Abstract
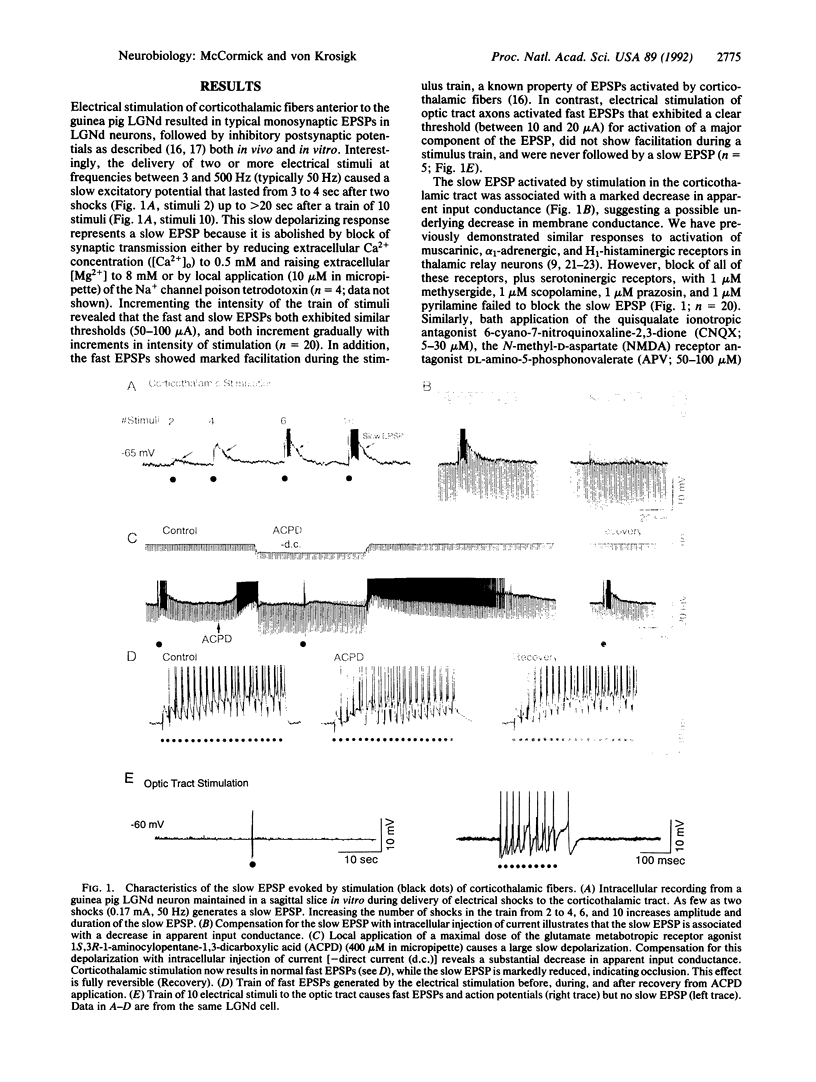
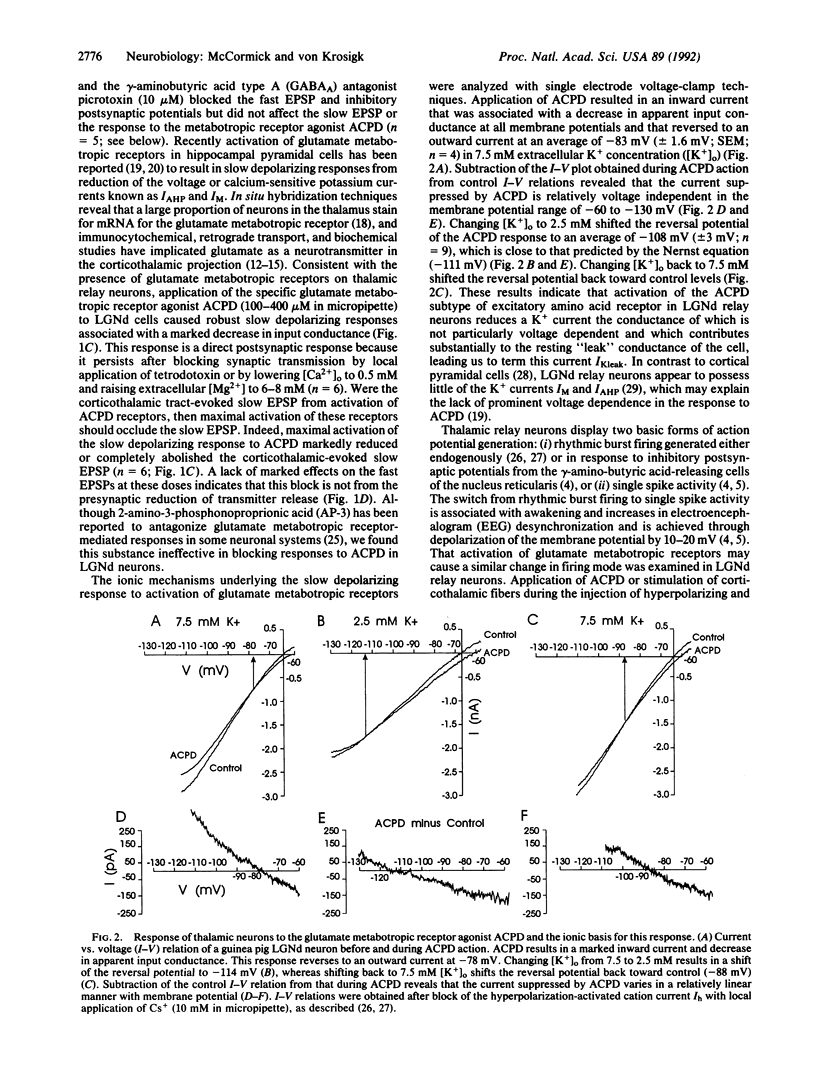
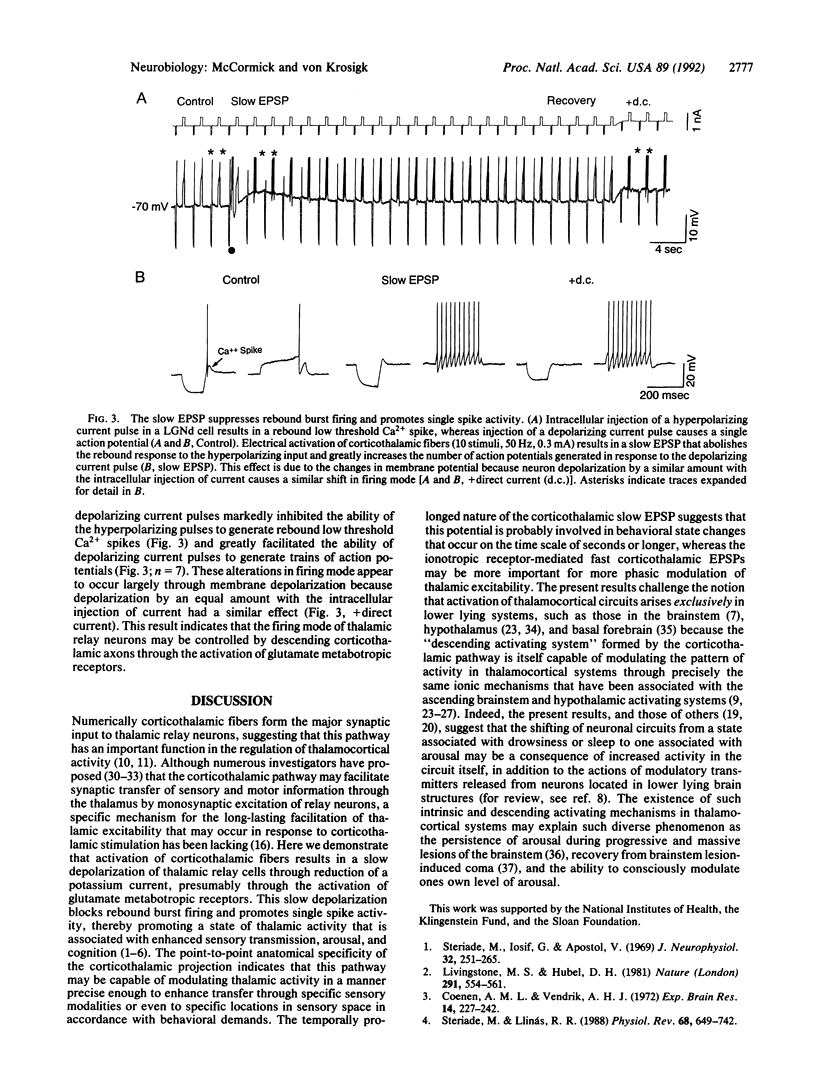
The mammalian thalamus forms an obligatory relay for nearly all sensory information that reaches the cerebral cortex. The transmission of sensory information by the thalamus varies in a state-dependent manner, such that during slow wave sleep or drowsiness thalamic responsiveness is markedly reduced, whereas during the waking, attentive state transmission is enhanced. Although activation of brainstem inputs to thalamic neurons has long been assumed to underlie this gating of sensory transfer through the thalamus, numerically the largest input to thalamic relay neurons derives from layer VI cells of the cerebral cortex. Here we report that activation of corticothalamic fibers causes a prolonged excitatory postsynaptic potential in guinea pig dorsal lateral geniculate relay neurons resulting from the reduction of a potassium conductance, consistent with the activation of glutamatergic "metabotropic" receptors. This slow depolarization can switch firing of thalamic neurons from the burst firing mode, which is prevalent during slow wave sleep, to the single spike mode, which is prevalent during waking, thereby facilitating transmission of sensory information through the thalamus. This prolonged enhancement of thalamic transfer may allow the cerebral cortex to gate or control selective fields of sensory inputs in a manner that facilitates arousal, attention, and cognition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlsen G., Grant K., Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982 Feb 25;234(2):454–458. doi: 10.1016/0006-8993(82)90886-1. [DOI] [PubMed] [Google Scholar]
- Baughman R. W., Gilbert C. D. Aspartate and glutamate as possible neurotransmitters of cells in layer 6 of the visual cortex. Nature. 1980 Oct 30;287(5785):848–850. doi: 10.1038/287848a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Bickford R. G., Ponomareff G., Thal L. J., Mandel R., Gage F. H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988 Nov;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H. Glutamate mediates a slow synaptic response in hippocampal slice cultures. Proc Biol Sci. 1991 Mar 22;243(1308):221–226. doi: 10.1098/rspb.1991.0035. [DOI] [PubMed] [Google Scholar]
- Coenen A. M., Vendrik A. J. Determination of the transfer ratio of cat's geniculate neurons through quasi-intracellular recordings and the relation with the level of alertness. Exp Brain Res. 1972;14(3):227–242. doi: 10.1007/BF00816160. [DOI] [PubMed] [Google Scholar]
- Denoyer M., Sallanon M., Buda C., Kitahama K., Jouvet M. Neurotoxic lesion of the mesencephalic reticular formation and/or the posterior hypothalamus does not alter waking in the cat. Brain Res. 1991 Jan 25;539(2):287–303. doi: 10.1016/0006-8993(91)91633-c. [DOI] [PubMed] [Google Scholar]
- Deschêenes Martin, Hu Bin. Electrophysiology and Pharmacology of the Corticothalamic Input to Lateral Thalamic Nuclei: an Intracellular Study in the Cat. Eur J Neurosci. 1990 Feb;2(2):140–152. doi: 10.1111/j.1460-9568.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F., Storm-Mathisen J., Divac I. Biochemical evidence for glutamate as neurotransmitter in corticostriatal and corticothalamic fibres in rat brain. Neuroscience. 1981;6(5):863–873. doi: 10.1016/0306-4522(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Hirsch J. C., Fourment A., Marc M. E. Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 1983 Jan 24;259(2):308–312. doi: 10.1016/0006-8993(83)91264-7. [DOI] [PubMed] [Google Scholar]
- Hull E. M. Corticofugal influence in the macaque lateral geniculate nucleus. Vision Res. 1968 Oct;8(10):1285–1298. doi: 10.1016/0042-6989(68)90050-3. [DOI] [PubMed] [Google Scholar]
- Izzo P. N. A note on the use of biocytin in anterograde tracing studies in the central nervous system: application at both light and electron microscopic level. J Neurosci Methods. 1991 Feb;36(2-3):155–166. doi: 10.1016/0165-0270(91)90041-w. [DOI] [PubMed] [Google Scholar]
- Kalil R. E., Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol. 1970 May;33(3):459–474. doi: 10.1152/jn.1970.33.3.459. [DOI] [PubMed] [Google Scholar]
- Leresche N., Lightowler S., Soltesz I., Jassik-Gerschenfeld D., Crunelli V. Low-frequency oscillatory activities intrinsic to rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:155–174. doi: 10.1113/jphysiol.1991.sp018744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S., Wróbel A. Frequency dependent corticofugal excitation of principal cells in the cat's dorsal lateral geniculate nucleus. Exp Brain Res. 1990;79(2):313–318. doi: 10.1007/BF00608240. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981 Jun 18;291(5816):554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci. 1992 Jan;12(1):278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989 Jun;12(6):215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Actions of acetylcholine in the guinea-pig and cat medial and lateral geniculate nuclei, in vitro. J Physiol. 1987 Nov;392:147–165. doi: 10.1113/jphysiol.1987.sp016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol. 1988 Mar;59(3):978–996. doi: 10.1152/jn.1988.59.3.978. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991 Oct;11(10):3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero V. M. A quantitative study of synaptic contacts on interneurons and relay cells of the cat lateral geniculate nucleus. Exp Brain Res. 1991;86(2):257–270. doi: 10.1007/BF00228950. [DOI] [PubMed] [Google Scholar]
- Montero V. M., Wenthold R. J. Quantitative immunogold analysis reveals high glutamate levels in retinal and cortical synaptic terminals in the lateral geniculate nucleus of the macaque. Neuroscience. 1989;31(3):639–647. doi: 10.1016/0306-4522(89)90429-6. [DOI] [PubMed] [Google Scholar]
- Scharfman H. E., Lu S. M., Guido W., Adams P. R., Sherman S. M. N-methyl-D-aspartate receptors contribute to excitatory postsynaptic potentials of cat lateral geniculate neurons recorded in thalamic slices. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4548–4552. doi: 10.1073/pnas.87.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G. Comparison of excitatory amino acid-stimulated phosphoinositide hydrolysis and N-[3H]acetylaspartylglutamate binding in rat brain: selective inhibition of phosphoinositide hydrolysis by 2-amino-3-phosphonopropionate. J Neurochem. 1989 Jul;53(1):273–278. doi: 10.1111/j.1471-4159.1989.tb07324.x. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Steriade M., Iosif G., Apostol V. Responsiveness of thalamic and cortical motor relays during arousal and various stages of sleep. J Neurophysiol. 1969 Mar;32(2):251–265. doi: 10.1152/jn.1969.32.2.251. [DOI] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Wada H., Inagaki N., Yamatodani A., Watanabe T. Is the histaminergic neuron system a regulatory center for whole-brain activity? Trends Neurosci. 1991 Sep;14(9):415–418. doi: 10.1016/0166-2236(91)90034-r. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Friedlander M. J., Sherman S. M. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proc R Soc Lond B Biol Sci. 1984 Jun 22;221(1225):411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]




