Abstract
Aims
To determine whether herpes simplex virus–based vectors can efficiently transduce mouse trigeminal ganglion (TG) neurons and attenuate preexisting nerve injury–induced whisker pad mechanical hypersensitivity in a trigeminal inflammatory compression (TIC) neuropathic pain model.
Methods
Tissue transduction efficiencies of replication-conditional and replication-defective vectors to mouse whisker pads after topical administration and subcutaneous injection were assessed using quantitative real-time PCR (qPCR). Tissue tropism and transgene expression were assessed using qPCR and reverse-transcriptase qPCR following topical application of the vectors. Whisker pad mechanical sensitivities of TIC-injured mice were determined using graduated von Frey fibers before and after application of human preproenkephalin expressing replication-conditional vector (KHPE). Data were analyzed using one-way analysis of variance (ANOVA) and post hoc tests.
Results
Transduction of target TGs was 8- to 50-fold greater after topical application than subcutaneous injection and ≥ 100-fold greater for replication-conditional than replication-defective vectors. Mean KHPE loads remained constant in TGs (4.5–9.8 × 104 copies/TG) over 3 weeks but were below quantifiable levels (10 copies/tissue) within 2 weeks of application in other nontarget cephalic tissues examined. Transgene expression in TGs was maximal during 2 weeks after topical application (100–200 cDNA copies/mL) and was below quantifiable levels (1 cDNA copy/mL) in all nontarget tissues. Topical KHPE administration reduced TIC-related mechanical hypersensitivity on whisker pads 4-fold (P < .05) for at least 1 week.
Conclusion
Topically administered KHPE produced a significant antinociceptive effect in the TIC mouse model of chronic facial neuropathic pain. This is the first report in which a gene therapeutic approach reduced trigeminal pain–related behaviors in an established pain state in mice.
Keywords: gene therapy, mechanical allodynia, nerve injury, neuropathic pain, trigeminal ganglia
Use of virus-based vectors for the treatment of chronic pain has been reported extensively and systematically reviewed.1–3 However, the methodology that affords efficient transfer of vectors to orofacial tissues, using simple clinical techniques, has not been extensively utilized. Delivery of vectors encoding nonopioid genes (ie, neurotrophins, neurotransmitters, and immune modulatory genes) or opioid genes (ie, enkephalin, β-endorphin, endomorphin, and μ-opioid receptor genes) via multiple routes—including subcutaneous, intra-articular, intradermal, intramuscular, neural, pancreas, parenchymal spinal cord, and intrathecal spinal cord injections, as well as topical application to abraded skin—has proven efficacious in many animal models of chronic pain.2 Remarkably, only three reports have described the use of virus-based vector therapy for trigeminal region–associated pain. Two of these studies involved the direct injection of vectors into the mouse and rat trigeminal ganglion (TG),4,5 whereas the third indirectly transduced rat TGs via nerves innervating the whisker pads.6 In all three cases, vectors were applied prior to injury and therefore evaluated the ability of gene therapy to hinder the development of induced orofacial pain.
Although many vectors are available for gene transfer, vectors based on recombinant herpes simplex virus (HSV) are uniquely suited to deliver therapeutic transgenes following subcutaneous inoculation to peripheral neurons because HSV is naturally taken up by peripheral nerve endings and is neurotropic.7–9 In addition, HSV-based vectors can be constructed to be replication-defective (ie, cannot replicate in any noncomplementing cell line) or replication-conditional (ie, can replicate in dividing cells but not in terminally differentiated, nondividing cells). Thus, the nonreplicative state of the vector remains in the neuron and precludes vector access to adjacent nonneuronal cells.10–16 By peripheral delivery of an HSV-based replication-conditional vector prior to injury, Meunier and colleagues demonstrated that the overproduction of rat proenkephalin A in TG neurons, before the establishment of chronic orofacial neuropathic pain, significantly attenuated the development of mechanical hypersensitivity in rats.6 However, whether replication-conditional vectors can be efficiently delivered to, and maintained in, TG neurons of mice after cutaneous delivery, and whether such vectors can ameliorate mechanical hypersensitivity after nerve injury, has not been reported.
The aims of this study were to determine whether HSV-based vectors can efficiently transduce mouse TG neurons and attenuate preexisting nerve injury–induced whisker pad mechanical hypersensitivity in a trigeminal inflammatory compression (TIC) neuropathic pain model.17
Materials and Methods
Animals
Experiments were carried out in accordance with the guidelines established by the National Institute of Health regarding the care and use of animals for experimental procedures. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky. Male Balb/C mice (Harlan Laboratories) were primarily used to optimize delivery of replication-conditional and replication-defective HSV-based vectors to the TG. CD-1, Webster, and C57bl/6 mice (Harlan Laboratories) were also used to validate the delivery methods, to assess potential spread, and to verify lack of strain differences. Male mice (n = 103) aged 5 to 6 weeks at the start of the study were accommodated in ventilated animal housing with a 12/12-hour light/dark cycle.
Vectors
Two types of HSV-based vectors were used. The replication-defective vector expressing green fluorescent protein (D3GFP) was provided by Dr Darren Wolfe (PeriphaGen). It has a backbone similar to that of the NP2 vector described by Fink and colleagues.18 The defective vectors are deleted for two essential immediate early (IE) genes (ICP4 and ICP27) and the promoters of two additional IE genes (ICP22 and ICP47), and they can only replicate in complementing cells. D3GFP was used at the concentration provided. Replication-conditional vectors that express the human preproenkephalin (hENK) gene (KHPE, experimental vector) or β-galactosidase (KHZ, vector control) replicate in dividing cells but not in terminally differentiated nondividing neurons due to insertional inactivation of the viral thymidine kinase gene, which is only required in nondividing cells.16 These vectors were obtained from Dr Steven Wilson19 and were propagated and titrated on Vero cells as previously described.20–22
Surgery
Induction of a peripheral trigeminal neuropathic pain model was performed as previously described.17 Briefly, mice were anesthetized with sodium pentobarbital (70 mg/kg, ip), shaved, and placed in a stereotaxic frame. Following a 15-mm skin incision, the orbicularis oculi muscle was gently dissected from the bone. The infraorbital nerve (trigeminal V2) was dissected free from the bone at its most rostral extent along the infraorbital canal. A 2-mm length of chromic gut suture (6-0) was inserted between the left infraorbital nerve and the maxillary bone. Skin incisions were sutured with 5-0 nylon nonabsorbable monofilament, and mice were allowed to recover for 3 days.
Assessment of Mechanical Allodynia on the Whisker Pad
The analgesic effect of KHPE vector, which has been shown to reduce mechanical and/or thermal sensitivity in mouse,19,23,24 rat,25 and primate26 pain models, was evaluated in the mouse TIC model. TIC induces mechanical hypersensitivity that becomes evident at day 3 post–nerve injury and persists for many weeks,17 thus allowing measurement of the efficacy of the vector over several weeks.
Whisker pad mechanical sensitivity was assessed using a series of eight von Frey fiber filaments (0.008, 0.02, 0.07, 0.16, 0.4, 1.0, 2.0, and 6.0 g; Stoelting) with a modified up-down method to calculate the 50% mechanical withdrawal threshold.17 During testing, one experimenter slightly restrained the mouse while another experimenter applied von Frey filaments onto the mouse whisker pad, both ipsilateral and contralateral to the surgery site. For consistency of results, each filament was applied five times with a few seconds interval between each application. The mouse was considered to be responsive to a filament when its head withdrawal occurred three times after probing with that filament. For this approach, whenever a positive response to the mechanical stimulus occurred, the next weaker von Frey filament was applied. If no positive response was evoked, the next stronger filament was applied. Mechanical thresholds on the whisker pads of both sides were measured 2 days prior to surgery (baseline), 3 days postsurgery, and twice a week for 17 days following vector administration, as previously described.17
Administration of HSV-Based Vectors
KHPE, KHZ, or D3GFP vectors or vehicle control (isotonic saline) were administered to mice. Mice were anesthetized with pentobarbital (70 mg/kg, ip) and the whisker pads were shaved with a sharp razor blade. HSV-based vectors (10 µL, 8–9 × 106 plaque-forming units [PFU]) were administered either by subcutaneous injection into the whisker pads or topical application following light abrasion of the whisker pads. Abrasion was produced with 20 strokes of a 25-gauge needle, a variable speed Dremel (Robert Bosch Tool Corporation) fitted with a small, fine-grit abrasive cylinder or a Dermapen (Dermapen) all of which lightly abraded the dermis at minimum depth without evidence of bleeding. For topical application, inoculums were gently rubbed onto the abraded surface by using the side of a polypropylene pipette tip and allowed to absorb/dry for 15 to 30 minutes.
Quantification of Vector DNA and RNA
Ipsilateral and contralateral TGs were collected from euthanized mice 3 days or ≥ 2 weeks postinoculation, based on previously reported gene expression kinetics of thymidine kinase minus (TK−) HSV-1 during the establishment of viral latency in mouse TGs,15 and then were frozen immediately or transferred to RNAlater (Life Technologies) prior to freezing. In some cases, the medulla, pons, cortex, midbrain, and lacrimal gland were also collected. QIAamp DNA and AllPrep DNA/RNA mini kits (Qiagen) were used to purify DNA from tissues frozen directly or DNA and RNA from tissues stored frozen in RNAlater, respectively, according to manufacturer’s instructions. Equivalent volumes of RNA per tissue type (11 µL from TG and 6 µL from all other tissues) were treated with DNAse by using DNA-free (Ambion) as recommended by the manufacturer. DNAse-treated RNA was reverse transcribed using random primers and SuperScript II (Life Technologies) as recommended by the manufacturer. The quantity of DNA and cDNA were determined by quantitative real-time PCR (qPCR) on a 7500 real-time PCR system (PE Applied Biosystems) in a 50-µL reaction volume consisting of final concentrations of 1× TaqMan Universal PCR master mix (PE Applied Biosystems), 900 nM primers, and 250 nM TaqMan probe, as previously described.27 Analysis of vector DNA was performed with HSV-1 polymerase gene primers and probe (forward 5'-AGAGGGACATCCAGGACTTTGT-3'; reverse 5'-CAGGCGCTTGTTGGTGTAC-3'; probe 5'-ACCGCCGAACTGAGCA-3'), as described elsewhere.28 Analysis of vector gene expression (cDNA) was performed using primers and probes specific to the HSV-1 latency-associated transcript (LAT)—a marker of HSV latency in neurons, as previously described,29,30 and specific to the human preproenkephalin gene (forward 5'-ACGTGCAGCTACCGCCTAGT-3'; reverse 5'-GGCAGTTTACCTTCACATTCCATT-3'; probe 5'-CCGACATCAACTTCCTGGCTTGCG-3'), designed with the Primer Express 1.0 software (PE Applied Biosystems).
Immunohistochemistry
Ipsilateral and contralateral TGs were collected from euthanized mice 3, 10, or 20 days post–vector inoculation, based on previously reported gene expression kinetics of TK− HSV-1 during establishment of viral latency in mouse TGs.15 After 7-hours fixation in 4% paraformaldehyde, TGs were switched to 70% sucrose and then embedded in paraffin. TGs were sectioned (5 µm) and mounted onto glass slides (Super Frost Plus, VWR, Radnor, PA). Slides were deparaffinized by two Citrisolv (Fisher) treatments, dehydration with graded ethanol followed by a tap water rinse. Sections were incubated with citrate buffer at 37°C for 45 minutes followed by 0.1 M phosphate-buffered saline (PBS, pH 7.4) wash for 5 minutes. Sections were then treated with methanol containing 0.65% hydrogen peroxide for 20 minutes followed by another wash with PBS. After the sections were blocked with 5% milk in PBS containing 0.25% NP-40 (Sigma) for 45 minutes, they were incubated overnight at room temperature with mouse anti-β-Galactosidase (1:10,000, Promega) or rabbit anti-HSV-1 (1:10,000, Dakocytomation) antibodies in PBS containing 1% milk for immunolocalization of β-galactosidase transgene and HSV-1 infected cell proteins. Sections were rinsed with PBS three times (10 minutes each) and incubated with anti-mouse or anti-rabbit secondary antibodies from VECTASTAIN ABC kit (Vector Laboratories) for 45 minutes, rinsed three times with PBS, and incubated with reagents A and B for 1 hour. Sections were developed with 3,3'-diaminobenzidine (DAB) followed by tap water wash. Counterstaining with hematoxylin was stopped by quick tap water rinse. Sections were dehydrated with graded ethanol, processed in Citrisolv and coverslipped with Permount (Fisher). Photographs were taken using a Nikon E1000 microscope (Nikon Instruments, Inc) equipped with Act-1 Digital Photography (Nikon) programs.
Statistical Analyses
Differences between treatment groups were determined by one-way analysis of variance (ANOVA) and Fisher’s least significance test, using Statview software (SAS Institute) for PCR quantification and Tukey's Multiple Comparison Analysis (Graph Pad Software, Inc) for behavioral measures. P values were considered significant at ≤ .05.
Results
Optimization of Vector Delivery to TG
Initial efforts were directed toward optimizing vector delivery to TG of mice by varying the vector type and route of application. Balb/C mice (n = 52) were used primarily for these experiments; however, other strains of mice (CD-1 [n = 7], Webster [n = 7], and C57bl/6 [n = 3]) were also used to assess and validate the efficiency of vector delivery. Since there was a negligible effect of mouse strain on vector delivery relative to the mode of delivery (topical vs injection), results were combined into application groups regardless of mouse strain. Replication-conditional and replication-defective vectors were administered by either subcutaneous injection into the whisker pad or topical application following light abrasion of the whisker pad. The quantity of vector DNA present in TGs, collected from euthanized mice 3 days postinoculation, was determined by qPCR.
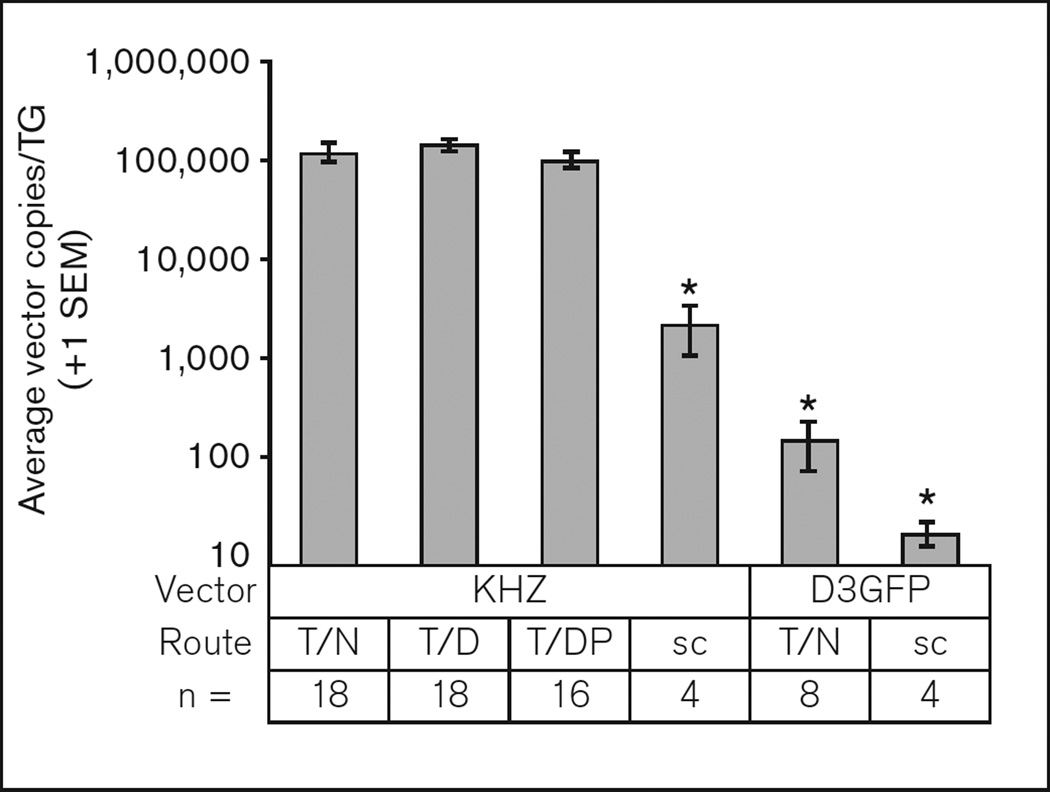
As shown in Fig 1, the viral load delivered by 3 to 4 days after topical application of the conditional vector (KHZ) was approximately 100,000 vector genomes/ipsilateral TG, regardless of the method of abrasion to the epidermis. In contrast, the amount of defective vector (D3GFP) DNA in the TG after topical application was nearly three orders of magnitude lower. Subcutaneous injection of either the conditional (KHZ) or defective (D3GFP) vectors resulted in substantially reduced loads detected in the TG (1–2 orders of magnitude) compared with topical delivery. These data indicate that the seeding of the TG benefits from conditional vector replication in the epithelium following topical treatment. Also, uptake from the nerve endings superficially in the epithelium rather than in subcutaneous tissue plays a role in optimal vector delivery to the TG.
Fig 1.
Efficiency of replication “conditional” KHZ and “defective” D3GFP vector delivery to the mouse trigeminal ganglion (TG). Mice were treated with replication-conditional vector KHZ (8.0 × 106 PFU in 10 µL) and replication-defective vector D3GFP (3.0 × 107 PFU in 10 µL) by subcutaneous injection (sc) into the whisker pad or topically following light abrasion of the whisker pad with a needle (T/N), Dremel (T/D), or Dermapen (T/DP). DNA was purified from ipsilateral TGs 3 to 4 days following administration. *P < .05 with respect to the first (left most) bar, ie, KHZ applied topically following light abrasion with the needle.
Anatomical Distribution of the Vector and Duration of Transgene Expression
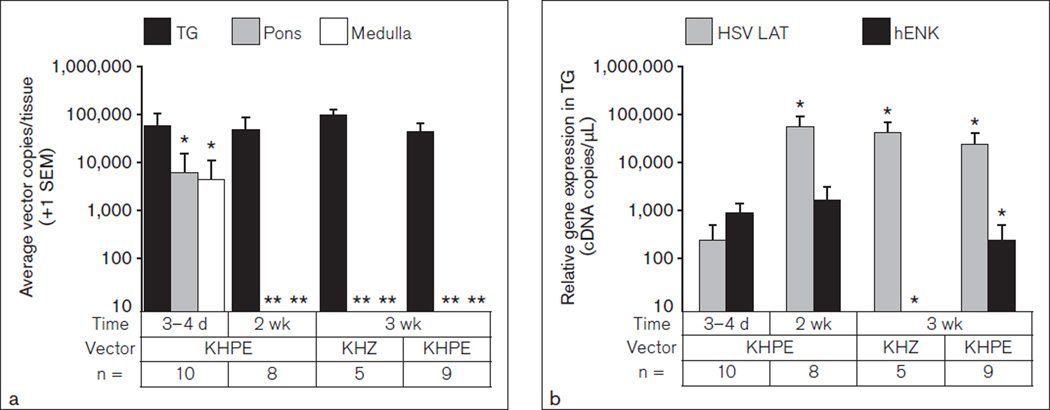
Since optimal transgene delivery to the TG was obtained with replication-conditional vectors following topical treatment, this method was employed for delivery of vectors to assess anatomical distribution. Neuronal tissues (TG, medulla, pons, cortex, midbrain) and the lacrimal gland, which is also innervated by the V2 branch of the trigeminal nerve, were harvested on day 3, week 2, and week 3 following topical application of KHZ and KHPE replication-conditional vectors. Total DNA was purified and the absolute vector DNA copies were determined. As shown in Fig 2a (dark bars), the amount of conditional vector DNA in the TG was stable over 3 weeks, with averages ranging from 45,000 to 98,000 copies per TG (average approximately 60,000 copies). In contrast, vector was detected in the pons (light bars) and medulla (empty bars) at day 3 postapplication but was below the level of quantification (ie, < 10 copies/tissue) 2 to 3 weeks postapplication (Fig 2a). Similarly, vector DNA was detected at substantial amounts at day 3 in the midbrain (3.5 × 103), cortex (5.1 × 103), and lacrimal glands (8.8 × 104) but decreased below the level of quantification by week 2 (data not shown).
Fig 2.
Tissue distribution of vector DNA. Vectors KHPE and KHZ were topically applied to the whisker pads of mice. Nucleic acid was purified from trigeminal ganglion (TG), pons, and medulla 3 days to 3 weeks after vector treatment. (a) Vector DNA was quantified by qPCR and average copies per ipsilateral tissue are shown as indicated. *P < .05 with respect to the TG day 3–4 data; **P < .01 with respect to pons or medulla day 3–4 day data. (b) cDNA was synthesized from RNA purified from the same tissues, and amounts of latency-associated transcript (LAT) and hENK cDNAs were quantified by qPCR. Average copies of LAT and hENK cDNA ± SEM derived from TGs are shown as indicated. *P < .05 with respect to day 3–4 data.
Since the primary objective was to deliver therapeutic genes to the TG, it was important to determine if the vector DNA identified in the TG was actively transcribed and for how long. In addition, assessment of vector gene expression in nontarget tissues could help explain the transitory nature of vector in these tissues. Total RNA was purified and cDNA was synthesized from the same tissues described in Fig 2a. The relative level of gene expression was determined by quantification of the cDNA complementary to the HSV-1 latency-associated transcript (LAT) and the KHPE vector-encoded hENK transgene. As shown in Fig 2b, the LAT transcript was detected in the TG, as expected, on day 3 after vector treatment and reached peak levels within 2 weeks. The LAT expression levels were 90 to 200 times higher at 2 and 3 weeks posttreatment than at day 3 posttreatment. In contrast, levels of hENK transgene mRNA were relatively similar in the TG at day 3 and week 2 postapplication of KHPE and declined by week 3, ie, hENK transgene expression levels at day 3 and week 2 were 3.5 to 6.5 times that of week 3 levels. Importantly, no hENK message was detected in animals treated with the control vector (KHZ) expressing β-galactosidase (Fig 2b) or vehicle controls (data not shown). Also, amounts of LAT and hENK cDNA were below the level of quantification (< 1 copy cDNA/mL) in cDNA generated from RNA derived from the pons, medulla, midbrain, cortex, and lacrimal gland on day 3 or week 2 after vector application (data not shown). These findings indicate that the vector is stably maintained and primarily expressed in the TG, whereas the vast majority of vector found in the pons, medulla, midbrain, cortex, and lacrimal gland remains cytoplasmic (ie, since it was transcriptionally inactive), where it is unstable and eliminated over a rather short period of time.
Transgene Expression Occurs in TG Neurons
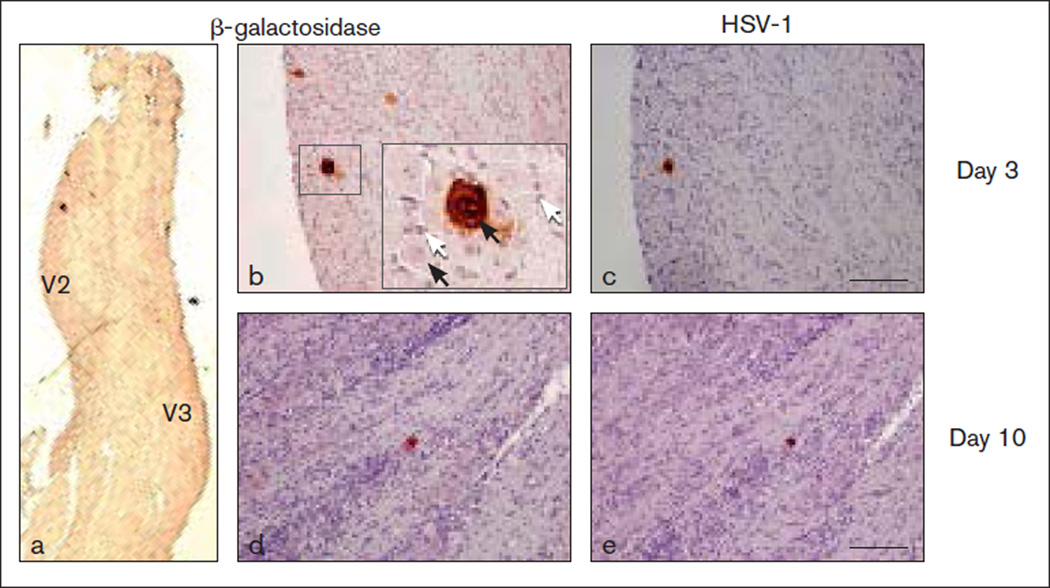
Since conditional vectors were primarily transcriptionally active in the TG, immunohistochemistry was used to determine (1) if transgene expression was limited to the innervating neurons; (2) the number of TG neurons producing detectable levels of transgene; and (3) if the vectors were capable of spreading to adjacent satellite glial cells. To avoid cross-reactivity with endogenous mouse enkephalin, these experiments were performed with the replication-conditional control vector expressing E coli β-galactosidase (KHZ) rather than the therapeutic vector expressing hENK (KHPE). At day 3 postapplication, β-galactosidase immunoreactivity was detected in 5 to 25 neurons per TG located in the V2 portion of the TG by using a β-galactosidase-specific antibody (Figs 3a and 3b). Although most sections were devoid of β-galactosidase staining, one to three positive-staining large cells consistent with the morphology of neurons were detected in several sections. Importantly, vector transgene was not detected in the smaller satellite glial cells and there was no evidence of vector spread to neighboring neurons. Immunoreactive HSV-1 infected cell protein was detected in the β-galactosidase positive-staining neuronal cells as determined in consecutive sections (Figs 3b and 3c); however, staining was generally of lower intensity than that for β-galactosidase. Between day 3 and day 10, the ability to detect vector-positive cells decreased. Only a single neuron producing detectable levels of β-galactosidase and HSV-1 infected cell proteins was observed among the TGs (n = 3) harvested 10 days after vector application (Figs 3d and 3e, respectively). Also, positively staining neurons were not detected in TGs (n = 3) 20 days postapplication (data not shown).
Fig 3.
Viral expression in the trigeminal ganglion (TG). Ipsilateral TGs were examined days 3 and 10 after vector KHZ application. Sections were immunostained for β-galactosidase (a to c) and respective consecutive sections for HSV-1 infected cell proteins (d and e). Few transduced neurons (dark-brown stain) were evident on days 3 (a to c) and 10 (d and e) in formalin-fixed, paraffin-embedded sections. Hematoxylin & eosin counterstain. Black arrows: neurons. White arrows: satellite glial cells. Calibration: (a) 500 µm; (b to e) 100 µm.
Topical Application of hENK-Expressing Vector Attenuates Mechanical Allodynia Induced by TIC
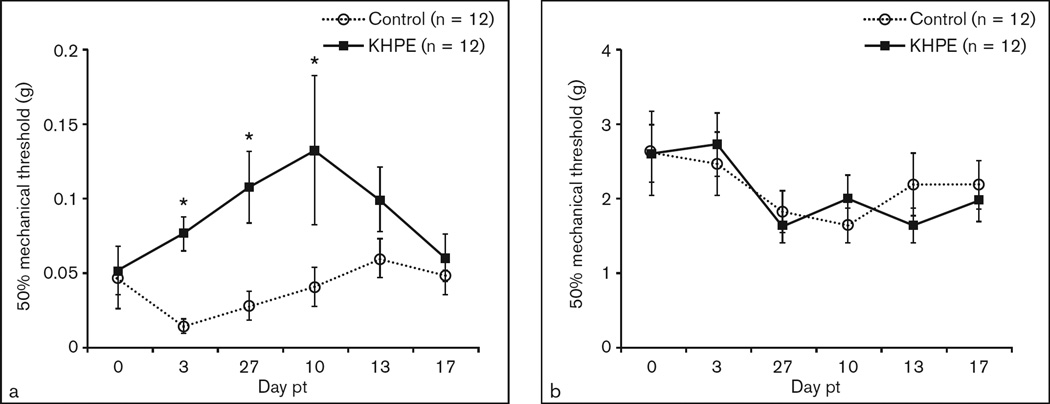
As a final step, the efficacy of proenkephalin overexpression, via a single application of KHPE to mouse whisker pads, for alleviating chronic orofacial pain–related behavior was determined. In this experiment, KHPE served as the treatment group and KHZ and vehicle as controls. As expected, by day 7 following compression nerve injury, the 50% mechanical threshold of the ipsilateral whisker pad was reduced to 0.05 g compared to 2.75 g on the contralateral side, indicating hypersensitivity (data not shown). On day 8 (ie, 1 day after confirmation of mechanical allodynia), the ipsilateral whisker pads of TIC-injured mice were lightly abraded and treated with the KHPE vector (experimental group), KHZ vector (vector control group), or isotonic saline (vehicle control group). As shown in Fig 4a, KHPE-treated mice demonstrated attenuated pain thresholds, ie, decreased facial mechanical hypersensitivity on days 3 to 10 posttreatment, whereas contralateral whisker pads were unaffected (Fig 4b). There was no significant difference in mechanical threshold between the KHZ vector control and saline vehicle control groups, so the two combined groups served as the control group. The average mechanical thresholds of KHPE-treated mice were 5.3, 3.8, and 3.2 times that of control mice on days 3, 7, and 10 posttreatment, respectively. Alleviation of mechanical hypersensitivity waned by day 13 and day 17 posttreatment.
Fig 4.
KHPE reduced mechanical hypersensitivity. Mechanical allodynia was evident on the ipsilateral whisker pad prior to vector delivery. Ipsilateral whisker pads of trigeminal inflammatory compression (TIC) mice were then treated topically with experimental vector KHPE (n = 12), control vector KHZ (n = 7), or vehicle control (n = 5) at 8 days post-TIC. Behavioral changes were determined on ipsilateral (a) and contralateral (b) whisker pads twice a week for 17 days after vector treatment (pt) (ie, 25 days post-TIC) and are presented as means ± SEM. *P < .05. Note scale differences.
Discussion
Identification of methods that provide relief for individuals suffering from chronic neuropathic pain in trigeminally innervated regions that are unresponsive to contemporary treatment is important. Although efficacy of gene therapy for alleviating pain occurring “below the neck” has been evaluated rather extensively, few studies have described efforts targeting facial areas innervated by the trigeminal nerve. The primary objective of this study was to exploit the natural ability of HSV-1 to infect terminal nerve peripheral endings, travel to the nerve cell nucleus via retrograde axonal transport, and be maintained in a nonreplicative state long-term (Fig 5), as has been previously shown.10–16 The study findings have demonstrated that HSV-1 based replication-conditional vectors (1) are more efficient in delivery to TG neurons than replication-defective vectors; (2) are more efficiently delivered to TG neurons following topical application to slightly abraded whisker pads compared to subcutaneous injection; (3) are maintained and remain transcriptionally active for at least 3 weeks in TG neurons; (4) are transiently present in a nonexpression state in other tissues; and (5) can significantly reduce ongoing pain-related behavior caused by previous nerve injury in the mouse.
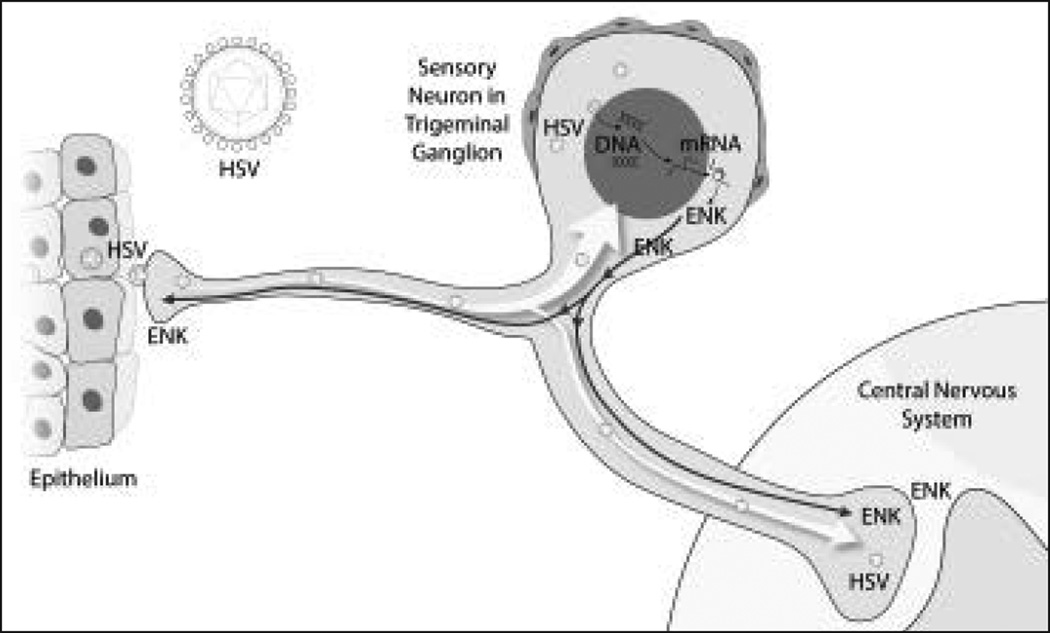
Fig 5.
A viral vector pump delivered to the mouse trigeminal ganglion by vector at peripheral application to facial tissues produced KHPE for 2 to 3 weeks and inhibited mechanical allodynia in whisker pad caused by trigeminal inflammatory compression injury. ENK = enkephalin; HSV = herpes simplex virus.
The natural properties of HSV-1 have been exploited to deliver therapeutic genes to nerve cells by targeting their peripheral nerve endings, as previously reviewed.8,9 In order for such neuronal-targeting vectors to be used for treatment of chronic oral and facial pain, it is important to determine if the most replication-compromised HSV-based vector (ie, replication-defective) and the least invasive route of delivery (ie, subcutaneous injection vs topical application to abraded skin) could be used to efficiently deliver vector to the TG. Findings from this study have shown that the replication-defective vector D3GFP can be delivered to the mouse TG following subcutaneous injection into the whisker pad, but the amount of vector may be two orders of magnitude less than subcutaneously injected replication-conditional vector KHZ. Delivery of defective vector D3GFP to the TG was enhanced 10-fold by applying the vector to the whisker pads after light abrasion with a 25-gauge needle. Remarkably, the amount of replication-conditional vector KHZ in the TG following topical application was nearly three orders of magnitude more than that of the defective vector and 50 times that of the conditional vector after subcutaneous injection. Application of KHZ to whisker pads after light skin perforation with a Dermapen or brief treatment with a Dremel resulted in similar TG vector loads as that following abrasion with a 25-gauge needle. Importantly, topical application of the control replication-conditional vector KHZ as well as that of the experimental vector KHPE resulted in delivery of vector DNA to the TG, which was stably maintained for at least 3 weeks. These results demonstrate that replication of the conditional vector in the epithelium near nerve endings rather than their axons is important for maximal delivery of vector to the TG, and that topical application, regardless of the method of light abrasion, is substantially superior to subcutaneous injection.
The requirement of TK− HSV-1 for viral replication in terminally differentiated TG and dorsal root ganglionic neurons is well established.10–16 However, the ability of TK− HSV-based vectors to persist and/or replicate in other tissues has not been reported. Since TK− HSV-based vectors, after administration at peripheral sites, can replicate in epithelium and migrate, it was important to examine the fate of such vectors at sites innervated by the V2 branch of the trigeminal nerve. An average of approximately 60,000 copies of KHPE vector DNA were detected in mouse TGs 3 days posttreatment and were maintained at high levels over the 3-week period of analysis. Notably, substantial amounts of KHPE vector DNA were also detected in the pons, medulla, midbrain, cortex, and lacrimal gland at days 3 and 4 after vector application. However, the presence of vector in these nontarget tissues was transitory, such that vector DNA was only rarely detected in trace quantities by 2 weeks posttreatment and beyond. Although it is not clear how vector DNA deposited in the lacrimal gland, it is possible that this may have resulted from vector transfer from the whisker pad to the eye during animal grooming.
To determine the kinetics of vector gene expression in the TG, cDNA was generated from RNA isolated from the same tissues used for vector DNA quantification. As expected, the expression of HSV-1 LAT and the hENK transgene were readily detected in TGs of KHPE-treated mice. LAT expression levels were 90 to 200 times higher at 2 and 3 weeks posttreatment than at day 3 posttreatment, whereas hENK transgene expression levels at day 3 and week 2 were 3.5 to 6.5 times that of week 3 levels. Only trace amounts of vector-transcribed RNA were detected in the pons, medulla, and lacrimal gland examined on day 3 posttreatment, indicating that the TK− HSV-based vector was only poorly, if at all, transported to, or transcribed in, the nuclei of cells of these tissues. Immunohistochemical staining of the KHZ-treated TGs demonstrated that relatively few neurons (0 to 3 per section) produced detectable levels of HSV-infected cell peptide or transgene (β-galactosidase) antigen, consistent with that found by others with TK− HSV-1 recombinant viruses following peripheral inoculation.10–16 Similarly, there was no indication of vector expression in satellite cells or replication and spread of the HSV-based vector in the TGs at the limits of detection with immunohistochemistry. The finding that the replication-conditional HSV-1 vectors were limited to the sensory neurons is consistent with several previous studies.10–16 Also the minimal, but statistically significant, analgesic effect of KHPE delivered by HSV-1 vector in the TIC model is likely reflective of the small number of vector-transduced neurons found in the TG.
The basis for this investigation is that (1) few studies have targeted vector delivery to the trigeminal region and (2) no studies involving orofacial pain gene therapy have applied vectors after the establishment of pain. Therefore, this study has provided insight into the efficacy of gene therapy for alleviating ongoing facial pain following its establishment. Here it was demonstrated that a single application of a replication-conditional HSV-based vector can significantly diminish pain-related behavior induced by injury to the mouse infraorbital nerve, as indicated by mechanical hypersensitivity of the whisker pad. The effect observed was 3 to 5 times less mechanical hypersensitivity than that of control TIC mice. Although the effects were transitory, lasting from 3 to 10 days after vector application, this is the first report in which a gene therapeutic approach reduced pain-related behavior in an established chronic facial pain model in mice. Whether the cutaneous neuronal nerve endings are the correct target site for vector delivery and whether other transgenes may prove more beneficial are questions to be addressed in future studies.
An important feature of the TIC model in mice is the minimal nerve damage that causes ipsilateral hypersensitivity. As described previously, the TIC model causes edema and myelin injury only in the nerve fascicles adjacent to the 2-mm chromic gut suture and does not impede blood flow. A similar report in mice also found a loosely tied infraorbital nerve (ie CCI-ION model) provided only insignificant hypersensitivity contralaterally.32 These mice models are in contrast to the original and many subsequent reports of bilateral hypersensitivity with the CCI-ION model in rats, which causes more demyelination and nerve injury after the entire nerve is tied.33 The anti-nociceptive effectiveness for the mechanical sensitivity relief is also consistent with that described in the literature.34
A limitation of the study was the use of male mice only. Data from female mice could be confounded by an increase in variability influenced by daily hormonal fluctuations. Female mice should be included in future studies in which such potential confounds are controlled.
Acknowledgments
The authors thank Hejab Malik, Michael Rechtin, and Dr Li Ping Zhang for their help in this research, and Matt Hazzard for creating the Fig 5 illustration. The investigation was supported by NIH COBRE grant 2P20RR020145-06 and from internal institutional sources from the University of Kentucky President’s Research Professorship Fund (KNW) and the College of Medicine Dean’s Start-up Fund (KNW).
Footnotes
The authors report no conflicts of interest related to this study.
Contributor Information
Fei Ma, Department of Physiology, University of Kentucky, Lexington, Kentucky, USA.
Chunmei Wang, College of Dentistry, University of Kentucky, Lexington, Kentucky, USA.
William E. Yoder, Department of Oral Health Practice, College of Dentistry, University of Kentucky, Lexington, Kentucky, USA.
Karin N. Westlund, Department of Physiology, University of Kentucky, Lexington, Kentucky, USA.
Charles R. Carlson, Department of Psychology, University of Kentucky, Lexington, Kentucky, USA.
Craig S. Miller, Department of Psychology, University of Kentucky, Lexington, Kentucky, USA.
Robert J. Danaher, Department of Oral Health Practice, University of Kentucky, Lexington, Kentucky, USA.
References
- 1.Glorioso JC, Mata M, Fink DJ. Therapeutic gene transfer to the nervous system using viral vectors. J Neurovirol. 2003;9:165–172. doi: 10.1080/13550280390193984. [DOI] [PubMed] [Google Scholar]
- 2.Goins WF, Cohen JB, Glorioso JC. Gene therapy for the treatment of chronic peripheral nervous system pain. Neurobiol Dis. 2012;48:255–270. doi: 10.1016/j.nbd.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Ruchi R, James SR, Chidiac EJ. Gene therapy for chronic neuropathic pain: How does it work and where do we stand today? Pain Med. 2011;12:808–822. doi: 10.1111/j.1526-4637.2011.01120.x. [DOI] [PubMed] [Google Scholar]
- 4.Tzabazis AZ, Klukinov M, Feliciano DP, Wilson SP, Yeomans DC. Gene therapy for trigeminal pain in mice. Gene Ther. 2014;21:422–426. doi: 10.1038/gt.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vit JP, Ohara PT, Sundberg C, et al. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meunier A, Latremoliere A, Mauborgne A, et al. Attenuation of pain-related behavior in a rat model of trigeminal neuropathic pain by viral-driven enkephalin overproduction in trigeminal ganglion neurons. Mol Ther. 2005;11:608–616. doi: 10.1016/j.ymthe.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Glorioso JC. Herpes simplex viral vectors: Late bloomers with big potential. Hum Gene Ther. 2014;25:83–91. doi: 10.1089/hum.2014.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glorioso JC, Fink DJ. Herpes vector-mediated gene transfer in the treatment of chronic pain. Mol Ther. 2009;17:13–18. doi: 10.1038/mt.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goins WF, Krisky D, Marconi P, et al. Herpes simplex virus vectors for gene transfer to the nervous system. J Neurovirol. 1997;3(suppl 1):S80–S88. [PubMed] [Google Scholar]
- 10.Coen DM, Kosz-Vnenchak M, Jacobson JG, et al. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hay KA, Gaydos A, Tenser RB. The role of herpes simplex thymidine kinase expression in neurovirulence and latency in newborn vs. adult mice. J Neuroimmunol. 1995;61:41–52. doi: 10.1016/0165-5728(95)00071-9. [DOI] [PubMed] [Google Scholar]
- 12.Tenser RB. Role of herpes simplex virus thymidine kinase expression in viral pathogenesis and latency. Intervirology. 1991;32:76–92. doi: 10.1159/000150188. [DOI] [PubMed] [Google Scholar]
- 13.Tenser RB, Miller RL, Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979;205:915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- 14.Thompson RL, Sawtell NM. Replication of herpes simplex virus type 1 within trigeminal ganglia is required for high frequency but not high viral genome copy number latency. J Virol. 2000;74:965–974. doi: 10.1128/jvi.74.2.965-974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davar G, Kramer MF, Garber D, et al. Comparative efficacy of expression of genes delivered to mouse sensory neurons with herpes virus vectors. J Comp Neurol. 1994;339:3–11. doi: 10.1002/cne.903390103. [DOI] [PubMed] [Google Scholar]
- 16.Tenser RB, Hay KA, Edris WA. Latency-associated transcript but not reactivatable virus is present in sensory ganglion neurons after inoculation of thymidine kinase-negative mutants of herpes simplex virus type 1. J Virol. 1989;63:2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma F, Zhang L, Lyons D, Westlund KN. Orofacial neuropathic pain mouse model induced by Trigeminal Inflammatory Compression (TIC) of the infraorbital nerve. Mol Brain. 2012;5:44. doi: 10.1186/1756-6606-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fink DJ, Wechuck J, Mata M, et al. Gene therapy for pain: Results of a phase I clinical trial. Ann Neurol. 2011;70:207–212. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson SP, Yeomans DC, Bender MA, Lu Y, Goins WF, Glorioso JC. Antihyperalgesic effects of infection with a preproenkephalin-encoding herpes virus. Proc Natl Acad Sci U S A. 1999;96:3211–3216. doi: 10.1073/pnas.96.6.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danaher RJ, Jacob RJ, Miller CS. Establishment of a quiescent herpes simplex virus type 1 infection in neurally-differentiated PC12 cells. J Neurovirol. 1999;5:258–267. doi: 10.3109/13550289909015812. [DOI] [PubMed] [Google Scholar]
- 21.Miller CS, Danaher RJ, Jacob RJ. ICP0 is not required for efficient stress-induced reactivation of herpes simplex virus type 1 from cultured quiescently infected neuronal cells. J Virol. 2006;80:3360–3368. doi: 10.1128/JVI.80.7.3360-3368.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller CS, Smith KO. Enhanced replication of herpes simplex virus type 1 in human cells. J Dent Res. 1991;70:111–117. doi: 10.1177/00220345910700020301. [DOI] [PubMed] [Google Scholar]
- 23.Yeomans DC, Jones T, Laurito CE, Lu Y, Wilson SP. Reversal of ongoing thermal hyperalgesia in mice by a recombinant herpesvirus that encodes human preproenkephalin. Mol Ther. 2004;9:24–29. doi: 10.1016/j.ymthe.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Wilson SP, Yeomans DC. Virally mediated delivery of enkephalin and other neuropeptide transgenes in experimental pain models. Ann N Y Acad Sci. 2002;971:515–521. doi: 10.1111/j.1749-6632.2002.tb04516.x. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, McNearney TA, Wilson SP, Yeomans DC, Westlund KN. Joint capsule treatment with enkephalin-encoding HSV-1 recombinant vector reduces inflammatory damage and behavioural sequelae in rat CFA monoarthritis. Eur J Neurosci. 2008;27:1153–1165. doi: 10.1111/j.1460-9568.2008.06076.x. [DOI] [PubMed] [Google Scholar]
- 26.Yeomans DC, Lu Y, Laurito CE, et al. Recombinant herpes vector-mediated analgesia in a primate model of hyperalgesia. Mol Ther. 2006;13:589–597. doi: 10.1016/j.ymthe.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 27.Danaher RJ, Cook RK, Wang C, Triezenberg SJ, Jacob RJ, Miller CS. C-terminal trans-activation sub-region of VP16 is uniquely required for forskolin-induced herpes simplex virus type 1 reactivation from quiescently infected-PC12 cells but not for replication in neuronally differentiated-PC12 cells. J Neurovirol. 2013;19:32–41. doi: 10.1007/s13365-012-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gussow AM, Giordani NV, Tran RK, et al. Tissue-specific splicing of the herpes simplex virus type 1 latency-associated transcript (LAT) intron in LAT transgenic mice. J Virol. 2006;80:9414–9423. doi: 10.1128/JVI.00530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danaher RJ, Jacob RJ, Steiner MR, Allen WR, Hill JM, Miller CS. Histone deacetylase inhibitors induce reactivation of herpes simplex virus type 1 in a latency-associated transcript-independent manner in neuronal cells. J Neurovirol. 2005;11:306–317. doi: 10.1080/13550280590952817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lyons DN, Kniffin TC, Zhang LP, et al. Trigeminal Inflammatory Compression (TIC) injury induces chronic facial pain and susceptibility to anxiety-related behaviors. Neuroscience. 2015;295:126–138. doi: 10.1016/j.neuroscience.2015.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krzyzanowska A, Pittolo S, Cabrerizo M, et al. Assessing nociceptive sensitivity in mouse models of inflammatory and neuropathic trigeminal pain. J Neurosci Methods. 2011;201:46–54. doi: 10.1016/j.jneumeth.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goss JR, Mata M, Goins WF, Wu HH, Glorioso JC, Fink DJ. Antinociceptive effect of a genomic herpes simplex virus-based vector expressing human proenkephalin in rat dorsal roof ganglion. Gene Ther. 2001;8:551–556. doi: 10.1038/sj.gt.3301430. [DOI] [PubMed] [Google Scholar]