Background
Although pain commonly accompanies infections caused by bacterial, fungal, and viral pathogens, the mechanisms responsible are not well understood. Until recently it was thought that pain was secondary to the inflammation produced by pathogen invasion but the discovery that nociceptor neurons are directly activated by pathogens and their molecular ligands has led to the appreciation that this interaction contributes to pain during infection. Here, we highlight the distinct mechanisms discovered so far for mediating pathogen recognition by sensory neurons.
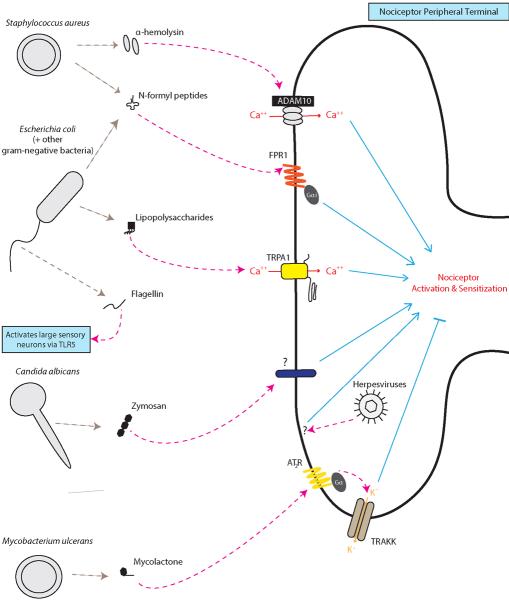
Bacterial Pathogens
Staphylococcus aureus, a gram-positive bacterial pathogen, is a leading cause of painful skin infections. S. aureus produces pain through α-hemolysin, a pore-forming toxin which directly activates nociceptor neurons through membrane pore-formation, leading to ion influx and the generation of action potentials, contributing to mechanical and thermal allodynia during infection [1].
Gram-negative bacteria such as Salmonella enterica and Escherichia coli are responsible for painful gastrointestinal and urinary tract infections. Lipopolysaccharide (LPS), a major component of gram-negative cell walls, directly activates the TRPA1 ion channel on nociceptors to produce pain [3]. This nociceptor activation requires Lipid A, the membrane anchored moiety of LPS [3]. LPS may also signal through a Toll-like receptor (TLR4) mediated sensitization of the capsaicin receptor TRPV1 [4,8,11].
N-formyl peptides are metabolic byproducts of all bacteria, and nociceptors detect these peptides through the G-protein coupled receptor FPR1 [1]. Flagellin, the major protein in bacterial flagella, activates A-fiber sensory neurons through TLR5 [9].
Mycobacterium ulcerans in contrast, produces large, painless skin ulcers during infection, and secretes a mycolactone which signals through the type 2 angiotensin receptor and potassium channels to produce its characteristic analgesia [7].
Viral and Fungal Pathogens
Viral pathogens also produce pain during infection, such as the common viral sore throat, but the mechanisms of nociceptor activation/sensitization produced by these pathogens are less well understood. TLR3 and TLR7, which recognize double-stranded and single-stranded RNA, respectively, are expressed by pruriceptor neurons and contribute to chronic itch [2,5], but whether they generate sensory disturbances in response to viral infection is not yet known.
HSV-1, HSV-2, and VZV infect nociceptor neurons to produce a variety of painful syndromes including cold sores and shingles [10]. However, it is unknown whether specific intracellular or extracellular viral recognition mechanisms by the sensory neurons mediate this pain.
The fungal pathogen Candida albicans and its cell wall component zymosan directly induce calcium influx into nociceptors [6] and this may contribute to the pain of thrush. Interestingly, nociceptors mediate a protective skin immune response against C. albicans [6].
Pain and infection: Beneficial for the Host or Pathogen?
Pain in response to pathogen invasion may serve its usual essential defense against danger role by warning the host of the presence of pathogenic invasion. Conversely, pathogens may have evolved mechanisms to manipulate nociceptor activity, and the pain it causes, to facilitate spread from host-to-host or within infected tissues. Nociceptors release molecular mediators from their peripheral terminals, such as the neuropeptides CGRP and substance P, that have powerful effects on immune cell recruitment and activation during infection [1,3,6]. Further work is required to determine the beneficial or harmful nature of pain in host defense against specific types of pathogens, nevertheless as every physician knows, pain is a prominent early feature of most infections.
Figure 1.
References
- 1.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501:52–57. doi: 10.1038/nature12479. doi:10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diogenes A, Ferraz CC, Akopian AN, Henry MA, Hargreaves KM. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J Dent Res. 2011;90:759–764. doi: 10.1177/0022034511400225. doi:10.1177/0022034511400225. [DOI] [PubMed] [Google Scholar]
- 3.Kashem SW, Riedl MS, Yao C, Honda CN, Vulchanova L, Kaplan DH. Nociceptive sensory fibers drive interleukin-23 production from CD301b+ dermal dendritic cells and drive protective cutaneous immunity. Immunity. 2013;43:515–526. doi: 10.1016/j.immuni.2015.08.016. doi:10.1016/j.immuni.2015.08.016 (2015). Nature 501, 52–57, doi:10.1038/nature12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Adamek P, Zhang H, Tatsui CE, Rhines LD, Mrozkova P, Li Q, Kosturakis AK, Cassidy RM, Harrison DS, Cata JP, Sapire K, Zhang H, Kennamer-Chapman RM, Jawad AB, Ghetti A, Yan J, Palecek J, Dougherty PM. The Cancer Chemotherapeutic Paclitaxel Increases Human and Rodent Sensory Neuron Responses to TRPV1 by Activation of TLR4. J Neurosci. 2015;35:13487–13500. doi: 10.1523/JNEUROSCI.1956-15.2015. doi:10.1523/JNEUROSCI.1956-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu T, Berta T, Xu ZZ, Park CK, Zhang L, Lü N, Liu Q, Liu Y, Gao YJ, Liu YC, Ma Q, Dong X, Ji RR. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J Clin Invest. 2012;122:2195–2207. doi: 10.1172/JCI45414. doi:10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu T, Xu ZZ, Park CK, Berta T, Ji RR. Toll-like receptor 7 mediates pruritus. Nat Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. doi:10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marion E, Song OR, Christophe T, Babonneau J, Fenistein D, Eyer J, Letournel F, Henrion D, Clere N, Paille V, Guérineau NC, Saint André JP, Gersbach P, Altmann KH, Stinear TP, Comoglio Y, Sandoz G, Preisser L, Delneste Y, Yeramian E, Marsollier L, Brodin P. Mycobacterial toxin induces analgesia in buruli ulcer by targeting the angiotensin pathways. Cell. 2014;157:1565–1576. doi: 10.1016/j.cell.2014.04.040. doi:10.1016/j.cell.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn JA, Fernández-Peña C, Talavera A, Kichko T, Navia B, Sánchez A, Señarís R, Reeh P, Pérez-García MT, López-López JR, Voets T, Belmonte C, Talavera K, Viana F. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun. 2014;5:3125. doi: 10.1038/ncomms4125. doi:10.1038/ncomms4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Min H, Lee H, Lim H, Jang YH, Chung SJ, Lee CJ, Lee SJ. TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol Brain. 2014;7:59. doi: 10.1186/s13041-014-0059-9. doi:10.1186/s13041-014-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagel MA, Gilden D. Neurological complications of varicella zoster virus reactivation. Curr Opin Neurol. 2014;27:356–360. doi: 10.1097/WCO.0000000000000092. doi:10.1097/WCO.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu ZZ, Kim YH, Bang S, Zhang Y, Berta T1, Wang F, Oh SB, Ji RR. Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat Med. 2015;21:1326–1331. doi: 10.1038/nm.3978. doi:10.1038/nm.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]