Abstract
Background
Several previous studies have found inverse associations between glioma susceptibility and a history of allergies or other atopic conditions. Some evidence indicates that respiratory allergies are likely to be particularly relevant with regard to glioma risk. Using data from the Glioma International Case-Control Study (GICC), we examined the effects of respiratory allergies and other atopic conditions on glioma risk.
Methods
The GICC contains detailed information on history of atopic conditions for 4533 cases and 4171 controls, recruited from 14 study sites across five countries. Using two-stage random-effects restricted maximum likelihood modeling to calculate meta-analysis odds ratios, we examined the associations between glioma and allergy status, respiratory allergy status, asthma, and eczema.
Results
Having a history of respiratory allergies was associated with an approximately 30% lower glioma risk, compared to not having respiratory allergies (mOR: 0.72, 95% CI: 0.58–0.90). This association was similar when restricting to high-grade glioma cases. Asthma and eczema were also significantly protective against glioma.
Conclusions
A substantial amount of data on the inverse association between atopic conditions and glioma has accumulated, and findings from the GICC study further strengthen the existing evidence that the relationship between atopy and glioma is unlikely to be coincidental.
Impact
As the literature approaches a consensus on the impact of allergies in glioma risk, future research can begin to shift focus to what the underlying biological mechanism behind this association may be, which could, in turn, yield new opportunities for immunotherapy or cancer prevention.
Keywords: glioma, glioblastoma, allergies, asthma, eczema
Introduction
Over the past several decades, a history of allergies and atopic conditions has been consistently reported to be associated with decreased glioma risk (1–7). With some exceptions (8–10), the majority of studies have found that allergic conditions may reduce glioma risk by as much as 20–40% (1–4, 7). These associations have been examined using single- [i.e., (11)], multi-site [i.e., (12–14)], and nested case-control studies [i.e., (15, 16)], prospective cohort studies [i.e., (3)], and meta-analyses [i.e., (1, 2, 7)]. Additionally, the observed inverse association between allergies and glioma has remained consistent across studies with different exposure assessment strategies, such as self-reported allergy status (3), self-reported physician-diagnosed allergies (12, 13, 17–19), number of allergy types (12, 17), and allergy-related biomarkers, such as Immunoglobulin E [overall (4, 11, 16, 20), pre-diagnostic (16, 21), and/or allergen-specific (11, 15, 16)], soluble CD23 levels (22), and polymorphisms in allergy-related genes (23–26). As the literature approaches a consensus on the relationship between allergies and glioma risk, our large consortium, the Glioma International Case-Control Study (GICC), provides an unprecedented opportunity to not only confirm the previously reported associations between atopy and glioma in the largest available study population, but also to hone in on the specific role of respiratory allergies.
In studies that have examined specific allergy types, the observed associations between glioma and respiratory allergies (hay fever/allergic rhinitis) or asthma tend to be among the more robust (12, 15, 16, 20, 27–29). While reactions to food allergens are often limited to the gut (or systemic in the worst cases), inhaled allergens activate mucosal mast cells in the nasal passage and respiratory tract, and usually induce a localized response (30). The nasal passage may be of particular interest in studies of glioma, as some intranasally administered peptides or chemicals can cross the blood-brain barrier (BBB) (20, 31). Furthermore, particles of a certain size, charge, and configuration may enter the brain directly from the nasal passage through the trigeminal nerve sheath, bypassing the BBB (32, 33). Thus, it has been hypothesized that intranasal exposures are more likely to directly impact intracranial immune responses than food or contact allergies (16, 20).
In this international multi-site consortium study, we assessed the role of allergies (particularly respiratory allergies), asthma, and eczema on glioma risk. We also evaluated whether regular oral antihistamine use or respiratory allergy treatment type was associated with glioma risk. Our study represents the largest study of these associations to date (n= 4533 cases and 4171 controls), with the exception of a few meta-analyses of the previous literature (1, 7).
Materials and Methods
Study Population
Detailed information on the GICC study can be found elsewhere (34). Briefly, the GICC is an international consortium with 14 recruitment sites across five countries: Brigham and Women’s Hospital (MA, USA), Case Western Reserve University (Ohio, USA), Columbia University (NY, USA), Danish Cancer Society Research Centre (Copenhagen, Denmark), The Gertner Institute (Tel Hashomer, Israel), Duke University (NC, USA), University of Texas MD Anderson Cancer Center (TX, USA), Memorial Sloan Kettering Cancer Center (NY, USA), Mayo Clinic (MN, USA), NorthShore HealthSystem ( IL, USA), Umeå University (Umeå, Sweden), University of California, San Francisco (CA, USA), University of Southern California (CA, USA) and The Institute of Cancer Research (London, United Kingdom). Cases were defined as individuals within 18–80 years of age (at diagnosis) who had a histologically-confirmed, supra-tentorial, intracranial glioma [fibrillary astrocytoma (9420/3), protoplasmic astrocytoma (9410/3), gemistocytic astrocytoma (9411/3), oligodendroglioma (9450/3), oligoastrocytoma (9382/3), anaplastic astrocytoma (9401/3), anaplastic oligodendroglioma (9451/3), anaplastic oligoastrocytoma (9382/3), gliosarcoma (9442/3), and glioblastoma (9440/3)]. They were recruited within a year of diagnosis and consented at their clinic visits.
Controls were eligible for the study if they were between 18 and 80 years old. Because it was not feasible for all sites to recruit controls using identical methods, seven sites recruited visitors accompanying cancer patients as controls, four sites recruited clinic-based controls, and three sites used population-based controls.
All sites received Institutional Review Board (IRB) or ethical board approval to conduct the study, and informed consent was obtained from participants.
Data Collection
All sites adhered to a common study protocol and administered the same questionnaire. Study coordinators were centrally trained to help standardize data collection procedures. Data were stored in a centralized database. More information on data collection and reliability is provided elsewhere (34).
The GICC risk factor questionnaire included information on demographics, past medical/medication history, and occupational exposure history. Questionnaires were administered in-person and/or by phone, or through mailed self-administered forms. Regarding allergies and atopy specifically, the participants were asked about their experiences ≥1 year prior to brain tumor diagnosis (or enrollment). Allergy status was assessed by asking if the person experienced certain symptoms (skin, respiratory, watery eyes, digestive problems, anaphylaxis, or other) and whether he/she demonstrated allergic reactions to any of a list of potential allergens (dust/mold, plants/pollens, foods, animals, medications, soaps/cosmetics, or other). For each allergen, the participant was asked the age at first allergic episode (age <12, 12–20, or >20 years) and how they treated that particular allergy (medication, desensitization shots, epinephrine shots, avoidance, etc.). Participants were also asked about asthma and eczema. Similar to allergies, they reported their age at first diagnosis and treatment method for each.
Detailed information on antihistamines and other allergy treatments was also collected. Participants were asked if they took antihistamines or decongestants regularly (once a month or more) for at least six months of their lives. We provided a list of the most commonly used allergy medications and also collected information on age at first allergy medication use.
Statistical Analysis
The overall GICC analysis plan (including sensitivity analyses) and a table of population demographics by study site has previously been published (34). For the current analyses, we compared cases and controls on relevant characteristics, overall, by study site, and by tumor grade (high-grade: WHO Grade IV; lower-grade: Grade II and III) among cases. Exposures of interest included: any allergies; respiratory allergies (defined as allergic rhinitis symptoms or allergic reactions to dust/mold or plants/pollens); allergies to animals/insects, food, medications, or soaps/cosmetics; allergy treatment severity; history of asthma; history of eczema; and long-term (≥6 months) oral antihistamine use. Analyses for each exposure of interest were conducted in the overall study population and separately by tumor grade.
Site-specific crude and adjusted odds ratios (ORs), along with their corresponding 95% Wald confidence intervals (CIs), were calculated for each exposure-outcome relationship, using unconditional logistic regression. Sites with less than five cases or controls in the exposed or unexposed groups were excluded from the meta-analyses. Data from Columbia University were omitted from the analyses due to a suspected data collection error that resulted in the prevalence of atopy being substantially lower among Columbia controls (6.4%) compared to all other sites and the national average.
Two-stage random-effects restricted maximum likelihood (REML) modeling was used to aggregate estimates across study sites into overall meta-analysis ORs (mORs) (34). For each REML model, the I2 statistic was calculated to assess the percentage of variability in the effect estimates due to heterogeneity, rather than sampling error. The tau2 statistic was used to evaluate the between-site variance, and the heterogeneity test p-value was computed. Forest plots were constructed as a visual representation of the site-specific (and overall meta-analysis) estimates. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) and R version 3.1.2.
We decided a priori to control for age and sex in our multivariable models, although doing so made virtually no difference to the effect estimates. Throughout our analyses, we also considered education, cigarette smoking, family history of brain tumors, and race/ethnicity as potential confounders, but none of these factors proved to be confounders in our data (based on a 10% change-in-estimate criterion) and were, thus, not included in the final models. Through stratified analyses, we explored the possibility of effect modification of allergy status by each of the following factors: sex, geographic location, asthma status, and long-term antihistamine use. We also conducted several sub-analyses stratifying by category of age at first allergic episode.
Because we had no direct measure of allergy severity, we examined the effects of treatment type, as an indirect measure of allergy severity. We constructed three treatment groups: high (treatments for pre-anaphylaxis/anaphylactic shock), medium (treatments for more moderate symptomologies), and mild/none (treatments for either mild or no symptoms). The high treatment group was defined by use of at least one of the following: desensitization shots, hospitalization, or epinephrine shot. The medium group was defined by use of allergy medications. The mild/no treatment group was defined by allergen avoidance or no treatment.
Although our analyses focused on the history of respiratory allergies, other allergy types (food, animal, medication, soap/cosmetic), asthma, and eczema were also evaluated separately. The impact of regular long-term oral antihistamine use on glioma risk was explored among those with and without allergies, as some individuals may take antihistamines regularly for indications other than allergic conditions (i.e., as sleep aids or antiemetics).
Despite our large sample size, our exploratory analyses of the potential interaction between respiratory allergies and asthma were underpowered, and thus, had to be analyzed by pooling the data from all sites, rather than by meta-analysis. While we acknowledge that pooling is not entirely appropriate given the inter-site heterogeneity present in our large consortium, these pooled analyses were exploratory in nature and were used to ascertain whether there may be some indication of an interaction at play that should be examined in future studies.
We conducted sensitivity analyses including and excluding proxy respondents and comparing the results to ensure that there were no meaningful discrepancies in ORs. Potential differences in ORs between sites by the different control types (visitor, clinic-, or population-based) were also examined to confirm that patterns by control type were not present in our results.
Results
The GICC includes a total of 4533 cases and 4171 controls recruited across 14 study sites. Table 1 presents the distributions of demographics and relevant attributes by case-control status and tumor grade. The age distribution between cases and controls was similar, but high-grade cases tended to be older than lower-grade cases, as expected. Cigarette smoking distribution was similar among cases and controls, with a slightly higher preponderance of current smokers among controls.
Table 1.
Selected population characteristics from The Glioma International Case-Control Study (GICC) by case-control status and glioma grade
| Glioma Cases | Controls | High-Grade Glioma Casesa | Lower-Grade Glioma Casesa | |||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Sex | 2679 | 59.1 | 2351 | 56.37 | 1728 | 62.29 | 916 | 54.3 |
| Male | ||||||||
| Female | 1854 | 40.9 | 1820 | 43.63 | 1046 | 37.71 | 771 | 45.7 |
| Diagnosis/enrollment age | 308 | 6.79 | 294 | 7.05 | 62 | 2.24 | 228 | 13.52 |
| 18–29 years | ||||||||
| 30–39 years | 521 | 11.49 | 473 | 11.34 | 108 | 3.89 | 398 | 23.59 |
| 40–49 years | 813 | 17.94 | 680 | 16.3 | 417 | 15.03 | 384 | 22.76 |
| 50–59 years | 1150 | 25.37 | 1079 | 25.87 | 796 | 28.7 | 338 | 20.04 |
| 60–69 years | 1239 | 27.33 | 1098 | 26.32 | 993 | 35.8 | 238 | 14.11 |
| 70–80 years | 502 | 11.07 | 547 | 13.11 | 398 | 14.35 | 101 | 5.99 |
| Educationb | ||||||||
| High School or less | 1126 | 27.67 | 911 | 22.47 | 716 | 28.66 | 392 | 25.99 |
| Some college | 1104 | 27.13 | 1293 | 31.89 | 651 | 26.06 | 433 | 28.71 |
| Bachelor’s degree | 1020 | 25.06 | 955 | 23.55 | 595 | 23.82 | 409 | 27.12 |
| Advanced degree | 808 | 19.85 | 892 | 22 | 530 | 21.22 | 268 | 17.77 |
| Missing | 12 | 0.29 | 4 | 0.1 | 6 | 0.24 | 6 | 0.4 |
| Smoking | 2458 | 54.22 | 2271 | 54.45 | 1504 | 54.22 | 914 | 54.18 |
| Never | ||||||||
| Current | 440 | 9.71 | 468 | 11.22 | 236 | 8.51 | 195 | 11.56 |
| Former | 1581 | 34.88 | 1413 | 33.88 | 998 | 35.98 | 562 | 33.31 |
| Missing | 54 | 1.19 | 19 | 0.46 | 36 | 1.3 | 16 | 0.95 |
| Respiratory allergy | 1547 | 34.13 | 1462 | 35.05 | 912 | 32.88 | 601 | 35.63 |
| Yes | ||||||||
| No | 2966 | 65.43 | 2682 | 64.3 | 1847 | 66.58 | 1081 | 64.08 |
| Missing | 20 | 0.44 | 27 | 0.65 | 15 | 0.54 | 5 | 0.3 |
| Animal/insect allergies | 641 | 14.14 | 556 | 13.33 | 357 | 12.87 | 270 | 16 |
| Yes | ||||||||
| No | 3759 | 82.93 | 3468 | 83.15 | 2340 | 84.35 | 1362 | 80.74 |
| Don’t know | 63 | 1.39 | 79 | 1.89 | 36 | 1.3 | 26 | 1.54 |
| Missing | 70 | 1.54 | 68 | 1.63 | 41 | 1.48 | 29 | 1.72 |
| Food Allergies | 398 | 8.78 | 437 | 10.48 | 223 | 8.04 | 162 | 9.6 |
| Yes | ||||||||
| No | 4013 | 88.53 | 3599 | 86.29 | 2478 | 89.33 | 1476 | 87.49 |
| Don’t know | 57 | 1.26 | 67 | 1.61 | 35 | 1.26 | 22 | 1.3 |
| Missing | 65 | 1.43 | 68 | 1.63 | 38 | 1.37 | 27 | 1.6 |
| Medication allergies | 985 | 21.73 | 828 | 19.85 | 600 | 21.63 | 366 | 21.7 |
| Yes | ||||||||
| No | 3412 | 75.27 | 3202 | 76.77 | 2098 | 75.63 | 1262 | 74.81 |
| Don’t know | 71 | 1.57 | 76 | 1.82 | 40 | 1.44 | 30 | 1.78 |
| Missing | 65 | 1.43 | 65 | 1.56 | 36 | 1.3 | 29 | 1.72 |
| Soap/cosmetics allergies | 317 | 6.99 | 327 | 7.84 | 187 | 6.74 | 121 | 7.17 |
| Yes | ||||||||
| No | 4076 | 89.92 | 3691 | 88.49 | 2505 | 90.3 | 1508 | 89.39 |
| Don’t know | 67 | 1.48 | 79 | 1.89 | 39 | 1.41 | 28 | 1.66 |
| Missing | 73 | 1.61 | 74 | 1.77 | 43 | 1.55 | 30 | 1.78 |
| Long-term antihistamine use | 772 | 17.03 | 731 | 17.53 | 466 | 16.8 | 288 | 17.07 |
| Yes | ||||||||
| No | 3750 | 82.73 | 3433 | 82.31 | 2304 | 83.06 | 1392 | 82.51 |
| Don’t know | 10 | 0.22 | 4 | 0.1 | 4 | 0.14 | 6 | 0.36 |
| Missing | 1 | 0.02 | 3 | 0.07 | 0 | 0 | 1 | 0.06 |
Sum of high-grade and lower-grade cases does not equal total number of cases, due to cases with unclassified tumors.
Educational attainment information was not collected by one study site (The Institute of Cancer Research, London, UK).
Overall, a history of any allergy was associated with a 21% lower risk of glioma, adjusting for age and sex, though this association was of borderline statistical significance (mOR: 0.79, 95% CI: 0.61–1.02) [data not shown]. UCSF was the only site in which a significant positive association was observed. Stratified by tumor grade, the association between any allergies and glioma was only significant among high-grade cases (mOR: 0.75, 95% CI: 0.58–0.98; among lower-grade, mOR: 0.84, 95% CI: 0.63–1.11). Geographic differences in the impact of allergies on glioma risk were not observed.
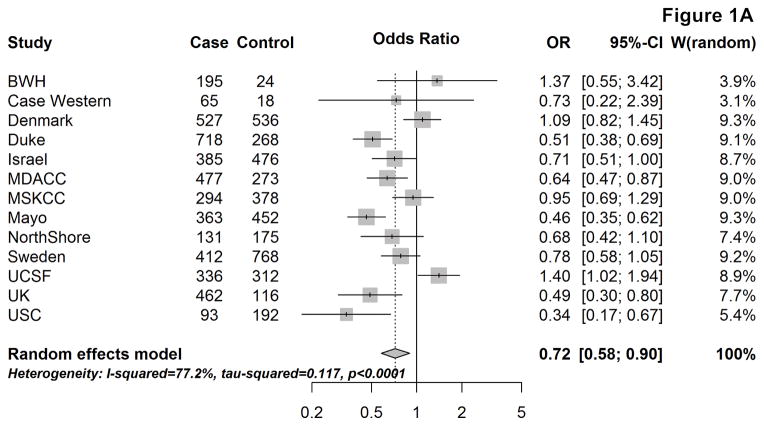
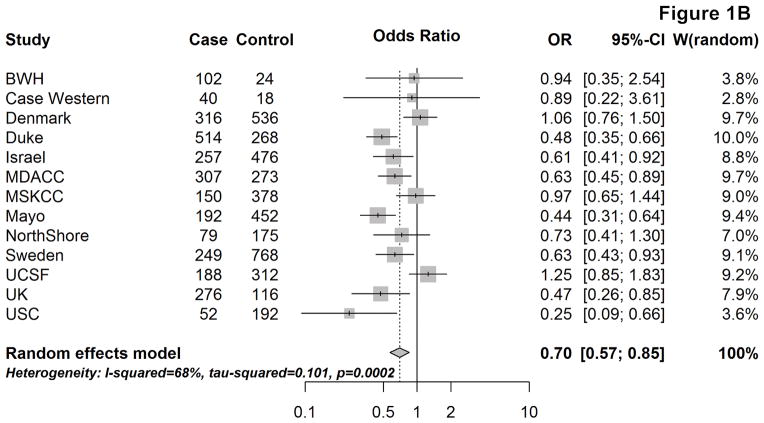
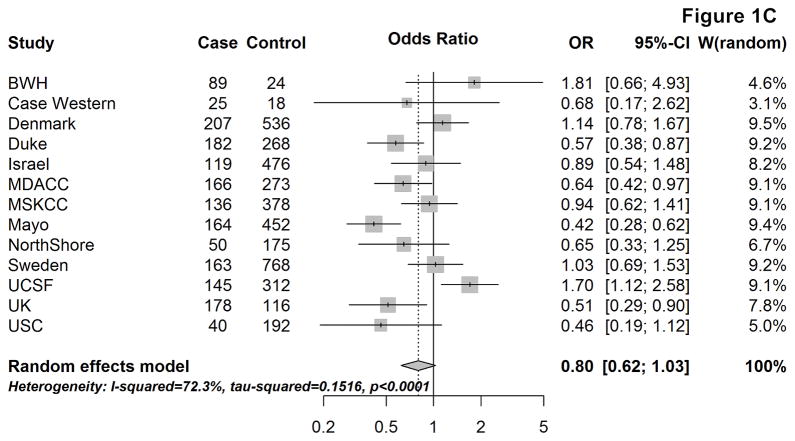
Figure 1 provides the site-specific and meta-OR for the association between respiratory allergies and glioma risk, overall (A) and by tumor grade (B & C). Overall, having respiratory allergies was associated with an approximately 30% lower glioma risk, compared to not having respiratory allergies (mOR: 0.72, 95% CI: 0.58–0.90). When stratifying by sex, the mOR remained remarkably similar among males (mOR:0.69, 95% CI:0.53–0.89), but among females, the effect was slightly attenuated (mOR:0.79, 95% CI:0.63–0.98) [not shown].
Figure 1.
Site-specific and meta-analysis odds ratios and 95% confidence intervals from the Glioma International Case-Control Study (GICC) for the association between history of respiratory allergies and glioma risk: overall (A), for high-grade glioma (B), and for lower-grade glioma (C). Meta-analysis odds ratio was calculated using restricted maximum likelihood modelling.
The association between respiratory allergies and glioma risk remained similar when restricting to high-grade gliomas (mOR:0.70, 95% CI: 0.57–0.85), whereas the magnitude of the effect was closer to the null and not statistically significant among lower-grade gliomas (mOR: 0.80, 95% CI: 0.62–1.03) [Fig. 1B&C]. When stratifying by age at glioma diagnosis/enrollment (<40, 40–59, and ≥60 years), the confidence intervals for the estimates overlapped between the three age groups and no obvious trends were seen (overall or by tumor grade).
Age of diagnosis of respiratory allergies was also considered in our analyses (Suppl. Table 1). When stratifying by age at allergy diagnosis prior to 20 years, the mOR between respiratory allergies and glioma was still in the inverse direction (mOR= 0.76, 95% CI: 0.58–1.00) and the confidence intervals largely overlapped with those of the mOR among allergies diagnoses at ages ≥20 (mOR= 0.67, 95% CI:0.54–0.83). Restricting the analyses to respiratory allergies diagnosed in early childhood (<12 years of age) yielded similar, though attenuated, results (mOR: 0.82, 95% CI: 0.64–1.06).
None of the other allergy types were significantly associated with glioma risk (Table 2), even among individuals without respiratory allergies [not shown].
Table 2.
Associations between various allergy types and glioma risk: Results from the Glioma International Case-Control Study (GICC)
| Case | Control | Adjusted Meta-Analysis Odds Ratios (95% Confidence Intervals)a | Heterogeneity Test | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | I2 (%) | Tau2 | P-value | ||||
| Animal/insect allergiesb | 59.30 | 0.0761 | 0.0063 | |||||||
| No | 3783 | 85.55 | 3407 | 86.08 | 1.00 | |||||
| Yes | 639 | 14.45 | 551 | 13.92 | 0.98 | 0.80 | 1.21 | |||
| Food allergiesb | 66.10 | 0.1411 | 0.0011 | |||||||
| No | 4038 | 91.11 | 3540 | 89.17 | 1.00 | |||||
| Yes | 394 | 8.89 | 430 | 10.83 | 0.78 | 0.59 | 1.03 | |||
| Medication allergies | 68.90 | 0.1004 | 0.0001 | |||||||
| No | 3443 | 77.91 | 3147 | 79.41 | 1.00 | |||||
| Yes | 976 | 22.09 | 816 | 20.59 | 0.98 | 0.80 | 1.21 | |||
| Soap/cosmetics allergiesc | 75.50 | 0.2607 | <0.0001 | |||||||
| No | 4098 | 92.82 | 3629 | 91.83 | 1.00 | |||||
| Yes | 317 | 7.18 | 323 | 8.17 | 0.79 | 0.55 | 1.12 | |||
Full two-stage random-effect restricted maximum likelihood model adjusting for glioma diagnosis/enrollment age and sex.
Case Western and USC excluded from meta-analysis due to cell frequencies <5.
Brigham and Women’s, Case Western, and USC excluded from meta-analysis due to cell frequencies <5.
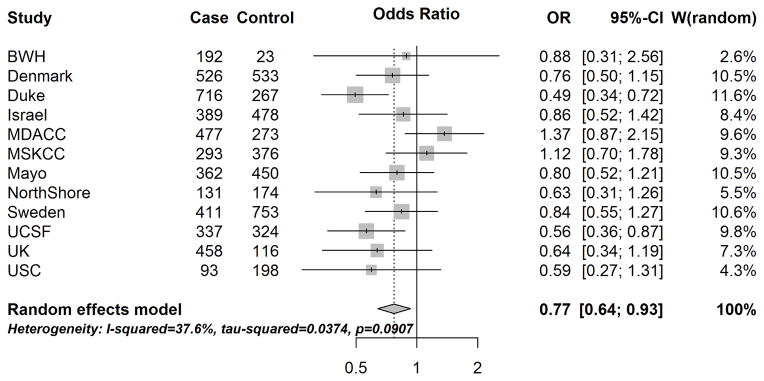
The site-specific and overall associations between asthma status and glioma risk are provided in Figure 2. Fewer of the site-specific ORs were significantly protective against glioma, compared to the results for respiratory allergies, but overall, asthma was associated with a statistically significant 23% decreased glioma risk (mOR: 0.77, 95% CI: 0.64–0.93). Results were similar stratified by tumor grade (among high-grade, mOR: 0.76, 95% CI: 0.60–0.97; among lower-grade, mOR:0.73, 95% CI:0.58–0.92).
Figure 2.
Site-specific and meta-analysis odds ratios and 95% confidence intervals from the Glioma International Case-Control Study (GICC) for the associations between history of asthma and glioma risk. Meta-analysis odds ratio was calculated using restricted maximum likelihood modelling.
Due to small numbers, we could not examine the potential joint effects of asthma and respiratory allergies using meta-regression. However, when the data were pooled, our results suggested that having both asthma and respiratory allergies together (compared to having neither) may potentially confer slightly greater protection than having only asthma or only respiratory allergies [not shown]. However, as the pooled analysis does not account for inter-site heterogeneity, those results may be due to statistical fluctuations in our data.
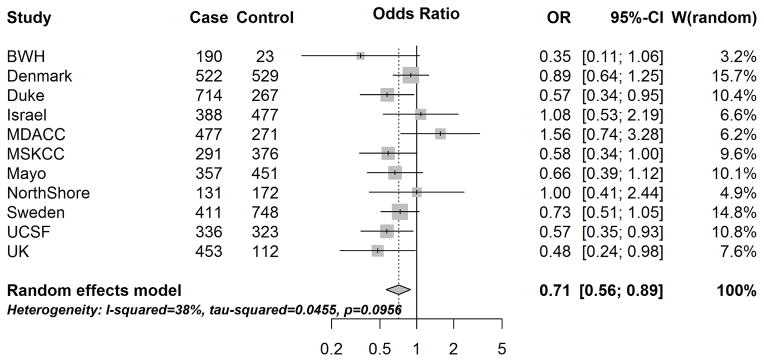
A history of eczema was significantly associated with a decreased glioma risk, adjusting for age and sex (mOR:0.71, 95% CI: 0.56–0.89) [Fig. 3]. The effect of eczema was similar stratified by tumor grade (among high-grade, mOR:0.70, 95% CI:0.52–0.95; among lower-grade, mOR:0.69, 95% CI:0.53–0.90).
Figure 3.
Site-specific and meta-analysis odds ratios and 95% confidence intervals from the Glioma International Case-Control Study (GICC) for the associations between history of eczema and glioma risk. Meta-analysis odds ratio was calculated using restricted maximum likelihood modelling.
The association between long-term antihistamine use and glioma risk was not statistically significant, adjusting for age, sex, and respiratory allergy status (mOR:0.87, 95% CI:0.71–1.07). Restricting to individuals with respiratory allergies did not meaningfully change the mOR between long-term antihistamine use and glioma risk (mOR:0.90, 95% CI:0.74–1.09). We could not reliably evaluate this association among those without respiratory allergies due to small numbers. No differences by treatment type were observed.
Discussion
To our knowledge, our study represents the largest study to date on the role of allergic conditions in glioma risk. Using data from our international consortium, we found that respiratory allergies, asthma, and eczema were all significantly associated with reduced glioma risk. Our results are concordant with three previously published meta-analyses (1, 2, 7), as well as another large international multi-site consortium study (12), all estimating a 20–40% lower risk of glioma associated with having allergic conditions. The mORs reported in our study for overall allergies and respiratory allergies fall within this range. Similarly, our effect estimates for asthma and eczema are also consistent with these and other smaller studies (14, 27, 28, 35). Based on the growing body of evidence in the literature, the scientific community may be approaching a consensus on the role of allergies in glioma risk (36, 37).
While our study confirms several previous reports of the protective effect of allergies against glioma (1, 2, 5, 7), our data suggest that respiratory allergies may largely be driving the association we observed between general self-reported allergy status and glioma risk. Prior studies that have examined specific allergy types have also described similar protective effects associated with respiratory allergy, allergic rhinitis, or hay fever (1, 3, 18, 27). For example, combining effect estimates from five different studies, Chen et al. provided a meta-analysis OR of 0.78 (95% CI: 0.70–0.87) for hay fever and glioma risk (1). A series of cohort studies have also suggested that hay fever/allergic rhinitis may be protective against glioma, although results from these studies did not reach statistical significance (likely due to small numbers of cases) (3). Additionally, a case-control study nested in the European Prospective Investigation into Cancer and Nutrition Cohort (EPIC) found an OR of 0.73 for having specific IgE against the eight most common respiratory allergens (using pre-diagnostic specimens), though this finding was not statistically significant (95% CI: 0.51–1.06) (15). Similarly, Wiemels et al. reported an odds ratio of 0.73 (95% CI: 0.56–0.96) associated with a positive history of at least one self-reported respiratory allergy (20). Their results also implied that elevated respiratory IgE may be protective against glioma (OR: 0.80, 95% CI: 0.60–1.06). Although the latter finding did not attain statistical significance, their overall findings on respiratory allergies were more robust than those on food allergies, leading the authors to conclude that future research should focus specifically on the effects of respiratory allergies.
Like other studies (12, 15, 21), our study found that the protective effect of respiratory allergies (and any allergies) was stronger among high-grade glioma cases than it was for lower-grade cases. However, we have a larger sample size of high-grade cases (n=2722 versus n=1664 lower-grade), and the mOR for respiratory allergies was borderline significant among lower-grade cases. Nevertheless, as other studies have also observed this pattern, future research should investigate why the effect of atopy may be more pronounced for high-grade gliomas.
Asthma and eczema were also found to be significantly protective against glioma risk in our study. Two major meta-analyses have estimated a 30% reduction in glioma risk for a positive history of either of these conditions (1, 2). Allergic rhinitis and asthma are both induced by inhaled allergens (30). Allergic rhinitis occurs when mucosal mast cells in the nasal epithelium are activated, whereas allergic asthma results from activation of the submucosal mast cells of the lower airways. Pathophysiologically, these two conditions both involve the respiratory tract, but asthma becomes characterized by chronic inflammation (even after the triggering allergen is no longer present). Eczema (or atopic dermatitis) is an allergic reaction in the skin, and like asthma, often involves persistent chronic inflammation. The fact that eczema demonstrates a similar inverse association with glioma as asthma and rhinitis may argue against that idea that reactions localized in the respiratory tract are more relevant to glioma etiology.
In our study, long-term antihistamine use was not significantly associated with glioma risk, adjusting for respiratory allergy status. The impact of antihistamine use is difficult to disentangle from that of allergies, as these factors are highly correlated, and few individuals without allergies use antihistamines regularly. Previously, McCarthy et al. reported an OR of 0.76 for the association between any oral antihistamine use versus none (95% CI: 0.59–0.99), but they did not adjust for allergy status and their result could, therefore, be confounded by the effect of allergies (17). In our prior studies, we have observed an increased risk for glioma associated with antihistamine use, particularly among individuals with allergic conditions (38–40); however, other studies have found either no association or a protective effect (18, 41). More detailed analyses on antihistamine use (accounting for frequency, duration, and type) are planned, and may help clarify this relationship.
As the literature approaches a consensus on the impact of allergies in glioma risk, future research can begin to shift focus to what the underlying biological mechanism behind this association may be, and eventually, whether this mechanism may be exploited to provide new avenues for immunotherapy or cancer prevention. One commonly proposed hypothesis on how allergies may confer protection against glioma revolves around the idea that allergies and other atopic conditions may represent a heightened state of immunosurveillance (2, 11, 17, 42). The presence of a hyperactive immune system may subsequently prohibit abnormal cell growth or proliferation, but the specific mechanism by which heightened immunosurveillance could help abate tumor growth remains unexplained. While the fact that allergies appear to reduce risk of some other cancers, such as pancreatic cancer, may lend credibility to this hypothesis, it is unclear why, then, allergies would increase risk for certain other cancers, such as bladder cancer (4, 6).
Another proposed explanation for the inverse association between allergies and glioma risk is that IgE antibodies against certain allergens may display some cross-reactivity to brain tumor antigens (16, 43). Most studies on IgE levels and antigen-specific IgE have isolated these antibodies from serum. Thus, information on whether serum IgE levels and subtypes reflect the IgE levels and subtypes in the brain would be useful in assessing the plausibility of this hypothesis.
Allergies (and IgE) are thought to have evolved as a defense against macro-parasite infestation, but it also has been argued that the Th2-IgE mediated allergic response has a key role in protecting against environmental toxins (i.e., irritants, venoms, and other harmful xenobiotics) (44–46). Therefore, another possible explanation for the protective effect of allergies against glioma involves the idea that individuals with stronger allergic responses are more successful at expelling and/or disarming environmental toxins or carcinogens over the course of their lives (16, 45). This hypothesis would be especially interesting if future analyses continue to find that respiratory allergies, specifically, are of key importance in decreasing glioma risk.
Epidemiologic studies are also still warranted to clarify other related topics. For example, what proportion of the protective effect of allergies against glioma risk is attributable specifically to respiratory allergies deems clarification. Although a few well-designed studies have examined respiratory allergen-specific IgE levels (15, 20), many additional studies are needed before the impact of respiratory versus other allergy types can truly be disentangled. Furthermore, innovative markers of respiratory-specific allergies may be needed, as circulating IgE has a serum half-life of about two days (47) and can be difficult to measure during the relevant etiologic time period (which itself remain unknown and should be examined in prospective studies).
There are also many questions remaining regarding the role of antihistamines. Future research should investigate the effects of different generations of antihistamines and should also separately examine H2 receptor antagonists (i.e., cimetidine), given that the brain has H1, H2, and H3 receptors (31). The joint effects of using antihistamines in conjunction with steroids, decongestants, inhalers, or nasal sprays also warrant elucidation.
Our study has some limitations inherent to multi-site consortia. There is a substantial amount of site-to-site heterogeneity between our 14 sites; thus, we have provided the site-specific effect estimates for key analyses. Due to differences in infrastructure, resources, and institutional policies, different types of controls and questionnaire administration methods had to be used across sites. However, we conducted a series of sensitivity analyses [detailed in (34)] to ensure that control type or questionnaire administration method did not discernably bias the results presented here. Nonetheless, we are unsure why the site-specific OR from UCSF demonstrates a significant adverse association between respiratory allergies and glioma here, as previous UCSF studies have reported inverse associations similar to those observed at our other sites (11, 20, 42).
A common limitation in retrospective studies of glioma is the use of proxy respondents for cases who have cognitive impairment. The proportion of proxy responses in our study is low (<10%), and exclusion of proxy responses did not meaningfully change the results of our analyses. Furthermore, Chen et al. has previously provided evidence that the associations observed in case-control studies of atopy and glioma are unlikely to be due to bias from proxy reporting (1). A related issue is the possibility that glioma cases may not remember past exposures accurately due to cognitive deficits. However, the associations reported here have also been found in prospective cohort studies, nested case-control studies, and meta-analyses of the existing literature (1–3, 7, 21).
A substantial amount of data on the inverse association between atopic conditions and glioma has accumulated (36, 37). In 2011, Davis and Al-Alem delineated the available evidence for each of Bradford-Hill’s causal criteria in their commentary, suggesting that the current knowledge on atopy and glioma supports a potentially causal underlying relationship (36). Two of the most important concerns about the atopy-glioma relationship relate to the temporality of reported allergies (or lack of allergies) relative to glioma development and the idea that glioma cases may not accurately remember having atopic conditions, thus under-reporting them compared to controls. However, given the series of different study designs and exposure assessment tactics used to evaluate these associations, there is now at least some evidence to allay both of these concerns. The findings from the GICC study support the existing evidence that the relationship between atopy and glioma is unlikely to be coincidental. Thus, future research should begin to focus on clarifying the biological mechanisms contributing to this long-observed inverse relationship.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants from the National Institutes of Health, Bethesda, Maryland: R01CA139020 (M.L. Bondy) and R01CA52689 (M.R. Wrensch). All authors received support from R01CA139020 (M.L. Bondy). Additional support was provided by the McNair Medical Institute and the Population Sciences Biorepository at Baylor College of Medicine (P30CA125123, M.E. Scheurer).
Abbreviations
- GICC
Glioma International Case-Control Study
- OR
odds ratio
- mOR
meta-analysis odds ratio
- BBB
blood-brain barrier
Footnotes
Notes: Written informed consent was obtained from each subject or from his or her guardian. Approval from local institutional review boards was received at each Gliogene participating institution. This study was conducted in accordance with the Declaration of Helsinki.
Conflicts of Interest: The authors declare no competing financial interests.
References
- 1.Chen C, Xu T, Chen J, Zhou J, Yan Y, Lu Y, et al. Allergy and risk of glioma: a meta-analysis. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2011;18:387–95. doi: 10.1111/j.1468-1331.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 2.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. Journal of the National Cancer Institute. 2007;99:1544–50. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzbaum J, Jonsson F, Ahlbom A, Preston-Martin S, Lonn S, Soderberg KC, et al. Cohort studies of association between self-reported allergic conditions, immune-related diagnoses and glioma and meningioma risk. International journal of cancer Journal international du cancer. 2003;106:423–8. doi: 10.1002/ijc.11230. [DOI] [PubMed] [Google Scholar]
- 4.Turner MC. Epidemiology: allergy history, IgE, and cancer. Cancer immunology, immunotherapy : CII. 2012;61:1493–510. doi: 10.1007/s00262-011-1180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner MC, Chen Y, Krewski D, Ghadirian P. An overview of the association between allergy and cancer. International journal of cancer Journal international du cancer. 2006;118:3124–32. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 6.Merrill RM, Isakson RT, Beck RE. The association between allergies and cancer: what is currently known? Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2007;99:102–16. doi: 10.1016/S1081-1206(10)60632-1. quiz 17–9, 50. [DOI] [PubMed] [Google Scholar]
- 7.Zhao H, Cai W, Su S, Zhi D, Lu J, Liu S. Allergic conditions reduce the risk of glioma: a meta-analysis based on 128,936 subjects. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2014;35:3875–80. doi: 10.1007/s13277-013-1514-4. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki K, Forsti A, Fallah M, Sundquist J, Sundquist K, Ji J. Risk of cancer in patients with medically diagnosed hay fever or allergic rhinitis. International journal of cancer Journal international du cancer. 2014;135:2397–403. doi: 10.1002/ijc.28873. [DOI] [PubMed] [Google Scholar]
- 9.Gousias K, Markou M, Arzoglou V, Voulgaris S, Vartholomatos G, Kostoula A, et al. Frequent abnormalities of the immune system in gliomas and correlation with the WHO grading system of malignancy. Journal of neuroimmunology. 2010;226:136–42. doi: 10.1016/j.jneuroim.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Berg-Beckhoff G, Schuz J, Blettner M, Munster E, Schlaefer K, Wahrendorf J, et al. History of allergic disease and epilepsy and risk of glioma and meningioma (INTERPHONE study group, Germany) European journal of epidemiology. 2009;24:433–40. doi: 10.1007/s10654-009-9355-6. [DOI] [PubMed] [Google Scholar]
- 11.Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, et al. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer research. 2004;64:8468–73. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 12.Turner MC, Krewski D, Armstrong BK, Chetrit A, Giles GG, Hours M, et al. Allergy and brain tumors in the INTERPHONE study: pooled results from Australia, Canada, France, Israel, and New Zealand. Cancer causes & control : CCC. 2013;24:949–60. doi: 10.1007/s10552-013-0171-7. [DOI] [PubMed] [Google Scholar]
- 13.Lachance DH, Yang P, Johnson DR, Decker PA, Kollmeyer TM, McCoy LS, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. American journal of epidemiology. 2011;174:574–81. doi: 10.1093/aje/kwr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamachari B, Il’yasova D, Scheurer ME, Bondy M, Zhou R, Wrensch M, et al. A pooled multisite analysis of the effects of atopic medical conditions in glioma risk in different ethnic groups. Annals of epidemiology. 2015;25:270–4. doi: 10.1016/j.annepidem.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlehofer B, Siegmund B, Linseisen J, Schuz J, Rohrmann S, Becker S, et al. Primary brain tumours and specific serum immunoglobulin E: a case-control study nested in the European Prospective Investigation into Cancer and Nutrition cohort. Allergy. 2011;66:1434–41. doi: 10.1111/j.1398-9995.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 16.Schwartzbaum J, Ding B, Johannesen TB, Osnes LT, Karavodin L, Ahlbom A, et al. Association between prediagnostic IgE levels and risk of glioma. Journal of the National Cancer Institute. 2012;104:1251–9. doi: 10.1093/jnci/djs315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy BJ, Rankin K, Il’yasova D, Erdal S, Vick N, Ali-Osman F, et al. Assessment of type of allergy and antihistamine use in the development of glioma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:370–8. doi: 10.1158/1055-9965.EPI-10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoemaker MJ, Swerdlow AJ, Hepworth SJ, McKinney PA, van Tongeren M, Muir KR. History of allergies and risk of glioma in adults. International journal of cancer Journal international du cancer. 2006;119:2165–72. doi: 10.1002/ijc.22091. [DOI] [PubMed] [Google Scholar]
- 19.Il’yasova D, McCarthy B, Marcello J, Schildkraut JM, Moorman PG, Krishnamachari B, et al. Association between glioma and history of allergies, asthma, and eczema: a case-control study with three groups of controls. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1232–8. doi: 10.1158/1055-9965.EPI-08-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiemels JL, Wilson D, Patil C, Patoka J, McCoy L, Rice T, et al. IgE, allergy, and risk of glioma: update from the San Francisco Bay Area Adult Glioma Study in the temozolomide era. Int J Cancer. 2009;125:680–7. doi: 10.1002/ijc.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calboli FC, Cox DG, Buring JE, Gaziano JM, Ma J, Stampfer M, et al. Prediagnostic plasma IgE levels and risk of adult glioma in four prospective cohort studies. Journal of the National Cancer Institute. 2011;103:1588–95. doi: 10.1093/jnci/djr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou M, Wiemels JL, Bracci PM, Wrensch MR, McCoy LS, Rice T, et al. Circulating levels of the innate and humoral immune regulators CD14 and CD23 are associated with adult glioma. Cancer research. 2010;70:7534–42. doi: 10.1158/0008-5472.CAN-10-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amirian E, Liu Y, Scheurer ME, El-Zein R, Gilbert MR, Bondy ML. Genetic variants in inflammation pathway genes and asthma in glioma susceptibility. Neuro-oncology. 2010;12:444–52. doi: 10.1093/neuonc/nop057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Backes DM, Siddiq A, Cox DG, Calboli FC, Gaziano JM, Ma J, et al. Single-nucleotide polymorphisms of allergy-related genes and risk of adult glioma. Journal of neuro-oncology. 2013;113:229–38. doi: 10.1007/s11060-013-1122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoemaker MJ, Robertson L, Wigertz A, Jones ME, Hosking FJ, Feychting M, et al. Interaction between 5 genetic variants and allergy in glioma risk. American journal of epidemiology. 2010;171:1165–73. doi: 10.1093/aje/kwq075. [DOI] [PubMed] [Google Scholar]
- 26.Wiemels JL, Wiencke JK, Kelsey KT, Moghadassi M, Rice T, Urayama KY, et al. Allergy-related polymorphisms influence glioma status and serum IgE levels. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1229–35. doi: 10.1158/1055-9965.EPI-07-0041. [DOI] [PubMed] [Google Scholar]
- 27.Wigertz A, Lonn S, Schwartzbaum J, Hall P, Auvinen A, Christensen HC, et al. Allergic conditions and brain tumor risk. American journal of epidemiology. 2007;166:941–50. doi: 10.1093/aje/kwm203. [DOI] [PubMed] [Google Scholar]
- 28.Brenner AV, Linet MS, Fine HA, Shapiro WR, Selker RG, Black PM, et al. History of allergies and autoimmune diseases and risk of brain tumors in adults. International journal of cancer Journal international du cancer. 2002;99:252–9. doi: 10.1002/ijc.10320. [DOI] [PubMed] [Google Scholar]
- 29.Schwartzbaum J, Ahlbom A, Malmer B, Lonn S, Brookes AJ, Doss H, et al. Polymorphisms associated with asthma are inversely related to glioblastoma multiforme. Cancer research. 2005;65:6459–65. doi: 10.1158/0008-5472.CAN-04-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janeway CA, Travers P, Walport M. Immunobiology: The Immune System in Health and Disease. 5. New York: Garland Science; 2001. [Google Scholar]
- 31.Montoro J, Sastre J, Bartra J, del Cuvillo A, Davila I, Jauregui I, et al. Effect of H1 antihistamines upon the central nervous system. Journal of investigational allergology & clinical immunology. 2006;16(Suppl 1):24–8. [PubMed] [Google Scholar]
- 32.Tonelli LH, Postolache TT. Airborne inflammatory factors: “from the nose to the brain”. Frontiers in bioscience. 2010;2:135–52. doi: 10.2741/s52. [DOI] [PubMed] [Google Scholar]
- 33.Djupesland PG, Mahmoud RA, Messina JC. Accessing the brain: the nose may know the way. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:793–4. doi: 10.1038/jcbfm.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amirian ES, Armstrong GN, Zhou R, Lau CC, Claus EB, Barnholtz-Sloan JS, et al. The Gliogene Consortium: A Report from the Glioma International Case-Control Study (GICC) American journal of epidemiology. 2015 In press. [Google Scholar]
- 35.Deckert S, Kopkow C, Schmitt J. Nonallergic comorbidities of atopic eczema: an overview of systematic reviews. Allergy. 2014;69:37–45. doi: 10.1111/all.12246. [DOI] [PubMed] [Google Scholar]
- 36.Davis FG, Al-Alem U. Allergies and adult gliomas: cohort results strengthen evidence for a causal association. Journal of the National Cancer Institute. 2011;103:1562–3. doi: 10.1093/jnci/djr397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin M. Research reinforces potential allergies-glioma connection. Journal of the National Cancer Institute. 2012;104:353–6. doi: 10.1093/jnci/djs153. [DOI] [PubMed] [Google Scholar]
- 38.Scheurer ME, Amirian ES, Davlin SL, Rice T, Wrensch M, Bondy ML. Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. International journal of cancer Journal international du cancer. 2011;129:2290–6. doi: 10.1002/ijc.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheurer ME, El-Zein R, Thompson PA, Aldape KD, Levin VA, Gilbert MR, et al. Long-term anti-inflammatory and antihistamine medication use and adult glioma risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:1277–81. doi: 10.1158/1055-9965.EPI-07-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amirian ES, Marquez-Do D, Bondy ML, Scheurer ME. Antihistamine use and immunoglobulin E levels in glioma risk and prognosis. Cancer epidemiology. 2013;37:908–12. doi: 10.1016/j.canep.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlehofer B, Blettner M, Preston-Martin S, Niehoff D, Wahrendorf J, Arslan A, et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. International journal of cancer Journal international du cancer. 1999;82:155–60. doi: 10.1002/(sici)1097-0215(19990719)82:2<155::aid-ijc1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 42.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. International journal of cancer Journal international du cancer. 2002;98:609–15. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 43.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–66. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman PW, Holland E, Sherman JS. Allergies: their role in cancer prevention. The Quarterly review of biology. 2008;83:339–62. doi: 10.1086/592850. [DOI] [PubMed] [Google Scholar]
- 46.Profet M. The function of allergy: immunological defense against toxins. The Quarterly review of biology. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 47.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. The Journal of allergy and clinical immunology. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.