Abstract
Purpose
Previous research has suggested that a failure of opioid inhibition may contribute to chronic bladder pain. The purpose of the present study was to determine how acute adult and/or prior early-in-life (EIL) exposure to bladder inflammation alters adult content of endogenous opioid peptides in the bladder, spinal cord, and blood.
Materials and Methods
Inflammation was induced by intravesical administration of zymosan. Female Sprague-Dawley rats were exposed to anesthesia only or zymosan EIL (P14-P16) and anesthesia only or zymosan as adults (12-17 weeks). Thoracolumbar and lumbosacral segments of the spinal cord, blood, and bladders were collected 24 hours after adult treatment. Opioid peptide content was measured using enzyme-linked immunosorbent assay (ELISA).
Results
EIL bladder inflammation alone produced a chronic increase in dynorphin A (1-17) in the lumbosacral spinal cord. When EIL inflammation was followed by adult re-inflammation, spinal cord dynorphin remained unchanged, but bladder dynorphin was decreased. In addition, EIL inflammation combined with adult bladder inflammation decreased endomorphin-2 content in the thoracolumbar spinal cord. Neither EIL nor adult bladder inflammation affected thoracolumbar dynorphin, serum dynorphin, lumbosacral endomorphin-2, or plasma β-endorphin.
Conclusions
Several opioid peptides were measured using ELISA following EIL and adult bladder inflammation. The changes observed are consistent with the view that EIL bladder inflammation alone can chronically alter spinal cord peptide content. When coupled with adult re-inflammation, these changes could set the neurochemical stage to support bladder hypersensitivity.
Keywords: Opioid, Visceral Pain, Inflammation, Painful Bladder Syndrome, Animals, Newborn
Introduction
There is a mounting interest in the long-term effects of exposure to early-in-life (EIL) noxious insults. Neonatal infants often receive repeated heel lancings, events that result in visceral pain, and other painful encounters. The evidence suggests that these events alter the development of pain processing systems and can result in changes in pain perception lasting into adulthood1. EIL exposure to noxious stimulation of the bladder may contribute to the development of painful functional bladder disorders as an adult. For example, childhood urinary tract infections (UTIs) are a relatively common occurrence2, and bladder pain syndrome/interstitial cystitis (BPS/IC) is epidemiologically associated with childhood bladder infections3. In rats, EIL exposure to bladder inflammation produces chronic changes in bladder function lasting into adulthood including increased adult baseline micturition frequency, decreased micturition threshold to bladder filling, and enhanced sensitivity to intravesical infusion of ice cold saline4-6. EIL exposure to bladder inflammation followed by adult re-inflammation also produces bladder hypersensitivity as manifested by increased visceromotor reflexes (VMRs) and cardiovascular responses to graded distension of the bladder (urinary bladder distension; UBD)6. These outcomes parallel some of the symptoms manifested by patients with BPS/IC including bladder pain, increased urinary frequency and urgency, and pain upon exposure to ice cold saline7,8. Therefore, this animal model has been proposed as potentially useful for the study of the etiology underlying BPS/IC.
Alterations in opioid inhibitory systems appear to contribute to some of the changes in nociceptive processing from the bladder produced by EIL inflammation. For example, EIL bladder inflammation decreases reactive opioid inhibition that would otherwise be engaged in an adult rat in response to adult bladder inflammation9. Specifically, adult rats naïve to any other treatment which received acute bladder inflammation show enhanced VMRs to UBD when tested in the presence of either intraperitoneal (i.p.) or intrathecal (i.t.) naloxone, but this does not occur in animals that had received prior EIL exposure to bladder inflammation. Preliminary data from our laboratory10 suggest changes in endogenous opioid peptides dynorphin and endomorphin-2 might be responsible for the changes in bladder sensitivity observed in this EIL animal model of BPS/IC. Therefore, the current study used enzyme-linked immunosorbent assay (ELISA) to measure spinal, serum, and bladder content of dynorphin, as well as spinal content of endomorphin-2 and plasma content of β-endorphin, to determine whether these neurochemical substrates were altered by EIL and/or adult bladder inflammation produced by zymosan11.
Materials and Methods
Animal Subjects
Female pups from timed-pregnant female Sprague-Dawley rats were used. The light-dark cycle was 7:00 to 7:00 for all rats. This study was approved by the University of Alabama at Birmingham Animal Care and Use Committee and conformed to NIH guidelines for the care and use of laboratory animals.
EIL Intravesical Treatments
Treatments of pups with either anesthesia only or intravesical zymosan began on P14. Anesthesia was induced with 5% isoflurane and the external urethra was swabbed with Betadine. The urinary bladders of animals in the zymosan group were catheterized transurethrally with a 24-gauge angiocatheter (Cardinal Health, Dublin, OH). Rats were maintained on 2% inhaled halothane and administered zymosan (0.1 ml, 1% in saline). The solution was allowed to dwell in the bladder for 30 minutes and drained. Body temperature was maintained with a warmed heating pad. All rats received subcutaneous (s.c.) ampicillin (0.05 ml, 100 mg/ml) at the end of the procedure. Three successive 30 min daily treatments were performed (P14-P16).
Adult Intravesical Treatments
Animals at 12-17 weeks of age were anesthetized with inhaled isoflurane and oxygen (5% for induction, 2% for maintenance). Rats were treated with anesthesia only or zymosan. Zymosan-treated animals had their bladders catheterized with a 22-gauge angiocatheter via the urethra. Zymosan (0.5 ml, 1% in saline) was administered intravesically for 30 min and drained. All animals received ampicillin at the end of the procedure (0.2 ml, 100 mg/ml, s.c.). EIL and adult intravesical treatments resulted in 4 groups: animals receiving anesthesia both EIL and as adults (AA), those receiving anesthesia EIL and zymosan as adults (AZ), those receiving zymosan EIL and anesthesia as adults (ZA), and those receiving zymosan both EIL and as adults (ZZ). Control groups with animals administered intravesical saline EIL and/or as an adult were not included in this study because previous studies have shown that the effects of these treatments are not significantly different from those of the anesthesia alone control procedure6.
Vaginal Cytology
Vaginal smears were obtained via vaginal lavage. Animals demonstrated at least 1 complete cycle before testing. All animals underwent adult intravesical treatments during proestrus, as characterized by a preponderance of nucleated epithelial cells. Therefore, most animals were in estrus on the day of tissue collection. Collecting during this phase optimized the ability to detect changes in the opioid system, since the largest magnitude of opioid inhibition, as measured by potentiation of VMRs to UBD by naloxone, is present in estrus12. A final sample was taken after completion of tissue collection.
Tissue collection, processing, and analysis
Cardiac blood and bladders were removed 24 hours following adult intravesical treatments, after which animals were euthanized via decapitation. Spinal cords were removed, and thoracolumbar (T13-L2) and lumbosacral (L4-S2) sections were isolated. Protein concentrations were determined using the Pierce BCA Assay Reagent Kit (Thermo Fisher Scientific, Rockford, IL). Spinal, serum, and bladder dynorphin A (1-17) content, spinal endomorphin-2 content, and serum β-endorphin content were measured using ELISA (Phoenix Pharmaceuticals, Burlingame, CA). Samples and serial dilutions of peptide standard were incubated with the appropriate primary antibody and corresponding biotinylated peptide on a plate precoated with secondary antibody. Incubations with strepavidin-horseradish peroxidase, 3,3′,5,5′-tetramethylbenzidine, and hydrochloric acid (2N) followed. Absorbance optical density was read at 450 nm (Fluostar Omega microplate reader; BMG Labtech, Ortenberg, Germany). The concentration of peptide was calculated from a standard curve (MARS Data Analysis Software; BMG Labtech). Bladder endomorphin-2 content was not measured because to our knowledge there is currently no evidence for its existence in the bladder. Spinal met-enkephalin content was not measured because no commercially available kits are available for the detection of met-enkephalin, and efforts to measure met-enkephalin in our laboratory without a kit have been unsuccessful. In addition, leu-enkephalin content was measured, but the kit used (Phoenix Pharmaceuticals EK-024-21) was not sensitive enough to detect measurable amounts of this peptide in the spinal cord.
Statistical analysis
One-way ANOVAs and Fisher's LSD followed by Holm's correction13 were conducted as appropriate. Any outliers (values more extreme than 1.5× the interquartile range from the first or third quartile) were excluded.
Results
Lumbosacral Dynorphin
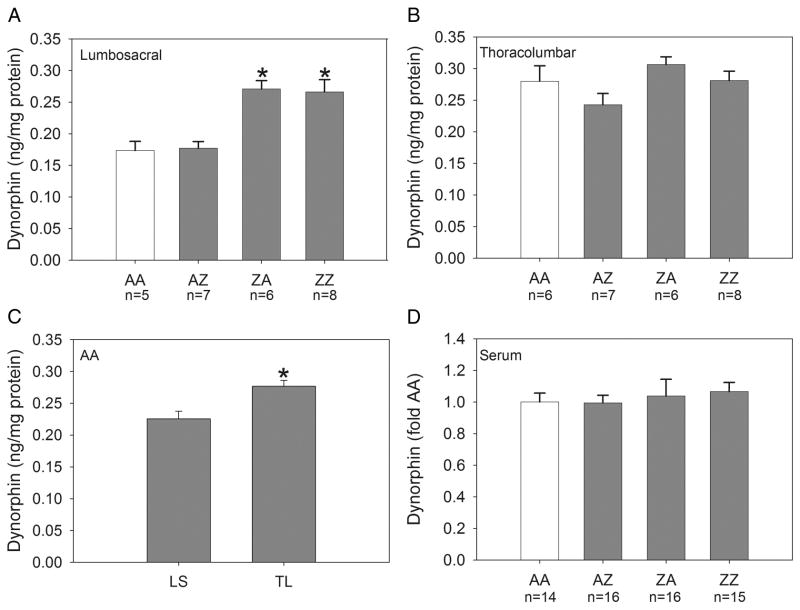
Dynorphin content normalized to total protein for the lumbosacral spinal cord is shown in Panel A of Figure 1. An ANOVA revealed a significant effect of intravesical treatment group [F(3,22)=11.59, p<0.001]. Dynorphin content of groups ZA and ZZ was increased relative to groups AA and AZ (p≤0.001). The latter groups were not significantly different from one another.
Figure 1.
Group mean content of dynorphin in the lumbosacral and thoracolumbar spinal cord and serum. Panel A: Dynorphin content was significantly increased in the lumbosacral spinal cord of groups ZA and ZZ compared to groups AA and AZ (*p≤0.001; n=5-8/group). Panel B: Dynorphin content in the thoracolumbar spinal cord was not significantly altered by the treatments (n=6-8/group). Panel C: Dynorphin content in the thoracolumbar spinal cord of AA controls was significantly greater than in the lumbosacral spinal cord (p=0.006). Panel D: Dynorphin content in serum was not altered by the treatments (n=14-16/group). AA control data are shown in open bars.
Thoracolumbar Dynorphin
Dynorphin content in the thoracolumbar spinal cord is shown in Panel B of Figure 1. An ANOVA revealed no significant effect of intravesical treatment group. Panel C of Figure 1 shows dynorphin content in the lumbosacral and thoracolumbar spinal cord of AA controls. Dynorphin content in the thoracolumbar spinal cord of AA controls was significantly greater than that in the lumbosacral spinal cord (p=0.006).
Serum Dynorphin
Dynorphin content in the serum is shown in Panel D of Figure 1. An ANOVA revealed no significant effect of intravesical treatment group. These data are expressed as fold of AA controls because results were pooled from two separate ELISA kits.
Bladder Dynorphin
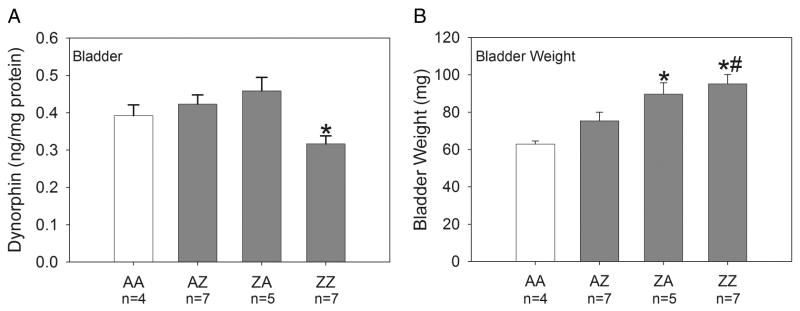
Dynorphin content in the bladder is shown in Panel A of Figure 2. An ANOVA revealed a significant effect of intravesical treatment group [F(3,19)=4.66, p=0.013]. Dynorphin content in group ZZ was significantly decreased compared to group ZA (p=0.002).
Figure 2.
Group mean content of dynorphin in the bladder and bladder weights (n=4-7/group). Panel A: Bladder dynorphin content was significantly decreased in group ZZ compared to group ZA (*p=0.002). Panel B: Bladder weights were significantly increased in groups ZA and ZZ compared to AA controls (*p≤0.003). Bladder weights of group ZZ were also significantly greater than those of group AZ (#p=0.005). AA control data are shown in open bars.
Bladder weight
Group mean bladder weights are shown in Panel B of Figure 2. An ANOVA revealed a significant effect of intravesical treatment group [F(3,19)=7.82, p=0.001]. Bladder weights were increased in groups ZA (p=0.003) and ZZ (p≤0.001) compared to AA controls. Bladder weights of ZZ animals were also significantly greater than those of group AZ (p=0.005).
Lumbosacral Endomorphin-2
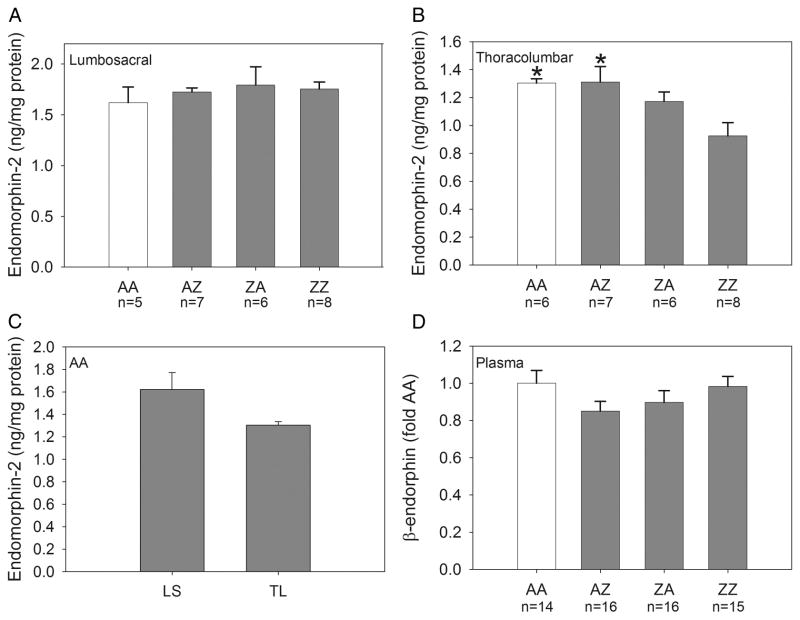
Endomorphin-2 content in the lumbosacral spinal cord is shown in Panel A of Figure 3. An ANOVA revealed no significant effect of intravesical treatment group.
Figure 3.
Group mean content of endomorphin-2 in the lumbosacral and thoracolumbar spinal cord and β-endorphin content in the plasma. Panel A: Endomorphin-2 content in the lumbosacral spinal cord was not significantly altered by the treatments (n=5-8/group). Panel B: Endomorphin-2 content was significantly decreased in the thoracolumbar spinal cord of group ZZ compared to groups AA and AZ (*p≤0.009). Panel C: Endomorphin-2 content in the thoracolumbar spinal cord of AA controls was not significantly different from that in the lumbosacral spinal cord. Panel D: β-endorphin content in plasma was not altered by the treatments (n=14-16/group). AA control data are shown in open bars.
Thoracolumbar Endomorphin-2
Endomorphin-2 content in the thoracolumbar spinal cord is shown in Panel B of Figure 3. An ANOVA revealed a significant effect of intravesical treatment group [F(3,23)=4.33, p=0.015]. Endomorphin-2 content of group ZZ was decreased relative to groups AA (p=0.009) and AZ (p=0.004). The latter groups were not significantly different from one another or from group ZA. Panel C of Figure 3 shows endomorphin-2 content in the lumbosacral and thoracolumbar spinal cord of AA controls. Endomorphin-2 content in the thoracolumbar spinal cord of AA controls was not significantly different from that in the lumbosacral spinal cord.
Plasma B-endorphin
β-endorphin content in the plasma is shown in Panel D of Figure 3. An ANOVA revealed no significant effect of intravesical treatment group. These data are expressed as fold of AA controls because results were pooled from two separate ELISA kits.
Discussion
The current study addressed two questions: 1) does EIL and/or adult bladder inflammation alter spinal and peripheral opioid peptide content and 2) can these alterations help to explain the bladder hypersensitivity and impairment of opioid inhibition that develops after EIL bladder inflammation and suggest a possible contributing mechanism underlying BPS/IC.
Dynorphin content in the spinal cord
EIL bladder inflammation alone (group ZA) significantly increased dynorphin content in the lumbosacral spinal cord when tissue was collected 3-4 months after inflammation compared to animals inflamed only as adults (group AZ) and anesthesia alone (group AA) controls. This indicates that EIL bladder inflammation alone chronically increases spinal dynorphin, and a previous study4 demonstrated that this occurs in the absence of significant changes in the thickness, fibrosis, or mast cells in the bladder. Dynorphin content of group ZZ was also increased relative to the same groups, but did not significantly differ from that of group ZA indicating that EIL bladder inflammation alone was a necessary and sufficient condition for increasing spinal dynorphin content. These increases were restricted to lumbosacral segments. It was surprising that group AZ did not show altered dynorphin content compared to AA controls because other studies have shown that hindpaw14,15, joint16,17, and abdominal18 inflammation increase dynorphin mRNA and peptide. It may be that we did not observe an increase in group AZ because tissue collection occurred 24 hours after inflammation of the bladder and prior to the synthesis of new dynorphin which in some studies requires up to 3 days19. Alternatively, it is possible that this represents an important difference in the effects of inflammation on somatic versus visceral tissues.
The importance of the changes in spinal dynorphin content following EIL bladder inflammation alone is reinforced by evidence showing that spinal dynorphin can act to maintain chronic pain states. Increases in spinal dynorphin due to nerve injury or exogenous administration lead to tactile, cold, and thermal hypersensitivities that can be reduced by NMDA and bradykinin antagonists but not naloxone20,21. Spinal dynorphin plays a similar role in the maintenance of chronic inflammatory nociception19. In the current study, an increase in lumbosacral spinal dynorphin after EIL bladder inflammation could permanently facilitate nociceptive transmission from the bladder and contribute to bladder hypersensitivity, perhaps through a bradykinin and/or NMDA-dependent mechanism.
Dynorphin content in the bladder
The dynorphin content of the bladder was significantly decreased after adult re-inflammation (group ZZ) compared to group ZA. We believe this difference may have important implications for understanding the role of dynorphin in the bladder for the following reasons. Su et al.22,23 found that U50,488H inhibited responses of decentralized pelvic afferents and VMRs to UBD. Systemic administration of other κ-opioid agonists, such as dynorphin, may similarly inhibit primary bladder afferent activity and VMRs to UBD. However, this suggestion is not supported by the finding that dynorphin (1–13) does not alter activity of decentralized colon primary afferents during colorectal distension24, and further studies are required to determine the functional impact of systemic dynorphin on the bladder.
Bladder content of dynorphin presumably reflects some changes in dynorphin derived from bladder afferents. Adult re-inflammation may have promoted release of dynorphin in group ZZ, leading to degradation and a reduction in dynorphin relative to group ZA prior to tissue collection. Depletion of dynorphin content in group ZZ may reduce the inhibitory effect of bladder dynorphin on bladder afferent activity (disinhibition) when compared to animals inflamed only EIL (group ZA) thereby enhancing bladder hypersensitivity relative to this group.
Endomorphin-2 content in the spinal cord
We observed a significant decrease in the thoracolumbar levels of endomorphin-2 in group ZZ relative to AA controls and group AZ. Endomorphin-2 is generally viewed as having inhibitory influences on spinal nociceptive transmission. Therefore, a reduction in thoracolumbar endomorphin-2 may reduce endogenous opioid inhibition of excitatory bladder input.
Relation to previous studies
Studies of the VMR to UBD have indicated that group ZZ displays markedly enhanced bladder sensitivity relative to group AZ and animals that receive zymosan EIL but saline as adults6. However, dynorphin content in the lumbosacral spinal cord of group ZZ was not significantly different from that of group ZA. Therefore, increased dynorphin content alone cannot explain hypersensitivity in group ZZ. A decreased inhibitory influence of endomorphin-2 in the thoracolumbar spinal cord could act in an additive or synergistic manner with the enhanced facilitatory influence of increased spinal dynorphin in the lumbosacral spinal cord to markedly enhance bladder hypersensitivity. In addition, a functional disinhibition of primary afferent input in group ZZ due to decreased bladder dynorphin would enhance bladder sensitivity even further. Collectively, these changes would explain bladder hypersensitivity6 and the lack of effectiveness of the opioid receptor antagonist naloxone9 in group ZZ in previous studies.
Clinical Implications
The purpose of the present and previous studies was to provide a consistent picture about how bladder insults like inflammation may help explain BPS/IC. Our data are consistent with the view that EIL bladder inflammation may increase susceptibility for the development of BPS/IC in a subset of patients through changes in dynorphin and endomorphin-2. This hypothesis is supported by evidence showing that women with BPS/IC are more likely to recall recurrent UTIs and antibiotic use3 and a relatively high incidence of UTIs in children under 8 weeks of age2. Experiencing a second adult UTI could trigger symptom onset in these individuals by further changing bladder dynorphin and endomorphin-2, and interventions might target these changes. For example, a gene therapy approach could upregulate spinal endomorphin-2 and downregulate spinal dynorphin using a similar concept to the first human trial of gene therapy for chronic pain25.
Conclusions
Several opioid peptides were measured using ELISA following EIL and adult bladder inflammation. The changes observed are consistent with the view that EIL bladder inflammation alone can chronically alter spinal cord peptide content. When coupled with adult re-inflammation, these changes could set the neurochemical stage to support bladder hypersensitivity. Interventions that compensate for these changes could provide some relief of chronic bladder pain associated with BPS/IC.
Acknowledgments
Supported by grants R01-DK073218, R01-DK078655 and 3R01-DK078655-02S1 to A. Randich
References
- 1.Fitzgerald M, Millard C, McIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anesthesia. Pain. 1989;39(1):31. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- 2.Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics. 1990;86(3):363. [PubMed] [Google Scholar]
- 3.Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73(2):258–262. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 4.DeBerry J, Randich A, Shaffer AD, et al. Neonatal bladder inflammation produces functional changes and alters neuropeptide content in bladders of adult female rats. J Pain. 2010;11(3):247. doi: 10.1016/j.jpain.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randich A, Mebane H, Ness TJ. Ice water testing reveals hypersensitivity in adult rats that experienced neonatal bladder inflammation: implications for painful bladder syndrome/interstitial cystitis. J Urol. 2009;182(1):337. doi: 10.1016/j.juro.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 6.Randich A, Uzzell T, DeBerry JJ, et al. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7(7):469. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 7.Hanno PM, Burks DA, Clemens JQ, et al. AUA guideline for the diagnosis and treatment of interstitial cystitis/bladder pain syndrome. J Urol. 2011;185(6):2162. doi: 10.1016/j.juro.2011.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukerji G, Walters J, Chessell IP, et al. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006;6:31. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeBerry J, Ness TJ, Robbins MT, et al. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distension: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8(12):914. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer AD, Ness TJ, Robbins MT, et al. Early-in-life bladder inflammation alters opioid peptide content in the spinal cord and bladder of adult female rats. Society for Neuroscience Annual Meeting. 2011 Nov; doi: 10.1016/j.juro.2012.08.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato M, Sano H, Iwaki D, et al. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. J Immunol. 2003;171(1):417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- 12.Ball CL, Ness TJ, Randich A. Opioid blockade and inflammation reveal estrous cycle effects on visceromotor reflexes evoked by bladder distension. J Urol. 2010;184(4):1529. doi: 10.1016/j.juro.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 13.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65. [Google Scholar]
- 14.Iadarola MJ, Brady LS, Draisci G, et al. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35(3):313. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- 15.Millan MJ, Czlonkowski A, Morris B, et al. Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat. Pain. 1988;35(3):299. doi: 10.1016/0304-3959(88)90140-6. [DOI] [PubMed] [Google Scholar]
- 16.Millan MJ, Millan MH, Pilcher CW, et al. Spinal cord dynorphin may modulate nociception via a kappa-opioid receptor in chronic arthritic rats. Brain Res. 1985;340(1):156. doi: 10.1016/0006-8993(85)90786-3. [DOI] [PubMed] [Google Scholar]
- 17.Przewlocka B, Lason W, Przewlocki R. Time-dependent changes in the activity of opioid systems in the spinal cord of monoarthritic rats—a release and in situ hybridization study. Neuroscience. 1992;46(1):209. doi: 10.1016/0306-4522(92)90020-3. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura F. Expression of preprodynorphin mRNA in the spinal cord after inflammatory abdominal stimulation in rats. Hokkaido Igaku Zasshi. 1994;69(1):95. [PubMed] [Google Scholar]
- 19.Lai J, Luo MC, Chen Q, Ma S, et al. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9(12):1534. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- 20.Vanderah TW, Laughlin T, Lashbrook JM, et al. Single intrathecal injections of dynorphin A or des-Tyr-dynorphins produce long-lasting allodynia in rats: blockade by MK-801 but not naloxone. Pain. 1996;69(2-3):275. doi: 10.1016/s0304-3959(96)03225-3. (1996) [DOI] [PubMed] [Google Scholar]
- 21.Luo MC, Chen Q, Ossipov MH, et al. Spinal dynorphin and bradykinin receptors maintain inflammatory hyperalgesia. J Pain. 2008;9(12):1096. doi: 10.1016/j.jpain.2008.06.005. (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su X, Riedel EX, Leon LA, et al. Pharmacologic evaluation of pressor and visceromotor reflex responses to bladder distension. Neurourol Urodyn. 2008;27(3):249. doi: 10.1002/nau.20469. (2008) [DOI] [PubMed] [Google Scholar]
- 23.Su X, Sengupta JN, Gebhart GF. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997;77(3):1566. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- 24.Su X, Joshi SK, Kardos S, Gebhart GF. Sodium channel blocking actions of the kappa-opioid receptor agonist U50,488 contribute to its visceral anti-nociceptive effects. J Neurophysiol. 2002;87(3):1271. doi: 10.1152/jn.00624.2001. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe D, Wechuck J, Kristy D, et al. A clinical trial of gene therapy for chronic pain. Pain Med. 2009;10(7):1325. doi: 10.1111/j.1526-4637.2009.00720.x. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]