis: skewing of T-cell receptor V beta-distributions in polyp-derived CD4+ and CD8+ T cells. Am J Rhinol. 2006;20:534–9. doi: 10.2500/ajr.2006.20.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conley DB, Tripathi A, Seiberling KA, et al. Superantigens and chronic rhinosinusitis II: analysis of T-cell receptor V beta domains in nasal polyps. Am J Rhinol. 2006;20:451–5. doi: 10.2500/ajr.2006.20.2880. [DOI] [PubMed] [Google Scholar]
- 52.Tripathi A, Conley DB, Grammer LC, et al. Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope. 2004;114:1822–6. doi: 10.1097/00005537-200410000-00027. [DOI] [PubMed] [Google Scholar]
- 53.Wang M, Shi P, Yue Z, et al. Superantigens and the expression of T-cell receptor repertoire in chronic rhinosinusitis with nasal polyps. Acta Otolaryngol. 2008;128:901–8. doi: 10.1080/00016480701760122. [DOI] [PubMed] [Google Scholar]
- 54.Patou J, Gevaert P, Van Zele T, et al. Staphylococcus aureus enterotoxin B, protein A, and lipoteichoic acid stimulations in nasal polyps. J Allergy Clin Immunol. 2008;121:110–5. doi: 10.1016/j.jaci.2007.08.059. [DOI] [PubMed] [Google Scholar]
- 55.Perez Novo CA, Jedrzejczak-Czechowicz M, Lewandowska-Polak A, et al. T cell inflammatory response, Foxp3 and TNFRS18-L regulation of peripheral blood mononuclear cells from patients with nasal polyps-asthma after staphylococcal superantigen stimulation. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2010;40:1323–32. doi: 10.1111/j.1365-2222.2010.03577.x. [DOI] [PubMed] [Google Scholar]
- 56.Langier S, Landsberg R, Sade K, Kivity S. Anti-IL-5 immunomodulates the effect of Staphylococcus aureus enterotoxin on T cell response in nasal polyps. Rhinology. 2011;49:570–6. doi: 10.4193/Rhino.11.090. [DOI] [PubMed] [Google Scholar]
- 57.Post JC, Hiller NL, Nistico L, et al. The role of biofilms in otolaryngologic infections: update 2007. Curr Opin Otolaryngol Head Neck Surg. 2007;15:347–51. doi: 10.1097/MOO.0b013e3282b97327. [DOI] [PubMed] [Google Scholar]
- 58.Sanderson AR, Leid JG, Hunsaker D. Bacterial biofilms on the sinus mucosa of human subjects with chronic rhinosinusitis. Laryngoscope. 2006;116:1121–6. doi: 10.1097/01.mlg.0000221954.05467.54. [DOI] [PubMed] [Google Scholar]
- 59.Suh JD, Ramakrishnan V, Palmer JN. Biofilms. Otolaryngol Clin N Am. 2010;43:521–30. doi: 10.1016/j.otc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 60.Sanclement JA, Webster P, Thomas J, Ramadan HH. Bacterial biofilms in surgical specimens of patients with chronic rhinosinusitis. Laryngoscope. 2005;115:578–82. doi: 10.1097/01.mlg.0000161346.30752.18. [DOI] [PubMed] [Google Scholar]
- 61.Calo L, Passali GC, Galli J, et al. Role of biofilms in chronic inflammatory diseases of the upper airways. Adv Otorhinolaryngol. 2011;72:93–6. doi: 10.1159/000324622. [DOI] [PubMed] [Google Scholar]
- 62.Foreman A, Holtappels G, Psaltis AJ, et al. Adaptive immune responses in Staphylococcus aureus biofilm-associated chronic rhinosinusitis. Allergy. 2011;66:1449–56. doi: 10.1111/j.1398-9995.2011.02678.x. [DOI] [PubMed] [Google Scholar]
- 63.Foreman A, Jervis-Bardy J, Wormald PJ. Do biofilms contribute to the initiation and recalcitrance of chronic rhinosinusitis? Laryngoscope. 2011;121:1085–91. doi: 10.1002/lary.21438. [DOI] [PubMed] [Google Scholar]
- 64.Boase S, Foreman A, Cleland E, et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis. 2013;13:210. doi: 10.1186/1471-2334-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abreu NA, Nagalingam NA, Song Y, et al. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003783. 151ra124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawley TD, Clare S, Walker AW, et al. Antibiotic treatment of clostridium difficile carrier mice triggers a supershedder state, spore-mediated transmission, and severe disease in immunocom-promised hosts. Infect Immun. 2009;77:3661–9. doi: 10.1128/IAI.00558-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes, and the host. Immunity. 2014;40:824–32. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Gallo RL. S. epidermidis influence on host immunity: more than skin deep. Cell Host Microbe. 2015;17:143–4. doi: 10.1016/j.chom.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review summarizes research regarding the interactions among microbes, microbial metabolites, and the human immune system are relatively well developed in gastrointestinal diseases and provides new perspectives on potential research paradigms for deciphering the CRS puzzle.
- 69.Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol. 2012;9:88–96. doi: 10.1038/nrgastro.2011.244. [DOI] [PubMed] [Google Scholar]
- 70•.Yan M, Pamp SJ, Fukuyama J, et al. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe. 2013;14:631–40. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is an observational study that demonstrates that the indigenous intranasal microbiota likely varies according to locations within the nose and according to carrier status of Staphylococcus aureus.
- 71.Ianiro G, Bibbo S, Scaldaferri F, et al. Fecal microbiota transplantation in inflammatory bowel disease: beyond the excitement. Medicine. 2014;93:e97. doi: 10.1097/MD.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roca-Ferrer J, Garcia-Garcia FJ, Pereda J, et al. Reduced expression of COXs and production of prostaglandin E(2) in patients with nasal polyps with or without aspirin-intolerant asthma. J Allergy Clin Immunol. 2011;128:66–72. doi: 10.1016/j.jaci.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 73.Perez-Novo CA, Waeytens A, Claeys C, et al. Staphylococcus aureus enterotoxin B regulates prostaglandin E2 synthesis, growth, and migration in nasal tissue fibroblasts. J Infect Dis. 2008;197:1036–43. doi: 10.1086/528989. [DOI] [PubMed] [Google Scholar]
- 74.Okano M, Fujiwara T, Haruna T, et al. Prostaglandin E(2) suppresses staphylococcal enterotoxin-induced eosinophilia-associated cellular responses dominantly through an E-prostanoid 2-mediated pathway in nasal polyps. J Allergy Clin Immunol. 2009;123:868–74. doi: 10.1016/j.jaci.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Moylan B, Leopold DA, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA. 2000;284:1814–9. doi: 10.1001/jama.284.14.1814. [DOI] [PubMed] [Google Scholar]
- 76.Wang X, Kim J, McWilliams R, Cutting GR. Increased prevalence of chronic rhinosinusitis in carriers of a cystic fibrosis mutation. Arch Otolaryngol Head Neck Surg. 2005;131:237–40. doi: 10.1001/archotol.131.3.237. [DOI] [PubMed] [Google Scholar]
- 77.Antunes MB, Gudis DA, Cohen NA. Epithelium, cilia, and mucus: their importance in chronic rhinosinusitis. Immunol Allergy Clin N Am. 2009;29:631–43. doi: 10.1016/j.iac.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 78.Chen B, Antunes MB, Claire SE, et al. Reversal of chronic rhinosinusitis-associated sinonasal ciliary dysfunction. Am J Rhinol. 2007;21:346–53. doi: 10.2500/ajr.2007.21.3029. [DOI] [PubMed] [Google Scholar]
- 79.Zuckerman JD, Lee WY, DelGaudio JM, et al. Pathophysiology of nasal polyposis: the role of desmosomal junctions. Am J Rhinol. 2008;22:589–97. doi: 10.2500/ajr.2008.22.3235. [DOI] [PubMed] [Google Scholar]
- 80.Rogers GA, Den Beste K, Parkos CA, et al. Epithelial tight junction alterations in nasal polyposis. Int Forum Allergy Rhinol. 2011;1:50–4. doi: 10.1002/alr.20014. [DOI] [PubMed] [Google Scholar]
- 81.Richer SL, Truong-Tran AQ, Conley DB, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–34. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012;130:1087–96. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 83.Den Beste KA, Hoddeson EK, Parkos CA, et al. Epithelial permeability alterations in an in vitro air-liquid interface model of allergic fungal rhinosinusitis. Int Forum Allergy Rhinol. 2013;3:19–25. doi: 10.1002/alr.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pothoven KL, Norton JE, Hulse KE, et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seshadri S, Rosati M, Lin DC, et al. Regional differences in the expression of innate host defense molecules in sinonasal mucosa. J Allergy Clin Immunol. 2013;132:1227–30. doi: 10.1016/j.jaci.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Psaltis AJ, Bruhn MA, Ooi EH, et al. Nasal mucosa expression of lactoferrin in patients with chronic rhinosinusitis. Laryngoscope. 2007;117:2030–5. doi: 10.1097/MLG.0b013e31812e01ab. [DOI] [PubMed] [Google Scholar]
- 87.Tieu DD, Peters AT, Carter RG, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–75. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Meyer JE, Harder J, Sipos B, et al. Psoriasin (S100A7) is a principal antimicrobial peptide of the human tongue. Mucosal Immunol. 2008;1:239–43. doi: 10.1038/mi.2008.3. [DOI] [PubMed] [Google Scholar]
- 89.Seshadri S, Lin DC, Rosati M, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67:920–8. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee RJ, Kofonow JM, Rosen PL, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wolk K, Kunz S, Witte E, et al. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 92.Wolk K, Witte E, Wallace E, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36:1309–23. doi: 10.1002/eji.200535503. [DOI] [PubMed] [Google Scholar]
- 93.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–81. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ramanathan M, Jr, Spannhake EW, Lane AP. Chronic rhinosinusitis with nasal polyps is associated with decreased expression of mucosal interleukin 22 receptor. Laryngoscope. 2007;117:1839–43. doi: 10.1097/MLG.0b013e31811edd4f. [DOI] [PubMed] [Google Scholar]
- 96.Hulse KE, Chaung K, Seshadri S, et al. Suppressor of cytokine signaling 3 expression is diminished in sinonasal tissues from patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2014;133:275–7. doi: 10.1016/j.jaci.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97•.Derycke L, Eyerich S, Van Crombruggen K, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS ONE. 2014;9:e97581. doi: 10.1371/journal.pone.0097581. [DOI] [PMC free article] [PubMed] [Google Scholar]; T cell cytokine patterns in control patients, CRSsNP, and CRSwNP are highlighted in this observational study.
- 98••.Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]; This studies identifies TSLP as a key epitelial cytokine drivin Type 2 responses in CRSwNP.
- 99.Shaw JL, Fakhri S, Citardi MJ, et al. IL-33-responsive innate lymphoid cells are an important source of IL-13 in chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2013;188:432–9. doi: 10.1164/rccm.201212-2227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100•.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]; This is a detailed review of constituents of the innate immune system that collaborate with each other, the adaptive immune system, and with non-hematopoietic cell types to not only promote immune responses, but also induce inflammation and tissue remodeling.
- 101.Allakhverdi Z, Comeau MR, Smith DE, et al. CD34+ hemopoietic progenitor cells are potent effectors of allergic inflammation. J Allergy Clin Immunol. 2009;123:472–8. doi: 10.1016/j.jaci.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 102.Mjosberg JM, Trifari S, Crellin NK, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–62. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 103.Kato A, Peters A, Suh L, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2008;121:1385–1392. 1392, e1381–1382. doi: 10.1016/j.jaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. JAllergy Clin Immunol. 2011;128:1198–206. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Van Zele T, Gevaert P, Holtappels G, et al. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2007;37:1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 106.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. The Journal of allergy and clinical immunology. 2013;131:933–957. doi: 10.1016/j.jaci.2013.02.023. quiz 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107•.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy : J Br Soc Allergy Clin Immunol. 2015;45:328–46. doi: 10.1111/cea.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review on the various etiologies of CRSwNP provides detailed summaries of the different cytokines and chemokines implicated in the pathogenesis of nasal polyposis.
- 108.Chandra RK, Lin D, Tan B, et al. Chronic rhinosinusitis in the setting of other chronic inflammatory diseases. Am J Otolaryngol. 2011;32:388–91. doi: 10.1016/j.amjoto.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–60. doi: 10.1016/j.jaci.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]