Abstract
Class I Human Leukocyte Antigens (HLA) mark infected cells for immune targeting by presenting pathogen encoded peptides on the cell surface. Characterization of viral peptides unique to infected cells is important for understanding CD8+ T cell responses and for the development of T cell-based immunotherapies. Having previously reported a series of West Nile virus (WNV) epitopes that are naturally presented by HLA-A*02:01, here we generated T cell receptor mimic monoclonal antibodies (TCRm mAbs) to three of these peptide/HLA complexes - the immunodominant SVG9 (E protein), the subdominant SLF9 (NS4B protein), and the immunorecessive YTM9 (NS3 protein) - and used these TCRm mAbs to stain WNV infected cell lines and primary antigen presenting cells. TCRm staining of WNV infected cells demonstrated that the immunorecessive YTM9 appeared several hours earlier and at 5 to 10 fold greater density than the more immunogenic SLF9 and SVG9 ligands. Moreover, staining following inhibition of the transporter associated with antigen processing (TAP) demonstrated that all three viral ligands were presented in a TAP-dependent manner despite originating from different cellular compartments. This study represents the first use of TCRm mAbs to define the kinetics and magnitude of HLA presentation for a series of epitopes encoded by one virus, and the results depict a pattern whereby individual epitopes differ considerably in abundance and availability. The observation that immunodominant ligands can be found at lower levels and at later time points post-infection suggests that a reevaluation of the factors that combine to shape T cell reactivity may be warranted.
Introduction
Class I human leukocyte antigens alert the cellular immune system by presenting peptides derived from viral proteins on the surface of infected cells (1). The presentation of peptides by class I HLA then enables CD8+ T lymphocytes to target infected cells, clear infection, and prevent viral persistence (2–4). Although empiric and in silico approaches have identified a large number of T cell epitopes in the context of virus infections, our understanding of the dynamics of epitope presentation on the cell surface and the factors that shape T cell response continues to evolve. Furthermore, as virus-encoded class I HLA peptide epitopes represent possible targets for immunotherapies directed against virus infected cells (5), a better understanding of viral epitope presentation will be of interest from both a basic immunologic and translational perspective.
A considerable number of factors are poised to influence immune responses to a virus. Within the infected cell, the rate of viral protein translation and degradation (protein turnover), the activity of proteolytic mechanisms, the positioning of molecular chaperones, and HLA binding stability all contribute to the selection of viral peptides for class I HLA presentation (6, 7). Once a viral peptide has been extracted from its source antigen, formed a complex with class I HLA, and transited to the cell surface, another wave of factors influence T cell reactivity to these HLA/peptide complexes. The copy number of HLA/viral-peptide complexes that reach the cell surface, the timing post-infection that these peptides are presented to T cells, and the frequency of naïve T cell precursors that emerge from thymic selection have all been found to contribute to immune recognition (7, 8). Therefore, an abundance of intra and extracellular factors contribute to the availability and immune recognition of HLA/viral-peptide complexes.
Two pivotal factors that are clearly important for the T cell recognition of HLA/viral peptide complexes are the timing and level of antigen presentation on the surface of infected cells. In spite of the fact that MHC class I ligand immunogenicity has been studied in great detail, no clear consensus has been reached as to how the timing and levels of antigen availability impact T cell reactivity. For instance, increased levels of MHC/epitope expression greatly influence the T cell recognition of influenza, vaccinia and L. monocytogenes epitopes in, but for EBV the most immunogenic peptide has been reported as the least abundant one (8–11). Timing of antigen presentation post-infection is another factor that influences the immunogenicity of MHC class I ligands, and the data available here is surprisingly sparse. Studies using HIV-1, CMV, HCV and influenza have shown that epitopes that are presented early after infection facilitate the elimination of the infected cells and thus play a protective role in control of viral infection. In other words the earlier an epitope is presented the greater the chance it evokes an immunodominant T cell response in infected subjects (8, 12–14). Thus, at present it is difficult to definitively say how the kinetics and magnitude of epitope presentation influence anti-viral T cell immunogenicity.
When considering how timing and levels of epitope availability influence immunogenicity, one must acknowledge the considerable heterogeneity that exists among the experimental approaches used to study these factors. For example, a variety of means have been used to study the kinetics of presentation of viral epitope/MHC complexes on the surface of infected cells: Some experiments use epitope-specific T cell clones to quantitate the number of HLA/peptide complexes on the cell surface (11, 12), other studies use the 25-D1.16 monoclonal antibody (mAb) to track the MHC presentation of the ovalbumin T cell epitope (SIINFEKL) following its incorporation into recombinant viruses (9, 15), and yet other studies use biochemical methods to track epitope abundance and timing. In addition, these varied methods have been applied to a number of both acute and chronic viruses, in humans and murine models, with various MHC class I molecules that are expressed at different levels. Not only is it unclear how epitope abundance and timing influence immunogenicity, but resolution of this topic is further complicated by inter-study variabilities.
One direct approach that has shed considerable light on the mechanisms underlying processing and presentation of the viral epitopes is to track the presentation of the SIINFEKL ligand via cell surface staining with 25-D1.16 mAb. Recognizing that mAbs are powerful tools for measuring the expression of MHC/peptide complexes at the plasma membrane, additional studies have built upon these 25-D1.16 results by generating mAbs to single MHC/viral peptide epitopes. These mAbs that mimic the specificity of a T cell receptor (TCR), have been applied to different pathogens including HBV, CMV, HIV, HTLV-1 (16–19), allowing researchers to directly visualize and quantify the presentation of individual viral epitopes. Our group also has recently reported a TCRm mAb against an immunodominant WNV epitope presented by mouse class I MHC (20). Here we generated the first panel of mAbs to a series of ligands encoded by a single virus and used these mAbs to determine how viral ligands with well-characterized patterns of T cell immunogenicity compare in their timing and level of presentation. In the development and testing of mAbs to WNV E-SVG9/HLA-A*02:01, NS4B-SLF9/HLA-A*0201, and NS3-YTM9/HLA-A*02:01, we were able to explore differences in the kinetics and magnitudes of viral epitope presentation in WNV-infected cells. An important discussion point arising from these data is how immunodominance might be uncoupled from the timing and abundance of epitopes on the surface of the infected cell.
Materials and Methods
Cells, antibodies and viruses
The human lymphoblastoid cell line T2 (endogenous HLA-A*02:01), HeLa, Vero E6, C6/36, and myeloma cell line P3X63.Ag8.653 (used as the fusion partner in generating hybridomas) were purchased from the American Type Culture Collection (ATCC). HeLa and Vero cells were cultured according to ATCC instructions in DMEM F12K (Wisent, Inc.), 10% FBS (Serum Source International), and 1% penicillin/streptomycin (Invitrogen). T2 Cells were grown in RPMI 1640 supplemented with 10% FBS and the C6/36 Aedes albopictus cells were cultured in RPMI 1640 with 10% fetal bovine serum (FBS), 1% non-essential amino acids (NEAA), 1% L-glutamine, and 1% sodium pyruvate and 1% penicillin/streptomycin (Invitrogen). All the cells were maintained at 37°C in 5% CO2 incubator except for C6/36 cells, which were maintained at 28°C. The hybridoma cell line BB7.2 that produces the BB7.2 mAb (specific for the α2 domain of properly folded HLA-A2) was purchased from the ATCC. The murine IgG2a and IgG1 isotype control Abs were purchased from Sigma-Aldrich. E24 mAb (specific for the WNV envelope) was provided by Dr. Michael Diamond (Washington University, St. Louis, MO) and secondary Ab “R-PE-AffiniPure F(ab′)2 Fragment Goat Anti-Mouse IgG, F(ab′)2 Fragment Specific” was purchased from Jackson ImmunoResearch Laboratories. WNV strain New York 1999 was propagated in Vero E6 and/or C6/36 cells. After infection, culture supernatant was harvested, cleared of cell debris by centrifugation at 1,300 × g for 10 min at 4°C and stored aliquoted at −80°C (21).
Generation of HLA-A*02:01/peptide complexes
WNV peptides, SVG9, SLF9 and YTM9 were folded with monomeric HLA-A*02:01 molecules having a C-terminal BirA biotinylation recognition site (22). Refolded peptide/HLA-A2 monomers were biotinylated using the biotin ligase enzyme (Avidity) and free biotin was removed by size exclusion chromatography (Superdex 75 size exclusion column). Biotinylated peptide/HLA-A*02:01 complexes were mixed with streptavidin at a molar ratio of 4 to 1 to produce tetramers. At each step, protein concentration was determined by BCA protein assay (Pierce, Rockford, IL). Monomers were used for antibody affinity measurements (surface plasmon resonance (SPR)) and tetramers were used for mice immunization.
Generation and purification of RL14C, RL15A and RL26A TCRm mAbs
Female BALB/c mice were immunized with a solution of 50 μg of purified peptide/HLA-A*02:01 complexes and Quil-A adjuvant (Sigma) at 15 day intervals as described (23, 24). One week after the third immunization, serum samples were evaluated for polyclonal TCRm mAb responses. Mice that had responses showing a signal to noise ratio of > 2 fold in absorbance reading and a titer of > 1/600 were selected for hybridoma generation. Hybridomas were produced by fusing splenocytes with the P3X63.Ag8.653 myeloma cell line using the clonacell-HY Kit (Stem Cell Technologies). After two weeks in semi-solid medium, single clones were picked, transferred to 96-well tissue culture plates and grown for 3–4 days. Hybridoma supernatants were screened for specific mAb production by ELISA and cell-based flow cytometric assays. Hybridoma lines were cultured in Hybridoma-SFM (Serum Free Media) (Gibco) and the RL14C (anti SLF9/HLA-A2), RL15A (anti SVG9/HLA-A2) and RL26A (anti YTM9/HLA-A2) TCRm mAbs were purified from cell supernatants by affinity chromatography with protein A Sepharose (Amersham-GE, Boston, MA).
Specificity and detection sensitivity of RL14C, RL15A and RL26A TCRm mAbs
The T2 cell line, derived from the Epstein-Barr virus transformed B lymphoblastoid line has a large chromosomal deletion in the MHC class II region including genes for transporters associated with antigen presentation (TAPl/TAP2) as well as genes encoding proteasome subunits (LMP2/LMP7). Therefore, T2 that present only HLA-A*02:01 are defective in the presentation of endogenously synthesized antigens (25–27). Incubating these cells with peptides with high affinity for HLA-A*02:01 stabilizes the peptide/HLA complex on the cell surface and results in an increase in staining with the HLA-A*02:01-specific mAb BB7.2. In order to determine the specificity of each TCRm mAb toward their cognate peptide epitopes, T2 cells (5×105) were incubated overnight in RPMI 1640 medium with the specific peptides: SLF9, SVG9 and YTM9 as well as 20 irrelevant HLA-A*02:01 restricted peptides. The cells were washed to remove unbound peptide and stained with either BB7.2 antibody (1 μg/sample) to detect the level of HLA-A*02:01 molecules present on the surface (positive control), or RL14C, RL15A and RL26A TCRm mAbs (1 μg/sample). PE-conjugated goat anti-mouse IgG, F(ab′)2 fragment specific was used as the secondary antibody. This antibody reacts with light chain of primary antibodies independently of their subclass.
To determine the detection sensitivity of the TCRm mAbs, the experiments were performed with T2 cells pulsed with various concentrations of the specific peptide (1 – 20,000 nM) and stained with each TCRm mAb directly conjugated to PE. The fluorescence labeling was done using R-Phycoerythrin Conjugation Kit (Abcam) and the volume and concentration of each TCRm mAb was adjusted so that a PE:mAb ratio of 1:1 was reached. In order to quantitate the number of PE-labeled TCRm mAbs bound per cell at different peptide concentrations, PE fluorescence quantitation kit (QuantiBRITETM PE, BD Biosciences) was used on the same instrument with the same setting as the cellular assays and the number of PE molecules per cell was calculated as previously described by Pannu et al. (28) and following manufacturer’s instruction.
Surface Plasmon Resonance (SPR)
The interaction of each TCRm mAb, RL14C, RL15A, RL26A was tested against all three antigens (SLF9/HLA-A2, SVG9/HLA-A2 and YTM9/HLA-A2) using SensiQ Pioneer SPR instrument (SensiQ Technologies, Inc., Oklahoma City, OK). The assay was done by capturing two antibodies at a time, one on channel 1, and one on channel 3, and passing each antigen over all three channels, with channel 2 left empty, as a reference. All analyte injections were performed in duplicate. Briefly, a solution of Protein G was amine coupled on the three channels of a COOH2 sensor chip until ~300 RU (Response Unit) of protein was immobilized. After immobilization of the Protein G, injections were optimized for binding of the RL14C, RL15A and RL26A TCRm mAbs to protein G, binding of antigens (SLF9/HLA-A2, SVG9/HLA-A2 and YTM9/HLA-A2 monomers) to the captured antibodies, and regeneration of the Protein G surface. For each antigen injection cycle, between 250 and 500 RU of antibody were captured prior to the antigen injection. Antigens were injected using the OneStep gradient method (29, 30) followed by a dissociation period of four minutes and protein G was regenerated using 20 mM K3PO4 pH 12. Buffer used for this assay was 10 mM Hepes, pH 7.4, 150 mM NaCl, 0.005% Tween-20.
Staining of peptide pulsed cells
HeLa cells, transfected with full length HLA-A*02:01 (referred to as HeLa-A2) were seeded at 1×106 cells/well in 6-well plates in complete growth medium. The cells were washed with DMEM + 9% ITS cell culture supplement, containing recombinant human insulin, human transferrin, and sodium selenite (Wisent, Inc.) and pulsed with 20 μM of SLF9, SVG9 or YTM9 peptides overnight in the same medium. Cells then were washed to remove unbound peptides, trypsinized, resuspended in cell staining buffer (CSB) (PBS/1% FCS, 0.09% sodium azide, BioLegend) and then stained with 1 μg of RL14C, RL15A and RL26A TCRm mAb and BB7.2 in 100 μl of CSB for 30 min at 4°C. Cells were washed with 1 ml CSB, centrifuged at 300 × g for 10 min and the pellets were resuspended in 100 μl of CSB containing 1 μg of PE labeled goat anti-mouse IgG, F(ab′)2 fragment specific secondary antibody. After incubation at 4°C in dark for 30 min, cells were washed, resuspended in 0.5 ml PBS, analyzed on a FACScalibur instrument (BD Biosciences) and the results were evaluated using Cell Quest software (BD Biosciences).
Staining of the WNV infected cell lines
HeLa-A2 cells were seeded at 2×105 cells/well in 6-well plates in DMEM + 9% ITS. The cells were incubated with WNV at a multiplicity of infection (MOI) of 3. After 24 h the cells were washed twice with CSB and incubated with the RL14C, RL15A, RL26A TCRm mAbs and BB7.2 for 30 min on ice. Cells were washed and incubated with the secondary antibody (PE labeled goat anti-mouse IgG, F(ab′)2 fragment specific) for 30 min on ice in the dark. Prior to flow cytometry, cells were fixed with 2% PFA. To confirm infection, in all experiments aliquots of fixed and permeabilized cells were stained with anti-WNV E protein mAb, E24 (31). To study the timing and comparative level of peptide presentation cells were infected at MOI of 3 and stained with the TCRm mAbs at various time points (10 –25 h) post-infection.
Staining with BB7.2 represents the expression of the HLA-A*02:01 molecules on the cell surface and staining with each TCRm mAb indicates presentation of the specific viral epitope by HLA-A*02:01. In order to calculate the fraction of total surface HLA-A*02:01 molecules presenting each viral epitope (SLF9, SVG9 and YTM9), the MFI of staining with each TCRm mAb was divided by the MFI of BB7.2 mAb at different time points after infection. In all experiments the MFI of staining of uninfected cells with each TCRm mAb was subtracted as background.
Generation and staining of the WNV-infected dendritic cells
Primary human dendritic cells were derived from HLA-A*02:01 positive PBMC (kindly provided by Dr. Beatriz Carreno, Washington University in St. Louis). Briefly, peripheral blood mononuclear cells (PBMC) suspended in RPMI 1640 with 1% human pooled plasma at 5 × 106 cells/mL were dispersed into T175 culture flasks. After a 2 h culture at 37°C in 5% CO2, non-adherent cells were removed by two washes with PBS. Adherent cells were cultured in RPMI 1640 with 1% human pooled plasma, 10 mM HEPES/L-glutamine/ penicillin /streptomycin supplemented with GM-CSF (100 ng/ml, Bayer HC Pharmaceuticals) and IL-4 (20 ng/ml, CellGenix). Fresh medium containing GM-CSF and IL-4 was added every 3 days. On day 6 of culture, DCs were harvested, washed twice in serum free medium and analyzed by flow cytometry. DCs were stained with fluorescence isothiocyanate (FITC)-anti-HLA-A2, phycoerythrin-(PE)-anti-CD11c and allophycocyanin (APC)- anti-CD80, -CD86, -or -CD83. All staining antibodies were obtained from Invitrogen. Cells were analyzed in a FACScalibur flow cytometer using Flow-Jo software V9.6.2 (Supplementary Fig. 1) (32). On day 7, MoDCs were plated at 1×106 cells/well in a 6-well plate. The following day the cells were infected with WNV at an MOI of 3. After 48 h, cells were detached with EDTA and stained with RL14C, RL15A and RL26A TCRm mAbs, BB7.2 and E24 (2 μg/sample). Cells were washed and incubated with the PE labeled goat anti-mouse IgG, F(ab′)2 fragment specific secondary antibody. Prior to flow cytometry, cells were fixed with 2% PFA and read on a FACScalibur machine.
TAP-dependent processing and presentation of WNV T cell epitopes
To test the TAP dependence of SLF9, SVG9 and YTM9 antigen presentation, HeLa-A2 cells were transduced with the HCMV TAP inhibitor protein ICP47. The cells were infected with WNV as described above (MOI of 3 for 48 h) and presentation of the WNV peptides were compared with HeLa-A2 cells that were mock transduced. Stable cell lines expressing TAP inhibitor protein, ICP47, were generated by retroviral transduction of HeLa-A2 cells. For this purpose pMIP.ICP47-myc was constructed by subcloning the coding sequence of ICP47-myc from pcDNA.ICP47-myc (kindly provided by Dr. Steve Jennings, Louisiana State Univ.) into the retroviral expression vector pMSCV.IRES.puro (pMIP). As an internal control for TAP function, hCG-β peptide (GVLPALPQV derived from human chorionic gonadotropin-β), that binds HLA-A2, was expressed in the cytosol by transducing HeLa-A2 cells with pMIN.HA-Ub-hCG expression vector. pMIN.HA-Ub-hCG was generated by fusing a HA-tagged ubiquitin coding sequence in frame to the N terminus of the hCG-β peptide coding sequence and then subcloning it into the retroviral expression vector pMSCV.IRES.neo (pMIN). TCRm mAb, RL4D (33) was used to detect the expression of hCG-β peptide/HLA-A2 on the cell surface.
To produce retrovirus, human embryonic kidney cells (HEK 293T) were transfected transiently with pMIP.ICP47-myc and/or pMIN.HA-Ub-hCG using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer’s instruction. HeLa-A2 cells were transduced with culture supernatant from HEK 293T cells containing retrovirus that expresses the ICP47 and/or the hCG-β peptide, and selected in the culture containing appropriate antibiotics post-transduction.
Statistical analysis
Statistical significance was determined by one-way ANOVA followed by Tukey’s method. A P value of less than 0.05 was considered significant. Statistical analysis was performed using GraphPad Prism 5.0 software (GraphPad Software, Inc.)
Results
Generation of RL14C, RL15A and RL26A TCRm mAbs
Previously, we reported identification of six WNV-encoded, HLA-A*02:01 restricted peptides eluted from the HLA class I of WNV infected cells using comparative mass spectrometry (34). When these viral epitopes were tested in an IFN-γ ELISPOT assay with PBMCs isolated from WNV infected HLA-A*02:01 individuals, they responded strongly to the peptide presented from the WNV envelope (SVG9) and moderately to the peptide derived from the virus NS4B (SLF9). The other HLA-A2/viral ligands including YTM9 from NS3 demonstrated little to no T cell reactivity above the healthy donors (34). To better understand how these viral ligands with different T cell immunogenicity compare in their timing and level of presentation, here we generated the RL14C, RL15A, and RL26A TCRm mAbs by immunizing mice with recombinant HLA-A*02:01 tetramers loaded with the WNV peptides SLF9, SVG9, and YTM9 respectively. The soluble heavy chains of HLA-A*02:01 and the β2-microglobulin (β2m) were produced in E. coli in the form of inclusion bodies, which were then purified and refolded with the identified WNV peptides. Refolded A*02:01/peptide complexes were used as immunogens and as positive controls for screening of hybridoma supernatants. After immunization and fusion, 3,000 to 5,000 clones for each TCRm mAb were screened and the hybridomas that specifically recognized HLA-A*02:01 loaded with cognate peptide were subcloned at least once by limiting dilution and isotyped. RL14C was IgG1 whereas RL15A and RL26A both were IgG2a. All three TCRm mAbs had kappa light chain. To establish that the TCRm mAbs were HLA-A2 restricted and peptide-specific a series of experiments were performed to characterize their binding specificity, detection sensitivity and affinity.
TCRm mAbs demonstrate high specificity and detection sensitivity for their cognate peptide/HLA-A*02:01 complexes
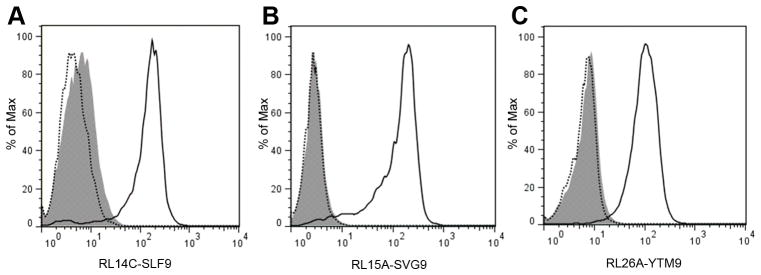
Two critical parameters for selecting a TCRm mAb for further characterization are the specificity and sensitivity of recognition of the cognate peptide/HLA complex. To test the specificity of RL14C, RL15A and RL26A TCRm mAbs, these antibodies were tested against a series of self-derived and viral peptides including other identified WNV peptides. T2 cells pulsed with 20 μM of the various peptides (Supplementary Table 1) were stained with RL14C, RL15A, or RL26A. For all three TCRm mAbs a significant shift in MFI was observed only when T2 cells were pulsed with the specific immunizing peptide. No shift in binding was observed with irrelevant viral or self-peptides (Fig. 1A–C). Thus, TCRm mAbs recognize specific peptide/HLA complex on the surface of T2 cells and do not cross-react with HLA-A*02:01 loaded with other irrelevant peptides or identified WNV peptides.
FIGURE 1.
Specificity, detection sensitivity and affinity of TCRm mAbs. (Upper panels) T2 cells were pulsed with 20 μM of irrelevant viral or cancer peptides and the specific peptide overnight. The cells were washed to remove unbound peptide and stained with RL14C (A), RL15A (B), or RL26A (C) followed by PE-conjugated Goat Anti-Mouse IgG, F(ab′)2 Fragment Specific antibody. All three TCRm mAbs showed a significant shift in the mean florescence intensity (MFI) only with the T2 cells pulsed with specific peptide and not with irrelevant viral or cancer peptides. Data are representative of three independent experiments. (Middle panels) T2 cells were pulsed with varying concentrations of the specific peptide (1 – 20,000 nM) and stained with PE-labeled RL14C (D), RL15A (E), and RL26A (F). With all three TCRm mAbs, MFI showed a dose dependent correlation with the amount of pulsed peptide which is displayed by a clear separation in the overlaid histograms. In all three panels solid lines indicate staining of the cells pulsed with varying concentrations of the specific peptide and shaded histograms indicate staining of the unpulsed cells. The number of specific peptide/HLA complexes corresponding to each peptide concentration was calculated using PE Fluorescence Quantitation Kit (QuantiBRITETM PE) and is presented in the accompanying tables. (Lower panels) The interaction of the RL14C (G), RL15A (H), and RL26A (I) with their respective binding antigens (SLF9/HLA-A2, SVG9/HLA-A2 and YTM9/HLA-A2) was characterized using SensiQ Pioneer FE SPR platform. All three TCRm mAbs showed high affinity (nanomolar level) toward their specific antigen. Data shown in the middle and lower panels are representative of two independent experiments.
To evaluate the detection sensitivity of RL14C, RL15A, and RL26A as it pertains to the density of peptide/HLA complexes on the cell surface, T2 cells were incubated with increasing concentrations of SLF9, SVG9 and YTM9 (1 – 20,000 nM) and stained with PE-labeled TCRm mAbs. With all three TCRm mAbs, the mean florescence intensity (MFI) showed a dose-dependent correlation with the amount of pulsed peptide (Fig. 1D–F). To quantify the number of specific peptide/HLA complexes on the cell surface at each peptide concentration, standard beads with known number of PE molecules were used. When pulsed with 1nM of the peptide, RL14C, RL15A, and RL26A were able to detect 73, 132 and 112 copies of their cognate peptide/HLA complex on the cell surface, respectively (Fig. 1D–F). Staining of T2 cells by TCRm mAbs therefore corresponds to the density of peptide/HLA-A*02:01 complexes present on the cell surface with a detection sensitivity of approximately 100 copies per cell.
Surface plasmon resonance
SPR was used to measure the binding affinity (KD) of TCRm mAbs to cognate peptide/HLA-A2 complexes. Affinity (KD) of an antibody is defined as the ratio of the antibody dissociation rate constant (kd), how quickly it dissociates from its antigen, to the antibody association rate constant (ka), how quickly it binds to its antigen. All three TCRm mAbs bound with high affinity (nanomolar level) to their cognate peptide/HLA-A2 complex. RL14C (KD: 22.9×10−9M) had the fastest association and dissociation rate constants, RL15A (KD: 67.7×10−9M) demonstrated the slowest association rate constant, and RL26A (KD: 9.71×10−9M) displayed the slowest dissociation rate constant (Fig. 1G–I). When the affinity of the TCRm mAbs was tested against the nonspecific antigens, RL14C and RL15A showed no affinity towards the nonspecific monomeric antigens. However, RL26A showed very weak binding, with 2680 and 1340 fold less affinity, to SLF9/HLA-A2 and SVG9/HLA-A2, respectively (data not shown). When a large number of antigens are screened against an antibody, there is a high probability that a cross-reacting antigen, which can be structurally related (our case) or unrelated to the immunogen used to raise the antibody, with similar or even higher affinity for the antibody, is found. This phenomenon that is called heterospecificity originates in that the antigens bind to different residues of the binding region (35). Therefore, Berzofsky and Schechter introduced the term selectivity instead, which is defined as the ratio of affinities of an antibody for binding to two different molecules. A selectivity of > 103 is considered to be highly specific (36). In the case of RL26A, the immunogens used to raise the antibodies share the HLA-A2 component (approximately 46 kDa compared to peptide which is about 1 kDa) and so they have 98% structural similarity. Therefore the weak binding of RL26A to other monomers was not surprising. However, RL26A is highly specific as it has >1000 times higher affinity towards YTM9/HLA-A2 than HLA-A2 loaded with other peptides (36).
These data establish that TCRm mAbs possess favorable binding characteristics for cognate peptide/HLA-A2 complex supporting their use for detection and characterization of naturally processed WNV epitopes presented on the surface of infected cells.
Specific recognition of viral peptide/HLA-A*02:01 complexes on pulsed cells
TCRm mAbs were next tested for their recognition of peptide/HLA-A2 complexes using the HeLa cell line expressing surface HLA-A*02:01. When HeLa-A2 cells were pulsed with specific and irrelevant peptides, each TCRm mAb stained only cells expressing the specific peptide/HLA-A*02:01 complex on their surface. HeLa cells that have not been peptide pulsed, although expressing considerable levels of endogenous peptide loaded A*02:01, were not stained with the TCRm mAbs (Fig. 2A–C). Once again, these data confirm that TCRm mAbs exclusively recognize their cognate peptide/HLA complex.
FIGURE 2.
TCRm mAbs recognize specific peptide on peptide-pulsed HeLa cells expressing surface HLA-A2. (A) Staining of the SLF9 pulsed HeLa-A2 FL cells with RL14C. (B) Staining of the SVG9 pulsed HeLa-A2 FL cells with RL15A. (C) Staining of the YTM9 pulsed HeLa-A2 FL cells with RL26A. In all three panels solid lines indicate staining of the cells pulsed with the specific peptide. Dotted lines indicate staining of the cells pulsed with the irrelevant peptide and, shaded histograms indicate unpulsed cells. Data are representative of three independent experiments.
Detection of endogenously presented viral epitopes on WNV infected cells
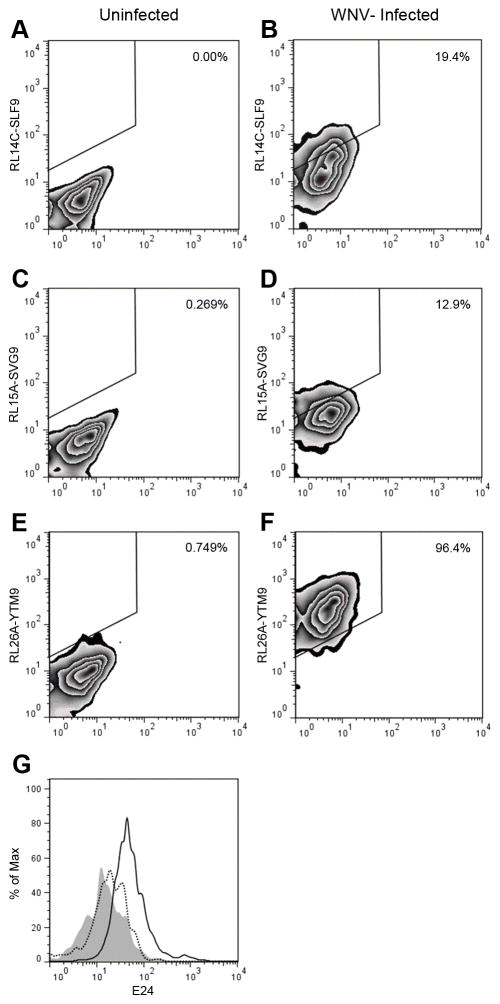
To test the ability of the TCRm mAbs to detect the viral epitopes in a natural setting, WNV-infected cells were stained with RL14C, RL15A and RL26A (Fig. 3). Initially, HeLa-A2 cells were infected with WNV at MOI of 3 for 24 hrs. To confirm that the cells were infected with WNV and track the surface expression of HLA-A*02:01, the virus infected HeLa-A2 cells were stained with the anti-WNV E protein mAb, E24 and anti HLA-A*02:01 mAb BB7.2. At this MOI, virtually all of the cells became infected (Fig. 3A). BB7.2 staining showed a substantial increase in the level of HLA-A*02:01 surface expression post-infection (MFI: 347) compared to uninfected cells (MFI: 184) (Fig. 3B), which is consistent with known effects of flaviviruses on class I MHC expression (37–39). For all three TCRm mAbs, there was a significant increase in MFI when infected cells were compared to uninfected cells, demonstrating the presentation of specific peptide HLA complexes on the infected cells (Fig. 3C–E). The shift in the MFI of staining with RL15A and RL14C was 8.5 and 13.0 respectively, while staining of infected cells with RL26A showed a much higher shift in the MFI of 63. BB7.2 mAb was used to determine total cell surface HLA-A2 expression and the fraction of total surface A2 molecules presenting each viral epitope was calculated as described in the methods. Taken together, these three WNV epitopes were presented by 24.3% of the total HLA-A2 complexes with YTM9 dominating at 18.1% and SVG9 and SLF9 representing 2.45% and 3.75% of the complexes, respectively (Fig. 3F).
FIGURE 3.
Epitope expression on WNV infected HeLa cells expressing surface HLA-A2. Cells were infected with WNV at MOI of 3 and stained with anti WNV E mAb, E24 (A), anti HLA-A2 mAb, BB7.2 (B) and each TCRm mAb (C–E) 24 h later. In (A–E) Solid lines represent WNV infected cells. Shaded histograms represent uninfected cells and the dotted lines indicate the isotype control. (F) The percent of surface HLA-A*02:01 complexes presenting each viral epitope was calculated by dividing the MFI of staining of each TCRm mAb by the MFI of BB7.2 staining. Together WNV epitopes represented 24.3% of the surface HLA-A*02:01 complexes. Percent of YTM9/A2 complexes (18.1%) was higher than SVG9/A2 and SLF9/A2 complexes representing 2.45% and 3.75% of all the HLA-A*02:01 complexes respectively. Data are representative of five independent experiments. Significance was determined by one-way ANOVA followed by Tukey’s test; P < 0.05.
To assess the expression pattern of viral epitopes in a more physiologically relevant cell type, human DCs from an HLA-A*02:01 positive individual were infected with WNV with the MOI of 3 for 48 hrs and stained with the TCRm mAbs. DCs are professional antigen presenting cells and a target cell type infected by WNV in vivo (20, 40). The TCRm mAbs detected the viral epitopes in the context of HLA-A*02:01 on the surface of infected DCs (Fig. 4A–F). Interestingly, the same order of abundance was observed again with YTM9 being presented significantly higher than the other 2 more immunogenic viral epitopes. In all experiments, viral infection was confirmed by staining permeabilized cells with anti-WNV E mAb E24 (Fig. 4G).
FIGURE 4.
Epitope expression on WNV infected primary DCs. Primary DCs from a HLA-A2 positive individual were infected with WNV at an MOI of 3. After 48 h, DCs were stained with RL14C, RL15A, RL26A (A–F) and E24 (G). (A, C and E) represent staining of uninfected cells with TCRm and (B, D and F) represent staining of the WNV infected DCs with TCRm mAbs. (G) Staining of the WNV infected DCs with anti-WNV E24 (solid line) or uninfected cells (dotted line). Shaded histogram indicates staining with the isotype control mAb. (A–F) Numbers indicate the percentages of TCRm-positive cells among total cells. Data are representative of two independent experiments.
Kinetics of endogenous peptide presentation on WNV infected cells
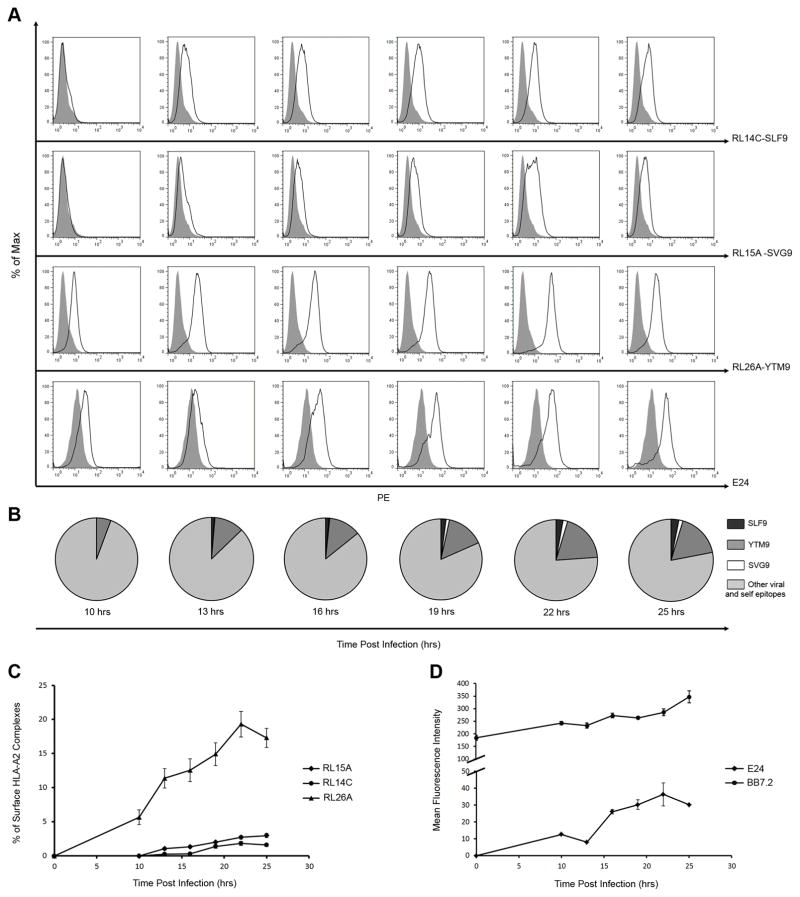
Next, we used the TCRm mAbs to study the kinetics of presentation of each viral epitopes on infected cells (MOI: 3) at different time points (10–25hrs) after infection. To date, most kinetic studies have analyzed the presentation of single epitopes (41) due to limited availability of reagents that recognize specific viral peptide/MHC complexes (16–20). Using these TCRm mAbs, we compared the kinetics and magnitude of presentation of three viral peptide epitopes (Fig. 5). YTM9 was presented earlier than SLF9 and SVG9 and was detectable above background at 10 hours post-infection and SLF9 was detected clearly above background at 13 hours post-infection. SVG9 while detected minimally above background at 13 and 16 hours post infection (Fig. 5A), was significantly above background 19 hours post-infection. Therefore, each of the viral peptides was presented at different time points after infection with YTM9 detected first followed by SLF9 and then SVG9 (Fig. 5A and B). The relative fraction of surface HLA-A2 presenting each of the viral epitopes was determined as described in the methods. A 5 to 10-fold greater level of YTM9 was observed on the cell surface compared to SLF9 and SVG9 at different time points (Fig. 5C), an observation that corresponds to the higher MFI of staining of infected cells with RL26A compared to RL14C and RL15A. The delay in presentation of viral epitopes was not due to a delay in infection as WNV E protein was detected at the earliest time point of 10 hours (Fig. 5D). Throughout the course of infection, a gradual increase in the level of HLA-A2 was observed on the surface of infected cells (Fig. 5D).
FIGURE 5.
Kinetics and level of presentation of WNV epitopes. Time course experiment where HeLa-A2 cells were infected at MOI of 3 and stained with the TCRm mAbs and anti-HLA-A2 antibody (BB7.2) and anti-WNV E mAb (E24) at different time points (0, 10, 13, 16, 19, 22, and 25 hours) post-infection. (A) Each plot shows the presentation of individual WNV epitope/HLA-A2 complexes at different time points during the first 25 hours post-infection. (B) Each pie chart shows the kinetics of presentation of all three WNV epitopes at certain time points during the course of experiment. The relative fraction of surface HLA-A2 complexes presenting each viral epitope was calculated by dividing the MFI of staining of infected cells with each TCRm mAb by the MFI of BB7.2 staining at indicated time points post-infection. Each sector represents the mean of the pooled samples with background at the time (0) being subtracted. (C) Changes in the relative fraction (percent) of surface HLA-A2 molecules presenting each viral epitope at different time points post-infection. (D) Changes in the level of surface HLA-A2 and WNV Envelope protein detected by BB7.2 and E24 antibodies respectively. All data are representative of five independent experiments. (C, D) Each data point shows the mean of the pooled samples from independent experiments and error bars indicate SDs.
TAP dependency of WNV epitopes
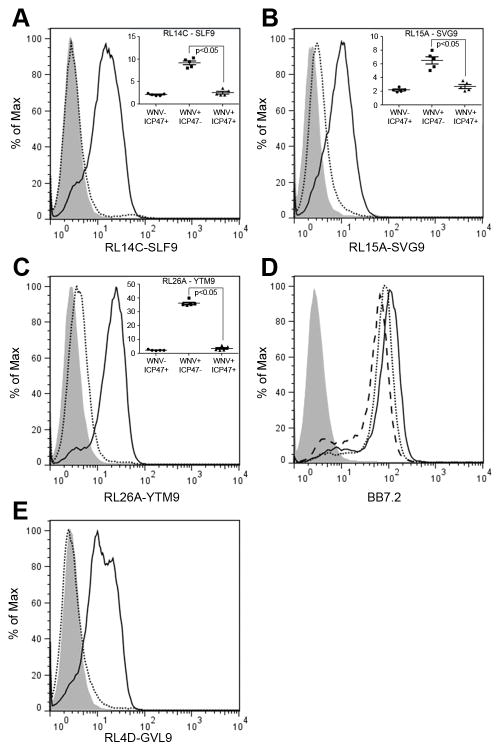
TAP plays an important role in the antigen processing and presentation pathway, transferring peptides degraded by proteasomes from the cytosol into the lumen of the endoplasmic reticulum (ER) (42). We used our TCRm mAbs to determine whether this common mechanism of antigen processing and presentation was used in the context of viral peptides during WNV infection. WNV epitopes SVG9, SLF9 and YTM9, arise from different parts of the WNV polyprotein: SVG9 originates from envelope that resides in the lumen of the ER (43), SLF9 originates from NS4B, a multi-pass transmembrane protein that spans the ER lumen, ER membrane, and cytosol. However, the SLF9 peptide is predicted to be expressed in the lumen of the ER (44–46), and YTM9 is processed from NS3, which resides on the cytosolic side of the ER (47). We hypothesized that due to their location in the lumen of the ER, SVG9 and SLF9 might directly load onto HLA without cytoplasmic processing by the proteasome and TAP transport back into the ER. To test this hypothesis, HeLa cells expressing surface A*02:01 were transduced with the HCMV TAP inhibitor protein ICP47. These cells were infected with WNV and antigen presentation was compared to mock-transduced cells. The expression of ICP47 abrogated surface expression of all three viral peptide/HLA complexes significantly (Fig. 6A–C). However, total expression of surface HLA-A2 was reduced only slightly after ICP47 transduction indicating that not all peptide/HLA complexes are TAP dependent (Fig. 6D), data consistent with previous studies showing TAP independent presentation of peptides from signal sequences as well as TAP independent presentation of hydrophobic peptide epitopes and exogenously acquired antigens (48). In each experiment, hCG-β peptide (GVL9) presentation by HLA-A*02:01 was used as an internal control for TAP function and RL4D TCRm mAb was used to detect GVL9 expression on the cell surface (Fig. 6E). These data show that, although WNV epitopes originate from proteins that localize in different subcellular compartments, they all are presented in a TAP-dependent manner. Thus, despite being localized to the ER lumen, proteasomes of the cytosol are required for the processing and presentation of WNV SVG9 and SLF9.
FIGURE 6.
Presentation of viral epitopes on the cell surface is TAP dependent. HeLa-A2 cells were transfected with the TAP inhibitor protein (ICP47+) or mock transfected (ICP47−). The ICP47+ and ICP47− cells were then infected with WNV and compared. In each experiment, hCG-β peptide (GVL9) presentation by HLA-A2 was used as an internal control for TAP function and RL4D TCRm mAb was used to detect GVL9 expression on the cell surface. (A–C) ICP47− and ICP47+ HeLa-A2 cells were infected with WNV at MOI of 3 for 48 h. Cells were then stained with the TCRm mAbs. Solid lines and dotted lines represent the TCRm staining of ICP47− and ICP47+ cells respectively. Shaded histograms show staining of uninfected ICP47+ HeLa-A2 cells. (D) Infected cells were stained with anti-HLA-A2 mAb, BB7.2. Solid line represents staining of ICP47− HeLa-A2 cells and dotted line represents staining of ICP47+ HeLa-A2 cells. Dashed line shows staining of uninfected ICP47+ HeLa-A2 cells and the shaded histogram shows uninfected HeLa cells. (E) ICP47− and ICP47+ HeLa-A2 cells expressing GVL9 peptide were stained with the specific TCRm mAb (RL4D). Solid lines and dotted lines represent the RL4D staining of ICP47− and ICP47+ cells respectively. Shaded histogram shows staining of HeLa-A2 cells without GVL9 peptide or mock transfected. Data are representative of five independent experiments. (A, B, C) Inset figures indicate that the expression of ICP47 abrogates the surface expression of all three viral peptides significantly. Significance was determined by one-way ANOVA followed by Tukey’s test; P < 0.05.
Discussion
While the innate immune response is accountable for the early control of WNV, adaptive humoral and T cell (CD4+ and CD8+) mediated immune responses combine to provide protective immunity and clear WNV infection (49). The vast majority of infected humans clear the virus asymptomatically, and West Nile virus therefore represents a model system to study how the dynamics of viral peptide presentation by class I HLA contributes to a successful and protective cellular immune response. A number of immunologic and biochemical approaches such as epitope-specific CTL clones, mass spectrometry, real time PCR, and assessment of naïve T cell precursor frequency have been used to evaluate the magnitude and kinetics of viral epitope presentation by HLA (8, 11, 12, 50). More recently, TCRm mAbs specific for viral peptide/HLA complexes have been made to directly track the presentation of viral epitopes on infected cells (16–19). These antibodies have contributed tremendously to our understanding of viral epitope processing, presentation, and localization. However, in all instances these HLA/peptide specific antibodies have been generated toward a single viral epitope, and the kinetics of presentation of one epitope may not represent all epitopes derived from that virus or even the same viral protein (50, 51). Having characterized the WNV epitopes that are presented by class I HLA following infection, here we produced TCRm mAbs to immunodominant, subdominant, and immunorecessive viral epitopes presented by HLA-A*02:01 (34). As no prior studies have used TCRm mAbs to track the HLA presentation of multiple viral epitopes during infection, we compared the magnitude and kinetics of WNV epitope presentation at different time points post-infection. The resulting data demonstrate a significant difference in presentation kinetics of immunodominant, subdominant, and recessive viral epitopes that is uncoupled from the magnitude of T cell response to these targets.
Rather unexpectedly, the immunodominant epitope was detected later and at a considerably lower cell surface level in comparison to the immunorecessive epitope. As the immunodominance and protective nature of envelope derived SVG9 epitope has been reported in several studies (5, 52, 53), we were surprised to find that the immunorecessive YTM9/HLA-A2 complex arrived at the surface of the infected cell several hours earlier and at much higher density. Moreover, the disparity between the levels of SVG9 and YTM9 at the cell surface persisted throughout the time course of the experiment. These findings differ from studies of hepatitis C virus where it has been reported that the earlier an epitope is presented the greater the chance that it provokes a protective immune response (14). An early-is-better posit has also been applied to HIV (12), where immune responses to late subdominant epitopes are thought to arise following the disappearance of early dominant viral epitopes via immune escape mechanisms such as mutation (54). As a point of discussion, it is conceivable that strong immunity to early/abundant ligands that have higher chance for mutation might distract or diminish immunity to key epitopes that are late, at lower abundance, and less likely to escape. Whatever the case for HIV and HCV, the immunodominance of SVG9 in humans and transgenic mice clearly demonstrates that successful viral immunity in WNV infection is directed toward the epitope that is fewer in number and fashionably late. It will be interesting to see if this pattern extends to other HLA alleles as well.
A review of the literature illustrates that controversy continues to surround the role of epitope abundance in priming CD8+ T cells, and a number of variables compound this debate including the study of different pathogens and strains thereof, the use of different host species (mice and humans), and the application of different experimental approaches for measuring epitope availability. For example, while studies of pathogens such as influenza, vaccinia and L. monocytogenes indicate a clear relationship between epitope abundance and T cell response hierarchies (8–10), the most immunogenic peptide associated with EBV infection is the least abundant (11). In most cases these epitope abundance data were collected at one time point post-infection and these experimental systems did not directly assess the expression of the epitope(s) in question. In a recent time course study of well-defined vaccinia immune epitopes presented by the mouse class I molecule H-2Kb, viral peptide ligand expression levels rose and fell considerably throughout the course of infection, underscoring the complex nature of the factors that work together to shape the T cell response (50). Here, the characterization of multiple CD8+ T cell targets via cell surface staining with TCRm mAbs demonstrates that the immunorecessive epitope YTM9 is the first to appear on the cell surface, is presented on more than 90% of the infected cells, and its expression remains high throughout the course of infection, suggesting that the parameters associated with successful immune recognition might differ from one pathogen to the next. As heterogeneity among experimental systems begins to diminish, assisted by the use of TCRm mAbs, the role of epitope timing and abundance in determining immune dominance will continue to emerge.
In this study, immortalized epithelial cells were initially used to examine the timing and abundance of HLA/viral epitopes at the cell surface. To verify that this epitope expression pattern extends to primary cells we next evaluated the presentation of these viral ligands on the surface of infected DCs. Dendritic cells are professional antigen presenting cells that process and present viral epitopes with different efficiency, and therefore at different levels, compared to cells in the periphery (55, 56). Interestingly, the same pattern of abundance was observed in WNV-infected primary DCs. These data confirm the uncoupling of HLA presented WNV epitope abundance and the magnitude of CTL response generated after infection. Important to the field of viral immunology is the consistency observed in antigen presentation between DC and epithelial cells derived from the periphery – immune effector cells trained in the lymph node by DC presenting a particular antigenic profile will better succeed in the periphery when presented with the same antigenic profile. Conversely, CD8+ T cells might struggle to recognize an antigenic profile in the periphery if it is markedly different than the lymph node training set. This panel of viral epitopes and their corresponding mAbs provide a clear view of how a protective immune response is formed in WNV infection. It will be exciting to see how this pattern compares to that of other viruses in regards to the role of epitope timing and abundance in determining immunodominance.
Next we used these mAbs to investigate if this distinct pattern of presentation is the outcome of being processed by different processing pathways. Previous studies using Epstein-Barr virus show that peptides derived from the EBV protein LMP2, which spans the ER membrane at multiple points, are presented by class I HLA in both TAP-dependent and TAP-independent manners (48, 57). Because WNV likewise spans the ER membrane such that its T cell epitopes originate from different cellular compartments (cytosol versus ER lumen), we studied whether the WNV peptide epitopes traffic via different intracellular routs. Our results indicate that unlike the EBV LMP2 ligands, the three WNV epitopes characterized here are all processed and presented in a TAP-dependent manner. The TAP dependency of YTM9 is logical as it is an epitope that originates from the NS3 protein (47), which resides in cytosol and is likely degraded through cytosolic proteasomes and then transported to ER through TAP. However, the finding that TAP is required for presentation of SVG9 and SLF9 was unexpected since these peptides are derived from ER resident portions of the viral E and NS4B proteins (43–45). Our data suggest that luminal segments of E and NS4B proteins are removed from ER by the ER associated degradation (ERAD) pathway and transported back into the cytoplasm (dislocation) for degradation by proteosomes, TAP translocation to the ER, and HLA class I presentation (58). ERAD substrates were originally thought to be exclusively misfolded proteins, however increasing evidence shows that certain folded, active proteins are also degraded via the ERAD pathway (59–61). In this case, the ERAD pathway appears to dislocate properly folded and/or misfolded WNV proteins from the ER for degradation in the cytosol after which the conventional TAP dependent transport of peptides for HLA presentation on the cell surface occurs (62, 63). Further investigation will be needed to determine if it is this longer route of ERAD dislocation prior to proteasomal degradation and presentation that causes the delayed appearance of SVG9 and SLF9 on the cell surface.
In summary, the current study reports the characterization of three HLA-A2/WNV T cell epitopes using a panel of TCRm mAbs. These viral epitopes differ in their immunogenicity and arise from different segments of the WNV polyprotein localized to different cellular compartments, and in a somewhat counterintuitive manner we found that the immunogenic epitopes (SVG9 and SLF9) are introduced to T cells later than the immune recessive viral ligand. In addition to becoming available for immune recognition at a later time point, they are also less abundant at the cell surface, and this copy number disparity persists over time. These data suggest that there are qualities inherent to T cell epitopes that extend well beyond the quantity and timing of availability, and the elucidation of these qualities might provide vaccine architects with more immunogenic ligands for therapeutic development. Lastly, TCRm mAbs represent a powerful tool for directly assessing the timing and levels of epitope availability. Increased use of these mAbs is poised to reduce system heterogeneity and provide for more consistent analyses of viral epitope availability as we continue to unravel the parameters that shape immunogenicity.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (www.nih.gov) Contract HHSN266200400027C (to W.H.H.) and National Institutes of Health grant U01AI082057 (to W.H.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank Dr. Kenneth Jackson from University of Oklahoma Health Sciences Center for technical assistance. We thank Dr. Rico Buchli from Pure Protein LLC. for making the viral peptide/HLA-A2 monomers. We sincerely thank Dr. Beatriz Carreno from Washington University in St. Louis for technical advice and helpful discussion.
References
- 1.Shastri N, Schwab S, Serwold T. Producing nature’s gene-chips: the generation of peptides for display by MHC class I molecules. Annu Rev Immunol. 2002;20:463–493. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
- 2.Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3:952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 3.Wahl A, Weidanz J, Hildebrand W. Direct class I HLA antigen discovery to distinguish virus-infected and cancerous cells. Expert Rev Proteomics. 2006;3:641–652. doi: 10.1586/14789450.3.6.641. [DOI] [PubMed] [Google Scholar]
- 4.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S, Li LJ, McMurtrey CP, Hildebrand WH, Weidanz JA, Gillanders WE, Diamond MS, Hansen TH. Single-Chain HLA-A2 MHC Trimers that incorporate an immundominant peptide elicit protective T cell immunity against lethal West Nile virus infection. J Immunol. 2010;184:4423–4430. doi: 10.4049/jimmunol.0903955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Akram A, Inman RD. Immunodominance: a pivotal principle in host response to viral infections. Clin Immunol. 2012;143:99–115. doi: 10.1016/j.clim.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 8.La Gruta NL, Kedzierska K, Pang K, Webby R, Davenport M, Chen W, Turner SJ, Doherty PC. A virus-specific CD8+ T cell immunodominance hierarchy determined by antigen dose and precursor frequencies. Proc Natl Acad Sci U S A. 2006;103:994–999. doi: 10.1073/pnas.0510429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. J Immunol. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 10.Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- 11.Crotzer VL, Christian RE, Brooks JM, Shabanowitz J, Settlage RE, Marto JA, White FM, Rickinson AB, Hunt DF, Engelhard VH. Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J Immunol. 2000;164:6120–6129. doi: 10.4049/jimmunol.164.12.6120. [DOI] [PubMed] [Google Scholar]
- 12.Kloverpris HN, Payne RP, Sacha JB, Rasaiyaah JT, Chen F, Takiguchi M, Yang OO, Towers GJ, Goulder P, Prado JG. Early antigen presentation of protective HIV-1 KF11Gag and KK10Gag epitopes from incoming viral particles facilitates rapid recognition of infected cells by specific CD8+ T cells. J Virol. 2013;87:2628–2638. doi: 10.1128/JVI.02131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ameres S, Mautner J, Schlott F, Neuenhahn M, Busch DH, Plachter B, Moosmann A. Presentation of an immunodominant immediate-early CD8+ T cell epitope resists human cytomegalovirus immunoevasion. PLoS Pathog. 2013;9:e1003383. doi: 10.1371/journal.ppat.1003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt J, Iversen AK, Tenzer S, Gostick E, Price DA, Lohmann V, Distler U, Bowness P, Schild H, Blum HE, Klenerman P, Neumann-Haefelin C, Thimme R. Rapid antigen processing and presentation of a protective and immunodominant HLA-B*27-restricted hepatitis C virus-specific CD8+ T-cell epitope. PLoS Pathog. 2012;8:e1003042. doi: 10.1371/journal.ppat.1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 16.Sastry KS, Too CT, Kaur K, Gehring AJ, Low L, Javiad A, Pollicino T, Li L, Kennedy PT, Lopatin U, Macary PA, Bertoletti A. Targeting hepatitis B virus-infected cells with a T-cell receptor-like antibody. J Virol. 2011;85:1935–1942. doi: 10.1128/JVI.01990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makler O, Oved K, Netzer N, Wolf D, Reiter Y. Direct visualization of the dynamics of antigen presentation in human cells infected with cytomegalovirus revealed by antibodies mimicking TCR specificity. Eur J Immunol. 2010;40:1552–1565. doi: 10.1002/eji.200939875. [DOI] [PubMed] [Google Scholar]
- 18.Nunoya J, Nakashima T, Kawana-Tachikawa A, Kiyotani K, Ito Y, Sugimura K, Iwamoto A. Short communication: generation of recombinant monoclonal antibodies against an immunodominant HLA-A*2402-restricted HIV type 1 CTL epitope. AIDS Res Hum Retroviruses. 2009;25:897–904. doi: 10.1089/aid.2009.0036. [DOI] [PubMed] [Google Scholar]
- 19.Cohen CJ, Sarig O, Yamano Y, Tomaru U, Jacobson S, Reiter Y. Direct phenotypic analysis of human MHC class I antigen presentation: visualization, quantitation, and in situ detection of human viral epitopes using peptide-specific, MHC-restricted human recombinant antibodies. J Immunol. 2003;170:4349–4361. doi: 10.4049/jimmunol.170.8.4349. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Pinto AK, Myers NB, Hawkins O, Doll K, Kaabinejadian S, Netland J, Bevan MJ, Weidanz JA, Hildebrand WH, Diamond MS, Hansen TH. A novel T-cell receptor mimic defines dendritic cells that present an immunodominant West Nile virus epitope in mice. Eur J Immunol. 2014;44:1936–1946. doi: 10.1002/eji.201444450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brien JD, Lazear HM, Diamond MS. Propagation, quantification, detection, and storage of West Nile virus. Curr Protoc Microbiol. 2013;31:15D 13 11–15D 13 18. doi: 10.1002/9780471729259.mc15d03s31. [DOI] [PubMed] [Google Scholar]
- 22.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 23.Weidanz JA, Nguyen T, Woodburn T, Neethling FA, Chiriva-Internati M, Hildebrand WH, Lustgarten J. Levels of specific peptide-HLA class I complex predicts tumor cell susceptibility to CTL killing. J Immunol. 2006;177:5088–5097. doi: 10.4049/jimmunol.177.8.5088. [DOI] [PubMed] [Google Scholar]
- 24.Weidanz JA, Piazza P, Hickman-Miller H, Woodburn D, Nguyen T, Wahl A, Neethling F, Chiriva-Internati M, Rinaldo CR, Hildebrand WH. Development and implementation of a direct detection, quantitation and validation system for class I MHC self-peptide epitopes. J Immunol Methods. 2007;318:47–58. doi: 10.1016/j.jim.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Parker KC, Wiley DC. Overexpression of native human beta 2-microglobulin in Escherichia coli and its purification. Gene. 1989;83:117–124. doi: 10.1016/0378-1119(89)90409-5. [DOI] [PubMed] [Google Scholar]
- 26.Wei ML, Cresswell P. HLA-A2 molecules in an antigen-processing mutant cell contain signal sequence-derived peptides. Nature. 1992;356:443–446. doi: 10.1038/356443a0. [DOI] [PubMed] [Google Scholar]
- 27.Demars R, Chang CC, Shaw S, Reitnauer PJ, Sondel PM. Homozygous Deletions That Simultaneously Eliminate Expressions of Class-I and Class-Ii Antigens of Ebv-Transformed B-Lymphoblastoid Cells 1. Reduced Proliferative Responses of Autologous and Allogeneic T-Cells to Mutant-Cells That Have Decreased Expression of Class-Ii Antigens. Hum Immunol. 1984;11:77–97. doi: 10.1016/0198-8859(84)90047-8. [DOI] [PubMed] [Google Scholar]
- 28.Pannu KK, Joe ET, Iyer SB. Performance evaluation of QuantiBRITE phycoerythrin beads. Cytometry. 2001;45:250–258. doi: 10.1002/1097-0320(20011201)45:4<250::aid-cyto10021>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Quinn JG. Modeling Taylor dispersion injections: determination of kinetic/affinity interaction constants and diffusion coefficients in label-free biosensing. Anal Biochem. 2012;421:391–400. doi: 10.1016/j.ab.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 30.Quinn JG. Evaluation of Taylor dispersion injections: determining kinetic/affinity interaction constants and diffusion coefficients in label-free biosensing. Anal Biochem. 2012;421:401–410. doi: 10.1016/j.ab.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremont DH, Diamond MS. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carreno BM, Becker-Hapak M, Linette GP. CD40 regulates human dendritic cell-derived IL-7 production that, in turn, contributes to CD8(+) T-cell antigen-specific expansion. Immunol Cell Biol. 2009;87:167–177. doi: 10.1038/icb.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma B, Neethling FA, Caseltine S, Fabrizio G, Largo S, Duty JA, Tabaczewski P, Weidanz JA. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol. 2010;184:2156–2165. doi: 10.4049/jimmunol.0902414. [DOI] [PubMed] [Google Scholar]
- 34.McMurtrey CP, Lelic A, Piazza P, Chakrabarti AK, Yablonsky EJ, Wahl A, Bardet W, Eckerd A, Cook RL, Hess R, Buchli R, Loeb M, Rinaldo CR, Bramson J, Hildebrand WH. Epitope discovery in West Nile virus infection: Identification and immune recognition of viral epitopes. Proc Natl Acad Sci U S A. 2008;105:2981–2986. doi: 10.1073/pnas.0711874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keitel T, Kramer A, Wessner H, Scholz C, Schneider-Mergener J, Hohne W. Crystallographic analysis of anti-p24 (HIV-1) monoclonal antibody cross-reactivity and polyspecificity. Cell. 1997;91:811–820. doi: 10.1016/s0092-8674(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 36.Berzofsky JA, Schechter AN. The concepts of crossreactivity and specificity in immunology. Mol Immunol. 1981;18:751–763. doi: 10.1016/0161-5890(81)90067-5. [DOI] [PubMed] [Google Scholar]
- 37.Mullbacher A, Lobigs M. Up-regulation of MHC class I by flavivirus-induced peptide translocation into the endoplasmic reticulum. Immunity. 1995;3:207–214. doi: 10.1016/1074-7613(95)90090-x. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, King N, Kesson A, Blanden RV, Mullbacher A. Flavivirus infection up-regulates the expression of class I and class II major histocompatibility antigens on and enhances T cell recognition of astrocytes in vitro. J Neuroimmunol. 1989;21:157–168. doi: 10.1016/0165-5728(89)90171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kesson AM, King NJ. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-kappaB activation. J Infect Dis. 2001;184:947–954. doi: 10.1086/323603. [DOI] [PubMed] [Google Scholar]
- 40.Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 41.Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 42.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorenz IC, Kartenbeck J, Mezzacasa A, Allison SL, Heinz FX, Helenius A. Intracellular assembly and secretion of recombinant subviral particles from tick-borne encephalitis virus. J Virol. 2003;77:4370–4382. doi: 10.1128/JVI.77.7.4370-4382.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller S, Sparacio S, Bartenschlager R. Subcellular localization and membrane topology of the Dengue virus type 2 Non-structural protein 4B. J Biol Chem. 2006;281:8854–8863. doi: 10.1074/jbc.M512697200. [DOI] [PubMed] [Google Scholar]
- 45.Wicker JA, Whiteman MC, Beasley DW, Davis CT, McGee CE, Lee JC, Higgs S, Kinney RM, Huang CY, Barrett AD. Mutational analysis of the West Nile virus NS4B protein. Virology. 2012;426:22–33. doi: 10.1016/j.virol.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wicker JA, Whiteman MC, Beasley DW, Davis CT, Zhang S, Schneider BS, Higgs S, Kinney RM, Barrett AD. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology. 2006;349:245–253. doi: 10.1016/j.virol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Yamshchikov VF, Compans RW. Processing of the intracellular form of the west Nile virus capsid protein by the viral NS2B-NS3 protease: an in vitro study. J Virol. 1994;68:5765–5771. doi: 10.1128/jvi.68.9.5765-5771.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lautscham G, Rickinson A, Blake N. TAP-independent antigen presentation on MHC class I molecules: lessons from Epstein-Barr virus. Microbes Infect. 2003;5:291–299. doi: 10.1016/s1286-4579(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 49.Suthar MS, Diamond MS, Gale M., Jr West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- 50.Croft NP, Smith SA, Wong YC, Tan CT, Dudek NL, Flesch IE, Lin LC, Tscharke DC, Purcell AW. Kinetics of antigen expression and epitope presentation during virus infection. PLoS Pathog. 2013;9:e1003129. doi: 10.1371/journal.ppat.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sacha JB, Reynolds MR, Buechler MB, Chung C, Jonas AK, Wallace LT, Weiler AM, Lee W, Piaskowski SM, Soma T, Friedrich TC, Wilson NA, Watkins DI. Differential antigen presentation kinetics of CD8+ T-cell epitopes derived from the same viral protein. J Virol. 2008;82:9293–9298. doi: 10.1128/JVI.00749-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piazza P, McMurtrey CP, Lelic A, Cook RL, Hess R, Yablonsky E, Borowski L, Loeb MB, Bramson JL, Hildebrand WH, Rinaldo CR. Surface phenotype and functionality of WNV specific T cells differ with age and disease severity. PLoS One. 2010;5:e15343. doi: 10.1371/journal.pone.0015343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith HL, Monath TP, Pazoles P, Rothman AL, Casey DM, Terajima M, Ennis FA, Guirakhoo F, Green S. Development of antigen-specific memory CD8+ T cells following live-attenuated chimeric West Nile virus vaccination. J Infect Dis. 2011;203:513–522. doi: 10.1093/infdis/jiq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson WE, Desrosiers RC. Viral persistence: HIV’s strategies of immune system evasion. Annu Rev Med. 2002;53:499–518. doi: 10.1146/annurev.med.53.082901.104053. [DOI] [PubMed] [Google Scholar]
- 55.Zehn D, Cohen CJ, Reiter Y, Walden P. Efficiency of peptide presentation by dendritic cells compared with other cell types: implications for cross-priming. Int Immunol. 2006;18:1647–1654. doi: 10.1093/intimm/dxl098. [DOI] [PubMed] [Google Scholar]
- 56.Bullock TN, Mullins DW, Engelhard VH. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J Immunol. 2003;170:1822–1829. doi: 10.4049/jimmunol.170.4.1822. [DOI] [PubMed] [Google Scholar]
- 57.Lautscham G, Haigh T, Mayrhofer S, Taylor G, Croom-Carter D, Leese A, Gadola S, Cerundolo V, Rickinson A, Blake N. Identification of a TAP-independent, immunoproteasome-dependent CD8+ T-cell epitope in Epstein-Barr virus latent membrane protein 2. J Virol. 2003;77:2757–2761. doi: 10.1128/JVI.77.4.2757-2761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 59.Varga K, Jurkuvenaite A, Wakefield J, Hong JS, Guimbellot JS, Venglarik CJ, Niraj A, Mazur M, Sorscher EJ, Collawn JF, Bebok Z. Efficient intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J Biol Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- 60.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruggiano A, Foresti O, Carvalho P. Quality control: ER-associated degradation: protein quality control and beyond. J Cell Biol. 2014;204:869–879. doi: 10.1083/jcb.201312042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dolan BP, Bennink JR, Yewdell JW. Translating DRiPs: progress in understanding viral and cellular sources of MHC class I peptide ligands. Cell Mol Life Sci. 2011;68:1481–1489. doi: 10.1007/s00018-011-0656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bacik I, Snyder HL, Anton LC, Russ G, Chen W, Bennink JR, Urge L, Otvos L, Dudkowska B, Eisenlohr L, Yewdell JW. Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol. J Exp Med. 1997;186:479–487. doi: 10.1084/jem.186.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.