Abstract
A subset of human regulatory T cells (Tregs) can secrete IFN-γ or IL-17 and thus share features of TH1 or TH17 effector cells, and lose suppressive function. The main factors driving this differentiation of Tregs towards a pro-inflammatory phenotype include IL-12 for TH1-like and IL-6 for TH17-type Tregs. In this study we show that Tregs of patients with de novo autoimmune hepatitis (dAIH) display increased frequencies of pro-inflammatory IFN-γ and IL-17 cytokines. Irrespective of a fully demethylated FOXP3 locus, Tregs of subjects with de novo autoimmune hepatitis are functionally impaired. In line with the observed Treg phenotype, we detected the presence of two dominant cytokines (IL-12 and IL-6) clustering with CD68+ monocyte/macrophage cells in livers of subjects with de novo autoimmune hepatitis and isolated monocytes of subjects with de novo autoimmune hepatitis secrete high levels of pro-inflammatory IL-12 and IL-6 suggesting that this inflammatory milieu is key for functional impairment of Tregs. Importantly, the blockade of IFN-γ partially restores suppressive function of Tregs of subjects with de novo autoimmune hepatitis indicating that monocyte/macrophage derived triggers might play a central role in Treg dysfunction and pathogenesis of de novo autoimmune hepatitis.
INTRODUCTION
Advances in liver transplantation have resulted in improved survival and better outcomes for most patients with liver disease. However, with these advances and longevity of liver allografts comes late allograft dysfunction. An important cause of late allograft dysfunction is de novo autoimmune hepatitis (dAIH). The etiology and underlying pathogenesis of de novo autoimmune remains unclear and while several risk factors such as preceding acute rejection episodes, steroid dependence (1), human lymphocyte antigen phenotype (2, 3), gender and age of the organ donor (4), have been reported and mechanisms proposed to explain the immune response observed (5-9), importantly, these risk factors have not been consistently observed in all patients.
CD25+FOXP3+ regulatory T cells (Tregs) mediate immunological tolerance and curb autoimmunity and exuberant immune responses (10-12). Over the last few years it has become clear that human Tregs can produce inflammatory cytokines (13-17), and some of these cytokine producing Tregs may have pathogenic potential as reports suggest they may contribute to mucosal disease and are implicated in the development of colon cancer (15, 18),inflammatory bowel disease (16) as well as autoimmune diseases such as multiple sclerosis and type 1 diabetes (TID) (19). There however remain unanswered questions about the function and role of cytokine producing Tregs in health and disease. As the key TH1 and TH17 effector cytokines, IFN-γ and IL-17, have been implicated in the immune pathogenesis of autoimmune hepatitis seen in non-transplanted individuals (20), and a multifaceted imbalance of T cells with regulatory function described as characterizing autoimmune hepatitis in non-transplanted individuals (21), we posited that pro-inflammatory cytokine secreting Tregs may be involved in the immune pathogenesis of de novo autoimmune hepatitis.
In an attempt to better understand the underlying immune pathogenesis of de novo autoimmune hepatitis, we conducted a cross sectional study involving liver transplanted recipients with: normal allograft function (LTC); de novo autoimmune hepatitis (dAIH); and healthy non-transplanted children (HC), where we sought to identify cell types prevalent in de novo autoimmune hepatitis and investigate how they might contribute towards disease pathogenesis; In addition, we sought to determine the role of cytokine producing FOXP3+ Tregs in the development of de novo autoimmune hepatitis. Our findings establish a framework to dissect the cell type(s) driving differentiation of Tregs towards a pro-inflammatory phenotype in human diseases as well as dissecting triggers that can induce local release of cytokines to direct Tregs cells towards the pro-inflammatory TH1/TH17-type differentiation pathway.
METHODS
Study subjects
This was a cross sectional study involving pediatric liver transplant recipients with a diagnosis of de novo autoimmune hepatitis (n=21), pediatric liver transplant recipients who had never been diagnosed with de novo autoimmune hepatitis and had normal allograft function at the time of enrollment and blood draw. These subjects acted as the liver transplant control group (LTC) (n=27), and healthy children who had no underlying immune mediated disorders and were not on any immunomodulatory agents. These subjects acted as the healthy control group (HC) (n=28). The transplanted subjects were enrolled as they attended their routine post-transplant clinics while the healthy non-transplanted subjects were enrolled using flyers posted in New Haven as well as from the help us discover database, a database of healthy children registered as research controls maintained by the Yale Center for Clinical Investigation (YCCI). The definition of de novo autoimmune hepatitis was as previously described (1, 22). We enrolled patients aged 0 through 20-years from January 2013 to June 2015. At enrollment, 30 ml of peripheral blood was obtained from each participant. Informed consent was obtained from parents or guardians and assent was obtained as necessary per institutional review board guidelines. The study protocol was reviewed and approved by the Institutional Review Board of Yale University.
Antibodies and reagents
The following monoclonal antibodies and reagents were used as follows: for cell surface staining: anti-CD4 (BD Pharmingen clone RPA-T4), anti-CD45RA (BD Pharmingen clone HI100), CD25 (BD Pharmingen clone M-A251), and CD127 (eBioscience clone eBioRDR5), anti-PDL1 (BD Pharmingen clone MIH1), anti-CD3 (BD Biosciences clone UCHT1), anti-CD14 (BD Biosciences clone MϕP9), anti-TIM3 (BioLegend clone F38-2E2), anti-CCR6 (BD Pharmingen clone 11A9), anti-CD161 (BD Pharmingen clone DX12), anti-CD49D (Miltenyi Biotec clone MZ18-24A9), anti-LAG3 (R&D systems clone POLY), anti-IL-23R (R&D systems clone 218213); for intracellular staining: anti-IFNγ (BioLegend clone 4S.B3), anti-IL-17A (BioLegend clone BL168 and BD Pharmingen clone N49-653), and Foxp3 (eBioscience clone PCH101), anti-IL-12 (BD Biosciences clone C11.5), anti-IL-1β (BD Biosciences clone AS10), anti-IL-6 (eBioscience clone MQ2-13A5); for effector cell (Tresp) stimulation in suppression assay: anti-CD2, anti-CD3 and anti-CD28 Treg Supression Inspector beads from Miltenyi Biotec (130-092-909), CFSE was obtained from Invitrogen (Carlsbad, CA), anti-PDL1 (BioLegend clone 29E.2A3), anti-PD-1 (BD Biosciences clone EH12.1); for immunofluorescence: anti-CD4 (Serotec MCA4846), anti-CD68 (Abcam cat #ab125157), Foxp3 (Abcam cat #ab20034), anti-IL17A (Abcam cat #ab136668), anti-IL-1β (Abcam cat #ab 104279), anti-IL-6 (Abcam cat # ab6672), anti-IL12 (R&D Systems MAB611), anti-IFN-γ (MAB285), AlexaFluor 488 and 594 (Life Technologies), DAPI (ProLong Antifade Gold P36934); for monocyte stimulation experiments: lipopolysaccharide LPS (Sigma L2654).
Cell isolation and FACS sorting of T cell populations
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood obtained from study participants at enrollment by Ficoll Hypaque gradient centrifugation. Total CD4+ T cells were isolated by negative selection using the CD4+ T cell isolation kit II (Miltenyi Biotec) and stained for fluorescence-activated cell sorting (FACS) with the following antibodies: anti-CD45RA, CD25, and CD127. The Treg (CD4+CD25hiCD127low/neg), Tmemory (CD4+CD25low/negCD45RA−) and Tnaive (CD4+CD25low/negCD45RA+) populations were sorted on a FACS Aria (BD Biosciences).
T cell activation and intracellular staining
T cell populations (50,000 cells) were stimulated with phobol-12-myristate-13-acetate (PMA) at 50ng/ml and ionomycin (250ng/ml) for 4-hours in the presence of GolgiStop 4μl/ml (BD Biosciences 554724), fixed and made permeable (Fix/Perm; eBioscience) and intracellular staining of cytokines and FOXP3 was performed with FOXP3 staining buffers (eBioscience) per manufacturer’s recommendations with the following antibodies: IFN-γ, IL-17A and FOXP3 for 30 – 45 minutes. Prior to fixation, cells were stained with LIVE/DEAD cell kit (Life Technologies) to exclude dead cells.
For all flow cytometry, data was acquired on a LSR II (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR).
Monocyte/regulatory T cell co-culture experiments
CD14+ monocytes were obtained by positive selection (StemCell #18058) from PBMCs’ following manufacturer’s guidelines and co-cultured with FACS sorted ex vivo isolated Tregs, in the presence of plate bound anti-CD3 (clone UCHT1) at 1μg/ml, and rIL-2 at 25U/ml Corning 356043), for five days at 37°C with 5% CO2. At the end of the culture period, cells were stimulated with PMA (50 ng/ml) (Sigma P8139)/ionomycin (250 ng/ml) (Sigma I0634) for 5-hours, with GolgiStop added in the last 4-hours. Cell surface staining with anti-CD3 and anti-CD14 was performed as well as intracellular staining of cytokines and FOXP3 as described above; IL-17A and IFN-γ production from FOXP3+ cells was assessed using flow cytometry.
Monocyte stimulation experiments
CD14+ monocytes were obtained by positive selection (StemCell #18058) from PBMCs’ following manufacturer’s guidelines, and plated in Xvivo15 media with LPS (1 μg/ml) for 24-hours at 37°C with 5% CO2. GolgiStop was added for the final 4-hours of culture according to the manufacturers instructions. Cells were then fixed and permeabilized using stainng buffer set (eBioscience 00-5523-00) according to manufacturers instructions. At the end of the culture period, cells were stained with anti-CD3 and anti-CD14 as well as for intracellular cytokine (IL-12, IL-1β, IL-6) production and analyzed using flow cytometry.
Quantification of mRNA expression levels by RT-PCR
RNA was isolated using Qiagen RNeasy Micro Kit (QIAGEN), following manufacturer’s guidelines and converted to cDNA by reverse transcription (RT) with random hexamers and Multiscribe RT (TQMN, Reverse Transcription Reagents; Applied Biosystems). For mRNA gene expression assays, probes were purchased from Applied Biosystems (Supplementary figure 1) and the reactions were set up following manufacturer’s guidelines and run on a 7500 Fast Real-Time PCR System (Applied Biosystems). Values are represented as the difference in Ct values normalized to β2-microglobulin for each sample as per the following formula: Relative RNA expression = (2−dCt) × 1000.
Suppression assays
Tregs were co-cultured with CFSE-labeled effector (responder) CD4+CD25−CD127+ T cells at Treg:Tresponder ratio of 1:1, 1:2, 1:4, 1:8 (i.e. 50,000 Tregs and 50,000 Tresp, or 25,000 Tregs and 50,000 Tresp, or 12,000 Tregs and 50,000 Tresp, or 6,000 Tregs and 50,000 Tresp). The stimuli used were anti-CD2, anti-CD3, anti-CD28 Treg Supression Inspector beads (Miltenyi Biotec 130-092-909) at manufacturer’s recommended concentration. At day 5, co-cultures were stained for viability with LIVE/DEAD stain kit (Life Technologies). Proliferation of viable responder T cells was analyzed on a LSR II flow cytometer (BD Biosciences). In parallel experiments, IFN-γ recombinant protein (Biolegend 713906) was added at 10 ng/ml to healthy control cultures and anti-IFN-γ (R&D Systems 25718) was added at 10 μg/ml to cultures of subjects with de novo autoimmune hepatitis when plating on Day 1. For cytolytic CD8 T cell function assay, Tregs were co-cultured with Cell Trace V450-labeled (Life Technologies) total PBMCs at a ratio of 1:2, 1:4, and 1:8. The stimuli used were immobilized anti-CD3 CD3 (BD Biosciences, BDB550368, clone UCHT1) and soluble anti-CD28 (BD Biosciences, BDB556620, clone CD28.2) monoclonal antibodies at 1ug/ml. At day 5, co-cultures were also stained for viability, fixed, permeabilized, and stained with anti-CD3 (BD Biosciences, BDB561417, clone UCHT1), anti-CD8 (BD Biosciences, BDB560662, clone RPA-T8), anti-Granzyme B (BD Biosciences, BDB561998, clone GB11), and anti-Perforin (eBioscience, 17-9994-42, clone dG9). Proliferation and Granzyme B/Perforin (cytolytic index) expression of viable CD8+ T cells was analyzed on a LSR II flow cytometer (BD Biosciences).
FOXP3 locus methylation analysis
DNA was harvested from ex vivo sorted Tregs using DNeasy Blood and Tissue Kit (Qiagen, cat #69504) according to manufacturers instructions and sent to Epiontis GmBH (Berlin, Germany), where DNA was analyzed by quantitative PCR (qPCR) for methylation status of the FOXP3 locus Treg Specific Demethylation Region (TSDR) using previously established protocols (23-25). Methylation status was expressed as percent demethylated and translated from qPCR expression as previously described (24).
Immunofluorescence
Immunohistochemical studies were carried out on formalin fixed, paraffin-embedded, 7-micron-thick sections of liver tissue obtained from 5 of the subjects with de novo autoimmune hepatitis during disease activity. The sections were deparafinized, and rehydrated; non-specific binding was blocked overnight in PBS containing 3% FBS at 4°C, and stained with primary antibody (IL-6, IL-1β, IL-12, IFN-γ, IL-17A, FOXP3, CD4, CD68) at 1:50 overnight at 4°C. The following day, slides were stained with appropriate secondary antibody at 1:50 for 2-hours at room temperature, stained with DAPI, mounted, cover slipped and sealed for microscopy.
Statistics
Summary statistics are reported overall and by group for Liver transplanted subjects without de novo autoimmune hepatitis (LTC), healthy non-transplanted children (HC) and subjects with de novo autoimmune hepatitis. For quantitative variables, mean, median, and standard deviation are reported; for categorical variables, counts and percentages are reported. Quantitative variables are compared between groups using the Kruskal-Wallis test for overall comparisons of more than two groups. Comparisons of a quantitative variable between two groups were performed using the Wilcoxon rank-sum test. Paired within-subject comparisons of quantitative variables were performed using the signed-rank test. Categorical variables are compared between groups using Fisher's exact test. P-values < 0.05 are considered statistically significant. Analyses were conducted using SAS 9.3.
RESULTS
The clinical characteristics of the three subject groups are as shown in Table I. Importantly, the mean duration from transplant at which a diagnosis of de novo autoimmune hepatitis was made was much earlier than the mean duration from transplant at the time of blood draw in our liver transplanted controls (2142.6 days vs. 3261.8 days), suggesting that our liver transplanted controls were true controls and not transplant recipients who had not yet developed de novo autoimmune hepatitis.
TABLE I.
Demographics of study participants
|
De novo AIH (n=21) |
LTC (n=27) |
HC (n=28) |
P value | |
|---|---|---|---|---|
| Age at blood draw mean ± SD (years) |
14.87 ± 4.60 | 10.48 ± 6.24 | 8.98 ± 5.28 | HC vs. LTC 0.3 HC vs. DAIH 0.002 LTC vs. DAIH 0.04 |
| Gender (m/f) | 11/10 | 18/9 | 13/15 | 0.3 |
| Duration from transplant at blood draw mean ± SD (days) |
4149.76 ± 1938.75 |
3261.80 ± 2363.39 |
n/a | 0.2 |
| Duration from transplant at de novo diagnosis mean ± SD (days) |
2142.65 ± 1175.85 |
n/a | n/a | |
| Pre transplant diagnosis: Biliary atresia Other |
13 8 |
17 10 |
n/a | 0.9 |
| CNI trough level at blood draw mean ± SD ( ) |
4.74 ± 1.88 | 3.39 ± 2.75 | n/a | 0.2 |
AIH: Autoimmune hepatitis
LTC: Liver transplanted control
HC: Healthy control
CNI: Calcineurin inhibitor
FOXP3+ Tregs from patients with de novo autoimmune hepatitis have a pro-inflammatory phenotype
In an attempt to identify pro-inflammatory cytokine profile from T cell subsets in de novo autoimmune hepatitis, total CD4+ T cells and highly purified FACS-sorted Tregs, memory (Tmem) and naïve (Tnaive) cells were stimulated with phobol-12-myristate-13-acetate (PMA) and ionomycin, and stained with FOXP3, IL-17 and IFN-γ antibodies as described in the methods section. (Gating strategy Supplementary Figure 2A-C, FOXP3 purity and FOXP3 mRNA expression Supplementary Figure 2D-E).
Of note, IFN-γ production was significantly higher from FOXP3− CD4+ T cells of subjects with de novo autoimmune hepatitis compared to FOXP3− CD4+ T cells of healthy non-transplanted children (p=0.02); and from liver transplanted subjects without de novo autoimmune hepatitis compared to healthy non-transplanted children (p=0.01) (Figure 1A) but IL-17 production from FOXP3− CD4+ T cells was not significantly different between the three subject groups (Figure 1A); IFN-γ production from memory CD4+ T cells was not significantly different between the three subject groups (Figure 1B).
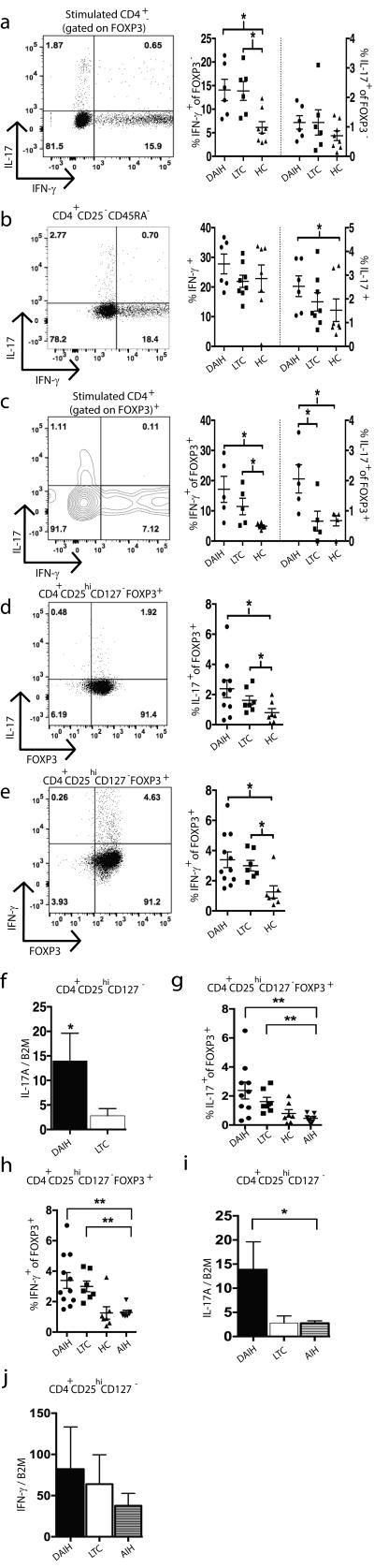
FIGURE 1.
FOXP3+ Tregs from patients with de novo autoimmune hepatitis display a pro-inflammatory phenotype. Total CD4+ T cells were isolated by negative selection and stained for fluorescence-activated cell sorting (FACS) with the following antibodies: anti-CD45RA, CD25, and CD127. The Treg (CD4+CD25hiCD127low/neg), Tmemory (CD4+CD25low/negCD45RA−) and Tnaive (CD4+CD25low/negCD45RA+) populations were sorted on a FACS Aria and stimulated with phobol-12-myristate-13-acetate (PMA) and ionomycin for 4-hours and intracellular staining of IFN-γ, IL-17A and FOXP3 was performed. RNA was isolated from the stimulated T cell populations and subjected to quantification of mRNA expression levels by RT-PCR.
A) IFN-γ secretion significantly higher from FOXP3− CD4+ T cells of subjects with de novo autoimmune hepatitis (dAIH) (n=6) compared to healthy non-transplanted subjects (HC) (n=8) (p=0.02); and from liver transplanted subjects without de novo autoimmune hepatitis (LTC) (n=6) compared to healthy non-transplanted subjects (p=0.01). IL-17 secretion from FOXP3− CD4+ T cells was not significantly different between the three subject groups.
B) IL-17 secretion from memory cells from subjects with de novo autoimmune hepatitis (n=6) significantly higher than that from memory cells of healthy non-transplanted subjects (n=7) (p=0.03). IFN-γ secretion from memory CD4+ T cells not significantly different between the three subject groups.
C) IFN-γ secretion from FOXP3+ CD4+ T cells from subjects with de novo autoimmune hepatitis (n=5) as well as liver transplanted subjects without de novo autoimmune hepatitis (n=5) significantly increased compared to healthy non-transplanted subjects (n=6) (p=0.01, p=0.03 respectively). IL-17 secretion from FOXP3+ CD4+ T cells from subjects with de novo autoimmune hepatitis (n=5) significantly increased compared to liver transplanted subjects without de novo autoimmune hepatitis (n=5) and healthy non-transplanted subjects (n=6) (p=0.04, p=0.01 respectively).
D) High IL-17 levels from Tregs isolated from subjects with de novo autoimmune hepatitis (n=10) compared to liver transplanted subjects without de novo autoimmune hepatitis (n=7). Tregs isolated from subjects with de novo autoimmune hepatitis as well as liver transplanted subjects without de novo autoimmune hepatitis secrete more IL-17 compared to Tregs from healthy non-transplanted subjects (n=7) (p=0.02).
E) High IFN-γ secretion from Tregs isolated from subjects with de novo autoimmune hepatitis (n=11) as well as liver transplanted subjects without de novo autoimmune hepatitis (n=7) compared to healthy non-transplanted subjects (n=7) (p=0.005; p=0.003 respectively).
F) Increased IL-17A mRNA expression in Tregs isolated from subjects with de novo autoimmune hepatitis (n=10) compared to liver transplanted subjects without de novo autoimmune hepatitis (n=12) (p=0.02).
G) High IL-17 production from Tregs isolated from liver transplanted subjects with/without de novo autoimmune hepatitis (n=10, n=7 respectively) compared to non-transplanted children with autoimmune hepatitis (AIH) (n=7) (p=0.009; p=0.004 respectively).
H) High IFN-γ levels from Tregs isolated from liver transplanted subjects with/without de novo autoimmune hepatitis (n=10, n=7 respectively) compared to non-transplanted children with autoimmune hepatitis (AIH) (n=7) (p=0.001 and 0.003 respectively).
I) Increased IL-17A mRNA expression in Tregs isolated from subjects with de novo autoimmune hepatitis (n=10) compared to non-transplanted children with autoimmune hepatitis (AIH) (n=7) (p=0.03).
J) IFN-γ mRNA expression not significantly different in Tregs isolated from subjects with de novo autoimmune hepatitis (n=9) vs. non-transplanted children with autoimmune hepatitis (AIH) (n=7).
IFN-γ production from FOXP3+ CD4+ T cells from subjects with de novo autoimmune hepatitis as well as liver transplanted subjects without de novo autoimmune hepatitis was significantly increased compared to healthy non-transplanted children (Figure 1C) (p=0.01, p=0.03 respectively); interestingly, IL-17 production from FOXP3+ CD4+ T cells from subjects with de novo autoimmune hepatitis was significantly increased compared to liver transplanted subjects without de novo autoimmune hepatitis and healthy non-transplanted children (p=0.04, p=0.01 respectively) (Figure 1C).
While IL-17 levels were higher from CD127-CD25hi….Tregs isolated from subjects with de novo autoimmune hepatitis compared to liver transplanted subjects without de novo autoimmune hepatitis, this difference did not achieve statistical significance (Figure 1D). However, Tregs from subjects with de novo autoimmune hepatitis as well as liver transplanted subjects without de novo autoimmune hepatitis did produce more IL-17 compared to Tregs from healthy non-transplanted children (p=0.02) (Figure 1D) and Tregs from non-transplanted children with autoimmune hepatitis (AIH) (p=0.009 and p=0.004 respectively) (Figure 1G); Similarly, IFN-γ production from Tregs isolated from subjects with de novo autoimmune hepatitis as well as liver transplanted subjects without de novo autoimmune hepatitis was significantly increased compared to healthy non-transplanted children (p=0.005; p=0.003 respectively) (Figure 1E) and non-transplanted children with autoimmune hepatitis (AIH) (p=0.001 and p=0.003 respectively) (Figure 1H). IL-17 production from memory cells from subjects with de novo autoimmune hepatitis was significantly higher than that from memory cells of healthy non-transplanted children (Figure 1B).
Not surprisingly, IL-17A mRNA levels were higher in Tregs isolated from subjects with de novo autoimmune hepatitis compared to liver transplanted subjects without de novo autoimmune hepatitis (p=0.02) (Figure 1F), and non-transplanted children with autoimmune hepatitis (p=0.03) (Figure 1I). In concordance with the protein data, IFN-γ mRNA expression in Tregs was not significantly different between the transplanted subjects with/without de novo autoimmune hepatitis (Supplementary Figure 2F). IFN-γ mRNA expression in Tregs was not significantly different between the transplanted subjects with de novo autoimmune hepatitis and non-transplanted children with autoimmune hepatitis (Figure 1J). Of note, anti-CD161, anti-TIM3, anti-LAG3 and anti-CD49D expression by Tregs was not significantly different between the transplanted subjects (data not shown).
Taken together, the above data suggests that increased frequencies of TH1-like Tregs are seen in the blood of liver transplanted recipients are similar to previous reports in kidney transplant recipients (26, 27) and classical autoimmune diseases like multiple sclerosis (28). Additionally, TH17-like Tregs are seen in the blood of liver transplanted recipients with de novo autoimmune hepatitis similar to previous reports in other human diseases (15, 16, 18, 19, 28). We therefore sought to characterize these potentially pro-inflammatory cytokine producing Tregs further in liver transplant recipients.
FOXP3+ Tregs from patients with de novo autoimmune hepatitis share phenotypic features with conventional effector T cells and are functionally impaired
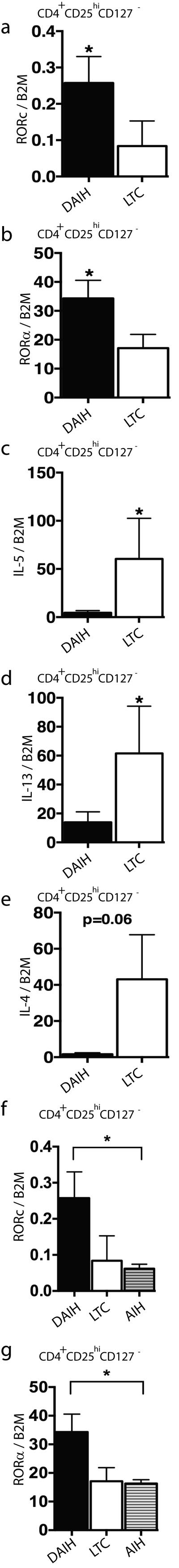
As IL-17 secretion has been associated with expression of RORC (29), our observation of production of IL-17 by Tregs isolated from subjects with de novo autoimmune hepatitis prompted us to assess the mRNA expression of RORC in isolated Treg populations. Tregs isolated from subjects with de novo autoimmune hepatitis had increased expression of RORC compared to Tregs from liver transplanted subjects without de novo autoimmune hepatitis (p=0.004) (Figure 2A) and Tregs from non-transplanted children with autoimmune hepatitis (p=0.03)(Figure 2F). RORA mRNA expression was also similarly increased in Tregs isolated from subjects with de novo autoimmune hepatitis compared to liver transplanted subjects without de novo autoimmune hepatitis (p=0.02) (Figure 2B) and non-transplanted children with autoimmune hepatitis (p=0.04) (Figure 2G). Not surprisingly, mRNA expression of the TH2 cytokines IL-5 and IL-13 was significantly higher in Tregs isolated from liver transplanted subjects without de novo autoimmune hepatitis compared to Tregs from subjects with de novo autoimmune hepatitis (p=0.02; p=0.03 respectively) (Figure 2C-D). IL-4 mRNA expression was also higher in Tregs isolated from liver transplanted subjects without de novo autoimmune hepatitis compared to Tregs from subjects with de novo autoimmune hepatitis however it did not achieve statistical significance (p=0.06) (Figure 2E). TBET and CXCR3-mRNA expression was not significantly different in Tregs isolated from liver transplanted subjects without de novo autoimmune hepatitis compared to Tregs from subjects with de novo autoimmune hepatitis (Supplementary Figure 2G-H).
FIGURE 2.
FOXP3+ Tregs from patients with de novo autoimmune hepatitis display some similarities with conventional TH17 cells. RNA was isolated from the stimulated T cell populations and subjected to quantification of mRNA expression levels by RT-PCR.
A-B) Increased RORC and RORA expression in Tregs isolated from subjects with de novo autoimmune hepatitis (dAIH) (n=10) compared to liver transplanted subjects without de novo autoimmune hepatitis (LTC) (n=12) (p=0.004 and 0.02 respectively).
C-E) IL-5 and IL-13 mRNA expression significantly higher in Tregs isolated from liver transplanted subjects without de novo autoimmune hepatitis compared to subjects with de novo autoimmune hepatitis (p=0.02; p=0.03 respectively). Similarly high IL-4 mRNA expression in Tregs isolated from liver transplanted subjects without de novo autoimmune hepatitis compared to subjects with de novo autoimmune hepatitis (p=0.06) (n=4 in each group).
F-G) Increased RORC and RORA expression in Tregs isolated from subjects with de novo autoimmune hepatitis (dAIH) (n=10) compared to Tregs from non-transplanted children with autoimmune hepatitis (AIH) (n=7) (p=0.03 and 0.04 respectively).
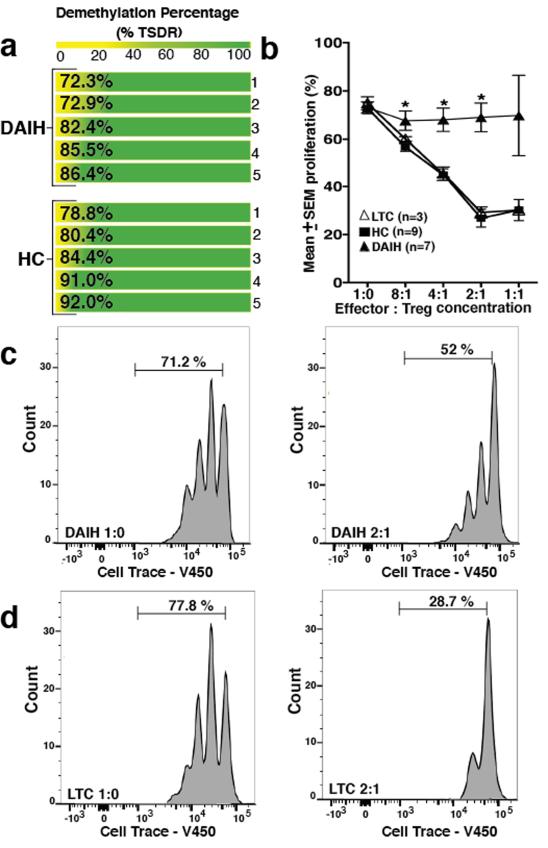
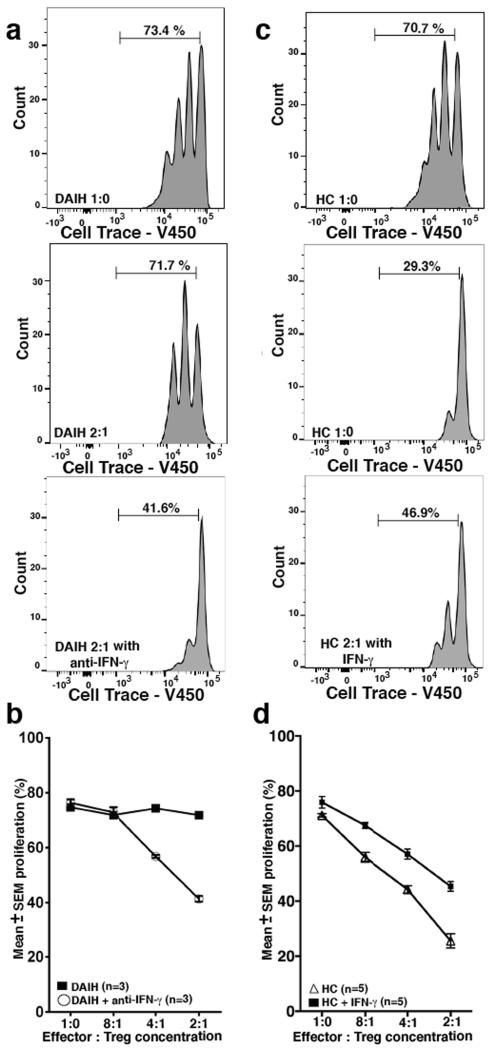
These observations of a pro-inflammatory signature in Tregs from transplant recipients with de novo autoimmune hepatitis led us to question the methylation status of the FOXP3 locus in these Tregs as well as the function of these Tregs. (FOXP3 DNA demethylation constitutes the most reliable criterion for natural Treg available at present (23). To address the above question about the methylation status of the FOXP3 locus as well as the function of these Tregs, we analyzed the FOXP3+ Treg cell-specific demethylated region (TSDR) in sorted Tregs from both healthy non-transplanted children and subjects with de novo autoimmune hepatitis. We also performed in vitro suppression assays and found that Tregs from both groups were similarly demethylated in the TSDR region (Figure 3A); but Tregs from subjects with de novo autoimmune hepatitis suppressed effector cell proliferation less efficiently compared to sorted Tregs from healthy non-transplanted children subjects and liver transplanted subjects without de novo autoimmune hepatitis (Figure 3B-D) (dAIH vs. LTC 2:1, 4:1, p=0.03, 0.02 respectively) (dAIH vs. HC 2:1, 4:1, 8:1, p=0.005, 0.002, 0.03 respectively). (Gating strategy Supplementary Figure 3A). They similarly inhibited cytolytic function of CD8 T cells less efficiently compared to sorted Tregs from a healthy non-transplanted child and a liver transplanted patient without de novo autoimmune hepatitis (Supplementary Figure 3B-D). Taken together, the above suggests that Tregs isolated from subjects with de novo autoimmune hepatitis are true Tregs and not significantly contaminated by activated effector T cells.
FIGURE 3.
Regulatory T cell function impaired in de novo autoimmune hepatitis. DNA harvested from sorted Tregs and analyzed by quantitative PCR (qPCR) for methylation status of the FOXP3 locus Treg Specific Demethylation Region (TSDR) (Epiontis GmBH). Sorted Tregs were also co-cultured with CFSE-labeled effector (responder) CD4+CD25−CD127+ T cells at 1:1, 2:1, 4:1, 8:1 Tresponder:Treg ratio and proliferation of viable responder T cells was analyzed on a LSR II flow cytometer.
A) Tregs from healthy non-transplanted subjects (HC) (n=5) as well as subjects with de novo autoimmune hepatitis (dAIH) (n=5) are similarly demethylated in the TSDR region.
B) Tregs from subjects with de novo autoimmune hepatitis (n=7) suppress effector cell proliferation less efficiently compared to sorted Tregs from healthy non-transplanted subjects (n=9) and liver transplanted subjects without de novo autoimmune hepatitis (LTC) (n=3) (dAIH vs. LTC 2:1, 4:1, p=0.03, 0.02 respectively) (dAIH vs. HC 2:1, 4:1, 8:1, p=0.005, 0.002, 0.03 respectively).
C) Representative histogram for dAIH. Tresponder:Treg ratio 2:1.
D) Representative histogram for LTC. Tresponder:Treg ratio 2:1.
Cytokine milieu in livers with de novo autoimmune hepatitis
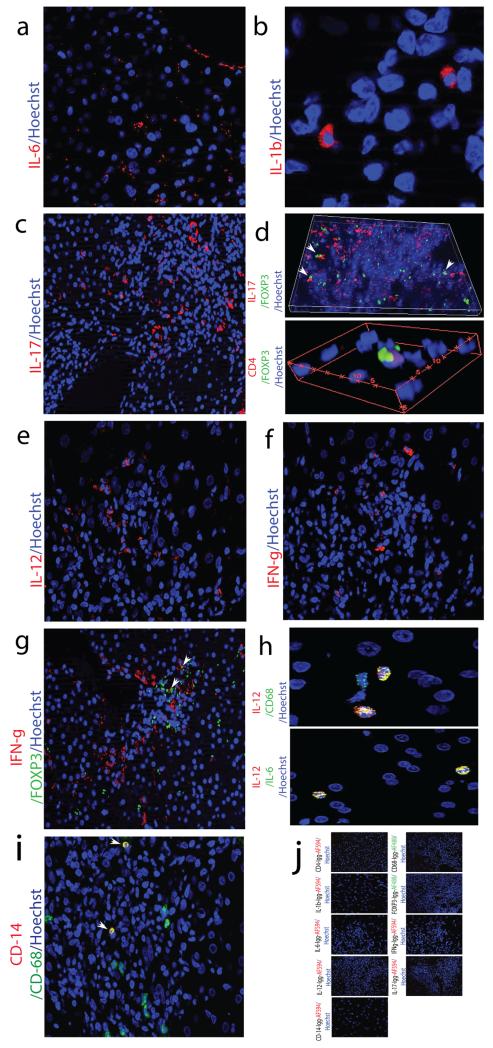
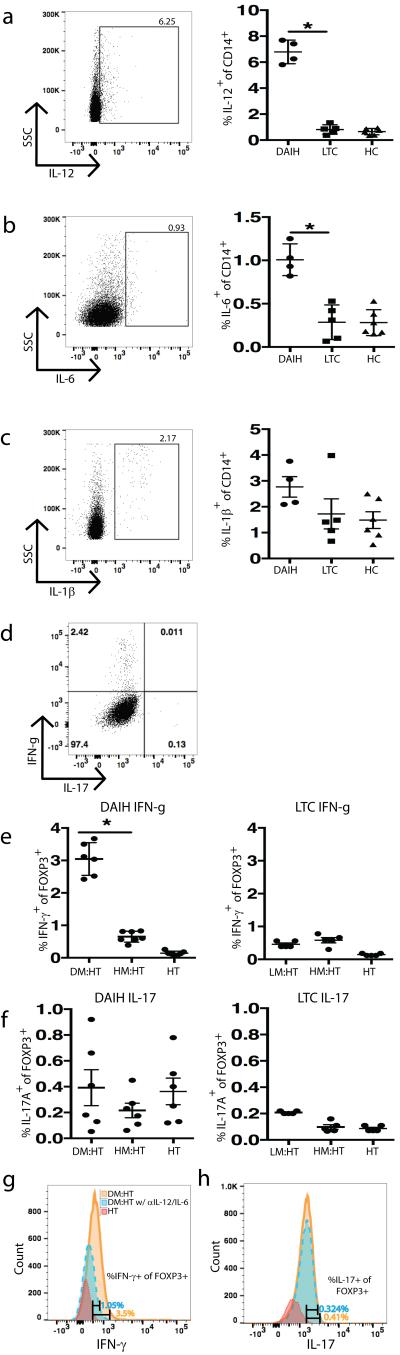
We next questioned if the loss of regulatory function noted could be a consequence of an inflammatory microenvironment? And thus could a pro-inflammatory cytokine milieu be contributing to the dysregulation of Tregs isolated from subjects with de novo autoimmune hepatitis, and driving the plasticity of Tregs towards an inflammatory phenotype? To investigate this, we first of all examined liver biopsies from subjects with de novo autoimmune hepatitis for pro-inflammatory cytokines using immunofluorescence. The TH17 polarizing cytokines (30, 31), IL-1β and IL-6 were present within portal inflammatory infiltrates, IL-6 more so than IL-1β (Figure 4A-B). Additionally, IL-17A was also abundantly present within portal inflammatory infiltrates and interestingly, both IL-17A and FOXP3 were co-expressed on the same cell (Figure 4C-D) suggesting a similar finding in the liver to our observation in the blood of IL-17 producing FOXP3+ Tregs. Interestingly, the TH1 polarizing cytokine, IL-12 as well as its signature cytokine, IFN-γ, was also present within portal inflammatory infiltrates of liver biopsies of subjects with de novo autoimmune hepatitis (Figure 4E-F). IFN-γ and FOXP3 were co-expressed on the same cell (Figure 4G). Of note, both IL-12 and CD68 were co-expressed on the same cell and IL-6 and IL-12 were also co-expressed on the same cell (Figure 4H) suggesting that the main sources of TH1 and TH17 promoting cytokines were coming from monocytes/macrophages. (Isotype controls provided Figure 4J).
FIGURE 4.
TH1 and TH17 polarizing cytokines and their key signature cytokines, IFN-γ and IL-17A, make up the cytokine milieu within livers with de novo autoimmune hepatitis. Formalin-fixed, paraffin-embedded 7-micron thick sections from liver biopsies of subjects with de novo autoimmune hepatitis (n=5) were stained for expression of CD4, FOXP3, CD68, IL-1β, IL-6, IL-17A, IL-12 and IFN-γ and examined using immunofluorescence. Representative slides from one patient are shown.
A) 40X magnification: a cluster of IL-6 positive cells within the portal tract (DAPI-blue, IL-6-red).
B) 40X magnification: very few IL-1β positive cells present within the portal tract (DAPI-blue, IL-1β-red).
C-D) 20X magnification: several IL-17A positive cells present within the portal tract (DAPI-blue, IL-17A-red) and co-expressed with FOXP3. Arrowheads point to FOXP3/IL17A co-expressing cells. (DAPI-blue, IL-17A-red, FOXP3 green). 60X magnification: Several CD4 positive cells seen that co-express with FOXP3 (DAPI-blue, CD4-green, FOXP3-red).
E-F) 20X magnification: the TH1 polarizing cytokine, IL-12 (DAPI-blue, IL-12-red) as well as its signature cytokine, IFN-γ (DAPI-blue, IFN-γ-red), present within portal inflammatory infiltrates.
G) 20X magnification: a few IFN-γ positive cells co-expressed with FOXP3 on same cell. Arrowheads point to FOXP3/IFN-γ co-expressing cells. (DAPI-blue, FOXP-green, IFN-γ-red).
(H) 60X magnification: IL-12 co-expressed with CD68 (DAPI-blue, IL-12-red, CD68-green); as well as IL-6 (40X magnification) (DAPI-blue, IL-12-red, IL-6-green).
(I) 20X magnification: CD14+ cells co-expressed with CD68+ cells (white arrows) (DAPI-blue, CD14-red, CD68-green).
(J) 20X magnification. Representative slides from isotype controls from one patient are shown.
Given the above observation of TH1 and TH17 polarizing cytokines being produced by monocytes/macrophages in liver biopsies of subjects with de novo autoimmune hepatitis, we questioned if monocytes/macrophages drive the inflammatory phenotype observed in Tregs of subjects with de novo autoimmune hepatitis using in vitro culture of monocytes and sorted Tregs.
Monocytes from subjects with de novo autoimmune hepatitis produce large amounts of pro-inflammatory cytokines and promote inflammatory Treg plasticity
To investigate if monocytes/macrophages display a pro-inflammatory profile and could thus drive the inflammatory phenotype observed in Tregs of subjects with de novo autoimmune hepatitis, CD14+ monocytes from healthy non-transplanted children, liver transplanted subjects without de novo autoimmune hepatitis and subjects with de novo autoimmune hepatitis were isolated and tested in vitro for their cytokine production. Importantly, monocytes from subjects with de novo autoimmune hepatitis were producing large amounts of the TH1 and TH17-type Treg promoting cytokines in contrast to monocytes from liver transplanted subjects without de novo autoimmune hepatitis and healthy non-transplanted children (Figure 5A-C) (p=0.01 for IL-12 and IL-6). (Gating strategy Supplementary Figure 4B).
FIGURE 5.
Monocytes from subjects with de novo autoimmune hepatitis secrete pro-inflammatory cytokines inducing TH1 cells. Monocytes from the subjects with de novo autoimmune hepatitis (n=4), liver transplanted subjects without de novo autoimmune hepatitis (n=5) and healthy non-transplanted subjects (n=6) were stimulated with LPS for 24-hours and stained with anti-CD3, anti-CD14, intracellular cytokines IL-12, IL-6 and IL-1β. Cytokine secretion analyzed using flow cytometry.
CD14+ monocytes from healthy non-transplanted subjects (n=6), liver transplanted subjects without de novo autoimmune hepatitis (n=5) and subjects with de novo autoimmune hepatitis (n=6) were co-cultured with sorted Tregs from healthy non-transplanted subjects in the presence of plate bound anti-CD3 for 5-days and IL-17A and IFN-γ secretion from FOXP3+ Tregs was assessed using flow cytometry. CD14+ monocytes from a subject with de novo autoimmune hepatitis were co-cultured with sorted Tregs (CD4+CD25hiCD127−) from a healthy non-transplanted subject in the presence of anti-IL-12, anti-IL-6, and plate bound anti-CD3 for 5-days and IL-17A and IFN-γ secretion from FOXP3+ Tregs was assessed using flow cytometry.
A-C) Increased IL-12 and IL-6 secretion from monocytes of subjects with de novo autoimmune hepatitis (dAIH) (p=0.01 for IL-12 and IL-6). IL-1β secretion not significantly different between the three patient groups.
D) Representative histogram for co-culture of Tregs isolated from healthy non-transplanted subjects (HC) with monocytes from subjects with dAIH.
E-F) monocytes from subjects with dAIH induce IFN-γ (but not IL-17A) secretion from FOXP3+ Tregs of HC (p=0.03), however monocytes from liver transplanted subjects without de novo autoimmune hepatitis (LTC) fail to induce significant amounts of IFN-γ and IL-17A secretion from FOXP3+ Tregs of HC. (DM: dAIH monocytes; HM: HC monocytes; HT: HC Tregs; LM: LTC monocytes)
G-H) monocytes from a subject with de novo autoimmune hepatitis induce IFN-γ (but not IL-17A) production from FOXP3+ Tregs of a healthy control, however in the presence of anti-IL12, this induction of IFN-γ is inhibited. Blockade with anti-IL-6 has no influence on IL-17A production.
To directly assess if monocytes from subjects with de novo autoimmune hepatitis impact the Treg phenotype, we co-cultured similar cells with sorted Tregs from healthy non-transplanted children in the presence of plate bound anti-CD3 for 5-days (32). At the end of the culture period, IL-17A and IFN-γ production from Foxp3+ Tregs was assessed using flow cytometry. (Gating strategy Supplementary Figure 4A). In line with our hypothesis, there was a strong tendency for increased IFN-γ production from FOXP3+ Tregs in the presence of monocytes from subjects with de novo autoimmune hepatitis but not when co-cultured with monocytes from healthy non-transplanted children or liver transplanted subjects without de novo autoimmune hepatitis. However, and of interest, IL-17 production by Tregs was not affected under similar conditions at the analyzed time point (Figure 5E-F) (p=0.03 for IFN-γ). To confirm that IFN-γ production from Tregs results from induction by IL-12 from monocytes, sorted Tregs from a healthy non-transplanted child were co-cultured with monocytes from a subject with de novo autoimmune hepatitis in the presence of plate bound anti-CD3 for 5-days following IL-12 and IL-6 blockade and as expected there was decreased IFN-γ production from FOXP3+ Tregs in the presence of monocytes from a subject with de novo autoimmune hepatitis, in the face of IL-12 blockade (Figure 5G). Also as expected, blockade of IL-6 did not result in a change in IL-17 production (Figure 5H). To directly assess the nature of these inflammatory macrophages/monocytes producing IL-12 and IL-6 and determine if they are tissue resident macrophages i.e. Kupffer cells or inflammatory monocytes recruited from the blood, paraffin embedded liver biopsy sections of patients with de novo autoimmune hepatitis was stained for both CD14 and CD68 using immunofluorescence. Indeed, CD14+CD68+ co-expressing cells could be detected (Figure 4I).
Taken together, the above suggests that monocytes/macrophages drive IFN-γ production from FOXP3+ Tregs and the TH1-like Treg phenotype in subjects with de novo autoimmune hepatitis. To exclude that cytokine production from FOXP3+ Tregs in the co-culture experiments with monocytes is only a result of allogeneity, we controlled the co-culture experiments in the presence and absence of plate bound anti-CD3 and confirmed the absence of cytokine production in the absence of plate bound anti-CD3 (Supplementary Figure 4C) (32).
Blockade of IFN-γ partially restores Treg function in de novo autoimmune hepatitis
The function of TH1-like Tregs has been variably reported as suppressive by some investigators (33-35) and non-suppressive by others (28). Having demonstrated that sorted Tregs from subjects with de novo autoimmune hepatitis lost their immunoregulatory function as well as demonstrating that a pro-inflammatory cytokine milieu within the liver in de novo autoimmune hepatitis can drive IFN-γ secretion from FOXP3+ Tregs, we next questioned if IFN-γ blockade would restore Treg function. To address this question, we performed suppression assays with FACS-sorted Tregs from subjects with de novo autoimmune hepatitis, co-cultured with CFSE-labeled effector cells, in the presence and absence of anti-IFN-γ.
Of note, the presence of anti-IFN-γ partially restored the suppressive capacity of Tregs from subjects with de novo autoimmune hepatitis, though not as fully as compared to Tregs from healthy non-transplanted children, indicating a significant role of the TH1-like Treg phenotype in the loss of suppression (Figure 6A-B). In line with this finding, the addition of IFN-γ to co-cultures of sorted Tregs from healthy non-transplanted children with effector cells, showed loss of suppression of effector cell proliferation by the Tregs in counter experiments (Figure 6C-D).
FIGURE 6.
Partial restoration of regulatory T cell function in Tregs of subjects with de novo autoimmune hepatitis following IFN-γ blockade. Sorted Tregs were co-cultured with CFSE-labeled effector (responder) CD4+CD25−CD127+ T cells at 1:1, 2:1, 4:1, 8:1 Tresponder:Treg ratio in the presence (n=3) and absence of anti-IFN-γ (n=3) and proliferation of viable responder T cells was analyzed on a LSR II flow cytometer. In counter experiments, Sorted Tregs were co-cultured with CFSE-labeled effector (responder) CD4+CD25−CD127+ T cells at 1:1, 2:1, 4:1, 8:1 Tresponder:Treg ratio in the presence (n=5) and absence (n=5) of IFN-γ.
A-B) blockade of IFN-γ secretion enhances suppressive function of Tregs from subjects with de novo autoimmune hepatitis (dAIH) however suppressive function is not completely restored to the level seen with Tregs isolated from healthy non-transplanted subjects (HC). Representative histogram for Tresponder:Treg ratio 2:1 without/with anti-IFN-γ and summary plot shown.
C-D) addition of IFN-γ to co-cultures of Tregs isolated from HC with effector cells, showed loss of suppression of effector cell proliferation by the Tregs. Representative histogram for Tresponder:Treg ratio 2:1 without/with IFN-γ and summary plot shown.
These results suggest that an inflammatory microenvironment as that seen in de novo autoimmune hepatitis livers could drive the induction of TH1-like Tregs, which seems to be partially responsible for the observed impaired immunoregulatory function. Of interest, a similar phenomenon has been described for several classical autoimmune diseases such as multiple sclerosis or T1D (28).
DISCUSSION
In this study we show that FOXP3+ Tregs isolated from peripheral blood of subjects with de novo autoimmune hepatitis display phenotypic and functional characteristics that are similar to many classical autoimmune diseases. Tregs from subjects with de novo autoimmune hepatitis are severely functionally impaired in in vitro suppression assays in line with a prominent TH1 and TH17-like pro-inflammatory phenotype, characterized by the production of inflammatory cytokines like IFN-γ and IL-17. Remarkably, these Tregs maintain a fully demethylated FOXP3 locus.
We further demonstrated that two cytokine clusters - the IL-12-IFN-γ axis and the IL-6-IL-17 axis are predominantly found in livers of patients with de novo autoimmune hepatitis and that the expression of IL-12 and IL-6 segregates with monocyte/macrophages. In line with this, monocytes isolated from the peripheral blood of patients with de novo autoimmune hepatitis produce significant amounts of inflammatory cytokines, which could drive the differentiation towards a pro-inflammatory Treg phenotype. In particular the production of IL-12 seems to promote the induction of IFN-γ producing TH1-like Tregs which seems to significantly contribute to the observed loss of suppression of Tregs from subjects with de novo autoimmune hepatitis in an IFN-γ dependent manner. The blockade of IFN-γ partially restored Treg function of patients with de novo autoimmune hepatitis. Thus monocytes/macrophages seem to be the key in promoting an inflammatory milieu that subsequently leads to the observed functional impairment of Tregs of patients with de novo autoimmune hepatitis and disease pathogenesis. This finding is further corroborated by the observation that cytokine co-expressing FOXP3+ cells are seen in the liver of subjects with de novo autoimmune hepatitis.
Taken together, these findings establish a framework to dissect the cell type(s) driving differentiation of Tregs towards a pro-inflammatory phenotype in de novo autoimmune hepatitis as well as dissecting triggers that can induce local release of cytokines to direct Tregs cells towards the TH1/TH17 differentiation pathway. Longhi et al have also elegantly shown vigorous activation of peripheral blood monocytes from non-transplanted children with autoimmune liver disease and inability of CD4+CD25+ and CD4+CD25+CD127− Tregs to restrain monocyte activation likely contributing to loss of immune tolerance and perpetuation of the autoimmune attack in autoimmune liver disease (36).
Our observation of TH1-like Tregs in liver transplanted recipients is consistent with reports of TH1-like Tregs in kidney transplant recipients where it’s been suggested that IFN-γ-mediated immune mechanisms contribute to long-term graft acceptance; indeed kidney transplant recipients with good graft function have been shown to have more CD4+CD25+FOXP3+IFN-γ+ T cells in peripheral blood compared to renal transplant recipients with impaired graft function. In the present study we show that TH1-like Tregs are present in peripheral blood of liver transplant recipients corroborating the finding in renal transplant recipients (26); Similarly, TH1-like Tregs from transplant recipients with normal allograft function in the present study had increased IL-4 expression (Figure 2E); however contrary to the report by Daniel et al, TH1-like Tregs was seen in both liver transplant recipients with normal allograft function as well as those with de novo autoimmune hepatitis, additionally, the frequency of TH1-like Tregs was not significantly higher in liver transplant recipients with normal allograft function compared to recipients with allograft dysfunction from de novo autoimmune hepatitis. This may be due to the difference in definition of TH1-like Tregs – (CD4+CD25hiCD127−FOXP3+IFN-γ+ in the present study vs. CD3+CD4+CD25+FOXP3+IFN-γ+ in the study by Daniel et al), as well as differences in the experiment design – (in our study IFN-γ production from sorted Tregs was measured in response to stimulation with PMA/ionomycin whereas peripheral blood lymphocytes were stained and not sorted and no stimulation was used in the study by Daniel et al).
Liver biopsies from subjects with de novo autoimmune hepatitis show all the features of a TH1 lesion (Figure 4F). The functional activity of TH1 cells is regulated by IL-12 and this polarizing cytokine is identified in liver biopsies from subjects with de novo autoimmune hepatitis (Figure 4E). The cellular source of IL-12 appears to be monocytes/macrophages (Figure 4H, 5A). It is unclear which triggers can induce local release of IL-12 to direct Treg cells towards the TH1 differentiation pathway however the role of microbial components and toll like receptors in initiating immune activation needs to be examined (37) since our results indicate that monocytes are key for the production of IFN-γ from Tregs through their production of IL-12 (Figures 4H, 5A, 5E). With regards to the nature of these inflammatory macrophages/monocytes, in humans, the major population of monocytes (~90%) expresses high levels of CD14 but no CD16 (CD14++CD16−) and are termed classical monocytes. The minor population (~10%) of human monocytes is divided into the intermediate subset with high CD14 and low CD16 (CD14++CD16+) and the non-classical subset with low CD14 and high CD16 (CD14+CD16++) (38). There is an enhanced ability of monocytes to enter the liver from blood via hepatic endothelium as reported by Liaskou et al who show monocyte transendothelial migration across inflamed hepatic sinusoidal endothelium in vitro (39). Monocytes circulate in blood for several days before migrating into tissues where they differentiate into tissue macrophages or dendritic cells (40). Co-expression of CD14, CD16 and CD68 on a population of large cells with the morphology of macrophages are present throughout the liver sinusoids. CD14+CD68− and CD16+CD68− cells as well as CD14+CD68+ and CD16+CD68+ cells are also detected, confirming that distinct monocyte-derived and sessile macrophage populations coexist in the liver (39). Similar to the report by Liaskou et al, we have shown co-expression of CD14+CD68+ cells in the liver of patients with de novo autoimmune hepatitis confirming coexistence of monocyte-derived and tissue macrophages in the liver as well as suggesting that these monocytes are recruited from the blood (Figure 4I).
Interestingly, while TH1-like Tregs maintained their immunomodulatory role in liver transplant recipients with normal allograft function, similar to previous reports (33-35), in the setting of inflammation (i.e. in transplant recipients with de novo autoimmune hepatitis), these Tregs lost the ability to suppress effector cell proliferation (Figure 3B-C). The presence of TH1-like Tregs which are fully demethylated at the FOXP3 locus, while possessing inflammatory features and lacking suppressive function has been previously reported in untreated patients with relapsing, remitting multiple sclerosis (RRMS) (28). Dominguez-Villar et al reported that human Tregs acquire a TH1-like phenotype when cultured in the presence of IL-12, and showed reduced suppressive activity in vitro (28). Similar to the report by Dominguez-Villar et al, non-suppressive activity of Tregs from transplant recipients with de novo autoimmune hepatitis could be partially reversed by anti-IFN-γ specific antibody (Figure 6A-B). Indeed blocking IFN-γ partially restored suppressive activity of Tregs of subjects with de novo autoimmune hepatitis suggesting that other mechanisms may also account for the defect in suppression. Our data provides a general mechanism by which proinflammatory cytokines such as IL-12 can rapidly alter the phenotype and function of Treg cells, decreasing their suppressive activity, and underscore the plasticity of Treg cells in a proinflammatory environment.
The TH17 effector cytokine, IL-17, has been implicated in the pathogenesis of several autoimmune diseases (41-45); Not surprisingly, TH17 polarizing cytokines IL-1β and IL-6 as well as the TH17 effector cytokine, IL-17A, (Figures 4A-C) are abundant in livers with de novo autoimmune hepatitis. In essence, a cluster of TH17 related cytokines participate in the inflammatory process in de novo autoimmune hepatitis. Of note, some of the IL-17A+ cells co-express CD4+ and FOXP3+ (Figure 4D) suggesting that some of the IL-17A seen in the liver comes from Tregs corroborating the finding of increased TH17-like Treg frequency in the blood.
Besides its role in inducing TH17 differentiation, IL-6 has also been implicated in regulating the induction of anti-inflammatory regulatory T cells. Specifically, the developmental pathways leading to the induction of TH17 and Treg cell seem reciprocal with IL-6, IL-21 and IL-23 blocking the Treg cell specifying transcription factor FOXP3 and instead up-regulating the TH17-inducing RORγt transcription factor (46, 47). Even though we observed similar FOXP3 mRNA expression in Tregs from both transplanted groups of patients (Supplementary figure 2E), Tregs of subjects with de novo autoimmune hepatitis had significant up-regulation of the TH17-inducing transcription factors RORC and RORA (Figure 2A-B). Thus IL-6 might have two major effects in de novo autoimmune hepatitis; promoting pro-inflammatory T cell immunity and paralyzing opposing anti-inflammatory T cells.
While IL-17 mRNA expression is significantly higher in Tregs from subjects with de novo autoimmune hepatitis compared to Tregs from transplanted subjects without de novo autoimmune hepatitis (Figure 1F), this difference is lost at the protein level (Figure 1D). There are several possible explanations for this. Protein expression is determined at many levels including (1) RNA expression, (2) translation efficiency and (3) protein stability (48). Indeed protein levels have been shown to often correlate poorly with RNA expression (49). Translation efficiency, i.e. the number of proteins synthesized per mRNA, has been suggested to account for a large component of the unexplained variation in protein levels (48). In mammalian cells, 30%-40% of the variance in protein abundance is explained by mRNA abundance. A similarly large fraction of variance can be explained by other factors, which is indicative of post-transcriptional and translational regulation and protein degradation (49).
With regards to the mechanism(s) underlying IL-17 production from Tregs, while IL-6 is produced by monocytes/macrophages of subjects with de novo autoimmune hepatitis, (Figures 4H, 5B); this (monocytes) does not appear to be the underlying mechanism accounting for IL-17 secretion from Tregs. We did however observe that memory cells from subjects with de novo autoimmune hepatitis produce significantly more IL-17 compared to memory cells from healthy non-transplanted children (Figure 1B) suggesting the need for further experiments to explore how memory cells are related to IL-17 production from Tregs. Additionally, it may also be worthwhile pursuing identification of genes underlying plasticity and cytokine production from these Tregs.
A pressing and unanswered question is the functional relevance of TH17-like Tregs in disease pathogenesis of de novo autoimmune hepatitis. TH17-like Tregs are reported to be suppressive (14, 16, 50) however in our hands we were unable to demonstrate suppression of effector cell proliferation (Figure 3B-C) and even following blockade of IFN-γ, we observed only partial restoration of suppressive function (Figure 6A-B) which makes it tempting to speculate if a pro-inflammatory milieu that includes IL-17 could contribute to Treg dysfunction and hence disease pathogenesis in de novo autoimmune hepatitis.
One of the limitations of our study is the fact that we couldn’t obtain liver biopsies from all subjects with de novo autoimmune hepatitis at the time of blood draw in an attempt to correlate inflammation and disease activity histologically with plasticity and function of Tregs. One could argue that aminotransferase levels would have been an adequate surrogate however it is well known that normal aminotransferase levels do not always correlate with absence of inflammation and disease activity histologically in de novo autoimmune hepatitis (51-56). Also, as the subjects with de novo autoimmune hepatitis did not receive anti-IFN-γ, we do not know if partial restoration of suppressive function of Tregs is associated with clinical improvement in disease activity. Another limitation is our inability to demonstrate function of Tregs isolated from liver tissue directly. This notwithstanding, the results from our immunofluorescence experiments suggest that they demonstrate a similar phenotype as seen in peripheral blood. Lastly, as HLA typing was not available in the recipients, and the donor HLA was also unknown, we are unable to say with any certainty if IL-17 producing Tregs is restricted by recipient/donor MHC.
In conclusion, we demonstrate that: (i) monocyte/macrophage derived cytokines of the IL-12-IFN-γ cluster and IL-6-IL-17 cluster are associated with de novo autoimmune hepatitis resulting in a pro-inflammatory milieu that could drive paralysis of regulatory function, and (ii) Tregs of patients with de novo autoimmune hepatitis display a pro-inflammatory phenotype and are functionally impaired akin to similar observations in many classical autoimmune diseases. Based on these findings, we hypothesize that monocytes/macrophages contribute significantly to the milieu in the liver that drives the induction of pro-inflammatory Tregs in patients with de novo autoimmune hepatitis and compromises their regulatory function (Figure 7). As this phenomenon plays a key role in several classical autoimmune diseases, it is highly likely that it contributes to the pathogenesis in de novo autoimmune hepatitis as well, and interference with this pathway could potentially offer new therapeutic options. However, more mechanistic studies applying pharmacological and genetic knockdown technologies in experimental systems are required to decipher mechanisms that underlie the involved induction of pro-inflammatory Tregs.
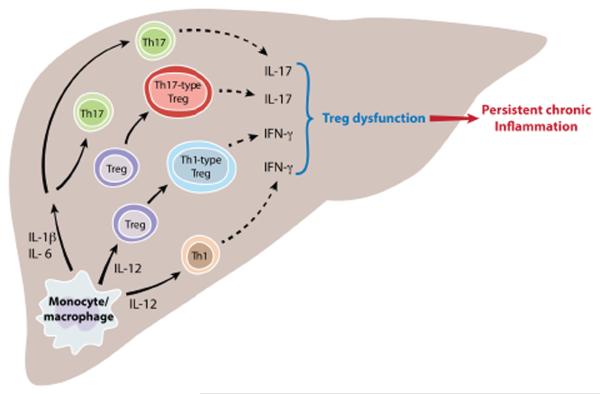
FIGURE 7. Working model for pathogenesis of de novo autoimmune hepatitis.
Two dominant cytokine clusters - the IL-12-IFN-γ axis and the IL-6-IL-17 axis contribute to de novo autoimmune hepatitis. Monocytes drive differentiation towards a TH1-like Treg phenotype through IL-12 secretion. The resulting pro-inflammatory milieu leads to paralysis of regulatory function.
Supplementary Material
ACKNOWLEDGEMENTS
CTSA Grant Number UL1 TR000142 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health.
National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number P30KD034989.
M.K. was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (640116).
4.Abbreviations
- (dAIH)
De novo autoimmune hepatitis
- (LTC)
Liver transplanted control
- (HC)
Healthy non-transplanted control
- (ROR)
Retinoid related orphan receptor
BIBLIOGRAPHY
- 1.Venick RS, McDiarmid SV, Farmer DG, Gornbein J, Martin MG, Vargas JH, Ament ME, Busuttil RW. Rejection and steroid dependence: unique risk factors in the development of pediatric posttransplant de novo autoimmune hepatitis. Am J Transplant. 2007;7:955–963. doi: 10.1111/j.1600-6143.2006.01717.x. [DOI] [PubMed] [Google Scholar]
- 2.Salcedo M, Vaquero J, Banares R, Rodriguez-Mahou M, Alvarez E, Vicario JL, Hernandez-Albujar A, Tiscar JL, Rincon D, Alonso S, De Diego A, Clemente G. Response to steroids in de novo autoimmune hepatitis after liver transplantation. Hepatology. 2002;35:349–356. doi: 10.1053/jhep.2002.31167. [DOI] [PubMed] [Google Scholar]
- 3.Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Farmer DG, Busuttil RW, McDiarmid SV, Reed EF. Donor-specific HLA Antibodies Are Associated With Late Allograft Dysfunction After Pediatric Liver Transplantation. Transplantation. 2015;99:1416–1422. doi: 10.1097/TP.0000000000000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montano-Loza AJ, Vargas-Vorackova F, Ma M, Bain VG, Burak K, Kumar T, Mason AL. Incidence and risk factors associated with de novo autoimmune hepatitis after liver transplantation. Liver Int. 2012;32:1426–1433. doi: 10.1111/j.1478-3231.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 5.Aguilera I, Wichmann I, Sousa JM, Bernardos A, Franco E, Garcia-Lozano JR, Nunez-Roldan A. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with de novo immune hepatitis following liver transplantation. Clin Exp Immunol. 2001;126:535–539. doi: 10.1046/j.1365-2249.2001.01682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguilera I, Sousa JM, Gavilan F, Bernardos A, Wichmann I, Nunez-Roldan A. Glutathione S-transferase T1 mismatch constitutes a risk factor for de novo immune hepatitis after liver transplantation. Liver Transpl. 2004;10:1166–1172. doi: 10.1002/lt.20209. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Mahou M, Salcedo M, Fernandez-Cruz E, Tiscar JL, Banares R, Clemente G, Vicario JL, Alvarez E, Rodriguez-Sainz C. Antibodies against glutathione S-transferase T1 (GSTT1) in patients with GSTT1 null genotype as prognostic marker: long-term follow-up after liver transplantation. Transplantation. 2007;83:1126–1129. doi: 10.1097/01.tp.0000259963.47350.da. [DOI] [PubMed] [Google Scholar]
- 8.Aguilera I, Sousa JM, Gavilan F, Gomez L, Alvarez-Marquez A, Nunez-Roldan A. Complement component 4d immunostaining in liver allografts of patients with de novo immune hepatitis. Liver Transpl. 2011;17:779–788. doi: 10.1002/lt.22302. [DOI] [PubMed] [Google Scholar]
- 9.Huguet S, Vinh J, Johanet C, Samuel D, Gigou M, Zamfir O, Duclos-Vallee JC, Ballot E. Identification by proteomic tool of atypical anti-liver/kidney microsome autoantibodies targets in de novo autoimmune hepatitis after liver transplantation. Ann N Y Acad Sci. 2007;1109:345–357. doi: 10.1196/annals.1398.041. [DOI] [PubMed] [Google Scholar]
- 10.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 13.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blatner NR, Mulcahy MF, Dennis KL, Scholtens D, Bentrem DJ, Phillips JD, Ham S, Sandall BP, Khan MW, Mahvi DM, Halverson AL, Stryker SJ, Boller AM, Singal A, Sneed RK, Sarraj B, Ansari MJ, Oft M, Iwakura Y, Zhou L, Bonertz A, Beckhove P, Gounari F, Khazaie K. Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci Transl Med. 2012;4:164ra159. doi: 10.1126/scitranslmed.3004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. Journal of immunology. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 19.McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmuller U, Baron U, Olek S, Bluestone JA, Brusko TM. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. Journal of immunology. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mieli-Vergani G, Vergani D. Autoimmune hepatitis. Nat Rev Gastroenterol Hepatol. 2011;8:320–329. doi: 10.1038/nrgastro.2011.69. [DOI] [PubMed] [Google Scholar]
- 21.Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ, Ma Y, Lenzi M, Mieli-Vergani G, Bianchi FB, Vergani D, Muratori L. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. doi: 10.1002/hep.23792. [DOI] [PubMed] [Google Scholar]
- 22.Banff Working, G. Demetris AJ, Adeyi O, Bellamy CO, Clouston A, Charlotte F, Czaja A, Daskal I, El-Monayeri MS, Fontes P, Fung J, Gridelli B, Guido M, Haga H, Hart J, Honsova E, Hubscher S, Itoh T, Jhala N, Jungmann P, Khettry U, Lassman C, Ligato S, Lunz JG, 3rd, Marcos A, Minervini MI, Molne J, Nalesnik M, Nasser I, Neil D, Ochoa E, Pappo O, Randhawa P, Reinholt FP, Ruiz P, Sebagh M, Spada M, Sonzogni A, Tsamandas AC, Wernerson A, Wu T, Yilmaz F. Liver biopsy interpretation for causes of late liver allograft dysfunction. Hepatology. 2006;44:489–501. doi: 10.1002/hep.21280. [DOI] [PubMed] [Google Scholar]
- 23.Baron U, Floess S, Wieczorek G, Baumann K, Grutzkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, Turbachova I, Hamann A, Olek S, Huehn J. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol. 2007;37:2378–2389. doi: 10.1002/eji.200737594. [DOI] [PubMed] [Google Scholar]
- 24.Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K, Pratschke J, Hamann A, Loddenkemper C, Stein H, Volk HD, Hoffmuller U, Grutzkau A, Mustea A, Huehn J, Scheibenbogen C, Olek S. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res. 2009;69:599–608. doi: 10.1158/0008-5472.CAN-08-2361. [DOI] [PubMed] [Google Scholar]
- 25.Sehouli J, Loddenkemper C, Cornu T, Schwachula T, Hoffmuller U, Grutzkau A, Lohneis P, Dickhaus T, Grone J, Kruschewski M, Mustea A, Turbachova I, Baron U, Olek S. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics. 2011;6:236–246. doi: 10.4161/epi.6.2.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel V, Naujokat C, Sadeghi M, Weimer R, Renner F, Yildiz S, Opelz G. Observational support for an immunoregulatory role of CD3+CD4+CD25+IFN-gamma+ blood lymphocytes in kidney transplant recipients with good long-term graft outcome. Transpl Int. 2008;21:646–660. doi: 10.1111/j.1432-2277.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 27.Daniel V, Sadeghi M, Wang H, Opelz G. CD4(+)CD25(+)Foxp3(+)IFNgamma(+) Treg are immunosuppressive in vitro and increase with intensity of the alloresponse in pretransplant MLC. Transpl Immunol. 2012;27:114–121. doi: 10.1016/j.trim.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 30.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 31.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nature immunology. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradshaw EM, Raddassi K, Elyaman W, Orban T, Gottlieb PA, Kent SC, Hafler DA. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. Journal of immunology. 2009;183:4432–4439. doi: 10.4049/jimmunol.0900576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng T, Cao AT, Weaver CT, Elson CO, Cong Y. Interleukin-12 converts Foxp3+ regulatory T cells to interferon-gamma-producing Foxp3+ T cells that inhibit colitis. Gastroenterology. 2011;140:2031–2043. doi: 10.1053/j.gastro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesenacker AM, Bending D, Ursu S, Wu Q, Nistala K, Wedderburn LR. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood. 2013;121:2647–2658. doi: 10.1182/blood-2012-08-443473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pesenacker AM, Wedderburn LR. T regulatory cells in childhood arthritis--novel insights. Expert Rev Mol Med. 2013;15:e13. doi: 10.1017/erm.2013.14. [DOI] [PubMed] [Google Scholar]
- 36.Longhi MS, Mitry RR, Samyn M, Scalori A, Hussain MJ, Quaglia A, Mieli-Vergani G, Ma Y, Vergani D. Vigorous activation of monocytes in juvenile autoimmune liver disease escapes the control of regulatory T-cells. Hepatology. 2009;50:130–142. doi: 10.1002/hep.22914. [DOI] [PubMed] [Google Scholar]
- 37.Jo J. Toll-like receptor 8 agonist and bacteria trigger potent activationof innate immune cells in human liver. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 39.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, Qureshi O, Lalor PF, Shaw J, Syn WK, Curbishley SM, Adams DH. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology. 2013;57:385–398. doi: 10.1002/hep.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology. 2006;211:609–618. doi: 10.1016/j.imbio.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Golden JB, McCormick TS, Ward NL. IL-17 in psoriasis: implications for therapy and cardiovascular co-morbidities. Cytokine. 2013;62:195–201. doi: 10.1016/j.cyto.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nylander A, Hafler DA. Multiple sclerosis. The Journal of clinical investigation. 2012;122:1180–1188. doi: 10.1172/JCI58649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–887. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jandus C, Bioley G, Rivals JP, Dudler J, Speiser D, Romero P. Increased numbers of circulating polyfunctional Th17 memory cells in patients with seronegative spondylarthritides. Arthritis Rheum. 2008;58:2307–2317. doi: 10.1002/art.23655. [DOI] [PubMed] [Google Scholar]
- 46.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 47.Barbi J, Pardoll D, Pan F. Metabolic control of the Treg/Th17 axis. Immunol Rev. 2013;252:52–77. doi: 10.1111/imr.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cenik C, Cenik ES, Byeon GW, Grubert F, Candille SI, Spacek D, Alsallakh B, Tilgner H, Araya CL, Tang H, Ricci E, Snyder MP. Integrative analysis of RNA, translation, and protein levels reveals distinct regulatory variation across humans. Genome Res. 2015;25:1610–1621. doi: 10.1101/gr.193342.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13:227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beriou G, Costantino CM, Ashley CW, Yang L, Kuchroo VK, Baecher-Allan C, Hafler DA. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bucuvalas J. Long-term outcomes in pediatric liver transplantation. Liver Transpl. 2009;15(Suppl 2):S6–11. doi: 10.1002/lt.21915. [DOI] [PubMed] [Google Scholar]
- 52.Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, Alonso EM. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008;14:1582–1587. doi: 10.1002/lt.21549. [DOI] [PubMed] [Google Scholar]
- 53.Evans HM, Kelly DA, McKiernan PJ, Hubscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006;43:1109–1117. doi: 10.1002/hep.21152. [DOI] [PubMed] [Google Scholar]
- 54.Fouquet V, Alves A, Branchereau S, Grabar S, Debray D, Jacquemin E, Devictor D, Durand P, Baujard C, Fabre M, Pariente D, Chardot C, Dousset B, Massault PP, Bernard D, Houssin D, Bernard O, Gauthier F, Soubrane O. Long-term outcome of pediatric liver transplantation for biliary atresia: a 10-year follow-up in a single center. Liver Transpl. 2005;11:152–160. doi: 10.1002/lt.20358. [DOI] [PubMed] [Google Scholar]
- 55.Herzog D, Soglio DB, Fournet JC, Martin S, Marleau D, Alvarez F. Interface hepatitis is associated with a high incidence of late graft fibrosis in a group of tightly monitored pediatric orthotopic liver transplantation patients. Liver Transpl. 2008;14:946–955. doi: 10.1002/lt.21444. [DOI] [PubMed] [Google Scholar]
- 56.Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009;49:880–886. doi: 10.1002/hep.22686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.