Abstract
Research on nanoscale semiconductor devices will elicit a novel understanding of biological systems. First, we discuss why it is necessary to build interfaces between cells and semiconductor nanoelectronics. Second, we describe some recent molecular biophysics studies with nanowire field effect transistor sensors. Third, we present the use of nanowire transistors as electrical recording devices that can be integrated into synthetic tissues and targeted intra- or extracellularly to study single cells. Lastly, we discuss future directions and challenges in further developing this area of research, which will advance biology and medicine.
1. Introduction
Biological systems are rich with electrical activity. Alongside the well known pathways of biochemical regulation, there exists additional pathways of biological communication governed not by chemical reagents, but by electrical signals1, 2. Recent in vitro experiments have shown that electromagnetic fields (EMFs) can act as epigenetic signals, controlling important cell behaviors1–4, such as the direction of cell migration and the orientation of cell division. Besides being able to be affected by EMFs, biological systems can also serve as the source of EMFs at several levels2, 4, 5. For example, mitochondria6 are a source of strong static electric fields – in the range of 106–107 V/m. Similarly, Microtubules (MTs), composed of electrically polar tubulin heterodimer subunits, have also been suggested as the source of cellular EMFs5. In this regard, bioelectric signals form an epigenetic pathway that can potentially be another network for understanding and controlling single cell behavior3 (Fig. 1). While other methods, such as glass microelectrodes7, 8 and voltage sensitive dyes9, 10 can be used to study these systems, this review will focus on advances in nanoscale semiconductor devices11–18, and how they offer a promising new approach to both studying, and altering the behavior of electrical activity in a biological context.
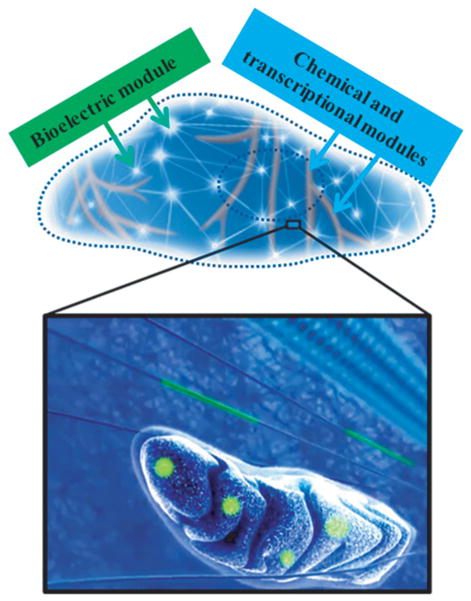
Figure 1. Bioelectric networks inside single cells are epigenetic, and could be the next target for studying and controlling cellular signaling.
The top panel depicts how bioelectric, and chemical and transcriptional modules form networks inside cells. Shown in the lower panel are single mitochondrion and microtubule bundles containing these modules, both of which can be used as intracellular electrical interfaces with nanoscale semiconductor devices shown in green.
1.1. ‘Nano’ is the natural length-scale for electronic interfaces with biological systems
Before continuing to examine how semiconducting materials can address bioelectric activity, let us briefly pause to consider why nanoscale devices are the ‘natural’ length scale for addressing biological electrical signals (Fig. 1). Biological systems are organized hierarchically, with unique characteristics and functionalities spanning multiple length scales; some examples including collagen fibers, metabolic networks, and even chromosome organization. Therefore, it is important to select the right organizational length scale for device and biointerface design. In the case of sub-cellular organization, this length scale is designated by the size of individual organelles which are on the order of tens to hundreds of nanometeres19. A probe must be able to distinguish between individual organelles, either for sensing or stimulation, providing a ‘natural length scale’ at which a sensor must operate, requiring a design capable of extreme spatial resolution (Fig. 1). In this regard, semiconductor nanomaterials are a good fit as they have proven detection capabilities, and have device designs down to a ~10 nm regime20.
1.2. New tools and opportunities, from Biophysics to Healthcare
The ability to interact electrically within a single cell or throughout the entire 3D volume of a tissue in a targeted fashion has many important implications for electrophysiology and biomedical sciences, however very few studies to date have experimentally examined the electrophysiology of sub-cellular organelles in complete cellular settings21, such as the endoplasmic reticulum or mitochondrion. While fluorescent dyes can act as point like voltage sensitive probes10, such markers tend to be confined to the plasma membrane, and can interfere with natural cell functionality, limiting their range of application. The patch clamp technique, in which a pulled glass micropipette filled with electrolyte is inserted into a cell, offers intracellular electrical measurements with high signal-to-noise ratio (S/N) and single ion channel recording capability13. Ideally, the micropipette should be as small as possible to increase the spatial resolution and reduce the invasiveness of the measurement. However, the overall performance of the technique also depends on the impedance of the interface between the micropipette and the cell interior (i.e., the smaller the probe tip size, the larger the junction impedance), which sets limits on the temporal resolution and S/N of the micropipette-based electrical probes13. Advanced techniques that involve inserting metal or carbon microelectrodes or nanoelectrodes into cells or tissues could be subject to similar dilemma, because all these tools are single terminal devices and electrochemical thermodynamics and kinetics must be considered for device operation13. Therefore a new set of tools is required to explore electrical dynamics in this regime, with nanoscale semiconductors appearing as a promising candidate.
2. Nanoscale semiconductor devices
Semiconductor devices have a rich set of physical properties that make them desirable targets for the design of next generation biomedical devices. In addition to a small intrinsic size which gives rise to both high spatial resolution and minimal invasiveness, nanoscale semiconductor devices show extreme chemical and electrical sensitivity, bio-marker selectivity, multiplexed signal detection, and flexible device configuration11, 13, 22.
2.1. Sensitivity and selectivity
Nanoscale semiconductor devices, particularly nanowire field effect transistors (NWFETs) are a highly sensitive and selective platform for detecting minute changes in chemical concentrations and electrochemical potentials. A FET device uses electrons or holes as the carriers, which exhibits a conductance change in response to variations in the charge or potential at the surface of the channel region. A FET device sensitivity is related to its transconductance, which is inversely scaled to the detectors dimensions, suggesting that nanoscopic devices can yield better sensitivities that are appropriate for resolving minute cellular signals13. The state-of-the-art NWFETs show detection sensitivities down to femto-molar concentrations23, 24 (i.e. parts-per-quadrillion (ppq) detection) and switching speeds as fast as 2 THz25, 26, allowing for responses on the picosecond timescale. For instance intracellular calcium concentrations, an important secondary messenger, are on the order of 100 nM for resting cells, a concentration well above the detection limit of NW devices. Additionally, NWFETs are also capable of operating under physiological conditions in a non-invasive manner11, 15. This unique capability makes them a particularly promising candidate for in-vivo studies.
2.2. Multiplex sensing
Multiple bio-marker detection, such as nucleic acids, proteins and ions, is a vital tool in the life sciences, with techniques such as the enzyme-linked immunosorbent assay (ELISA)27. Semiconducting nanomaterials offer a promising analog to these types of assays, as multiplexed devices can monitor for a variety of signals within a single sample with high sensitivity and in a reusable fashion23. Multiplexing, the use of multiple semiconductor devices for the simultaneous measurement of a single sample, is an important step in achieving this goal, as correlated detection can cut down on electrical cross-talk and/or false-positives, while individual nanoscale detectors can be configured through surface modification to monitor for distinct targets23, 24, 28, 29. This allows for the simultaneous measurement of multiple biomarkers and can give insight into how chemical systems dynamically evolve in real-time28, 30. While there are certain practical challenges in device implementation preventing the current commercialization of these devices, recent advancements in fabrication techniques such as patterned positioning31 present promising opportunities for future implementations.
2.3. Flexible electronics
Nanoscopic devices are capable of extreme flexibility when compared to bulk materials allowing for the construction of uniquely pliable electronic devices11, 14, 32–37 (Fig. 2). This enables the design of free standing three dimensional device configurations and allows for the dynamic response to changes in tissue positioning and conformation. In an analogous fashion to existing engineered active components in tissue culture, flexible nanoelectronics allows for the observation and modulation of tissue behavior in a three dimensional volume14.
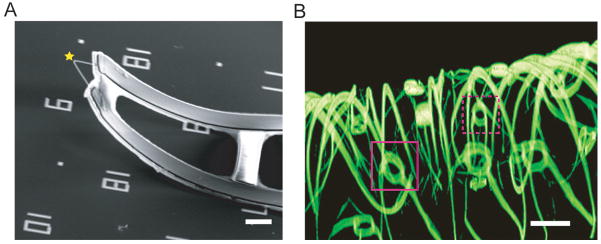
Figure 2. Flexible and three dimensional nanoelectronic devices.
(A) Scanning electron microscopy image of a single kinked nanowire probe used for intracellular potential recording. The yellow star highlights the position of a field effect transistor. Scale bar, 5 μm. (B) Confocal fluorescence microscopy image of a macroporous nanoelectronic scaffold used for sensing from engineered tissues. Magenta boxes demarcate two field effect transistor devices. Scale bar, 20 μm.
2.4. A new library of bio-orthogonal tools
One of the most important properties of semiconductor materials is the diverse range of configurations, allowing for interrogation with biological systems in a bio-orthogonal fashion13, 38. During the past several decades, many such materials have been designed and realized, including colloidal nanoparticles39, semiconductor nanowires (NWs) and carbon nanotubes40–42, with scale dependent properties distinct from the bulk. Among all semiconductor nanosystems, silicon based materials and devices are particularly important given they are biocompatible and biodegradable32, 43, 44. Nevertheless, other semiconductor components can be chemically engineered to reduce their cytotoxicities under physiological conditions45, 46. This diverse set of materials provides a wide range of nanoscopic “building blocks” that can be applied in a biological context leading to a host of possible applications, with some examples including nanoscale biosensors23, 24, 28, drug delivery systems38, 47–49, intracellular pressure sensors50 and engineered tissue scaffolds14.
In regards to diverse functionality through synthetic control, silicon nanowires (SiNWs) have been one of the most successful nanoscopic platforms. SiNW structures can be designed and synthetically realized with complex, yet controlled, modulations in composition, doping, defects, and even topography13, 41, 42 (Fig. 3A). Recent progress has also observed the synthesis of other meso- or nanostructured silicon materials, such as silicon ‘diatoms’ 51(Fig. 3B) and nanoporous silicon membranes52 (Fig. 3C). This high degree of synthetic control enables the creation of building blocks with predictable physical properties and the assembly of hybrid or multicomponent functional materials in novel layouts and configurations, in turn allowing for the rational exploration of the silicon/biology interface38, creating new opportunities and technologies for a library of bio-orthogonal tools.
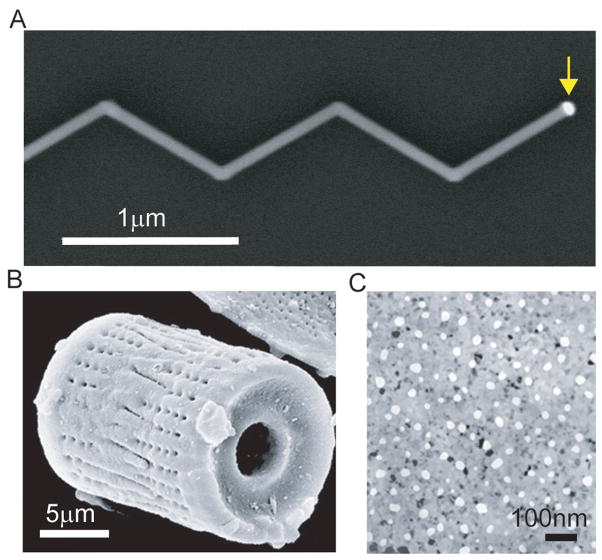
Figure 3. Complex silicon-based nanostructured materials.
(A) Scanning electron microscopy image of a kinked nanowire; yellow arrow highlights the gold catalyst used for VLS growth. (B) A silicon ‘diatom’ synthesized by magnesium reduction. (C) A nanoporous silicon membrane used for molecular separation. B and C are adapted from Reference 51 and 52, respectively, with the permission from Nature Publishing Group.
3. Silicon nanowire sensors for molecular biophysics studies
3.1. Study of molecule kinetics and activities
Nanowire field effect transistor based devices can be designed to examine protein dynamics with high precision at both an ensemble and single protein level24, 30. Selective protein discrimination can be achieved by the modification of a detector’s surface, in an analogous fashion to biomarker detection24, 28, 29. When multiple binding domains are present on a single detector, this approach yields an ensemble measurement and can be used to examine kinetics information, however this approach can also be adapted to the single protein level, reporting on processes such as folding and unfolding53. To study single protein dynamics, only a single protein may be present on an individual detector. Achieving this can pose a significant challenge, but could be addressed through point defect methods as demonstrated in carbon nanotube based sensors54, 55. Single protein dynamics can offer insight into the different stages of the enzymatic process, such as protein specific turnover rates, and the cause of enzymatic deactivation at the single molecule level information not readily available from ensemble measurements,.
3.2. DNA Detection with NWFET pores
As the demand for DNA sequencing increases, new high-throughput methods are needed to reduce consumer prices and achieve faster sequencing rates. To meet this challenge, a variety of methods have been explored, including translocation through nanotube devices56 and solid state nanopore devices57–59. Nanopore based platform is one of the most promising techniques, sequencing DNA by measuring the conductance through nanoscopic pores as DNA transports between two aqueous compartments58, 59. However the membrane translocation speed, ~ 1μs/base, can be too fast for signal amplification in small ion currents and can result in the detection lag60. One proposed solution is the use of NWFETs which can detect DNA in an analogous fashion to proteins and pathogens, but with faster temporal resolution. In 2011 the Lieber group demonstrated that NWFETs could potentially be configured as DNA sequencing devices when used in conjunction with a nanoscopic membrane pore60, combing the advantages of both techniques.
4. Silicon nanowire sensors for cellular biophysics studies
4.1. Extracellular electrical recordings
Monitoring extracellular electrical processes is important in understanding both intra and intercellular signaling, or how cells communicate across large networks. To study these processes, multiplexed NW arrays have been used both on the single cell level and as detectors for clustered groups of cells, allowing for the spatially resolved detection, stimulation and inhibition of extracellular signal propagation11, 13. NW biosensors can interface with single cells extracellularly, which sense changes in electric field potential as ionic species transverse the cell membrane (Fig. 4A). Multiple NWFETs can also be arranged along different points in the culture allowing for measurement of signal transduction speeds13. Moreover, because these nanowires can be placed within a confined region without apparent cross-talk, differences between long distance and short distance signaling can be discerned11, 13. So far, NWFETs have already been used to explore electrical signal propagation in neuronal cells and cardiomyocytes11, 13, although we note that they also hold potential in the study of several other cell types that use electrical signals as an activation mechanism.
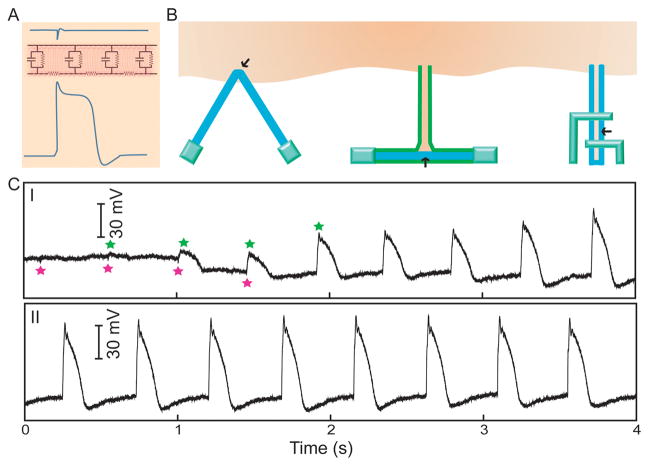
Figure 4. Intracellular electrical recording with field effect transistors.
(A) Plot comparing the amplitude of intracellular (lower) vs extracellular (upper) FET recordings, with shape ‘distortions’ due to the resistor-capacitor (RC) components from plasma membrane (middle). (B) Several FET configurations for intracellular recording. The black arrows indicate the sensing domains. (C) Electrical recording traces from a kinked nanowire probe as it transitions (I) from an extracellular (magenta stars) to intracellular (green stars) space, and (II) reaches an intracellular steady state.
4.2 Intracellular electrical recordings
Lipid membranes serve as electrical barriers which attenuate transmembrane signal amplitude and produce signal distortions13 (Fig. 4A). As a result, extracellular sensors are limited in their capacity to detect intracellular signals. Recent progress has shown that NWFETs can be brought into contact with intracellular domains in order to directly record intracellular activities in a localized and tunable fashion, with three examples depicted in Fig. 4B: kinked NWFET11, branched intracellular nanotube NWFET15 and active nanotube NWFET16. The representative electrical recordings from a spontaneously beating cardiomyocyte using a kinked NWFET are shown in Figure 4C11.
This process is relatively non-invasive when compared to traditional intracellular recording probes such as voltage-sensitive optical dyes and single-terminal glass or carbon microelectrodes13. Such electrodes are limited as intracellular species are exposed to the probe’s electrolyte solution (Fig. 5A) and current is passed directly through the cytosol (Fig. 5B), both of which may induce irreversible changes to the cells, calling into question the physiological relevance of these recordings and preventing long-term non-invasive studies. Semiconductor devices are able to circumvent this by using a fundamentally different circuit configuration (Fig. 5C); processing cellular information without the need for direct communication with cellular ions thus minimizing junction impedance and cellular invasiveness issues13.
Figure 5. A comparison between conventional intracellular electrical recording tools and a kinked nanoscale field effect transistor (nanoFET) probe.
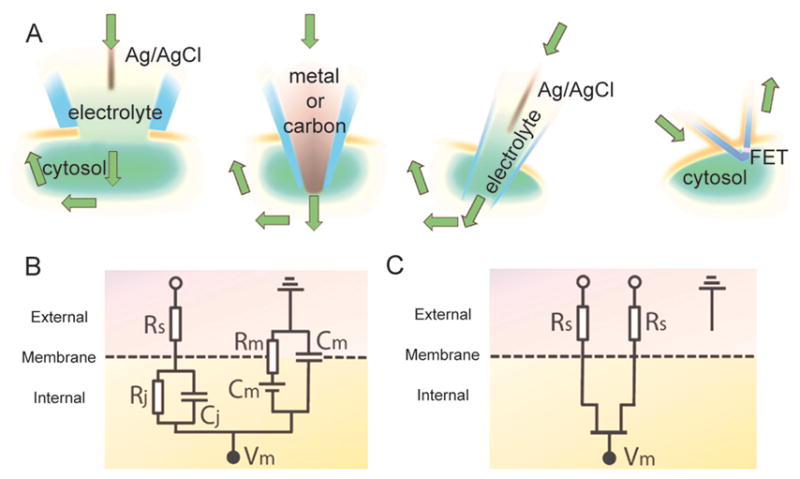
(A) Four intracellular recordings are depicted: glass micropipette, metal or carbon micro/nanoelectrode, glass micropipette, and nanoFET (from left to right). The green arrows indicate the current flows. (B) and (C) are the equivalent circuits of the intracellular junctions established through conventional devices and nanoFET, respectively. Abbreviations: Cj, junction capacitance; Cm, membrane capacitance; Rs, series resistance; Rj, junction resistance; Rm, membrane resistance; Vm, intracellular potential.
4.3 Electrical recordings from synthetic tissues
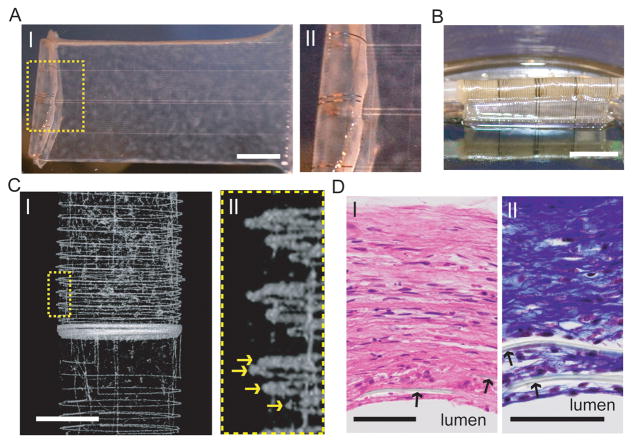
The use of semiconductors for recording electrical information can be further extended towards the development of synthetic tissues with embedded nanoelectronic sensory capabilities. In 2012, the Lieber and Kohane groups designed vascular nanoelectronic scaffolds (nanoES) constructs for use in tissue-engineering blood vessels14. Hybrid human aortic smooth muscle cell (HASMC) nanoES sheets were fabricated by culturing the cells on a 2D mesh nanoES with an agent that promotes natural ECM deposition on the mesh (Fig, 6A). The hybrids were then rolled into 3D tubular structures and allowed to mature (Fig. 6B). Micro-computed tomography (micro-CT) was used to visualize the distribution of the nanoES mesh in the tubular structure and it was shown that metal interconnects were regularly spaced (Fig. 6C, I) with at least four revolutions (Fig. 6C,II)14. The hybrid tissues were subsequently stained with Hematoxylin-eosin and Masson-trichome stains revealing healthy 200 micron thick smooth muscle with embedded polymer (SU-8) ribbons from the nanoES confirming the 3D integration of the NWFET with the smooth muscle tissue (Figure 6D). The ability to successfully integrate NW sensors into 3D tissues represents a new direction for merging nanoelectronics with biological systems including incorporating nanoscale stimulatory elements into the tissue-nanoES hybrids13. With future engineering approaches, sensing capabilities could be broadened to address various disease states, in vitro (lab-on-a-chip, 3D tissue-based therapeutic assays) or in vivo. Cell or tissue interactions with nanoES could be fine-tuned by modification with cell growth determinants47. The elements in nanoES could be expanded to incorporate nanoscale stimulators and stretchable designs35 to provide electrical and mechanical stimulation to enhance cell culture; in vivo these properties could provide functionalities such as pacing, and moduli that match those of host tissues.
Figure 6. Nanoelectronics integrated into synthetic tissue.
(A) (I) Photograph of a single HASMC sheet cultured with sodium L-ascorbate on a nanoES. (II) Zoomed-in view of the dashed area in (I), showing metallic interconnects macroscopically integrated with cellular sheet. (B) Photograph of the vascular construct after rolling into a tube and maturation in a culture chamber for 3 weeks. (C) (I) Micro-computed tomograph of a tubular construct segment. (II) Zoomed-in view of (I). Yellow arrows mark the individual nanowire FET-containing layers of the rolled construct. Scale bar, 1 mm. (D) (I) Hematoxylin & eosin and (II) Masson Trichrome (;collagen is blue) stained sections of nanoelectronic-HASMC hybrid (~ 6 μm thick) cut perpendicular to the tube axis; lumen regions are labeled. Black arrows mark the positions of SU-8 ribbons of the nanoES. Scale bars, 50 μm.
5. Outlook
As minimally invasive and highly sensitive detectors, nanoscale semiconductor devices offer a promising new approach to studying the behavior of electrical activity in a biological context. Notably, there are emerging challenges and opportunities in FET based electrical sensing of biological systems. For example, although pH sensing is readily achievable, the sensitivity for proteins and other macromolecules under physiological conditions need to be improved significantly with new operation schemes61 or surface chemistry62. Additionally, FET is currently limited to the detection of potential variations and charges, and there is still a significant need for ionic current sensing in order to understand more quantitatively the signaling of biological systems63, 64.
This mini-review has highlighted some of the key aspects that make semiconductors good detectors, however there are many unexplored opportunities for also influencing the behavior of cells via electrical or optoelectronic stimulation. The mechanisms by which proteins sense voltage changes are diverse65. Ion channels, for example, have a conserved, positively charged transmembrane region that moves in response to changes in membrane potential65. Additionally, some G-protein coupled receptors possess a specific voltage-sensing motif while some membrane pumps and transporters use the ions that they transport across membranes to sense membrane voltage65. The charged groups of proteins, their arrangements, local field strength, disposition and movements of the charges or dipoles can be variable; however, the final result is that changes in the electric field are transduced into a conformational change that alters the proteins function, thereby ultimately controlling a single cells behavior65. This important feature of proteins not only strongly indicates that the intracellular bioelectric networks may be very important in cell signaling (Fig. 1), but that such protein responses can be controlled using localized electrical or optoelectronic stimulations through nanostructured semiconductor devices. This gives rise to the possibility of ‘Cyborg Cells’ and a technique based on semiconductor analog of ‘optogenetics’66–68, cells with internalized semiconducting materials capable of tunable behavior, controlled using external optical and electrical stimulus (Fig. 1).
Acknowledgments
BT acknowledges the financial support from National Science Foundation (NSF CAREER, Award Number 1254637) and Searle Scholars Foundation. RP acknowledges support from Interdisciplinary Scientist Training Program - T32 NIH Training grant (GM007281).
Biographies
John Zimmerman
Born and raised in Seattle, WA, John Zimmerman graduated from Whitman college in 2011, receiving his bachelor’s in chemistry. During his undergraduate career, he investigated protein purification platforms for use in microbatch x-ray crystallography under Tim Machonkin. He is currently a third year graduate student in the University of Chicago’s Chemistry Ph.D program studying the interface between silicon nanomaterials and biological systems. In his free time he enjoys painting, trombone, sailing and board games.
Ramya Parameswaran
Ramya Parameswaran grew up in Moraga, CA. She received a BS with Honors in Chemical Engineering in 2010 and an MS in Chemical Engineering in 2011 from Stanford University. As an undergraduate, she worked in the Felsher Laboratory at Stanford University studying mouse models of lymphoma. Prior to joining the University of Chicago’s Medical Scientist Training Program (MSTP) in 2012, she worked in the Weiss Laboratory at UCSF studying B cell development and anergy. She is currently a second year in the MSTP doing her Ph.D in Biophysical Sciences. Her other hobbies include playing the violin, drawing, dancing, and running.
Bozhi Tian
Dr. Tian received his Ph. D degree in physical chemistry from Harvard University in 2010. His Ph.D. research with Professor Charles Lieber included new nanowire materials synthesis, the fundamental study of high performance nanowire photovoltaics and the application of novel nanowire devices in cells and tissue. He worked with Professors Robert Langer and Daniel Kohane as a postdoctoral scholar in regenerative medicine. He is now an assistant professor at the University of Chicago, working on semiconductor based cellular biophysics. Dr. Tian’s accolades include 2013 NSF CAREER award, 2013 Searle Scholar award, and 2012 TR35 honoree. He enjoys painting and calligraphy.
References
- 1.Levin M. Seminars in Cell & Developmental Biology. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin M, Stevenson CG. In: Annual Review of Biomedical Engineering. Yarmush ML, editor. Vol. 14. 2012. pp. 295–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levin M. Bioelectromagnetics. 2003;24:295–315. doi: 10.1002/bem.10104. [DOI] [PubMed] [Google Scholar]
- 4.Cifra M, Fields JZ, Farhadi A. Progress in Biophysics & Molecular Biology. 2011;105:223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Cifra M, Pokorny J, Havelka D, Kucera O. Biosystems. 2010;100:122–131. doi: 10.1016/j.biosystems.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Kann O, Kovacs R. American Journal of Physiology-Cell Physiology. 2007;292:C641–C657. doi: 10.1152/ajpcell.00222.2006. [DOI] [PubMed] [Google Scholar]
- 7.Spira ME, Hai A. Nature Nanotechnology. 2013;8:83–94. doi: 10.1038/nnano.2012.265. [DOI] [PubMed] [Google Scholar]
- 8.Grewer C, Gameiro A, Mager T, Fendler K. In: Annual Review of Biophysics. Dill KA, editor. Vol. 42. 2013. pp. 95–120. [DOI] [PubMed] [Google Scholar]
- 9.Knopfel T. Nature Reviews Neuroscience. 2012;13:687–700. doi: 10.1038/nrn3293. [DOI] [PubMed] [Google Scholar]
- 10.Grinvald A, Hildesheim R. Nature Reviews Neuroscience. 2004;5:874–885. doi: 10.1038/nrn1536. [DOI] [PubMed] [Google Scholar]
- 11.Timko BP, Cohen-Karni T, Qing Q, Tian BZ, Lieber CM. Ieee Transactions on Nanotechnology. 2010;9:269–280. doi: 10.1109/TNANO.2009.2031807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian BZ, Lieber CM. Pure and Applied Chemistry. 2011;83:2153–2169. doi: 10.1351/PAC-CON-11-08-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian BZ, Lieber CM. In: Annual Review of Analytical Chemistry. Cooks RG, Pemberton JE, editors. Vol. 6. 2013. pp. 31–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian BZ, Liu J, Dvir T, Jin LH, Tsui JH, Qing Q, Suo ZG, Langer R, Kohane DS, Lieber CM. Nature Materials. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan XJ, Gao RX, Xie P, Cohen-Karni T, Qing Q, Choe HS, Tian BZ, Jiang XC, Lieber CM. Nature Nanotechnology. 2012;7:174–179. doi: 10.1038/nnano.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao RX, Strehle S, Tian BZ, Cohen-Karni T, Xie P, Duan XJ, Qing Q, Lieber CM. Nano Letters. 2012;12:3329–3333. doi: 10.1021/nl301623p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qing Q, Pal SK, Tian BZ, Duan XJ, Timko BP, Cohen-Karni T, Murthy VN, Lieber CM. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1882–1887. doi: 10.1073/pnas.0914737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Z, Qing Q, Xie P, Gao RX, Lieber CM. Nano Letters. 2012;12:1711–1716. doi: 10.1021/nl300256r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitesides GM. Nature Biotechnology. 2003;21:1161–1165. doi: 10.1038/nbt872. [DOI] [PubMed] [Google Scholar]
- 20.Leong M, Doris B, Kedzierski J, Rim K, Yang M. Science. 2004;306:2057–2060. doi: 10.1126/science.1100731. [DOI] [PubMed] [Google Scholar]
- 21.Brenner C, Moulin M. Circulation Research. 2012;111:1237–1247. doi: 10.1161/CIRCRESAHA.112.265942. [DOI] [PubMed] [Google Scholar]
- 22.Duan XJ, Fu TM, Liu J, Lieber CM. Nano Today. 2013;8:351–373. doi: 10.1016/j.nantod.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patolsky F, Zheng GF, Lieber CM. Nature Protocols. 2006;1:1711–1724. doi: 10.1038/nprot.2006.227. [DOI] [PubMed] [Google Scholar]
- 24.Zheng GF, Patolsky F, Cui Y, Wang WU, Lieber CM. Nature Biotechnology. 2005;23:1294–1301. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- 25.Hu YJ, Xiang J, Liang GC, Yan H, Lieber CM. Nano Letters. 2008;8:925–930. doi: 10.1021/nl073407b. [DOI] [PubMed] [Google Scholar]
- 26.Xiang J, Lu W, Hu YJ, Wu Y, Yan H, Lieber CM. Nature. 2006;441:489–493. doi: 10.1038/nature04796. [DOI] [PubMed] [Google Scholar]
- 27.MacBeath G. Nature Genetics. 2002;32:526–532. doi: 10.1038/ng1037. [DOI] [PubMed] [Google Scholar]
- 28.Stern E, Klemic JF, Routenberg DA, Wyrembak PN, Turner-Evans DB, Hamilton AD, LaVan DA, Fahmy TM, Reed MA. Nature. 2007;445:519–522. doi: 10.1038/nature05498. [DOI] [PubMed] [Google Scholar]
- 29.Stern E, Vacic A, Rajan NK, Criscione JM, Park J, Ilic BR, Mooney DJ, Reed MA, Fahmy TM. Nature Nanotechnology. 2010;5:138–142. doi: 10.1038/nnano.2009.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan XX, Li Y, Rajan NK, Routenberg DA, Modis Y, Reed MA. Nature Nanotechnology. 2012;7:401–407. doi: 10.1038/nnano.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao J, Yan H, Lieber CM. Nature Nanotechnology. 2013;8:329–335. doi: 10.1038/nnano.2013.55. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SW, Tao H, Kim DH, Cheng HY, Song JK, Rill E, Brenckle MA, Panilaitis B, Won SM, Kim YS, Song YM, Yu KJ, Ameen A, Li R, Su YW, Yang MM, Kaplan DL, Zakin MR, Slepian MJ, Huang YG, Omenetto FG, Rogers JA. Science. 2012;337:1640–1644. doi: 10.1126/science.1226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webb RC, Bonifas AP, Behnaz A, Zhang YH, Yu KJ, Cheng HY, Shi MX, Bian ZG, Liu ZJ, Kim YS, Yeo WH, Park JS, Song JZ, Li YH, Huang YG, Gorbach AM, Rogers JA. Nature Materials. 2013;12:938–944. doi: 10.1038/nmat3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim DH, Lu NS, Ghaffari R, Kim YS, Lee SP, Xu LZ, Wu JA, Kim RH, Song JZ, Liu ZJ, Viventi J, de Graff B, Elolampi B, Mansour M, Slepian MJ, Hwang S, Moss JD, Won SM, Huang YG, Litt B, Rogers JA. Nature Materials. 2011;10:316–323. doi: 10.1038/nmat2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim DH, Lu NS, Ma R, Kim YS, Kim RH, Wang SD, Wu J, Won SM, Tao H, Islam A, Yu KJ, Kim TI, Chowdhury R, Ying M, Xu LZ, Li M, Chung HJ, Keum H, McCormick M, Liu P, Zhang YW, Omenetto FG, Huang YG, Coleman T, Rogers JA. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- 36.Rogers JA, Lagally MG, Nuzzo RG. Nature. 2011;477:45–53. doi: 10.1038/nature10381. [DOI] [PubMed] [Google Scholar]
- 37.Viventi J, Kim DH, Vigeland L, Frechette ES, Blanco JA, Kim YS, Avrin AE, Tiruvadi VR, Hwang SW, Vanleer AC, Wulsin DF, Davis K, Gelber CE, Palmer L, Van der Spiegel J, Wu J, Xiao JL, Huang YG, Contreras D, Rogers JA, Litt B. Nature Neuroscience. 2011;14:1599–U1138. doi: 10.1038/nn.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine D, Grattoni A, Goodall R, Bansal SS, Chiappini C, Hosali S, van de Ven AL, Srinivasan S, Liu XW, Godin B, Brousseau L, Yazdi IK, Fernandez-Moure J, Tasciotti E, Wu HJ, Hu Y, Klemm S, Ferrari M. Adv Healthc Mater. 2013;2:632–666. doi: 10.1002/adhm.201200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin Y, Alivisatos AP. Nature. 2005;437:664–670. doi: 10.1038/nature04165. [DOI] [PubMed] [Google Scholar]
- 40.Hu JT, Odom TW, Lieber CM. Accounts of Chemical Research. 1999;32:435–445. [Google Scholar]
- 41.Li Y, Qian F, Xiang J, Lieber CM. Materials Today. 2006;9:18–27. [Google Scholar]
- 42.Lu W, Lieber CM. Journal of Physics D-Applied Physics. 2006;39:R387–R406. [Google Scholar]
- 43.Cassidy MC, Chan HR, Ross BD, Bhattacharya PK, Marcus CM. Nature Nanotechnology. 2013;8:363–368. doi: 10.1038/nnano.2013.65. [DOI] [PubMed] [Google Scholar]
- 44.Tasciotti E, Liu XW, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MMC, Decuzzi P, Tour JM, Robertson F, Ferrari M. Nature Nanotechnology. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 45.Dobrovolskaia MA, Germolec DR, Weaver JL. Nature Nanotechnology. 2009;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 46.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nature Materials. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 47.Dvir T, Timko BP, Kohane DS, Langer R. Nature Nanotechnology. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Timko BP, Dvir T, Kohane DS. Advanced Materials. 2010;22:4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 49.Shalek AK, Robinson JT, Karp ES, Lee JS, Ahn DR, Yoon MH, Sutton A, Jorgolli M, Gertner RS, Gujral TS, MacBeath G, Yang EG, Park H. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1870–1875. doi: 10.1073/pnas.0909350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Martinez R, Hernandez-Pinto AM, Duch M, Vazquez P, Zinoviev K, de la Rosa EJ, Esteve J, Suarez T, Plaza JA. Nature Nanotechnology. 2013;8:517–521. doi: 10.1038/nnano.2013.118. [DOI] [PubMed] [Google Scholar]
- 51.Bao ZH, Weatherspoon MR, Shian S, Cai Y, Graham PD, Allan SM, Ahmad G, Dickerson MB, Church BC, Kang ZT, Abernathy HW, Summers CJ, Liu ML, Sandhage KH. Nature. 2007;446:172–175. doi: 10.1038/nature05570. [DOI] [PubMed] [Google Scholar]
- 52.Striemer CC, Gaborski TR, McGrath JL, Fauchet PM. Nature. 2007;445:749–753. doi: 10.1038/nature05532. [DOI] [PubMed] [Google Scholar]
- 53.Choi YK, Moody IS, Sims PC, Hunt SR, Corso BL, Perez I, Weiss GA, Collins PG. Science. 2012;335:319–324. doi: 10.1126/science.1214824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldsmith BR, Coroneus JG, Khalap VR, Kane AA, Weiss GA, Collins PG. Science. 2007;315:77–81. doi: 10.1126/science.1135303. [DOI] [PubMed] [Google Scholar]
- 55.Sorgenfrei S, Chiu CY, Gonzalez RL, Yu YJ, Kim P, Nuckolls C, Shepard KL. Nature Nanotechnology. 2011;6:125–131. doi: 10.1038/nnano.2010.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goldberger J, Fan R, Yang PD. Accounts of Chemical Research. 2006;39:239–248. doi: 10.1021/ar040274h. [DOI] [PubMed] [Google Scholar]
- 57.Venkatesan BM, Bashir R. Nature Nanotechnology. 2011;6:615–624. doi: 10.1038/nnano.2011.129. [DOI] [PubMed] [Google Scholar]
- 58.Branton D, Deamer DW, Marziali A, Bayley H, Benner SA, Butler T, Di Ventra M, Garaj S, Hibbs A, Huang XH, Jovanovich SB, Krstic PS, Lindsay S, Ling XSS, Mastrangelo CH, Meller A, Oliver JS, Pershin YV, Ramsey JM, Riehn R, Soni GV, Tabard-Cossa V, Wanunu M, Wiggin M, Schloss JA. Nature Biotechnology. 2008;26:1146–1153. doi: 10.1038/nbt.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deamer D. In: Annual Review of Biophysics. Rees DC, Dill KA, Williamson JR, editors. Vol. 39. 2010. pp. 79–90. [DOI] [PubMed] [Google Scholar]
- 60.Xie P, Xiong QH, Fang Y, Qing Q, Lieber CM. Nature Nanotechnology. 2012;7:119–125. doi: 10.1038/nnano.2011.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kulkarni GS, Zhong ZH. Nano Letters. 2012;12:719–723. doi: 10.1021/nl203666a. [DOI] [PubMed] [Google Scholar]
- 62.Buriak JM. Chemical Reviews. 2002;102:1271–1308. doi: 10.1021/cr000064s. [DOI] [PubMed] [Google Scholar]
- 63.Buzsaki G, Anastassiou CA, Koch C. Nature Reviews Neuroscience. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid B, Nuccitelli R, Zhao M. Nature Protocols. 2007;2:661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- 65.Bezanilla F. Nature Reviews Molecular Cell Biology. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 66.Kim TI, McCall JG, Jung YH, Huang X, Siuda ER, Li YH, Song JZ, Song YM, Pao HA, Kim RH, Lu CF, Lee SD, Song IS, Shin G, Al-Hasani R, Kim S, Tan MP, Huang YG, Omenetto FG, Rogers JA, Bruchas MR. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martiradonna L. Nature Materials. 2013;12:472–472. [Google Scholar]
- 68.Deisseroth K. Nature Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]