Abstract
Abusive head trauma (AHT) is the most common cause of severe traumatic brain injury (TBI) in infants and the leading cause of child abuse-related deaths. For reasons that remain unclear, mortality rates after moderate AHT rival those of severe non-intentional TBI. The developing brain’s vulnerability to injury may be partially responsible for the poor outcomes observed after AHT. AHT is mechanistically more complex than non-intentional TBI. The acute-on-chronic nature of the trauma along with synergistic injury mechanisms that include rapid rotation of the brain, diffuse axonal injury, blunt force trauma, and hypoxia-ischemia make AHT challenging to treat. The anesthesiologist must understand the complex injury mechanisms inherent to AHT, as well as the pediatric TBI treatment guidelines, in order to decrease the risk of persistent neurologic disability and death. In this review we discuss the epidemiology of AHT, differences between AHT and non-intentional TBI, the severe pediatric TBI treatment guidelines in the context of AHT, anesthetic considerations, as well as ethical and legal reporting requirements.
Introduction
Abusive head trauma (AHT) is the most common cause of death from child abuse.1 Although most clinical studies and treatment guidelines combine AHT with other types of pediatric traumatic brain injury (TBI), AHT may actually encompass a complex disease process that warrants specific study and perhaps specific treatments. The anesthesiologist plays a critical role in the treatment of AHT and must be well versed in the pediatric TBI treatment guidelines.
Intentional injury should be considered in all children who present with trauma and have no clear history of accidental injury
AHT refers to brain injury from non-accidental, intentional, or inflicted trauma. It is distinct from non-intentional, non-inflicted, or accidental TBI. The “whiplash shaken infant syndrome” was described in a seminal article in 1974 that presented cases and autopsy findings of infants who had subdural and intraocular hemorrhages and long bone fractures but no other signs or history of accidental trauma to explain these findings.2 While this combination of injuries has become the stereotypic description of physical child abuse, the trauma can occur without shaking, and the abuse can result in a wide constellation of injuries. Survivors suffer permanent neurologic disabilities that include language and motor delays or attention disorders.2,3 Given the variety of mechanisms that can contribute to neurologic injury after intentional trauma, including shaking, blunt impact, spinal cord injury, and hypoxia-ischemia, the American Academy of Pediatrics recommended that the term AHT replace the previously used “shaken baby syndrome.”4
Estimating the incidence of AHT depends upon reporting accuracy, which relies on the caregivers’ disclosure of abuse or recognition of the abuse by clinicians and other authority figures. The risk of AHT increases in situations with premature birth, congenital birth defect, young maternal age, and socioeconomic and household stress.3,5,6 Children who suffer abuse often have vague clinical symptoms or a nonspecific clinical history. As a result, a significant proportion of child abuse cases remain undetected and some are first diagnosed at autopsy. The incidence of AHT is approximately 27.5 to 32.2 cases per 100,000 infants per year,7 but this number is probably a conservative estimate. Younger patients, particularly those less than 1–2 years-old, are at greatest risk of AHT. Although the incidence of AHT is highest during the early months of life, mortality after AHT increases in children ages 12 to 23 months. Retinal hemorrhages are associated with a higher risk of death.1 For reasons that remain unclear, outcomes after AHT are worse than those after accidental TBI when the injuries are of similar severity as measured by the Glasgow Coma Scale (GCS) and injury classification.8
AHT and intentional trauma must be considered in the diagnosis of all injured children, including older children and independent of the child’s functional status, when the mechanism of injury is unclear or if the provided clinical history does not match the injuries. Approximately 25% of children diagnosed with AHT are older than 1 year.9 In a case series of older children who died from AHT, retinal hemorrhages, subdural hematomas, diffuse axonal injury, and optic nerve sheath hemorrhages resulted from the abuse. These children were 3 to 7 years-old and weighed 12 to 22 kg, thereby illustrating that AHT does occur in older and heavier children. Of note, none of the victims had bone fractures on radiologic examination or autopsy.10 While this constellation of symptoms can be seen in accidental trauma, the consequences of misdiagnosing a child suffering from abuse can be severe.
AHT involves multiple mechanisms of injury
Overall, AHT is a more complex disease process than non-intentional TBI. In most cases of non-intentional TBI, a primary brain injury, such as a car accident, blunt trauma, or gunshot wound, is followed by secondary brain injury. Child abuse, by contrast, can manifest as multiple incidents of inflicted trauma over long periods of time. AHT can therefore represent recurrent primary brain injuries from repeat acute trauma upon a background of evolving secondary injury from prior abuse and chronic trauma.
The injury mechanisms that contribute to AHT are complex and synergistic. They include shaking, blunt force trauma, diffuse axonal injury, hypoxia-ischemia, as well as brainstem and spinal cord injuries. Rapid movement of the brain within the cranial vault can tear bridging vessels and cause subdural hemorrhages.3 Injury from these shear forces are sometimes observed in the orbit as retinal hemorrhages.3,11 The absence of retinal hemorrhage, however, should not exclude a diagnosis of AHT, and retinal hemorrhages are not diagnostic of abuse.12 The reported incidence of retinal hemorrhage in AHT varies from 30% to more than 80%.13–16 The acute-on-chronic nature of AHT results in both acute and chronic subdural hematomas, and the repeated abuse itself can cause acute subdural hemorrhages rather than hemorrhages from spontaneous re-bleeding. Children with acute and chronic subdural hemorrhages from AHT may present with asymptomatic macrocephaly, although more severe acute intracranial hemorrhages will cause acute neurologic symptoms.17 Multiple neuroimaging techniques may be necessary to diagnose the severity of the AHT and determine the timing of the injuries.18
Hypoxia-ischemia plays an important role in the pathology of AHT.19 The development of hypoxic-ischemic injury is related to a combination of aberrant cerebral blood flow and hypoxia. Rapid head rotation in piglets reduces blood flow through the carotid arteries and global blood flow to the brain.20 Brainstem and occipitocervical spinal cord injuries induce hypoventilation, and cervical spine ligamentous injuries correlate with brain ischemia in children with AHT.21 In addition, delays in seeking medical attention for the child by the caregiver increase the severity of the hypoxic-ischemic brain injury.
Many pediatric TBI studies include children with AHT, but relatively few specifically address AHT. In a study of 386 children with AHT, the mortality rate among children with moderate AHT (defined as GCS score 9–11) was similar to that of children with severe non-intentional TBI (defined as GCS score <9). Moreover, the children with AHT and GCS of 9–11 had a six-fold higher mortality rate than did those with a GCS of 12–15. Incremental decreases in the GCS score, cerebral edema, as well as retinal and intraparenchymal hemorrhages were independently associated with in-hospital mortality after AHT.22 The high mortality rate from moderate AHT, rivaling that of severe non-intentional TBI, suggests that treatments specifically for AHT should be investigated.
The young child is uniquely vulnerable to neurologic injury after AHT
The developing brain’s rapid growth, cell differentiation, and synaptogenesis increase a child’s vulnerability to permanent neurologic injuries after trauma. The biomechanics of the young child’s head and neck,23 including a large head with relatively poor cervical muscular control and lax ligaments,24 elevate the risk profile. In swine models of brain injury from rapid rotation, both neonatal and juvenile pigs exhibited subarachnoid hemorrhages and diffuse axonal injury.25 However, the axonal injuries were more severe in younger pigs than in older pigs and the intracranial hemorrhages took longer to resolve.26
A rapidly progressing form of brain atrophy has been described in young victims of AHT, but the exact etiology is unclear. Multifocal leukoencephalopathy or multicystic encephalopathy,19 which has also been coined “the big black brain,”27 occurs from synergistic injuries in the developing brain. This phenomenon appears limited to infants and children younger than 5 years and is independent of vessel occlusion. The atrophic regions are often supratentorial, span from the frontal to the occipital pole, and cross vascular territories. The loss of gray-white matter differentiation occurs unilaterally or bilaterally (Figure 1).27 Complex interactions between subdural hematomas, diffuse axonal injury, hypoxia-ischemia, injury to the cervicomedullary junction, cerebral edema, seizures, and hypotension in the developing brain may be responsible for this tragic disease process.
Figure 1.
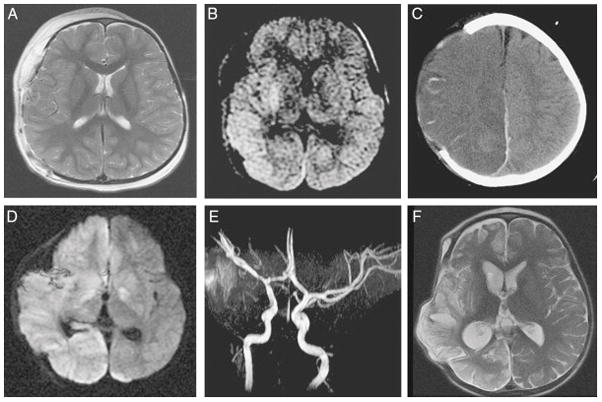
Example of widespread hemispheric damage in a 2-year-old girl with abusive head trauma and an acute right subdural hematoma. The hematoma was surgically evacuated, and a hemicraniectomy was performed. Thereafter, intracranial pressure was monitored and reported to be under good control with maintenance of cerebral perfusion pressure. A, Magnetic resonance imaging (MRI), T2 sequence, on postoperative day 1. The brain parenchyma appears symmetric. B, Diffusion-weighted sequence. Note the bright signal in the basal ganglia and temporal and occipital cortex. C, Computed tomography scan on postoperative day 3. Hypodensity has developed in the entire right hemisphere with progressive swelling. D, MRI, diffusion sequence, one week postoperatively. Bright signal has developed in the entire hemisphere as well as in the contralateral basal ganglia. E, Magnetic resonance angiogram. All arteries appear patent. F, MRI, fast spin-echo T2-weighted sequence, 2 months post-injury. Widespread damage to the right hemisphere is apparent. The patient developed persistent hemiparesis and hemianopsia. Reprinted with permission from Progress In Brain Research, volume 161, Duhaime AC, Durham S, Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”), pages 293–302, copyright 2007, with permission from Elsevier.
Anesthetic considerations for patients with AHT
The treatment of AHT as an entity separate from non-intentional TBI has not been well studied. Anecdotal evidence suggests that some clinicians are less aggressive when treating children with AHT because they assume the prognosis will be poor. We argue that clinicians should be more aggressive in treating these children to improve their chances for survival. For reasons previously discussed, investigations into whether children with AHT should be treated differently from those with non-intentional TBI are urgently warranted.
The anesthesiologist should consider a few specific steps that are unique to suspected abuse situations. Abused children may have orofacial injuries with bleeding in the mouth if a perpetrator forced an object into the child’s mouth, such as a bottle or eating utensil, or from a directed injury.28 While frenulum injuries3 are not conclusive of abuse, the anesthesiologist should conduct an evaluation of the oral cavity and document such injuries prior to intubation. An ophthalmology evaluation for retinal hemorrhages is helpful but not required preoperatively. The anesthesiologist should be aware if the patient received unilateral or bilateral mydriatic eye drops to dilate the pupils.
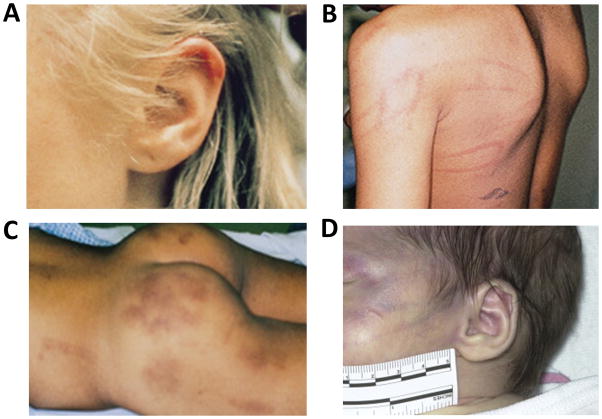
Anesthesiologists and other clinicians in the operating room have the opportunity to conduct a full skin examination. They may also be the first to see the child fully undressed. Bruises and other traumatic skin lesions evolve with time and may only become apparent as time progresses from the injury. Specific patterns within skin injuries should raise the clinician’s suspicion for abuse, including hand “slap” patterns in bruises and petechiae; lines or patterns from wires, belts, buckles, cables, or cords; bruises of different ages; and bruised or “boxed” ears (Figure 2).29,30 These injuries must be clearly documented in the medical record. To simplify documentation and if it is difficult to enter the examination findings into the electronic medical record, the anesthesiologist could draw an outline of a body on paper and mark on the picture where the child’s injuries are located. The injuries should be described by size and location using simple terminology, for example “two bruises, each larger than a quarter, on the right, lower back.” It is critical to document these injuries prior to surgery so the skin lesions cannot be blamed on events that occur in the operating room, such as pressure points from positioning or factors related to the surgery. Injuries to the anal and genital area31 must be documented prior to Foley catheter insertion. Postoperative surgical bandages and casts will interfere with future skin examinations. Moreover, it will take time for a child protection team or other investigative authority to evaluate the child, and the clarity of the skin or genital lesions may disappear. If documentation is conducted on paper, the anesthesiologist must ensure that the information is scanned into the electronic medical record for review by the child protection team and investigative authorities.
Figure 2.
Examples of skin injury patterns consistent with abuse. A, Ear bruising from being “boxed” is unlikely to occur through non-intentional trauma. B, Loop marks inflicted by a cord. C, Bruises of different ages inflicted at different times. D, Hand slap injury. Panels A–C are reprinted with permission from Evaluation of Physical Abuse in Children, May 15, 2000, Vol 61, No 10, American Family Physician Copyright © 2000 American Academy of Family Physicians. All Rights Reserved. Panel D is reprinted with permission from Pediatric Clinics of North America, volume 61, Petska HW, Sheets LK, Sentinal injuries: subtle findings of physical abuse, pages 923–35, copyright 2014, with permission from Elsevier.
The current recommendations for AHT are to follow the clinical guidelines for pediatric TBI, which were updated in 201232 (Table 1). In a study on the 2003 severe pediatric TBI treatment guidelines, adherence to the guidelines during the first 72 hours of hospital admission was associated with a favorable neurologic outcome and survival to hospital discharge. Approximately 25% of TBI cases in this study were from AHT, and the patients were on average 8 years-old (standard deviation [SD]: 6.3).33 Few studies specifically focus on severe TBI from AHT in young patients. This may be due to study enrollment criteria that require intracranial pressure (ICP) monitoring and the reluctance of some neurosurgeons to place ICP monitors in infants with incompletely ossified craniums. Nonetheless, the anesthesiologist should consider the predominance of infants and young children who suffer AHT when following the treatment guidelines.
Table 1.
Summary of recommendations from the 2012 Guidelines for the Acute Medical Management of Severe Traumatic Brain Injury (TBI) in Infants, Children, and Adolescents
| Physiologic parameters | Recommendations | Level of evidence |
|---|---|---|
|
| ||
| Intracranial pressure | Consider ICP monitoring in infants and children with severe TBI | III |
|
| ||
| Treatment of ICP may be considered at a threshold of 20 mmHg | III | |
|
| ||
| Cerebral perfusion pressure | A minimum CPP of 40 mmHg may be considered in children with TBI | III |
|
| ||
| A CPP threshold of 40–50 mmHg may be considered; age-specific thresholds may exist, with infants at the lower end of the range and adolescents at the upper end | III | |
|
| ||
| Brain oxygenation | If brain oxygen monitoring is used, maintenance of partial pressure of brain tissue oxygen tension ≥10 mmHg may be considered | III |
|
| ||
| Hyperosmolar therapy | Hypertonic saline should be considered to treat intracranial hypertension. Acute dosing range is 6.5 to 10 mL/kg. | II |
|
| ||
| 3% hypertonic saline (0.1 to 1 mL/kg/h infusion) should be considered for the treatment of intracranial hypertension. Serum osmolarity should be maintained at <360 mOsm/L. | III | |
|
| ||
| Note: no studies of mannitol met the inclusion criteria as evidence for this topic | ||
|
| ||
| Hyperventilation | Avoidance of prophylactic severe hyperventilation to a PaCO2 < 30 mmHg may be considered in the initial 48 h after injury | III |
|
| ||
| If hyperventilation is used in the management of refractory intracranial hypertension, advanced neuromonitoring for evaluation of cerebral ischemia may be considered | III | |
|
| ||
| Temperature control | Moderate hypothermia (32–33°C) beginning early after severe TBI for only 24 h duration should be avoided | II |
|
| ||
| Moderate hypothermia (32–33°C) beginning within 8 h after severe TBI for up to 48 h duration should be considered to reduce intracranial hypertension | II | |
|
| ||
| If hypothermia is induced for any reason, rewarming at a rate >0.5°C/h should be avoided | II | |
|
| ||
| Cerebrospinal fluid drainage | CSF drainage through an external ventricular drain may be considered for management of elevated ICP | III |
|
| ||
| The addition of a lumbar drain may be considered in patients with refractory intracranial hypertension, a functioning external ventricular drain, open basal cisterns, and no evidence of a mass lesion or shift on imaging studies | III | |
|
| ||
| Barbiturates | High-dose barbiturate therapy may be considered in hemodynamically stable patients with refractory intracranial hypertension despite maximal medical and surgical management | III |
|
| ||
| When high-dose barbiturate therapy is used to treat refractory intracranial hypertension, continuous arterial blood pressure monitoring and cardiovascular support are required to maintain adequate CPP | III | |
|
| ||
| Corticosteroids | The use of corticosteroids is not recommended to improve outcome or reduce ICP for children with severe TBI | II |
|
| ||
| Analgesics, sedatives, and neuromuscular blockade | Etomidate may be considered to control severe intracranial hypertension; however, the risks of adrenal suppression must be considered | III |
|
| ||
| Thiopental may be considered to control intracranial hypertension | III | |
|
| ||
| Note: the specific indications, choice, and dosing of analgesics, sedatives, and neuromuscular-blocking agents should be left to the treating physician | III | |
|
| ||
| As stated by the FDA, a continuous infusion of propofol for either sedation or management of refractory intracranial hypertension in infants and children with severe TBI is not recommended | III | |
|
| ||
| Antiseizure prophylaxis | Prophylactic use of antiseizure therapy is not recommended for preventing late post- traumatic seizures in children with severe TBI | III |
|
| ||
| Prophylactic antiseizure therapy may be considered as a treatment option to prevent early post-traumatic seizures in young pediatric patients and infants at high risk of seizures after head injury | III | |
|
| ||
| Nutrition | Evidence does not support the use of immune- modulating diet to improve outcome | II |
|
| ||
| Decompressive craniectomy | Decompressive craniectomy with duraplasty may be considered for patients who are showing early signs of neurologic deterioration or herniation or are developing intracranial hypertension refractory to medical management during the early stages of their treatment | |
ICP, intracranial pressure; TBI, traumatic brain injury; CPP, cerebral perfusion pressure; CSF, cerebrospinal fluid; FDA, Food and Drug Administration.
Adapted from: Hardcastle N, Benzon HA, Vavilala MS. Update on the 2012 guidelines for the management of pediatric traumatic brain injury-information for the anesthesiologist. Pediatr Anesth 2014; 24:703–10.
The anesthetic treatment of a child with AHT initially focuses on securing the airway with in-line stabilization of the cervical spine and minimizing secondary brain injury by preventing hypoxia, avoiding hypotension, maintaining normothermia, and treating seizures. Hyperthermia must be strictly avoided. Prophylactic hyperventilation is not recommended, and normocarbia should be maintained with PaCO2 >30 mmHg, particularly during the first 48 hours after injury.32,34 Hyperventilation should be reserved only for patients with refractory intracranial hypertension. Acute hyperventilation with a decrease in the PaCO2 induces cerebral vasoconstriction, decreases the intracranial cerebral blood volume, and subsequently lowers the ICP. Prolonged or severe hyperventilation induces cerebral vasoconstriction that risks cerebral ischemia. The requirement to closely regulate CO2 necessitates using a properly sized pediatric endotracheal tube. A pediatric cuffed endotracheal tube can be used with minimal air inside the cuff plus routine intracuff pressure checks.35
ICP monitoring is indicated in all infants and children with TBI and GCS <9, independent of whether the infant has open fontanelles or cranial sutures. The observations that children with moderate AHT (GCS 9–11) have poorer outcomes than would be expected based on GCS and compared to children with non-intentional TBI22 force us to consider whether ICP monitoring should be used in children with AHT and GCS≥9. Importantly, an open fontanel does not protect the infant brain from intracranial hypertension during cerebral swelling. In fact, a study of children with severe TBI (median age: 9.7 years; range: 2 months to 16 years) demonstrated that younger children are at greater risk of intracranial hypertension than are older children with severe TBI.36 ICP monitors can be placed into the brain parenchyma or ventricle. An extraventricular drain permits cerebrospinal fluid drainage to treat intracranial hypertension. Invasive arterial blood pressure monitoring is crucial for monitoring and maintaining cerebral perfusion pressure (CPP) within a range that supports cerebrovascular autoregulation. CPP is the difference between the mean arterial blood pressure (MAP) and ICP (or central venous pressure if it exceeds the ICP). Several factors, including hypovolemia, hemorrhage, brainstem injury, and pulmonary injury from neurogenic pulmonary edema or aspiration, can cause significant hemodynamic instability.
Titrating hemodynamic goals and other treatments based on the neurologic examination are not possible during general anesthesia. The pediatric TBI treatment guidelines provide level III recommendations that clinicians should maintain a patient’s ICP at less than 20 mmHg and CPP greater than 40–50 mmHg. The target CPP might need to be increased for older children and adolescents.32,34,37 Having a higher ICP and lower CPP during the first 6 hours after injury is associated with worse neurologic outcome in pediatric TBI.37 Although ICP and CPP are inherently connected, both ICP-directed and CPP-directed goals must be met concurrently. For example, it is not enough to maintain CPP by increasing MAP alone. Rather, treatment to decrease the ICP must be implemented as well. Intracranial compliance is lower when ICP is elevated. So any further increase in intracranial volume, such as from bleeding or vasodilation from hypercapnia, pain, or seizures, would significantly raise the ICP and risk cerebral herniation. Raising the MAP to accommodate intracranial hypertension increases myocardial oxygen demand and risks cardiovascular compromise. High doses of vasopressors can also reduce splanchnic blood flow. Because high ICP shifts the blood pressure lower limit of autoregulation to a higher pressure,38 decreasing the ICP would better support autoregulatory function at the same level of CPP. In a study of severe pediatric TBI that included 30% of cases with AHT, children with poor blood pressure cerebrovascular autoregulatory function were less likely to survive than those with better autoregulatory function. Age was similar among survivors (mean: 7.2 years; SD: 5.0) and non-survivors (mean: 3.3 years; SD: 3.6).39 Therefore, the anesthesiologist should use ICP- and CPP-guided treatments in the context of the autoregulation curve.
Because outcomes after AHT are worse than those after non-intentional TBI when the injuries are of similar severity based on GCS score,8 it can be reasonably argued that ICP-directed therapies should be more aggressive for children with AHT. Neural cell death from hypoxia-ischemia if the child suffered respiratory insufficiency or cardiac arrest, recurrent brain injuries from repeated abuse, evolution of secondary brain injury from a delay in seeking medical care, and young age may make the intracranial hypertension more complex to treat than in non-intentional TBI. While research is needed to clarify ICP treatment thresholds in AHT, the anesthesiologist could consider instituting medical therapies to maintain ICP ≤ 15 mmHg. However, whether keeping ICP below 15 mmHg will improve neurologic outcomes after AHT has not been well studied.
Several options are available to treat intracranial hypertension. Maintaining a deep plane of anesthesia to minimize the response to painful stimuli is critical while preventing hypotension. Hypertonic saline 3% is the preferred hyperosmolar therapy for intracranial hypertension in pediatric TBI.34 The risks and benefits of hypertonic saline for AHT are unclear. The pediatric TBI guidelines cite two class II studies to formulate the recommendation for hypertonic saline: one study did not include AHT, and the number of AHT cases was not apparent in the other.40–42 One single-institution pediatric TBI study with 29% of cases from AHT reported an association between higher cumulative volume of hypertonic saline 3%, greater peak sodium level, and deep vein thrombosis.43
Barbiturates can decrease the ICP, but they should be used with caution because resultant hemodynamic depression could lower the CPP. Inducing a barbiturate coma with or without decompressive craniectomy or cerebrospinal fluid drainage may be required to treat refractory intracranial hypertension.44,45 Barbiturate comas have primarily been reported for accidental TBI and not specifically for AHT. In one retrospective study of severe pediatric TBI with refractory intracranial hypertension,45 pentobarbital administered to achieve electrographic burst suppression decreased the ICP to less than 20 mmHg in some cases. Children with resolution of the intracranial hypertension by pentobarbital were older (median age: 10.7 years, interquartile range [IQR]: 2.7, 15.6) than those who had sustained intracranial hypertension despite pentobarbital (median age: 6.4 years, IQR: 2.2, 11.3). Five children in this study had AHT, and the ICP remained <20 mmHg with pentobarbital in only one child with AHT.45 Whether young children with AHT are less responsive to barbiturates for ICP control than children with accidental TBI is not clear.
Decompressive craniectomy must be considered for intracranial hypertension that is refractory to medical treatment and in patients with neurologic deterioration or signs of brain herniation.32 The evidence supporting decompressive craniectomy in pediatric TBI is primarily limited to single-institution studies and case series. Randomized controlled trials of decompressive craniectomy are inherently difficult to conduct in pediatric TBI.46 Moreover, outcomes after craniectomy may differ by TBI mechanism. In a study of 37 children with decompressive craniectomy for intracranial hypertension after TBI, children with AHT were more likely to die or have a poor outcome than those with non-inflicted TBI. Children with AHT were younger (mean age: 2.2 years; SD: 1.0) than children with non-inflicted TBI (mean age: 8.4 years; SD: 1.8).47 Therefore, decompressive craniectomy for AHT requires further study. When this surgical intervention is used, it should perhaps be done earlier or at lower ICP thresholds than what is generally considered for accidental TBI.
The anesthesiologist can consider moderate hypothermia to a core temperature of 32–33°C beginning within 8 hours after brain injury and for up to 48 hours’ duration to relieve intracranial hypertension.34 Therapeutic hypothermia after pediatric brain injury has been most extensively studied in neonatal hypoxic ischemic encephalopathy48 and pediatric cardiac arrest.49 The safety profile of hypothermia after TBI remains controversial. An international, multicenter study50 randomized 225 children with severe TBI to receive either 24 hours of hypothermia (goal 32.5°C) or normothermia. The mean ages of children randomized to hypothermia or normothermia were 9.8 years (SD: 4.9) and 10.2 years (SD: 4.8), respectively. Hypothermia was induced within 8 hours of injury. Hypothermic children had lower arterial blood pressures and required more vasoactive medications, particularly during rewarming, than did normothermic children. Normothermic children required more hypertonic saline to control the ICP. Of note, CPP was lower in the hypothermic group during rewarming when compared to the normothermic group. The risk of a poor neurologic outcome at 6 months was greater in children who received hypothermia. There was also a trend towards higher mortality rate in the hypothermia group compared to that of the normothermia group with a p-value of 0.06. The use of hyperventilation to PaCO2 <30 mmHg in more than 40% of children in the normothermic and hypothermic groups made the data somewhat difficult to interpret. Nonetheless, this study resulted in the recommendation to avoid short durations of hypothermia for only 24 hours after TBI.32,50 A separate multicenter, randomized control trial examined 55 children with severe TBI. After randomization to either 72 hours of hypothermia (goal 32–33°C) or normothermia, the hypothermic (mean age: 11.0 years; range: 6.9–14.2) and normothermic (mean age: 9.5; range: 5.2–13.8) children had similar neurologic outcomes at 12 months.51 Thus, while the option for therapeutic hypothermia remains in the pediatric TBI guidelines,32 this practice remains controversial.
TBI from AHT deserves special discussion when considering therapeutic hypothermia. AHT can incorporate aspects of both TBI and hypoxic-ischemic injury from cardiac arrest, respiratory insufficiency, or delay in obtaining medical care. In addition, the older age of children in the hypothermia and TBI trials 50,51 may make these studies less relevant to young AHT victims. Therapeutic hypothermia has become the standard of care for neonatal hypoxic-ischemic encephalopathy at many hospitals worldwide given its association with improved neurocognitive outcomes when compared to normothermia after an ischemic birth injury.52 However, the out-of-hospital cardiac arrest arm of the Therapeutic Hypothermia After Pediatric Cardiac Arrest trial did not show a difference in neurocognitive outcome between children randomized to post-arrest hypothermia or normothermia.49 The results of the in-hospital cardiac arrest arm of the Therapeutic Hypothermia After Pediatric Cardiac Arrest trial (www.thapca.org) were still pending at the time of writing this article. Given the poorer outcomes of AHT relative to non-intentional TBI of similar severity based on GCS score,8 clinicians could consider inducing therapeutic hypothermia in infants with AHT. There is little research in the use of therapeutic hypothermia specifically for AHT, but this topic warrants further study given the limited treatment options available for AHT.
The young age of many AHT victims must be considered during anesthesia. For example, young children have less cerebral autoregulatory reserve than do older children. In a study of 22 children younger than 2 years and with severe TBI, including 82% with AHT, more episodic decreases in CPP below 45 mmHg during the first 7 days after injury were associated with worse neurologic outcomes.53 Relatively little is known about the blood pressure lower limit of autoregulation during general anesthesia in children. Available data suggest that the lower limit of autoregulation is similar among healthy, ASA level I children of different ages without brain injury and may be at a MAP of approximately 50–65 mmHg.54 However, based on data from animal models, increases in ICP with or without acute trauma shift the lower limit of autoregulation to a higher CPP.38,55 It can be reasonably assumed that increases in ICP raise the lower limit of autoregulation after AHT in clinical situations, as well. The combination of elevated ICP with a concomitant decrease in CPP and increase in the lower limit of autoregulation after AHT place young, anesthetized children at significant risk of dysregulated cerebral blood flow, hypoperfusion, and ischemia. Whether general anesthesia provides some level of protection by decreasing the cerebral metabolic rate remains unclear. Moreover, moderate hypothermia may also affect the limits of autoregulation after brain injury.56
The specific vasopressor chosen to support CPP often depends upon institutional protocols and clinician preference. The current pediatric treatment guidelines from 2012 recommend maintaining CPP above 40 mmHg, noting that an age-related continuum for the optimal CPP is between 40 and 65 mmHg. CPP is often maintained within these levels by using vasopressors to increase CPP and optimize cerebral blood flow. However, vasoactive agents clinically used to elevate MAP after TBI, such as phenylephrine, dopamine and norepinephrine,57–62 have not been sufficiently compared regarding their effects on CPP, cerebral blood flow, autoregulation, and survival after TBI. A retrospective study at a single institution reported similar MAP and CPP levels in children with TBI who received dopamine, phenylephrine, or norepinephrine.63 Currently, clinical vasopressor use is variable and empiric.
Since ethical considerations constrain mechanistic studies in children with TBI, an established porcine model of fluid percussion injury that mimics TBI has been used to corroborate clinical observations regarding cerebral autoregulation after TBI.64 Newborn and juvenile pigs may approximate the human infant-to-child (6 months to 2 years) and pre-teen (8 to 10 years) age ranges, respectively.65 Marked sex differences with respect to the impact of vasopressor use on cerebral hemodynamics have been demonstrated with the piglet fluid percussion model. Phenylephrine protected autoregulation in newborn female piglets but aggravated cerebrovascular dysregulation in newborn male piglets after brain injury.66 Subsequent studies showed that vasoactive agents may enhance the impairment of cerebral autoregulation (phenylephrine),66 protect from impairment (dopamine),67 or have no effect on cerebral autoregulatory function (norepinephrine)68 in newborn male piglets after TBI. Dopamine, however, improved outcome after TBI equally in newborn male and female piglets.67 Based on these preclinical data, it is tempting to speculate that dopamine might improve neuroprotection after TBI independent of gender. However, additional clinical studies on the relationships between gender, type of vasopressor, and outcome after pediatric TBI and AHT are needed.
In addition to ICP monitoring, measuring the partial pressure of brain tissue oxygen should be considered, with a goal of maintaining the oxygen tension at ≥10 mmHg.32,34 Invasively measuring brain tissue oxygenation in infants with incompletely ossified craniums may be challenging, however, because the monitoring device could fracture the skull. Noninvasive technology for measuring cerebral oxygenation, such as near-infrared spectroscopy, has not been thoroughly tested in AHT.
Seizures are common after AHT, and they must be diagnosed and treated early. One study of more than 400 children with AHT, including 95% younger than 1 year, reported that more than 70% had clinical seizures. Seizures and abnormal electroencephalography recordings were associated with poor neurologic outcomes, including persistent motor or sensory deficits and psychomotor delay. Some children died with refractory status epilepticus or associated intracranial hypertension.69 Prophylactic antiseizure medications can be considered to prevent early posttraumatic seizures, but they should not be used to prevent late posttraumatic seizures.34
The choice of anesthetic regimen is at the discretion of the anesthesiologist. Although some evidence from animal models indicates that an IV technique with opiates and benzodiazepines may preserve cerebral blood flow autoregulation better than inhaled techniques with volatile agents,70 clinical research comparing IV to inhaled anesthesia in pediatric TBI has been inadequate to make a recommendation. Most IV induction agents, including etomidate, propofol, and barbiturates, decrease the cerebral metabolic rate and oxygen demand and induce cerebral vasoconstriction, thereby lowering the ICP. Etomidate has the benefit of maintaining MAP and therefore not reducing CPP, whereas bolus doses of propofol and barbiturate can cause significant hypotension that must be treated immediately. However, the future risk of adrenal suppression with etomidate must be considered. Ketamine is gaining in popularity for the treatment of pediatric TBI, especially during the induction of anesthesia and intubation. Ketamine does not increase the ICP, and in some situations it may decrease ICP while supporting the CPP in TBI patients.71 Investigations into preventing secondary neurologic injury with pharmacologic agents, including N-methyl-D-aspartate receptor antagonists, have not yet demonstrated a neuroprotective effect after TBI, but clinical trials continue.72 A rapid sequence induction is indicated for patients with a full stomach. Concerns about fasiculations from succinylcholine with a slight increase in ICP73 must be weighed against the risk of aspiration with a significant and potentially catastrophic increase in ICP.
Intraoperative hyperglycemia is common among children with TBI. Although some studies report an association between hyperglycemia and poor outcomes after pediatric TBI,74,75 the quality of evidence was insufficient to make recommendations regarding glycemic control in the pediatric TBI guidelines. Corticosteroids are not recommended for pediatric TBI.32,34
Multimodal neuromonitoring and cerebrovascular autoregulation
The Brain Trauma Foundation guidelines for the treatment of adult TBI76 recommend using ancillary monitors of cerebral blood flow and oxygenation to facilitate CPP management. The Neurocritical Care Society and the European Society of Intensive Care Medicine issued a statement supporting multimodality monitoring in patients with acute neurologic disorders.77 For example, clinicians can use ICP and brain tissue oxygenation levels as surrogate measures of cerebral blood volume and cerebral blood flow/oxygen metabolism, respectively, to assess cerebrovascular autoregulation. Metrics of autoregulatory vasoreactivity and cerebral blood flow autoregulation can be calculated by correlating the ICP or brain tissue oxygenation levels to blood pressure. The pressure reactivity index, for instance, correlates ICP to MAP and determines whether the cerebral vasculature is pressure-reactive (thereby indicating functional autoregulation) or pressure-passive (with impaired autoregulation) across changes in an individual patient’s blood pressure. Single institution studies of pressure reactivity index and TBI demonstrate that functional autoregulation and maintaining a patient’s CPP near the optimal CPP where autoregulation is most robust are associated with better neurologic outcomes in children39,78 and adults.79 Correlating the partial pressure of brain tissue oxygen and CPP to produce an index of cerebral blood flow autoregulation after TBI 80 may also clarify neuroprotective CPP goals.
When invasive intracranial monitoring is not available, calculating autoregulation indices from transcranial Doppler 81 or near infrared spectroscopy82–84 could clarify blood pressure ranges that optimize autoregulation after TBI. These methods are not Food and Drug Administration-approved for use in children and they are still being explored for pediatric TBI. While it can be assumed that preserving autoregulation would improve neurologic outcomes, this has not yet been demonstrated in children using noninvasive neurologic monitors.
Microdialysis is recommended for adults with neurologic injuries with risk of cerebral ischemia, hypoxia, energy failure, and glucose deprivation. Low brain glucose or elevated lactate/pyruvate ratio are associated with poor outcomes, and microdialysis monitoring can assist in titrating blood product transfusions, hypocapnea, and hyperoxia.77 While microdialysis measurements remain largely investigational in pediatric TBI, they may show promise in treating AHT. As with many bedside neuromonitors, these techniques are limited by regional brain measurements that may not reflect global cerebral autoregulation and metabolism.
Identifying other comorbid injuries
Children with AHT are at significant risk of injury to other organ systems in addition to the brain. Identifying these injuries can be challenging because the children may have only vague symptoms with nonspecific clinical histories. Anesthesiologists must work closely with surgeons, intensivists, emergency medicine physicians, and other members of their institutional trauma service to conduct a thorough trauma survey. When possible, obtaining a skeletal survey and full body imaging to diagnose non-brain injuries prior to neurosurgery would determine whether additional interventions are needed under the same anesthetic as well as alert the anesthesiologist to concomitant injuries. If the child must be emergently taken for neurosurgery, obtaining these scans after surgery and during the same anesthetic if the patient is hemodynamically stable would facilitate clinical care.
In a single institutional study of 188 children with abusive trauma and median age 1.1 years, 48% had multiple injuries. AHT was the most common, followed by lower extremity fracture, skull fracture, and retinal hemorrhage.85 The fact that 52% of the children presented with only one injury suggests that an absence of multiple injuries should not reduce the clinician’s concern for potential abuse. Moreover, children who come to the hospital injured on multiple occasions must be screened for possible abuse.
Spinal cord injuries require stabilization, particularly during airway management. Infants and young children have cervical ligamentous laxity as well as poor muscle development and control, which, when combined with the orientation of the spine to the large head, creates a greater range of motion and higher potential for cervical spinal cord injury than that in adults.24 Some children with cervical spine injuries will have normal plain radiographs.86 In a study of 67 children with AHT, 78% had cervical spine ligamentous injuries.21 Thoracolumbar subdural hemorrhages in the spinal canal were identified in 63% of children aged 0–2 years with AHT.87 Therefore, the anesthesiologist must take special precautions to protect against further spinal injury when managing the airway and positioning the child intraoperatively.
Although AHT remains the most common cause of death, abused children can also experience life-threatening abdominal injuries.88,89 Less than 10% of child abuse cases involve serious intraabdominal injuries.85,90 Nonetheless, all children with suspected abuse must be screened for abdominal injuries because even the most severe injuries can be difficult to diagnose. More than half of children with abdominal injuries will not have abdominal bruising, tenderness, distension, or abnormal bowel sounds.90,91 Abdominal trauma can include small bowel perforations or transections and hepatic, splenic, pancreatic, renal, bladder, gastric, or adrenal injuries. The duodenum is the most commonly injured section of bowel, and a posttraumatic hematoma or stricture can present as a bowel obstruction. Cardiovascular, pulmonary, esophageal, or pharyngeal injury may also occur.91,92
Bone fractures from abuse stereotypically involve the long bones, but fractures can occur anywhere in the body. Fractures from acute or chronic abuse may have subtle radiographic findings that require interpretation by an experienced radiologist. For example, faint fracture fragments may arise from the metaphysis, the growth plates may show subtle irregularities, and there may be signs of subperiosteal new bone formation.93
Controversy in “diagnosing” child abuse
Debates within the general public, legal, and medical communities have questioned the diagnostic accuracy of retinal and subdural hemorrhages for AHT. Attorneys in child abuse cases have argued that subdural hematomas are caused by birth injuries, hypoxia, cerebral venous thrombosis, and preexisting medical conditions rather than abuse. Retinal hemorrhages have been blamed on chest compressions and other resuscitation efforts in court cases. Physicians have been accused in court of being “rushed to judgement” in diagnosing AHT.94,95 Despite strong evidence that retinal hemorrhages are associated with abuse,15,96 some have even argued that vaccinations cause retinal hemorrhage. This myth was debunked in a retrospective cohort study.97
Retinal hemorrhages are rare in infants and children after the neonatal period.96 Subdural and intraocular or retinal hemorrhages can occur in accidental TBI, such as cranial crush injury,98 motor vehicle crash,99 or falls.100,101 But these cases should have clear histories to explain the accidental trauma, near immediate presentation of the child for emergency medical care, and coexisting injuries that corroborate the accidental trauma. Rare metabolic disorders that are associated with subdural hemorrhages, such as glutaric aciduria type 1, are diagnosed by characteristic neurologic lesions, urine abnormalities, and other screening tests.102 Some clinicians may worry that clinical findings suspicious for child abuse may actually be manifestations of a medical disease, such as Ehlers Danlos syndrome,103 Kasabach-Merritt syndrome,104 coagulopathy,105 infection,106 non-intentional brain trauma,107 or cardiopulmonary resuscitation.12
The anesthesiologist must suspect abuse when a clear history for accidental trauma or comorbid disease is missing, inconsistent histories are provided by the caregivers, there is a delay in seeking medical care, or the constellation of injuries cannot be easily explained. The anesthesiologist must immediately and thoroughly document clinical findings. Skin lesions and other injuries evolve and fade with time. Clinicians must keep child abuse in the differential diagnosis and alert the child protection team or other authorities while awaiting the results of diagnostic tests.
Reporting suspected child abuse
All clinicians have the ethical and legal responsibility to report suspected child abuse. The consequences of not reporting can be fatal. As long as the report is made in good faith, physicians are protected by law from potential ramifications of the report.108
Many medical institutions have child protection service teams, social services staff, or other personnel who can assist physicians in making a report to Child Protective Services or another investigative body. The process for filing a report differs in each state. Anesthesiologists must work with surgeons, intensivists, and other members of the clinical team to identify and report suspected cases of abuse. It is the responsibility of each physician to ensure that a report is made. It is not the physician’s responsibility to attempt to identify the perpetrator; only medical facts should be provided. Medical information should be stated in simple terminology that a person without a medical background can understand. For example, use the terms “bruise” and “bleeding” rather than “ecchymosis” and “hemorrhage/hematoma.” Social workers and other personnel can assist the medical team in making the child’s guardians aware of the report and in maintaining a positive doctor-patient-family relationship. Reporting suspected abuse could save a child’s life and protect other children in the same home and social environment.108
Conclusions
AHT involves complex injury mechanisms that distinguish it from non-intentional TBI. The young child’s developing brain is highly vulnerable to injury from abuse, which may partially explain the high mortality rates and poor neurologic outcomes observed in children with AHT.22 The anesthesiologist plays a critical role in caring for children with AHT and must be well versed in the current pediatric TBI treatment guidelines.32 Given the distinct injury mechanisms and poor neurologic prognoses for AHT, studies to investigate whether AHT should be treated differently than non-intentional TBI are needed.
Acknowledgments
Funding: Dr. Lee’s work was supported by grants from the NIH (K08 NS080984) and the American Heart Association. Dr. Lee also has clinical research funding from Medtronic.
We would like to thank Claire Levine for her editorial assistance and Dr. William Armstead for his contributions to this manuscript.
Footnotes
Reprints will not be available from the authors.
This manuscript was handled by: James A. DiNardo, MD
Disclosures
Name: Jennifer K. Lee, MD
Contribution: Dr. Lee contributed to the content and writing of the manuscript.
Attestation: Dr. Lee approved the final manuscript.
Conflicts of Interest: Dr. Lee has research funding from Medtronic.
Name: Ken M. Brady, MD
Contribution: Dr. Brady contributed to the content and writing of the manuscript.
Attestation: Dr. Brady approved the final manuscript.
Conflicts of Interest: None.
Name: Nina Deutsch, MD
Contribution: Dr. Deutsch contributed to the content and writing of the manuscript.
Attestation: Dr. Deutsch approved the final manuscript.
Conflicts of Interest: None.
Contributor Information
Jennifer K. Lee, Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology, Johns Hopkins University, Baltimore, Maryland.
Ken M. Brady, Department of Pediatrics, Anesthesia, and Critical Care, Texas Children’s Hospital, Baylor College of Medicine, Houston, Texas.
Nina Deutsch, Departments of Anesthesiology and Pediatrics, Children’s National Health System, Washington DC.
References
- 1.Nuno M, Pelissier L, Varshneya K, Adamo MA, Drazin D. Outcomes and factors associated with infant abusive head trauma in the US. J Neurosurg Pediatr. 2015:1–8. doi: 10.3171/2015.3.PEDS14544. [DOI] [PubMed] [Google Scholar]
- 2.Caffey J. The whiplash shaken infant syndrome: manual shaking by the extremities with whiplash-induced intracranial and intraocular bleedings, linked with residual permanent brain damage and mental retardation. Pediatrics. 1974;54(4):396–403. [PubMed] [Google Scholar]
- 3.Koe S, Price B, May S, Kyne L, Keenan P, McKay M, Nicholson AJ. Medical, social and societal issues in infants with abusive head trauma. Ir Med J. 2010;103(4):102–5. [PubMed] [Google Scholar]
- 4.Christian CW, Block R Committee on Child Abuse and Neglect, American Academy of Pediatrics. Abusive head trauma in infants and children. Pediatrics. 2009;123(5):1409–11. doi: 10.1542/peds.2009-0408. [DOI] [PubMed] [Google Scholar]
- 5.Gumbs GR, Keenan HT, Sevick CJ, Conlin AM, Lloyd DW, Runyan DK, Ryan MA, Smith TC. Infant abusive head trauma in a military cohort. Pediatrics. 2013;132(4):668–76. doi: 10.1542/peds.2013-0168. [DOI] [PubMed] [Google Scholar]
- 6.Wood JN, French B, Fromkin J, Fakeye O, Scribano PV, Letson MM, Makoroff KL, Feldman KW, Fabio A, Berger R. Association of Pediatric Abusive Head Trauma Rates With Macroeconomic Indicators. Acad Pediatr. 2015 doi: 10.1016/j.acap.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Ellingson KD, Leventhal JM, Weiss HB. Using hospital discharge data to track inflicted traumatic brain injury. Am J Prev Med. 2008;34(4 Suppl):S157–62. doi: 10.1016/j.amepre.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Beers SR, Berger RP, Adelson PD. Neurocognitive outcome and serum biomarkers in inflicted versus non-inflicted traumatic brain injury in young children. J Neurotrauma. 2007;24(1):97–105. doi: 10.1089/neu.2006.0055. [DOI] [PubMed] [Google Scholar]
- 9.Scribano PV, Makoroff KL, Feldman KW, Berger RP. Association of perpetrator relationship to abusive head trauma clinical outcomes. Child Abuse Negl. 2013;37(10):771–7. doi: 10.1016/j.chiabu.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Salehi-Had H, Brandt JD, Rosas AJ, Rogers KK. Findings in older children with abusive head injury: does shaken-child syndrome exist? Pediatrics. 2006;117(5):e1039–44. doi: 10.1542/peds.2005-0811. [DOI] [PubMed] [Google Scholar]
- 11.Longmuir SQ, Oral R, Walz AE, Kemp PS, Ryba J, Zimmerman BM, Abramoff MD. Quantitative measurement of retinal hemorrhages in suspected victims of child abuse. J AAPOS. 2014;18(6):529–33. doi: 10.1016/j.jaapos.2014.07.175. [DOI] [PubMed] [Google Scholar]
- 12.Longmuir SQ, McConnell L, Oral R, Dumitrescu A, Kamath S, Erkonen G. Retinal hemorrhages in intubated pediatric intensive care patients. J AAPOS. 2014;18(2):129–33. doi: 10.1016/j.jaapos.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Gaffar MA, Esernio-Jenssen D, Kodsi SR. Incidence of retinal hemorrhages in abusive head trauma. J Pediatr Ophthalmol Strabismus. 2013;50(3):169–73. doi: 10.3928/01913913-20130129-01. [DOI] [PubMed] [Google Scholar]
- 14.Jenny C, Hymel KP, Ritzen A, Reinert SE, Hay TC. Analysis of missed cases of abusive head trauma. JAMA. 1999;281(7):621–6. doi: 10.1001/jama.281.7.621. [DOI] [PubMed] [Google Scholar]
- 15.Binenbaum G, Mirza-George N, Christian CW, Forbes BJ. Odds of abuse associated with retinal hemorrhages in children suspected of child abuse. J AAPOS. 2009;13(3):268–72. doi: 10.1016/j.jaapos.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkhart ZN, Thurber CJ, Chuang AZ, Kumar KS, Davis GH, Kellaway J. Risk factors associated with retinal hemorrhage in suspected abusive head trauma. J AAPOS. 2015;19(2):119–23. doi: 10.1016/j.jaapos.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldman KW, Sugar NF, Browd SR. Initial clinical presentation of children with acute and chronic versus acute subdural hemorrhage resulting from abusive head trauma. J Neurosurg Pediatr. 2015;16(2):177–85. doi: 10.3171/2014.12.PEDS14607. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez E, Delgado I, Sanchez-Montanez A, Fabrega A, Cano P, Martin N. Imaging abusive head trauma: why use both computed tomography and magnetic resonance imaging? Pediatr Radiol. 2014;44(Suppl 4):S589–603. doi: 10.1007/s00247-014-3216-5. [DOI] [PubMed] [Google Scholar]
- 19.Kubat B, Bilo RA, van Rijn RR. Multicystic encephalopathy in abusive head trauma. Clin Neuropathol. 2014;33(4):299–307. doi: 10.5414/NP300700. [DOI] [PubMed] [Google Scholar]
- 20.Clevenger AC, Kilbaugh T, Margulies SS. Carotid artery blood flow decreases after rapid head rotation in piglets. J Neurotrauma. 2015;32(2):120–6. doi: 10.1089/neu.2014.3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhary AK, Ishak R, Zacharia TT, Dias MS. Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol. 2014;44(9):1130–40. doi: 10.1007/s00247-014-2959-3. [DOI] [PubMed] [Google Scholar]
- 22.Shein SL, Bell MJ, Kochanek PM, Tyler-Kabara EC, Wisniewski SR, Feldman K, Makoroff K, Scribano PV, Berger RP. Risk factors for mortality in children with abusive head trauma. J Pediatr. 2012;161(4):716, 722.e1. doi: 10.1016/j.jpeds.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan S, Coats B, Margulies SS. Biofidelic neck influences head kinematics of parietal and occipital impacts following short falls in infants. Accid Anal Prev. 2015;82:143–53. doi: 10.1016/j.aap.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanderhave KL, Chiravuri S, Caird MS, Farley FA, Graziano GP, Hensinger RN, Patel RD. Cervical spine trauma in children and adults: perioperative considerations. J Am Acad Orthop Surg. 2011;19(6):319–27. doi: 10.5435/00124635-201106000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim NG, Ralston J, Smith C, Margulies SS. Physiological and pathological responses to head rotations in toddler piglets. J Neurotrauma. 2010;27(6):1021–35. doi: 10.1089/neu.2009.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weeks D, Sullivan S, Kilbaugh T, Smith C, Margulies SS. Influences of developmental age on the resolution of diffuse traumatic intracranial hemorrhage and axonal injury. J Neurotrauma. 2014;31(2):206–14. doi: 10.1089/neu.2013.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duhaime AC, Durham S. Traumatic brain injury in infants: the phenomenon of subdural hemorrhage with hemispheric hypodensity (“Big Black Brain”) Prog Brain Res. 2007;161:293–302. doi: 10.1016/S0079-6123(06)61020-0. [DOI] [PubMed] [Google Scholar]
- 28.Starr M, Klein EJ, Sugar N. A Perplexing Case of Child Abuse: Oral Injuries in Abuse and Physician Reporting Responsibilities. Pediatr Emerg Care. 2015;31(8):581–3. doi: 10.1097/PEC.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 29.Pressel DM. Evaluation of physical abuse in children. Am Fam Physician. 2000;61(10):3057–64. [PubMed] [Google Scholar]
- 30.Petska HW, Sheets LK. Sentinel injuries: subtle findings of physical abuse. Pediatr Clin North Am. 2014;61(5):923–35. doi: 10.1016/j.pcl.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Muram D. Anal and perianal abnormalities in prepubertal victims of sexual abuse. Am J Obstet Gynecol. 1989;161(2):278–81. doi: 10.1016/0002-9378(89)90498-5. [DOI] [PubMed] [Google Scholar]
- 32.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR American Academy of Pediatrics-Section on Neurological Surgery, American Association of Neurological Surgeons/Congress of Neurological Surgeons, Child Neurology Society, European Society of Pediatric and Neonatal Intensive Care, Neurocritical Care Society, Pediatric Neurocritical Care Research Group, Society of Critical Care Medicine, Paediatric Intensive Care Society UK, Society for Neuroscience in Anesthesiology and Critical Care, World Federation of Pediatric Intensive and Critical Care Societies. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 33.Vavilala MS, Kernic MA, Wang J, Kannan N, Mink RB, Wainwright MS, Groner JI, Bell MJ, Giza CC, Zatzick DF, Ellenbogen RG, Boyle LN, Mitchell PH, Rivara FP Pediatric Guideline Adherence and Outcomes Study. Acute care clinical indicators associated with discharge outcomes in children with severe traumatic brain injury. Crit Care Med. 2014;42(10):2258–66. doi: 10.1097/CCM.0000000000000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardcastle N, Benzon HA, Vavilala MS. Update on the 2012 guidelines for the management of pediatric traumatic brain injury - information for the anesthesiologist. Paediatr Anaesth. 2014;24(7):703–10. doi: 10.1111/pan.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobias JD. Pediatric airway anatomy may not be what we thought: implications for clinical practice and the use of cuffed endotracheal tubes. Paediatr Anaesth. 2015;25(1):9–19. doi: 10.1111/pan.12528. [DOI] [PubMed] [Google Scholar]
- 36.Guerra SD, Carvalho LF, Affonseca CA, Ferreira AR, Freire HB. Factors associated with intracranial hypertension in children and teenagers who suffered severe head injuries. J Pediatr (Rio J) 2010;86(1):73–9. doi: 10.2223/JPED.1960. [DOI] [PubMed] [Google Scholar]
- 37.Chambers IR, Stobbart L, Jones PA, Kirkham FJ, Marsh M, Mendelow AD, Minns RA, Struthers S, Tasker RC. Age-related differences in intracranial pressure and cerebral perfusion pressure in the first 6 hours of monitoring after children’s head injury: association with outcome. Childs Nerv Syst. 2005;21(3):195–9. doi: 10.1007/s00381-004-1060-x. [DOI] [PubMed] [Google Scholar]
- 38.Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Czosnyka M, Smielewski P, Shaffner DH. The lower limit of cerebral blood flow autoregulation is increased with elevated intracranial pressure. Anesth Analg. 2009;108(4):1278–83. doi: 10.1213/ane.0b013e3181964848. [DOI] [PubMed] [Google Scholar]
- 39.Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, Jallo GI, Guerguerian AM. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124(6):e1205–12. doi: 10.1542/peds.2009-0550. [DOI] [PubMed] [Google Scholar]
- 40.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4(1):4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Bell MJ, Kochanek PM. Pediatric traumatic brain injury in 2012: the year with new guidelines and common data elements. Crit Care Clin. 2013;29(2):223–38. doi: 10.1016/j.ccc.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simma B, Burger R, Falk M, Sacher P, Fanconi S. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer’s solution versus hypertonic saline. Crit Care Med. 1998;26(7):1265–70. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 43.Webster DL, Fei L, Falcone RA, Kaplan JM. Higher-volume hypertonic saline and increased thrombotic risk in pediatric traumatic brain injury. J Crit Care. 2015;30(6):1267–71. doi: 10.1016/j.jcrc.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Glick RP, Ksendzovsky A, Greesh J, Raksin P. Initial observations of combination barbiturate coma and decompressive craniectomy for the management of severe pediatric traumatic brain injury. Pediatr Neurosurg. 2011;47(2):152–7. doi: 10.1159/000330709. [DOI] [PubMed] [Google Scholar]
- 45.Mellion SA, Bennett KS, Ellsworth GL, Moore K, Riva-Cambrin J, Metzger RR, Bratton SL. High-dose barbiturates for refractory intracranial hypertension in children with severe traumatic brain injury. Pediatr Crit Care Med. 2013;14(3):239–47. doi: 10.1097/PCC.0b013e318271c3b2. [DOI] [PubMed] [Google Scholar]
- 46.Adelson PD. Editorial: Severe traumatic brain injury and decompressive craniectomy. J Neurosurg Pediatr. 2015:1–3. doi: 10.3171/2014.11.PEDS14562. [DOI] [PubMed] [Google Scholar]
- 47.Oluigbo CO, Wilkinson CC, Stence NV, Fenton LZ, McNatt SA, Handler MH. Comparison of outcomes following decompressive craniectomy in children with accidental and nonaccidental blunt cranial trauma. J Neurosurg Pediatr. 2012;9(2):125–32. doi: 10.3171/2011.11.PEDS09449. [DOI] [PubMed] [Google Scholar]
- 48.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH National Institute of Child Health and Human Development Neonatal Research Network. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 49.Moler FW, Silverstein FS, Holubkov R, Slomine BS, Christensen JR, Nadkarni VM, Meert KL, Clark AE, Browning B, Pemberton VL, Page K, Shankaran S, Hutchison JS, Newth CJ, Bennett KS, Berger JT, Topjian A, Pineda JA, Koch JD, Schleien CL, Dalton HJ, Ofori-Amanfo G, Goodman DM, Fink EL, McQuillen P, Zimmerman JJ, Thomas NJ, van der Jagt EW, Porter MB, Meyer MT, Harrison R, Pham N, Schwarz AJ, Nowak JE, Alten J, Wheeler DS, Bhalala US, Lidsky K, Lloyd E, Mathur M, Shah S, Wu T, Theodorou AA, Sanders RC, Jr, Dean JM THAPCA Trial Investigators. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372(20):1898–908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, Joffe AR, Kirpalani HM, Meyer PG, Morris KP, Moher D, Singh RN, Skippen PW Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 51.Beca J, McSharry B, Erickson S, Yung M, Schibler A, Slater A, Wilkins B, Singhal A, Williams G, Sherring C, Butt W Pediatric Study Group of the Australia and New Zealand Intensive Care Society Clinical Trials Group. Hypothermia for Traumatic Brain Injury in Children-A Phase II Randomized Controlled Trial. Crit Care Med. 2015;43(7):1458–66. doi: 10.1097/CCM.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 52.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD TOBY Study Group. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N Engl J Med. 2014;371(2):140–9. doi: 10.1056/NEJMoa1315788. [DOI] [PubMed] [Google Scholar]
- 53.Mehta A, Kochanek PM, Tyler-Kabara E, Adelson PD, Wisniewski SR, Berger RP, Sidoni MD, Bell RL, Clark RS, Bell MJ. Relationship of intracranial pressure and cerebral perfusion pressure with outcome in young children after severe traumatic brain injury. Dev Neurosci. 2010;32(5–6):413–9. doi: 10.1159/000316804. [DOI] [PubMed] [Google Scholar]
- 54.Vavilala MS, Lee LA, Lam AM. The lower limit of cerebral autoregulation in children during sevoflurane anesthesia. J Neurosurg Anesthesiol. 2003;15(4):307–12. doi: 10.1097/00008506-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Nusbaum D, Clark J, Brady K, Kibler K, Sutton J, Easley RB. Alteration in the lower limit of autoregulation with elevations in cephalic venous pressure. Neurol Res. 2014;36(12):1063–71. doi: 10.1179/1743132814Y.0000000397. [DOI] [PubMed] [Google Scholar]
- 56.Lee JK, Brady KM, Mytar JO, Kibler KK, Carter EL, Hirsch KG, Hogue CW, Easley RB, Jordan LC, Smielewski P, Czosnyka M, Shaffner DH, Koehler RC. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med. 2011;39(10):2337–45. doi: 10.1097/CCM.0b013e318223b910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishikawa S, Ito H, Yokoyama K, Makita K. Phenylephrine ameliorates cerebral cytotoxic edema and reduces cerebral infarction volume in a rat model of complete unilateral carotid artery occlusion with severe hypotension. Anesth Analg. 2009;108(5):1631–7. doi: 10.1213/ane.0b013e31819d94e3. [DOI] [PubMed] [Google Scholar]
- 58.Malhotra AK, Schweitzer JB, Fox JL, Fabian TC, Proctor KG. Cerebral perfusion pressure directed therapy following traumatic brain injury and hypotension in swine. J Neurotrauma. 2003;20(9):827–39. doi: 10.1089/089771503322385764. [DOI] [PubMed] [Google Scholar]
- 59.Sookplung P, Siriussawakul A, Malakouti A, Sharma D, Wang J, Souter MJ, Chesnut RM, Vavilala MS. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. 2011;15(1):46–54. doi: 10.1007/s12028-010-9448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biestro A, Barrios E, Baraibar J, Puppo C, Lupano D, Cancela M, Borovich B, Pouso J. Use of vasopressors to raise cerebral perfusion pressure in head injured patients. Acta Neurochir Suppl. 1998;71:5–9. doi: 10.1007/978-3-7091-6475-4_2. [DOI] [PubMed] [Google Scholar]
- 61.Steiner LA, Johnston AJ, Czosnyka M, Chatfield DA, Salvador R, Coles JP, Gupta AK, Pickard JD, Menon DK. Direct comparison of cerebrovascular effects of norepinephrine and dopamine in head-injured patients. Crit Care Med. 2004;32(4):1049–54. doi: 10.1097/01.ccm.0000120054.32845.a6. [DOI] [PubMed] [Google Scholar]
- 62.Kroppenstedt SN, Thomale UW, Griebenow M, Sakowitz OW, Schaser KD, Mayr PS, Unterberg AW, Stover JF. Effects of early and late intravenous norepinephrine infusion on cerebral perfusion, microcirculation, brain-tissue oxygenation, and edema formation in brain-injured rats. Crit Care Med. 2003;31(8):2211–21. doi: 10.1097/01.CCM.0000080482.06856.62. [DOI] [PubMed] [Google Scholar]
- 63.Di Gennaro JL, Mack CD, Malakouti A, Zimmerman JJ, Armstead W, Vavilala MS. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 2010;32(5–6):420–30. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Armstead WM. Age-dependent cerebral hemodynamic effects of traumatic brain injury in newborn and juvenile pigs. Microcirculation. 2000;7(4):225–35. [PubMed] [Google Scholar]
- 65.Dobbing J. The later development of the brain and its vulnerability. In: Davis JA, Dobbing J, editors. Scientific Foundations of Pediatrics. London: Heineman Medical; 1981. pp. 744–59. [Google Scholar]
- 66.Armstead WM, Kiessling JW, Kofke WA, Vavilala MS. Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit Care Med. 2010;38(9):1868–74. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Armstead WM, Riley J, Vavilala MS. Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of Up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr Crit Care Med. 2013;14(2):e103–11. doi: 10.1097/PCC.0b013e3182712b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armstead WM, Riley J, Vavilala MS. Preferential protection of cerebral autoregulation and reduction of hippocampal necrosis with norepinephrine after traumatic brain injury in female piglets. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000603. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Bourgeois M, Di Rocco F, Garnett M, Charron B, Boddaert N, Soufflet C, Roujeau T, Zerah M, Sainte-Rose C, Plouin P, Renier D. Epilepsy associated with shaken baby syndrome. Childs Nerv Syst. 2008;24(2):169, 72. doi: 10.1007/s00381-007-0493-4. discussion 173. [DOI] [PubMed] [Google Scholar]
- 70.Bruins B, Kilbaugh TJ, Margulies SS, Friess SH. The anesthetic effects on vasopressor modulation of cerebral blood flow in an immature swine model. Anesth Analg. 2013;116(4):838–44. doi: 10.1213/ANE.0b013e3182860fe7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeiler FA, Teitelbaum J, West M, Gillman LM. The ketamine effect on ICP in traumatic brain injury. Neurocrit Care. 2014;21(1):163–73. doi: 10.1007/s12028-013-9950-y. [DOI] [PubMed] [Google Scholar]
- 72.McConeghy KW, Hatton J, Hughes L, Cook AM. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26(7):613–36. doi: 10.2165/11634020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 73.Minton MD, Grosslight K, Stirt JA, Bedford RF. Increases in intracranial pressure from succinylcholine: prevention by prior nondepolarizing blockade. Anesthesiology. 1986;65(2):165–9. doi: 10.1097/00000542-198608000-00006. [DOI] [PubMed] [Google Scholar]
- 74.Chong SL, Harjanto S, Testoni D, Ng ZM, Low CY, Lee KP, Lee JH. Early Hyperglycemia in Pediatric Traumatic Brain Injury Predicts for Mortality, Prolonged Duration of Mechanical Ventilation, and Intensive Care Stay. Int J Endocrinol. 2015;2015:719476. doi: 10.1155/2015/719476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Melo JR, Di Rocco F, Blanot S, Laurent-Vannier A, Reis RC, Baugnon T, Sainte-Rose C, Olveira-Filho J, Zerah M, Meyer P. Acute hyperglycemia is a reliable outcome predictor in children with severe traumatic brain injury. Acta Neurochir (Wien) 2010;152(9):1559–65. doi: 10.1007/s00701-010-0680-z. [DOI] [PubMed] [Google Scholar]
- 76.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care, AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, Wright DW. Guidelines for the management of severe traumatic brain injury. IX. Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 77.Le Roux P, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, Diringer MN, Stocchetti N, Videtta W, Armonda R, Badjatia N, Boesel J, Chesnut R, Chou S, Claassen J, Czosnyka M, De Georgia M, Figaji A, Fugate J, Helbok R, Horowitz D, Hutchinson P, Kumar M, McNett M, Miller C, Naidech A, Oddo M, Olson D, O’Phelan K, Provencio JJ, Puppo C, Riker R, Robertson C, Schmidt M, Taccone F Neurocritical Care Society, European Society of Intensive Care Medicine. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care : a statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(9):1189–209. doi: 10.1007/s00134-014-3369-6. [DOI] [PubMed] [Google Scholar]
- 78.Lewis PM, Czosnyka M, Carter BG, Rosenfeld JV, Paul E, Singhal N, Butt W. Cerebrovascular Pressure Reactivity in Children With Traumatic Brain Injury. Pediatr Crit Care Med. 2015 doi: 10.1097/PCC.0000000000000471. [DOI] [PubMed] [Google Scholar]
- 79.Dias C, Silva MJ, Pereira E, Monteiro E, Maia I, Barbosa S, Silva S, Honrado T, Cerejo A, Aries MJ, Smielewski P, Paiva JA, Czosnyka M. Optimal Cerebral Perfusion Pressure Management at Bedside: A Single-Center Pilot Study. Neurocrit Care. 2015;23(1):92–102. doi: 10.1007/s12028-014-0103-8. [DOI] [PubMed] [Google Scholar]
- 80.Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med. 2006;34(6):1783–8. doi: 10.1097/01.CCM.0000218413.51546.9E. [DOI] [PubMed] [Google Scholar]
- 81.Liu X, Czosnyka M, Donnelly J, Budohoski KP, Varsos GV, Nasr N, Brady KM, Reinhard M, Hutchinson PJ, Smielewski P. Comparison of frequency and time domain methods of assessment of cerebral autoregulation in traumatic brain injury. J Cereb Blood Flow Metab. 2015;35(2):248–56. doi: 10.1038/jcbfm.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, Brady KM, Hutchinson PJ, Menon DK, Pickard JD, Smielewski P. Noninvasive Monitoring of Cerebrovascular Reactivity with Near Infrared Spectroscopy in Head-Injured Patients. J Neurotrauma. 2010;27(11):1951–8. doi: 10.1089/neu.2010.1388. [DOI] [PubMed] [Google Scholar]
- 83.Highton D, Ghosh A, Tachtsidis I, Panovska-Griffiths J, Elwell CE, Smith M. Monitoring cerebral autoregulation after brain injury: multimodal assessment of cerebral slow-wave oscillations using near-infrared spectroscopy. Anesth Analg. 2015;121(1):198–205. doi: 10.1213/ANE.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler RC, Shaffner DH, Brady KM. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke. 2009;40(5):1820–6. doi: 10.1161/STROKEAHA.108.536094. [DOI] [PubMed] [Google Scholar]
- 85.Ward A, Iocono JA, Brown S, Ashley P, Draus JM., Jr Non-accidental Trauma Injury Patterns and Outcomes: A Single Institutional Experience. Am Surg. 2015;81(9):835–8. [PubMed] [Google Scholar]
- 86.Nigrovic LE, Rogers AJ, Adelgais KM, Olsen CS, Leonard JR, Jaffe DM, Leonard JC Pediatric Emergency Care Applied Research Network (PECARN) Cervical Spine Study Group. Utility of plain radiographs in detecting traumatic injuries of the cervical spine in children. Pediatr Emerg Care. 2012;28(5):426–32. doi: 10.1097/PEC.0b013e3182531911. [DOI] [PubMed] [Google Scholar]
- 87.Choudhary AK, Bradford RK, Dias MS, Moore GJ, Boal DK. Spinal subdural hemorrhage in abusive head trauma: a retrospective study. Radiology. 2012;262(1):216–23. doi: 10.1148/radiol.11102390. [DOI] [PubMed] [Google Scholar]
- 88.Kondolot M, Yagmur F, Yikilmaz A, Turan C, Oztop DB, Oral R. A life-threatening presentation of child physical abuse: jejunal perforation. Pediatr Emerg Care. 2011;27(11):1075–7. doi: 10.1097/PEC.0b013e3182360653. [DOI] [PubMed] [Google Scholar]
- 89.Dedouit F, Guilbeau-Frugier C, Capuani C, Sevely A, Joffre F, Rouge D, Rousseau H, Telmon N. Child abuse: practical application of autopsy, radiological, and microscopic studies. J Forensic Sci. 2008;53(6):1424–9. doi: 10.1111/j.1556-4029.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 90.Lindberg DM, Shapiro RA, Blood EA, Steiner RD, Berger RP ExSTRA investigators. Utility of hepatic transaminases in children with concern for abuse. Pediatrics. 2013;131(2):268–75. doi: 10.1542/peds.2012-1952. [DOI] [PubMed] [Google Scholar]
- 91.Maguire SA, Upadhyaya M, Evans A, Mann MK, Haroon MM, Tempest V, Lumb RC, Kemp AM. A systematic review of abusive visceral injuries in childhood--their range and recognition. Child Abuse Negl. 2013;37(7):430–45. doi: 10.1016/j.chiabu.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 92.Naik-Mathuria B, Akinkuotu A, Wesson D. Role of the surgeon in non-accidental trauma. Pediatr Surg Int. 2015;31(7):605–10. doi: 10.1007/s00383-015-3688-x. [DOI] [PubMed] [Google Scholar]
- 93.Perez-Rossello JM, McDonald AG, Rosenberg AE, Tsai A, Kleinman PK. Absence of rickets in infants with fatal abusive head trauma and classic metaphyseal lesions. Radiology. 2015;275(3):810–21. doi: 10.1148/radiol.15141784. [DOI] [PubMed] [Google Scholar]
- 94.Eggleston JC. In re Natalie AA. Appellate Division Third. 2015;130:50–4. [Google Scholar]
- 95.In re MaKenna S. West Law 2011;2011 WL 4447225:1–10.
- 96.Binenbaum G, Forbes BJ. The eye in child abuse: key points on retinal hemorrhages and abusive head trauma. Pediatr Radiol. 2014;44(Suppl 4):S571–7. doi: 10.1007/s00247-014-3107-9. [DOI] [PubMed] [Google Scholar]
- 97.Binenbaum G, Christian CW, Guttmann K, Huang J, Ying GS, Forbes BJ. Evaluation of Temporal Association Between Vaccinations and Retinal Hemorrhage in Children. JAMA Ophthalmol. 2015;133(11):1261–5. doi: 10.1001/jamaophthalmol.2015.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lueder GT, Turner JW, Paschall R. Perimacular retinal folds simulating nonaccidental injury in an infant. Arch Ophthalmol. 2006;124(12):1782–3. doi: 10.1001/archopht.124.12.1782. [DOI] [PubMed] [Google Scholar]
- 99.Kivlin JD, Currie ML, Greenbaum VJ, Simons KB, Jentzen J. Retinal hemorrhages in children following fatal motor vehicle crashes: a case series. Arch Ophthalmol. 2008;126(6):800–4. doi: 10.1001/archopht.126.6.800. [DOI] [PubMed] [Google Scholar]
- 100.Reddie IC, Bhardwaj G, Dauber SL, Jacobs MB, Moran KT. Bilateral retinoschisis in a 2-year-old following a three-storey fall. Eye (Lond) 2010;24(8):1426–7. doi: 10.1038/eye.2010.70. [DOI] [PubMed] [Google Scholar]
- 101.Ogrenci A, Eksi MS, Koban O, Karakus M. Spontaneous rapid resolution of acute subdural hematoma in children. Childs Nerv Syst. 2015;31(12):2239–43. doi: 10.1007/s00381-015-2910-4. [DOI] [PubMed] [Google Scholar]
- 102.Vester ME, Bilo RA, Karst WA, Daams JG, Duijst WL, van Rijn RR. Subdural hematomas: glutaric aciduria type 1 or abusive head trauma? A systematic review. Forensic Sci Med Pathol. 2015;11(3):405–15. doi: 10.1007/s12024-015-9698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castori M. Ehlers-Danlos syndrome(s) mimicking child abuse: Is there an impact on clinical practice? Am J Med Genet C Semin Med Genet. 2015 doi: 10.1002/ajmg.c.31460. [DOI] [PubMed] [Google Scholar]
- 104.Bouvet R, Pierre M, Toutain F, Beucher J, Dabadie A, Le Gall F, Francois-Chervet C, Pladys P, Bruneau B, Le Gueut M. Tufted angioma with Kasabach-Merritt syndrome mistaken for child abuse. Forensic Sci Int. 2014;245C:e15–7. doi: 10.1016/j.forsciint.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Paroskie A, Carpenter SL, Lowen DE, Anderst J, DeBaun MR, Sidonio RF., Jr A two-center retrospective review of the hematologic evaluation and laboratory abnormalities in suspected victims of non-accidental injury. Child Abuse Negl. 2014;38(11):1794–800. doi: 10.1016/j.chiabu.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salvatori MC, Lantz PE. Retinal haemorrhages associated with fatal paediatric infections. Med Sci Law. 2015;55(2):121–8. doi: 10.1177/0025802414527077. [DOI] [PubMed] [Google Scholar]
- 107.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114(3):633–9. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berger RP, Simon D, Wolford JE, Bell MJ. Chapter 62: Abusive head trauma. In: Nichols DG, Shaffner DH, editors. Rogers’ Textbook of Pediatric Intensive Care. 5. Amsterdam, The Netherlands: Wolters Kluwer; 2016. pp. 982–989. [Google Scholar]