Abstract
Activated phospholipase C (PLC*) generates 1,4,5-triphosphate (IP3) and diacylglycerol (DAG) from phosphatidyl inositol (PIP2). The DAG remains in the plasma membrane and co-activates conventional protein kinase C (PKC) with Ca2+. We have developed a mathematical model for the activation of the Ca2+-dependent PKC and its negative feedback on phospholipase C (PLC) and coupled it to the De Young-Keizer model for IP3 mediated Ca2+ oscillations. The model describes the cascade of reactions for the translocation of PKC to plasma membrane, and simulates activation of Ca2+ and diacylglycerol (DAG) oscillations. The model demonstrates that oscillations in Ca2+ and DAG are possible with or without a positive Ca2+ feedback on phospholipase C consistent with experiment. In many experimental studies, the timing of the peaks of the Ca2+ and IP3 oscillations have been used to suggest causality, i.e. that the IP3 oscillations cause the Ca2+ oscillations. The model is used to explore this question. To this end, the positive and negative feedback between Ca2+ and IP3 production are modulated, resulting in changes to the phase lag between the peaks in [Ca2+]cyt and [IP]cyt. The model simulates a possible experimental protocol that can be used to differentiate whether or not the positive feedback of Ca2+ on PLC is needed for the oscillations.
Keywords: protein kinase C (PKC), diacyl glycerol (DAG), phospholipase C (PLC), calcium oscillation, computational model
I. Introduction
In response to plasma membrane receptor-coupled G-protein activation, the specific proteins such as Gq (α and βγ subunits) activate phospholipase Cβ (PLCβ). PLCβ cleaves PIP2 to form DAG and IP3. In contrast, another isoform PLCγ is activated through phosphorylation by a tyrosine kinase to also produce DAG and IP3. The newly produced DAG remains in the plasma membrane while IP3 diffuses into the cytoplasm. The IP3 binds to the IP3 receptor in the endoplasmic reticulum (ER) to release Ca2+. The dynamics of Ca2+ often takes the form of oscillations that involve cycles of uptake and release from the ER.
These Ca2+ release events are thought to provide feedback on PLC by two mechanisms: 1) through protein kinase C (PKC) and 2) through calmodulin binding to PLC [5–9]. In the first mechanism, the increased cytosolic [Ca2+] binds to PKC, the PKC isoforms participate in a number of signaling transduction pathways in many cells. They are classified into three groups: conventional, novel, and atypical. The conventional PKCs have several isoforms (α, βI, βII, γ) and are regulated by Ca2+ and DAG. In contrast to conventional PKC, novel PKC isoforms (δ, ε, η, θ) are activated by DAG alone. Our computational study considers the role of a classic PKC. Before activation, PKC is found mainly in the cytosol. A conceptual “sequential model” has been proposed by others suggested that Ca2+ binding of C2 domain led to a conformational change and translocation to the cell membrane, which allows DAG binding of C1 domain [10, 11]. Many experiments have shown that PKC inhibits the activation of PLCβ [6, 9]. PKC inhibition of other isoforms of PLC has also been reported, for example PKC phosphorylates serine 1248 of PLCγ which may alter the interaction of PLCγ with protein tyrosine kinase and leads to a decrease in PLC activity [12]. The second proposed mechanism is based on more indirect evidence. Biden and co-workers observed in pancreatic β-cells that Ca2+ is necessary for IP3 production from PIP2[7]. Codazzi and colleagues (2001) observed that oscillating translocations of PKC are accompanied by oscillations in the indicators reporting both Ca2+ and DAG binding domain (GFP-C12δ) in astrocytes. The observed that the oscillations of the translocation of DAG binding domain lagged in phase behind the oscillations in the Ca2+ indicator and that the Ca2+ signal reached a threshold triggering the translocation of GFP-C12δ. Based on these phase lags a conceptual model in which there is fast activation of PLCβ by Ca2+ has been proposed. More recently, Thore and co-workers observed in pancreatic β-cells, activation of PLC-β required elevation of cytosolic Ca2+ due to CRAC channels [8]. In support of this hypothesis, recent experimental findings have shown that the affinity of PLCβ for calmodulin increases with increasing calcium [5]. For PLCγ no evidence of direct activation by Ca2+ has been observed. In this study, we utilize computational models to explore how Ca2+, IP3, DAG, PKC, and PLC interact to produce experimentally observed dynamics. It demonstrates that the phase lag between Ca2+ and IP3 peaks during agonist-induced oscillations can vary greatly and therefore should not be used to imply that one peak triggers the other (causality). Specifically, we study how the negative feedback of Ca2+ on PLC activity though the activation of PKC by both Ca2+ and DAG affects Ca2+, IP3, and DAG dynamics. The model first demonstrates that the experimentally observed oscillations in Ca2+ and DAG are possible both with and without fast positive feedback of Ca2+ on PLC. The model is also used to explore how the positive feedback of Ca2+ on PLC might affect Ca2+, IP3 and DAG dynamics for cells that have PLCβ. Finally the model suggests a test that might be used to explore whether this positive feedback is playing an essential role in the oscillations.
II. Methods
THE MODEL
A computational model was developed to explore the dynamics and interactions of Ca2+, DAG, IP3, PKC, and PLC during Ca2+ oscillations. The model includes the following features: 1) the model for intracellular Ca2+ dynamics used by De Young and Keizer [13]; 2) an IP3 production formulation is based on the model by Swillens and Mercan [1]; and 3) a new experimentally constrained computational model of the interactions of PKC with DAG, Ca2+ and PLC; and 4) fast activation of PLC by Ca2.
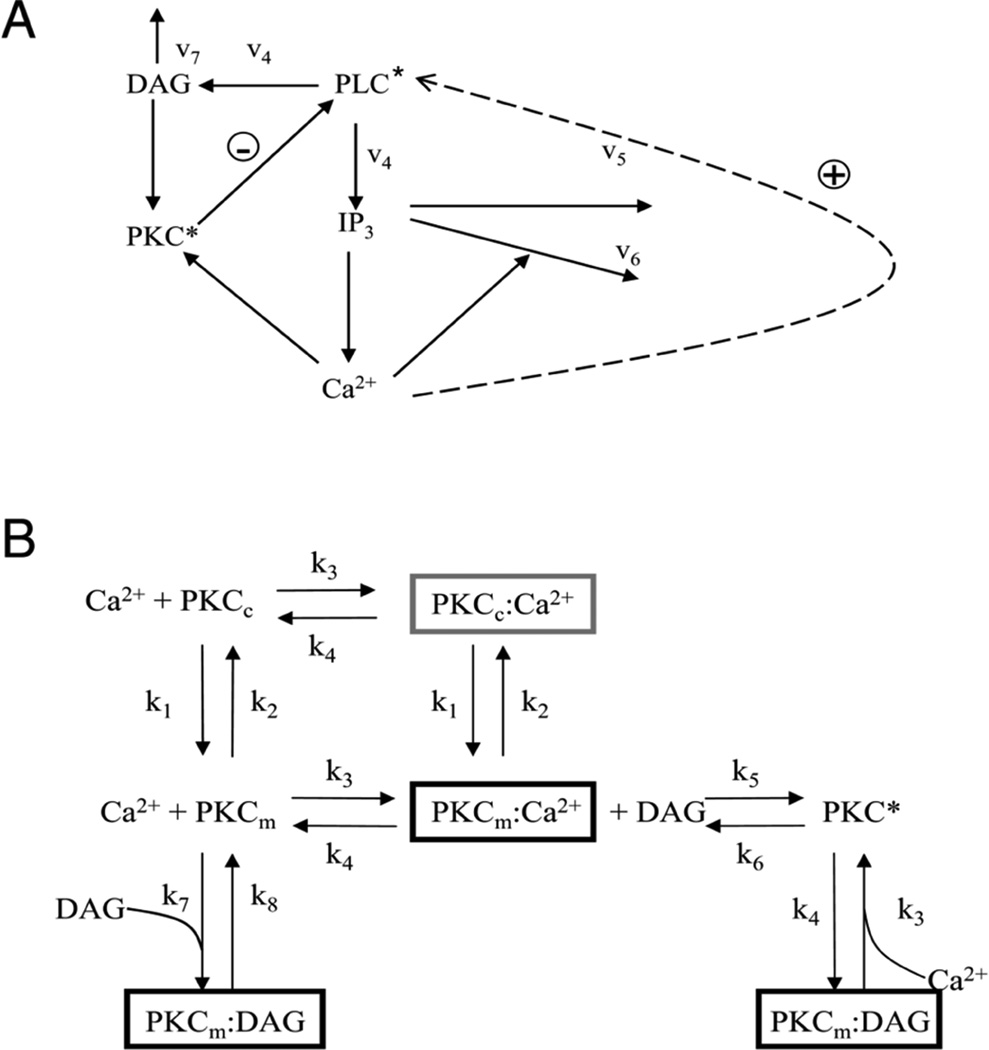
The De Young-Keizer model [6] was revised to include the dynamics of PKC activation, PLC activation, and IP3 production. The mathematical model for the action of PKC describes the binding of Ca2+ to the C2 domain and translocation and activation of PKC triggered by the binding of DAG to the C1 domain. A schematic diagram of mechanism is shown in Fig. 1A. Activated PLC (PLC*) generates IP3 and DAG (rate constant v4 in Fig. 1A). Ca2+ is released from the ER by changes in IP3 concentrations and positive feedback by Ca2+. The mechanism for IP3 degradation is through dephosphorylation by a 5-phosphatase and by phosphorylation to inositol 1,3,4,5-tetrakisphosphate [1]. Ca2+ and DAG bind to C2 and C1 domains of PKC, respectively, which cause PKC translocation. PKC is known to reduce PLC activity via negative feedback on PLC in Fig. 1A [6, 14]. The activation of PLC by Ca2+ is added to the model shown by the dotted arrow (rate constant v5 in Fig. 1A).
Fig. 1.
Model schematic. A. PLC activation pathway. (DAG – diacyl glycerol; PKC* - activated protein kinase C; PLC* – activated phospholipase C; IP3 – inositol 1,4,5-trisphosphate; Ca2+ - calcium ion, v1–v6 – reaction rates). The dashed line indicates the positive feedback of Ca2+ on PLC which is explored further in this study. B. Reaction scheme for PKC activation. (PKCc – cytoplasmic PKC; PKCm – membrane associated PKC; k1–k8 reaction rates).
A detailed reaction scheme of PKC activation is demonstrated in Fig. 1B. Here cytosolic species are shown in the upper half of the figure and given the subscript c. Membrane species are shown in the lower half of the figure and given the subscript m. At rest, PKC is located primarily in the cytosol (PKCc). Upon elevation of Ca2+, Ca2+ binds the C2 domain of PKCc in the cytoplasm resulting in its activated form, designated PKCc:Ca2+ (Fig. 1B - dark gray box). The activated C2 domain results in translocation of the entire complex to the plasma membrane and is designated PKCm:Ca2+ (Fig. 1B – black). Once in the membrane DAG binds the C1 domain of PKCm:Ca2+ resulting in fully activated PKC (PKC*). The model treats cytosolic and plasma membrane entities as distinct, and thus includes the 7 separate chemical species shown in Fig. 1B. The arrows between species represent forward and reverse chemical reactions, or membrane translocation. The rates of reaction, and of translocation, are represented by the 8 rate constants, designated k1 to k8 (Table I).
TABLE I.
PKC Rate Constants
The model consists of twelve differential equations: (1) the cytosolic Ca2+ concentration (2) the ER Ca2+ concentration (3) the cytosolic IP3 concentration; (4) the fraction of cytosolic IP3R subunit in the closed state with only IP3 bound; and (5) the fraction of cytosolic IP3R subunits in the open state with both IP3 and an activating Ca2+ bound, and (6)–(12) the system of 7 first order differential equations describing the mass action kinetics of the 7 states of PKC shown in the reaction scheme (Fig. 1B) (Appendix). The parameters in the equations include the 8 rate constants.
Two processes of the cytosolic calcium cycling from the ER in De Young-Keizer model is used in the model. The first is Ca2+ influx through the IP3 receptor (IP3R), and leak flux across the ER membrane (JIP3R) can be described by
| (1) |
where v1 is the maximal Ca2+ flux rate through the IP3R, X110 is fraction of IP3R subunits in the permissive state (both activating Ca2+ and IP3) bound, v2 is the leak flux rate, and c1 is the ER to cytosol volume ratio. The ER and cytosolic Ca2+ concentrations are represented by [Ca2+]ER and [Ca2+]cyt, respectively, and their difference describes the Ca2+ concentration gradient. The fraction X110 is cubed to reflect that three subunits need to be in the permissive state in order for the channel to be open. In the second process, Ca2+ is removed from the cytosol and sequestered into the ER via Ca2+ uptake by the SERCA pump (JSERCA), described by
| (2) |
Here v3 is the maximal SERCA pump rate and K3 is the affinity of SERCA for cytosolic Ca2+. The balance equations for [Ca2+]cyt is
| (3) |
where βcyt is a constant describing the fraction of Ca2+ flux not bound to Ca2+ buffers in the cytosol. The balance equations for ER Ca2+ concentration is given by
| (4) |
where the fluxes are described above and βER is the fraction of ER Ca2+ flux not bound to ER Ca2+ buffers.
The activation and inactivation states of cytosolic IP3 receptors by Ca2+ are given by following equations:
| (5) |
| (6) |
where X100 is the fraction of channels with the IP3 bound that is not in the activated state and X110 is the fraction of activated channels with both IP3 and activating Ca2+ bound [2]. The three subscripts indicate IP3 bound, activating Ca2+ bound, and inactivating Ca2+ bound by a “1” moving from left to right. A “0” indicated that the site is available (i.e. nothing is bound to it).
The formulation of IP3 production and degradation dynamics in the cytosol are based on Swillens-Mercan model [1]. The equation of free cytosolic concentration of IP3 ([IP3]cyt) is given by
| (7) |
where v4 is the rate of formation of IP3 from PLC. In the second term, v5 is the maximal rate of dephosphorylation IP3 by a 5-phosphatase governed by the binding constant K5. The third term represents that the rate of IP3 phosphorylation to inositol 1,3,4,5-tetrakisphosphate by a 3-kinase with maximal rate v6 and binding constant K6 that is increased by Ca2+ with binding constant K7 (Fig. 1A) [1].
In the model, there is a direct activation of PLC ([PLC*]) by Ca2+. It describes the Ca2+ activated PLC via positive feedback though inclusion of an additional factor in the first term in the equation for PLC activation and KCa describes the affinity of PLC for Ca2+.
| (8) |
Here, Kpkc is the dissociation constant for PKC inhibition. The parameter values and definitions are given in Tables II–III.
TABLE II.
Ca2+ Regulatory Mechanism Parameters
| Parameter | Definition | Value | Reference |
|---|---|---|---|
| c1 | Ratio of ER volume to cytosolic volume |
0.185 | [2] |
| c2 | Proportionary constant of X111 to [IP3] |
0.2 µM | [3] |
| v1 | Maximum IP3 receptor flux |
10.0 s−1 | [2] |
| v2 | Ca2+ leak rate constant | 2.0 s−1 | Rescaled from [2] |
| v3 | SERCA maximal pump rate |
80.0 µM s−1 | [2] |
| K3 | SERCA dissociation rate |
0.1 µM | Rescaled from [2] |
| a2 | Inhibitory receptor binding constant |
0.03 µM−1 s−1 | [2] |
| a5 | Activation receptor binding constant |
20.0 µM−1 s−1 | [2] |
| d2 | Inhibitory receptor binding constant |
1.049 µM | [2] |
| d5 | Activation receptor binding constant |
82.34 nM | [2] |
| βcyt | Ca2+ buffers in the cytosol |
0.01 | estimated |
| βER | ER Ca2+ buffers | 0.01 | estimated |
TABLE III.
IP3 Production Parameters
| Parameter | Definition | Value | Reference |
|---|---|---|---|
| v4 | PIP2 turnover rate by PLC |
0.12 µM s−1 |
Scaled from[1] |
| v5 | IP3 dephosphorylation rate |
0.625 µM s−1 |
Scaled from[1] |
| v6 | IP3 dissociation rate |
0.75 µM s−1 |
Scaled from[1] |
| K5 | IP3 dissociation constant |
25.0 µM | Scaled from[1] |
| K6 | IP3 dissociation constant |
0.2 µM | Scaled from[1] |
| K7 | Ca2+ dissociation constant |
1 µM | Scaled from[1] |
| h | Hill coefficient | 1 | [1] |
| Kpkc | Dissociation constant for PKC inhibition |
0.1 µM | estimated |
| KCa | Dissociation constant for calmodulin/PLCβ1 interaction |
0.2 µM | (Mccullar et al 2007) |
| kdag | Degradation rate | 0.1 s−1 | estimated |
| kplcon | Positive feedback | 0.2 s−1 | estimated |
The time course for the concentrations of each of the chemical species from a given initial condition is determined by numerical solution of the system using Euler’s Method. Table I lists the rate constants used in the model. Those rate constants were estimated from published data so that model variables and time courses conformed to experimental data. Parameter definitions and all values are given in Table I–IV. These are used in all the simulations unless otherwise specified in the text. The computer program was written in FORTRAN and run on an HP-UX workstation, and the results were visualized and graphs were made using MATLAB 7.0.1 by the Mathworks, Inc.
TABLE IV.
Resting State Concentrations
| Variable | Concentration (µM) |
|---|---|
| [Ca2+]cyt | 0.1 |
| [Ca2+]ER | 100.6 |
| X100 | 0.24 |
| X110 | 0.32 |
| [IP3]cyt | 0.25 |
| DAG | 1.0 |
| PKCm:DAG | 0.57 |
| PKCc:Ca2+ | 0.1 |
| PKCm:Ca2+ | 0.078 |
| PKC* | 0.01 |
| PKCc | 18 |
| PKCm | 17 |
III. Results
Using the parameter values in Tables I–III, the model is allowed to come to steady state at rest. The resting state concentrations are shown in Table IV. Codazzi and co-workers [14] have studied how conventional PKC isoforms affect Ca2+ oscillations in astrocytes using total internal reflection fluorescence (TIRF) microscopy. They observed oscillations in PKC translocation to the plasma membrane using GFP-PKCγ. They also observed that oscillations in DAG lagged behind Ca2+ oscillations in phase by measuring fluorescence of GFP-C1 bound to DAG and Ca2+ bound to the Ca2+ indicator Ca2+ crimson. They suggested that the phase lag might indicate that during the Ca2+ oscillations when Ca2+ concentration increased it activates PLC, which in turn generates more DAG.
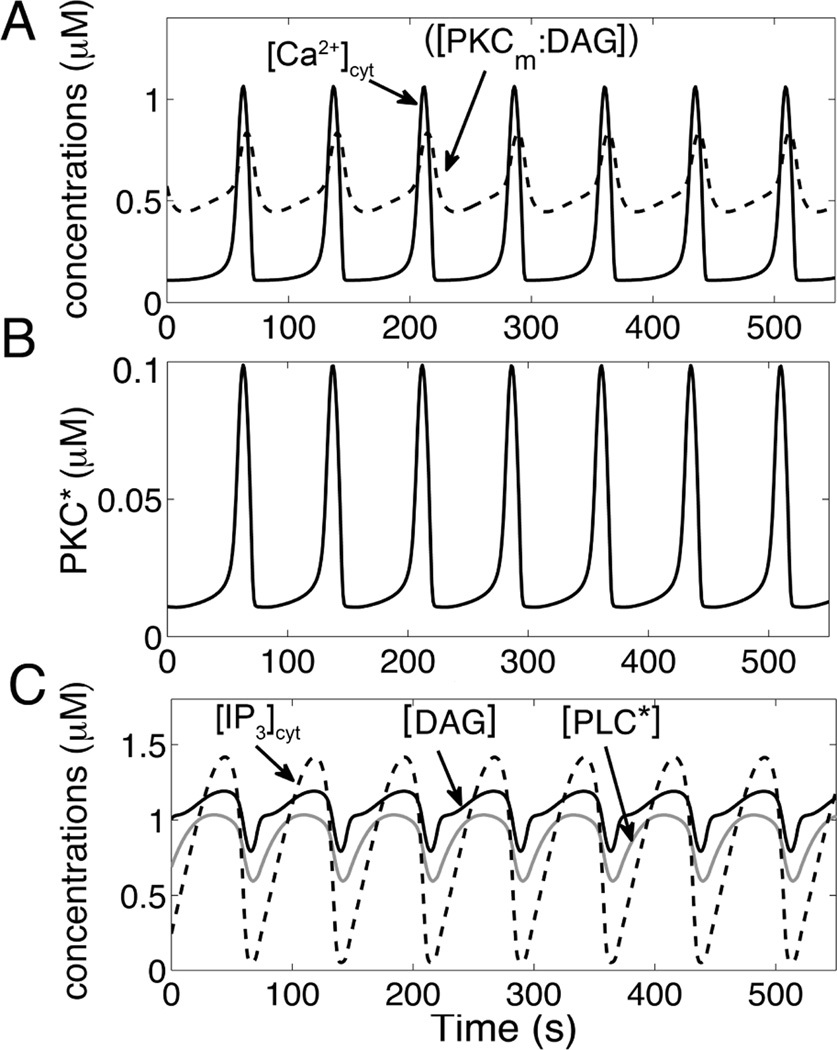
To this end, the model is used to simulate the experimental results. In order to simulate agonist stimulation and PLC activation v4 was increased to 0.15 µM s−1 causing production of IP3 and DAG and the ensuing Ca2+ oscillations. Fig. 2A shows our simulated train of Ca2+ oscillations. In these simulations, [Ca2+] is released from ER stores caused by fast activation of IP3R and with termination by depletion of the ER. Fig. 2B shows the activated PKC (PKC*) concentrations plotted as a function of time that seem to be almost in-phase with the Ca2+ oscillations with only a slight lag. The simulated PKC* is delayed by a few seconds compares to cytosolic Ca2+ oscillation similar to the experiments by Codazzi and co-workers (2001) measuring the C1 domain of PKC (GFP-C1). In addition, Matsu-ura and co-workers observed IP3 oscillations during agonist stimulation in HeLa cells [15]. Our results show the accompanying oscillations in IP3, DAG, and PLC in Fig. 2C. The IP3 concentration (dashed line) rises prior to the Ca2+ oscillations along with DAG (solid black line). The results show that the DAG and IP3 produced at the same rate from PLC as one would expect.
Fig. 2.
Simulated time courses of concentrations. A. Simulated intracellular Ca2+, DAG binding domains of PKC-C1 (PKCm:DAG) oscillations when PLC is active (top panel). The translocation of C1 is delayed by a few seconds. B. Activated PKC (PKC*) oscillations are almost in-phase with Ca2+ transit. C. Activated PLC ([PLC*]), DAG and IP3 show in-phase oscillations.
Fig. 3A shows free PKC concentrations in cytosol [PKCc] and plasma membrane [PKCm] behave in a similar manner; [PKCm] increases seconds after elevation of [PKCc]. The concentration of PKC binding of Ca2+ ([PKCc:Ca2+]) follows the reverse trend, increasing rapidly after [PKCc] reaches a certain level as shown in Fig. 3B. The translocation of PKC-C1 (Fig. 3C) appears to start seconds later after Ca2+ concentrations reached a threshold. These simulations suggest that binding process of PKC caused the delayed translocation of PKC-C1 because elevation of the cytosolic Ca2+ concentration is needed for Ca2+ to bind to PKC-C2 domains. The binding of Ca2+ to PKC-C2 results in its translocation to the plasma membrane, which then allows DAG to bind to PKC-C1 domains. This indicates that Ca2+ activation of PLC is not necessary to see the phase lag observed experimentally.
Fig. 3.
Simulated time courses of concentrations. A. Free PKC concentrations in the cytosol [PKCc] and PKC concentrations associated with the plasma membrane [PKCm] oscillate during PLC activation pathway. B. PKC bound to Ca2+ in cytoplasm ([PKCc:Ca2+]), and C. Ca2+ binding of PKC-C2 domains translocate to plasma membrane ([PKCm:Ca2+]) both oscillate during Ca2+ oscillations.
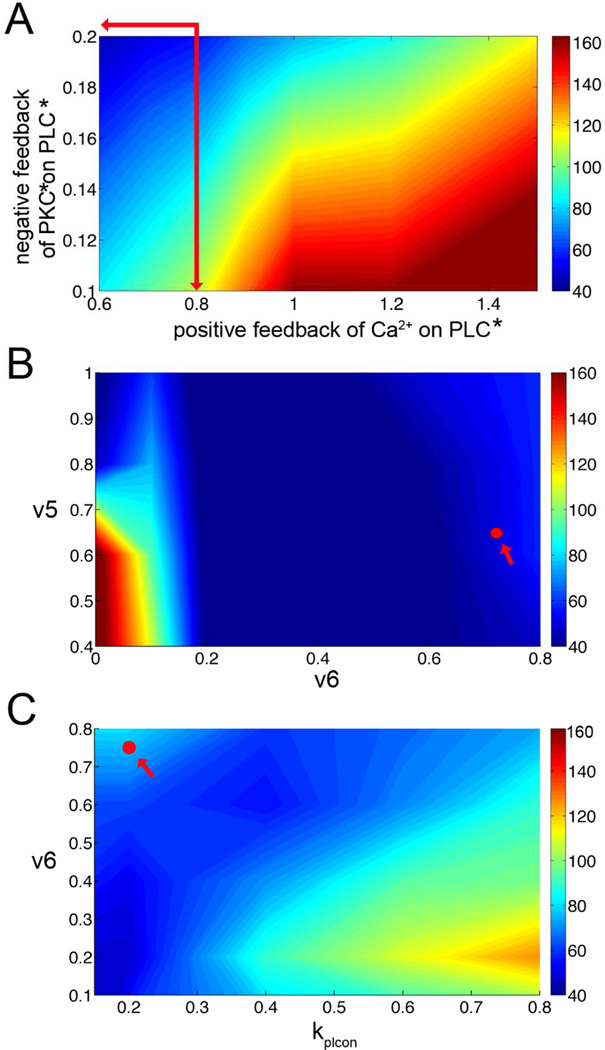
The next set of simulations tested how the phase lag between Ca2+ and IP3 oscillations is modulated by PLC dynamics. In order to do this, the positive feedback of Ca2+ on PLC and the negative feedback on PLC by Ca2+ through PKC were varied (Fig. 4A). Here the DAG peak is used as it corresponds with the IP3 peak and it is often used in experiments as an indicator of IP3. Depending upon the relative contributions of each feedback mechanism, the phase lag varies greatly. When the positive feedback is strong, the Ca2+ and IP3 oscillations can be 160° out of phase. However, when the negative feedback dominates, the phase lag is minimal. Note that the oscillations are never completely in phase. The default model values for these parameters are indicated at the intersection of the red arrows. Varying individual parameters such as the degradation rate of IP3 (v5) and the positive feedback of Ca2+ on PLC (v6) also can affect the phase lag (Fig. 4B). A reduction of both parameters promotes out of phase oscillations. The default model parameters are indicated by the solid red circle. In Fig. 4C the effect of v6 and the Ca2+ dependent rate kplcon of positive feedback is studied. The interplay between these two parameters shows once again that the phase lag can vary depending upon parameter choices. The default model parameters are indicated by the solid red circle. In summary, the phase lag between the Ca2+ and IP3 oscillations can vary depending upon the signaling strength of various components of the signaling network. Biologically, this might simply depend upon gene expression levels or the phosphorylation state of the system.
Fig. 4.
Phase maps. A. The effects of positive feedback of Ca2+ and negative feedback of PKC* on Ca2+ and DAG oscillations. These plots show the phase difference between the [Ca2+]cyt and [IP3]cyt oscillation peaks. The phase difference is calculate as 360*((time of Ca2+ peak) - (time of DAG peak))/(oscillation period), In this way when the periods are aligned the value is 0, when they are opposite in phase it is 180. The synchronization between Ca2+ and DAG oscillations varies with feedback strength. B. The changing of the degradation rate of IP3 (v5) affects the phase differences of Ca2+ and IP3. When v6 (Ca2+ dependent rate of PLC activation) large, the oscillations of calcium and IP3 are in phase. The default model parameter is shown by the red circle. C. The Ca2+ dependent rate of PLC activation (v6) and Ca2+ dependent rate kplcon for positive feedback affect the phase differences of Ca2+ and IP3. The default model parameter is shown by the red circle.
In earlier modeling work by Meyer and Stryer [16], there was an important interplay between the Ca2+ and IP3 concentrations that produced oscillations. In our model, when Ca2+ concentration is held fixed, the IP3 oscillations cease consistent with this idea (Fig. 5A). In the model when Ca2+ oscillations are present, there are oscillations in the amount of activated PLC ([PLC*]) (Fig. 5B – solid line). When DAG degradation is blocked the oscillations cease (Fig. 6B – dashed line).
Fig. 5.
Ca2+ oscillations affect IP3 and activated PLC ([PLC*]) oscillations. A. There is no IP3 oscillation when Ca2+ is set to a constant level (1.0 µM). B. Blocking DAG degradation eliminates [PLC*] oscillation (dashed line), whereas DAG degradation results in PLC oscillations (solid line).
Fig. 6.
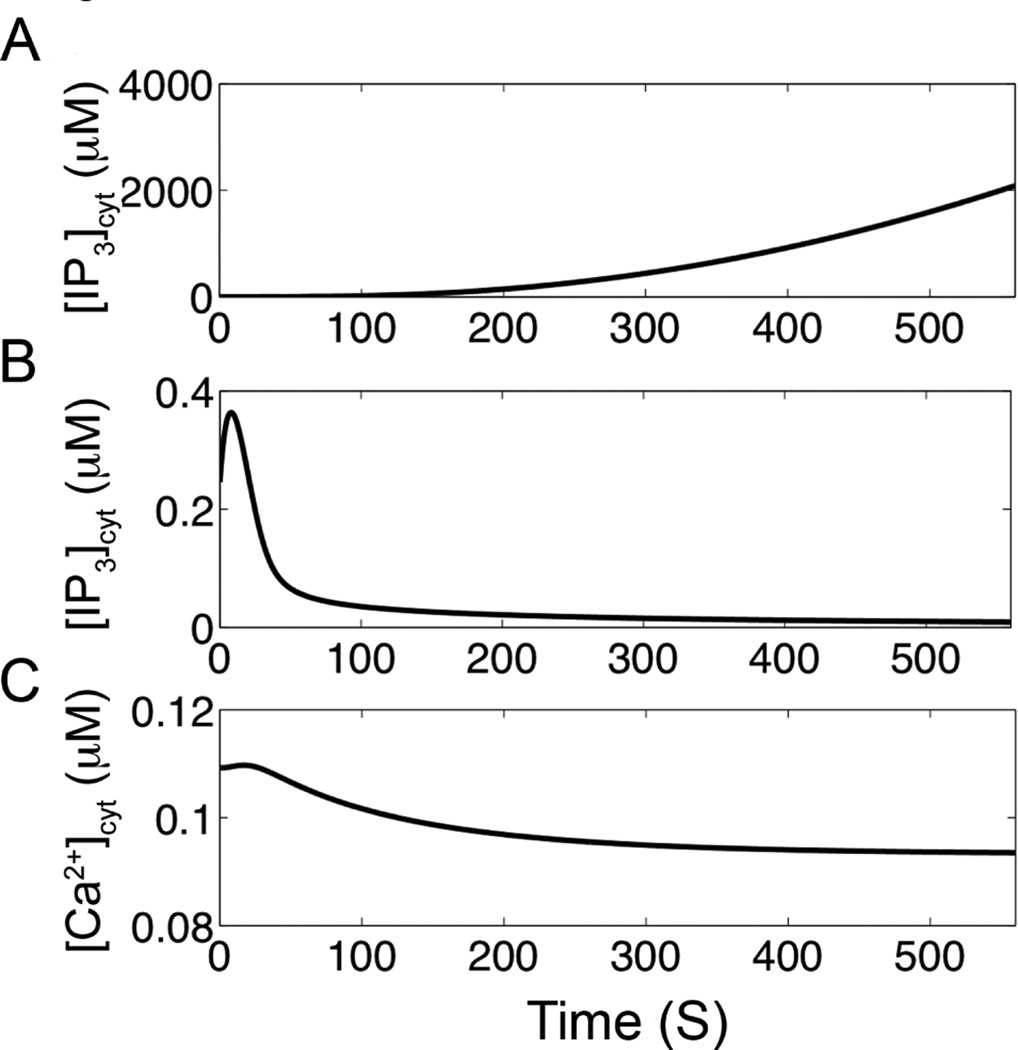
Blocking the feedbacks on PLC affect Ca2+ dynamics. A. There are no Ca2+ oscillations and the IP3 rise by blocking PKC. Furthermore, the IP3 concentration rises as there is no negative feedback on PLC. B. When the positive feedback of Ca2+ on PLC is eliminated the IP3 concentration returns to steady state. C. When the positive feedback of Ca2+ on PLC is eliminated the Ca2+ concentration also returns to steady-state level. However, if the mean [IP3]cyt is raised, [Ca2+]cyt oscillations can occur in the absence of positive feedback of Ca2+ on PLC (see Figure 8).
Additional simulations were performed to understand the interacting components on Ca2+ dynamics during agonist-induced signaling. To further understand the role of PKC, the action of PKC on PLC is blocked which terminated the oscillations (Fig. 6A). The IP3 concentration rises as the negative feedback on the production of IP3 by PLC has been removed. Similarly, the [DAG] concentration will also rise. The positive feedback of Ca2+ on PLC activation is blocked to assess its role in Ca2+ oscillations (Figs. 6B and 6C). In this case, the oscillations cease and the IP3 concentration (Fig. 6B) and the Ca2+ concentration (Fig. 6C) both return to baseline. However, it would be incorrect to conclude that this means that the positive feedback is needed for oscillations. The oscillations can be restored by adjusting parameters to increase the IP3 concentration slightly as shown in Fig. 7.
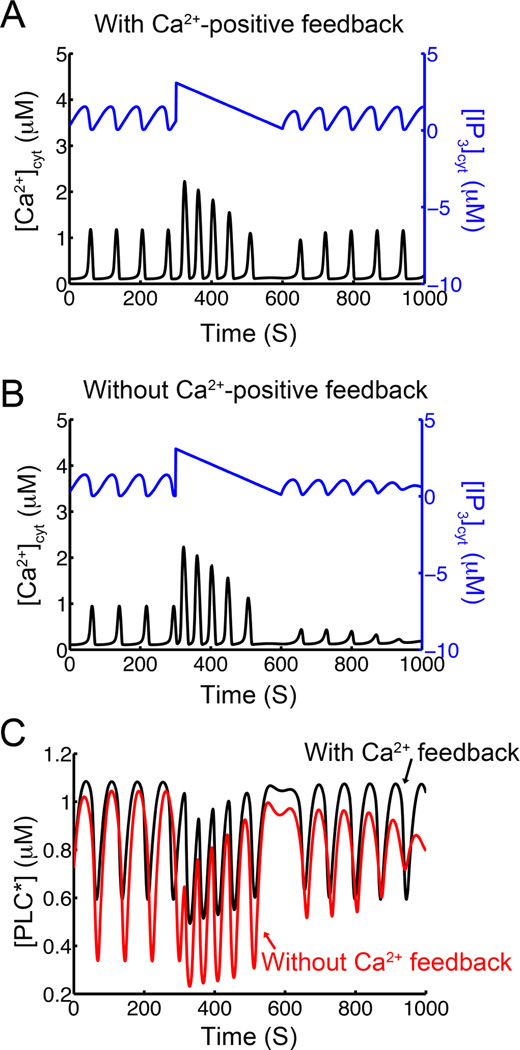
Fig. 7.
Test to see if positive feedback of Ca2+ on PLC is involved with [Ca2+]cyt oscillations. An applied IP3 pulse as shown results in different behaviors in the models with and without positive feedback of Ca2+ on PLC. In both cases the [Ca2+]cyt oscillation frequency increases with the increased [IP3]cyt. A. In the model with positive feedback of Ca2+ on PLC, the oscillations resume similar to those before the pulse. B. In the model without positive feedback of Ca2+ on PLC, the [Ca2+]cyt oscillations do not resume. C. The time course for activated [PLC] shows differing dynamics in the system with Ca2+ feedback on PLC (black) and without Ca2+ feedback on PLC (red).
The modeling results suggest that the phase lag between the [Ca2+]cyt and [IP3]cyt oscillations is not a good indicator of the presence of the positive feedback of Ca2+ on PLC. To provide a simple test that could be implemented in experiment, a pulse of IP3 was applied with a gradual decay back to baseline in as shown in Fig. 7. In the model that included the positive feedback of Ca2+ on PLC, the frequency and amplitude of the [Ca2+]cyt oscillations increase during the pulse and return to pre-pulse amplitude and frequency afterward (Fig. 7A). On the other hand, in the model that excluded the positive feedback of Ca2+ on PLC*, the increase in the [Ca2+]cyt oscillations is followed by oscillations of low amplitude after the IP3 pulse ended (Fig. 7B). Fig. 7C shows that in both cases the system with the positive feedback of Ca2+ on PLC, the amount of PLC activation is higher than in the case without positive feedback of Ca2+ on PLC. It is important to note that the only difference between the two models is that in the case without Ca2+ feedback on PLC, the term for [Ca2+]cyt is set to its resting level of 0.1 µM to eliminate the positive feedback. Therefore the pulse of IP3 provides a possible test for the role of the positive feedback of Ca2+ on PLC.
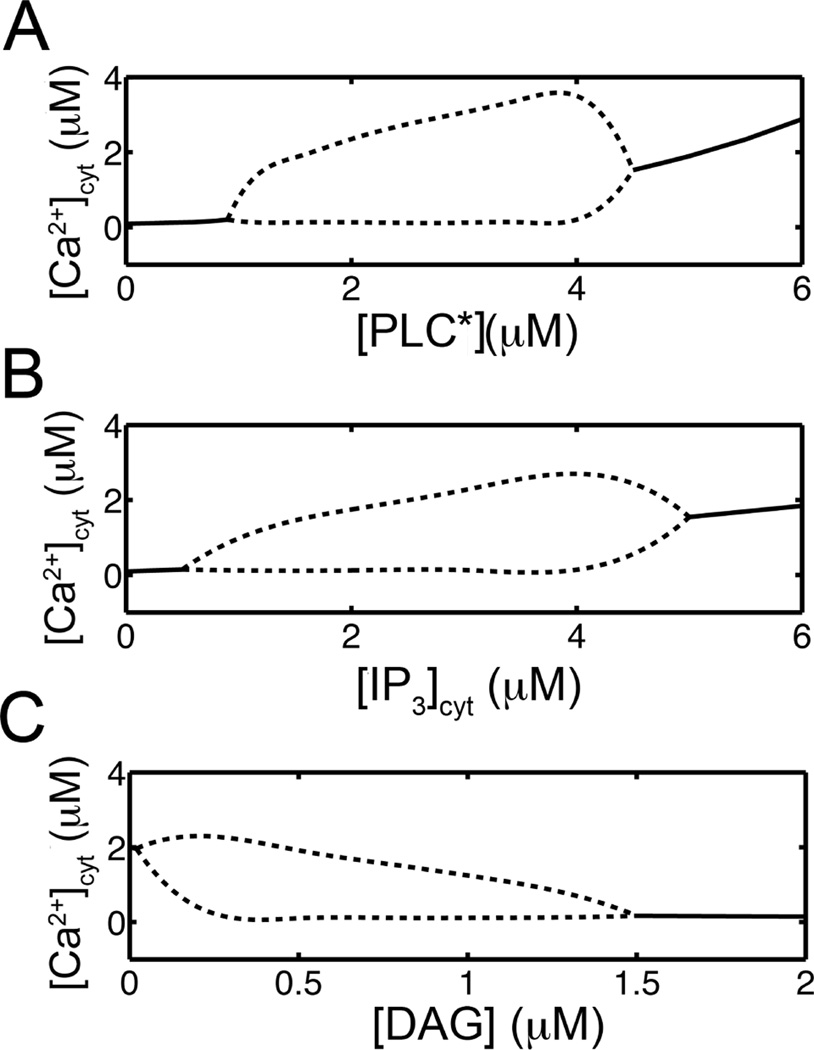
In order to understand the differing dynamics of the Ca2+ oscillations in the two systems shown in Fig. 7, a number of simulations were determined to construct bifurcation diagrams. Fig. 8 shows bifurcation diagrams for the concentrations of activated PLC, IP3, and DAG, in panels A, B, and C, respectively. In these diagrams the solid lines show the steady-state [Ca2+]cyt and the dotted lines show the maximum and minimal [Ca2+]cyt values during oscillations. This behavior is consistent with experiments and other models [16, 17]. However, the dynamics are significantly more complex as these quantities are not simple parameters. Instead, they are variables whose values are determined by the dynamics. This would be similar to the case found in living cells. In the system with the positive feedback of Ca2+ on PLC, the level of activated PLC after the pulse of IP3 rises to cross the bifurcation point for the advent of oscillation (Fig. 8A). On the other hand, in the case without feedback, the levels of activated PLC are too low to allow for oscillations. The reason that the former reaches higher level is that there is a positive feedback loop where Ca2+ activates PLC, which increases IP3 production resulting in further mobilization of Ca2+. On the other hand if Ca2+ does not activate PLC, then only the inhibiting effect of Ca2+ through PKC activation remains effectively attenuating the levels of activated PLC. Fig. 8B shows oscillations within a finite range similar to previous studies. Fig. 8C demonstrates that as [DAG] rises above a certain range oscillations terminate. Figs. 8B–C provide an explanation why the block of PKC in Fig. 6A results in the termination of oscillations, i.e. the [IP3] or [DAG] levels could rise out of the oscillatory regime with the block of the negative feedback of PKC on PLC.
Fig. 8.
Bifurcation diagrams for Ca2+ oscillations (——) indicates stable steady states and (-------) gives maximum and minimum [Ca2+]cyt during oscillations. A. The model generates oscillations in Ca2+ while [PLC*] between 1 and 4.2 µM. B. The constant IP3 ([IP3] + [IP3β]) between 0.8 and 4.4 µM can produce Ca2+ oscillations. C. [DAG] can affect Ca2+ oscillations when set [DAG] to a constant in between 0.0 and 2.0 µM.
IV. Discussion
In the past several models of agonist-induced Ca2+ oscillations have been proposed. Meyer and Stryer [18] proposed a model in which the Ca2+ positively activates PLCβ to increase IP3 concentrations and IP3 cause Ca2+ release that generates the next calcium transients. Swillens and Mercan [1] developed a model that suggested negative control of Ca2+ efflux through IP3 receptor contribute to Ca2+ oscillations, and also the activation of 3-kinase positively co-operates with the calcium for oscillating IP3. DeYoung and Keizer [2] presented a model that described the kinetics of the IP3 receptor along with ER sequestration of Ca2+ and found that its dynamics was sufficient to produce Ca2+ oscillations. While all these models have modeled different pieces of the Ca2+ signaling pathway, this is the first to model integrate the different parts into one model. As a result it shows how the different pieces modulate Ca2+ oscillations.
The Ca2+ and DAG dependent conventional PKC activation (PKC*) model presented here incorporates experimentally determined reaction pathways. The model reproduces experimentally observed behaviors of translocation of PKC to plasma membrane. Several parameter values were estimated to fit the experimental observations. The model simulates the PKC oscillations consistent with experimental results [10, 14]. Similar to their experiments, we observed oscillations in both DAG and IP3 in the model. The model presents a classic type of Ca2+ oscillations induced by IP3 leads to Ca2+ release and uptake of Ca2+ from intracellular ER stores, with a negative feedback of PKC on PLC in this manuscript. It suggests that the translocation of PKC to plasma membrane is delayed by a few second without a positive feedback of Ca2+ on PLC. A previous model by Pollitti and co-workers (2006) explored the effects of positive and negative feedback on Ca2+ oscillations[19]. They concluded that the model with coupled IP3 and Ca2+ oscillations displayed a greater frequency range than simpler models. Furthermore, they suggested that positive feedback of Ca2+ on PLC required fast degradation of IP3 and negative feedback requires slow degradation IP3 turnover.
The model demonstrates that the apparent lag of peak values of [Ca2+]cyt and [IP3]cyt oscillations is not a sufficient criterion to determine causality, that is, which process is triggering the other in the oscillatory regime. Moreover, the model suggests a test that can be used to determine whether or not Ca2+ feedback on PLC is playing a role in the oscillations. Another work by Sneyd and co-workers (2006) addressed a similar question with a combined modeling and experimental study [20, 21]. They sought to determine if Ca2+ oscillations depend upon IP3 oscillations. They considered class I models where the [Ca2+]cyt oscillation frequency is an increasing function of the parameter [IP3]cyt and class II models where [IP3]cyt is a variable that oscillates. Perturbation of the oscillations by IP3 injections in class I models results in a transient increase in oscillation frequency. On the other hand, injection of IP3 in class II models results in a delay in the oscillation phase, i.e. the next peak in [Ca2+]cyt is delayed. The model presents here is a class II model as IP3 concentration is a variable that can oscillate. Here, we demonstrate that the presence or absence of Ca2+ feedback on PLC is does not absolutely determine phase lag between [Ca2+]cyt and [IP3]cyt oscillations.
V. Conclusions
The model presented here integrates experimentally observed IP3 receptor and PLC dynamics to demonstrate their interplay during agonist induced Ca2+ signaling. It demonstrates that Ca2+ occurs through a complex interplay of factors. Furthermore, it suggests that the phase lag between Ca2+ and IP3 oscillations is not a good indicator of causality because the phase lag can be changed dramatically depending upon the strength of positive feedback of Ca2+ on PLC as well as the strength of the negative feedback of Ca2+ through PKC.
Acknowledgments
This material is based upon work supported by the National Science Foundation under Grant No. 0443843 (MSJ) and by National Institutes of Health (NIH) grant No. 5R01HL105239 (MSJ) and 5U01HL116321 (MSJ).
Biographies

Pei-Chi Yang received her PhD degree from George Mason University in 2007 from the Program in Bioinformatics and Computational Biology. She is currently an Assistant Project Scientist working with Colleen Clancy in the Department of Pharmacology, University of California, Davis, CA, USA. Her research interests include calcium signaling, cardiac electrophysiology, arrhythmia susceptibility, and pharmacology.

M. Saleet Jafri received the PhD degree from the Mount Sinai School of Medicine/City University of New York University, New York, NY, USA, in 1993 from the Department of Biomathematical Sciences. He is currently Professor and Chair of the Molecular Neuroscience Department at George Mason University, Fairfax, VA USA. He is an Adjunct Professor at the Center for Biomedical Engineering and Technology, University of Maryland, Baltimore, MD, USA. From 1999 to 2002 to 2009, he was Assistant Professor at the Department of Mathematical Sciences, The University of Texas at Dallas, Richardson, TX, USA. Prior to that he was a Research Associate at the Department of Biomedical Engineering, The Johns Hopkins University, Baltimore, MD, USA. He did his Postdoctoral work with Joel Keizer at the Institute for Theoretical Dynamics, University of California, Davis, CA, USA. His research interest includes the use of computational model to understand the cellular mechanism of normal and pathophysiology. His areas of expertise include calcium signaling, cardiac excitation-contraction coupling, mitochondrial physiology, and t-cell signaling.
APPENDIX
PKC Model Equations
Contributor Information
Pei-Chi Yang, Department of Pharmacology, University of California Davis, Davis CA 95616 USA; Molecular Neuroscience Department and School of Systems Biology, George Mason University, Fairfax, VA 22030 USA.
M. Saleet Jafri, Molecular Neuroscience Department and School of Systems Biology, George Mason University, Fairfax, VA 22030 USA; Center for Biomedical Engineering and Technology, University of Maryland School of Medicine, Baltimore, MD 20201 USA.
References
- 1.Swillens S, Mercan D. Computer simulation of a cytosolic calcium oscillator. Biochem J. 1990;271(3):835–838. doi: 10.1042/bj2710835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Young GW, Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jafri MS, Keizer J. On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophysical journal. 1995;69(5):2139–2153. doi: 10.1016/S0006-3495(95)80088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinnecker D, Schaefer M. Real-time analysis of phospholipase C activity during different patterns of receptor-induced Ca2+ responses in HEK293 cells. Cell calcium. 2004;35(1):29–38. doi: 10.1016/s0143-4160(03)00169-6. [DOI] [PubMed] [Google Scholar]
- 5.McCullar JS, Malencik DA, Vogel WK, Crofoot KM, Anderson SR, Filtz TM. Calmodulin potentiates Gβγ activation of phospholipase C-β3. Biochem Pharmacol. 2007;73(2):270–278. doi: 10.1016/j.bcp.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue C, Ku CY, Liu M, Simon MI, Sanborn BM. Molecular mechanism of the inhibition of phospholipase C beta 3 by protein kinase C. J Biol Chem. 2000;275(39):30220–30225. doi: 10.1074/jbc.M004276200. [DOI] [PubMed] [Google Scholar]
- 7.Biden TJ, Peter-Riesch B, Schlegel W, Wolheim CB. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987;262(6):3567–3571. [PubMed] [Google Scholar]
- 8.Thore S, Dyachok O, Gylfe E, Tengholm A. Feedback activation of phospholipase C via intracellular mobilization and store-operated influx of Ca2+ in insulin-secreting β-cells. J Cell Sci. 2005;118(19):4463–4471. doi: 10.1242/jcs.02577. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett PJ, Young KW, Nahorski SR, Challiss RA. Single cell analysis and temporal profiling of agonist-mediated inositol 1,4,5-trisphosphate, Ca2+, diacylglycerol, and protein kinase C signaling using fluorescent biosensors. J Biol Chem. 2005;280(23):21837–21846. doi: 10.1074/jbc.M411843200. [DOI] [PubMed] [Google Scholar]
- 10.Tanimura A, Nezu A, Morita T, Hashimoto N, Tojyo Y. Interplay between calcium, diacylglycerol, and phosphorylation in the spatial and temporal regulation of PKCalpha-GFP. J Biol Chem. 2002;277(32):29054–29062. doi: 10.1074/jbc.M201130200. [DOI] [PubMed] [Google Scholar]
- 11.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95(3):307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 12.Park DJ, Min HK, Rhee SG. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992;267(3):1496–1501. [PubMed] [Google Scholar]
- 13.Keizer J, De Young GW. Two roles of Ca2+ in agonist stimulated Ca2+ oscillations. Biophys J. 1992;61(3):649–660. doi: 10.1016/S0006-3495(92)81870-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codazzi F, Teruel MN, Meyer T. Control of astrocyte Ca(2+) oscillations and waves by oscillating translocation and activation of protein kinase C. Curr Biol. 2001;11(14):1089–1097. doi: 10.1016/s0960-9822(01)00326-8. [DOI] [PubMed] [Google Scholar]
- 15.Matsu-ura T, Michikawa T, Inoue T, Miyawaki A, Yoshida M, Mikoshiba K. Cytosolic inositol 1,4,5-trisphosphate dynamics during intracellular calcium oscillations in living cells. The Journal of cell biology. 2006;173(5):755–765. doi: 10.1083/jcb.200512141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li YX, Keizer J, Stojilkovic SS, Rinzel J. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations. The American journal of physiology. 1995;269(5 Pt 1):C1079–C1092. doi: 10.1152/ajpcell.1995.269.5.C1079. [DOI] [PubMed] [Google Scholar]
- 17.Hajnoczky G, Thomas AP. Minimal requirements for calcium oscillations driven by the IP3 receptor. The EMBO journal. 1997;16(12):3533–3543. doi: 10.1093/emboj/16.12.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer T, Stryer L. Molecular model for receptor-stimulated calcium spiking. Proc Natl Acad Sci U S A. 1988;85(14):5051–5055. doi: 10.1073/pnas.85.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Politti A, Gaspers LD, Thomas AP, Hofer T. Models of IP3 and Ca2+ oscillations: frequency encoding and Identification of underlying feedbacks. Biophys J. 2006;90:3120–3133. doi: 10.1529/biophysj.105.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sneyd J, Tsaneva-Atanasova K, Reznikov V, Bai Y, Sanderson MJ, Yule DI. A method for determining the dependence of calcium oscillations on inositol trisphosphate oscillations. Proc Natl Acad Sci U S A. 2006;103(6):1675–1680. doi: 10.1073/pnas.0506135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harootunian AT, Kao JP, Tsien RY. Agonist-induced calcium oscillations in depolarized fibroblasts and their manipulation by photoreleased Ins(1,4,5)P3, Ca++, and Ca++ buffer. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):935–943. doi: 10.1101/sqb.1988.053.01.108. [DOI] [PubMed] [Google Scholar]