Abstract
Introduction and hypothesis
The aim of our study was to assess the performance of levator ani muscle deficiency (LAD) evaluated by 3D endovaginal ultrasound (EVUS) to detect pelvic floor muscle function as assessed by digital examination.
Methods
This cross-sectional study was conducted among 77 patients referred to our urogynecology clinic for pelvic floor dysfunction symptoms. Patients underwent physical examinations including digital pelvic muscle strength assessment using the Modified Oxford scale (MOS). EVUS volumes were evaluated and levator ani muscles were scored according to a validated LAD scoring system. MOS scores were categorized as nonfunctional (scores 0–1) and functional (scores 2–5).
Results
Mean age of participants was 56 (SD± 12.5) and 71% were menopausal. Overall, 32.5% had nonfunctional muscle strength and 44.2% were classified as having significant LAD. LAD identified by ultrasound had a sensitivity of 60% (95% CI 41%–79%) for detecting nonfunctional muscle and a specificity of 63% (95% CI 50%–77%) for detecting functional muscle. Overall, LAD demonstrated fair ability to discriminate between patient with and without poor muscle function (area under the ROC curve = 0.70 (95% CI 0.58–0.83). Among patients with an LAD score of 16–18, representing almost total muscle avulsion, 70% had nonfunctional MOS scores. Whereas, in patients with normal/minimal LAD (scores of 0–4), 89.5% had functional MOS scores
Conclusions
LAD and MOS scales were moderately negatively correlated Among patients with normal morphology or the most severe muscle deficiency, LAD scores can identify the majority of patients with functional or non-functional MOS scores, respectively.
Keywords: Levator ani deficiency, Modified Oxford Scale, Endovaginal ultrasound
Introduction
Injury to levator ani muscle as a result of birth has been documented [1–3]. To detect such birth-related injuries to the levator ani muscles, modern imaging techniques such as magnetic resonance imaging (MRI) [4,5], transperineal ultrasonography [6,2,7] and endovaginal ultrasonography (EVUS) [8,9] have been used. The appearance of the levator ani muscle subdivisions and a scoring system for evaluation of levator ani muscle deficiency by endovaginal ultrasound has been described [10,9]. The degree of levator ani defects seen by high resolution 3D endovaginal automatic acquisition ultrasound (EVUS) has been described as “levator ani deficiency” (LAD) [9]. Unlike the terms “defect” and “avulsion” which may imply an all or none phenomenon, the term “levator ani deficiency” implies a measurable gradient of muscle loss. The scoring system for grading LAD is based on visualization of each levator ani muscle subdivisions on each side of the pelvic floor. Each muscle subdivision is scored based on its thickness and attachment to the pubic bone. This scoring system has been demonstrated to have excellent inter and intra-rater reliability [11]. Severity of LAD detected by EVUS has been positively correlated with severity of pelvic organ prolapse [9]. Severe LAD was more prevalent in women with severe anal incontinence [12] and its role as a risk factor for urinary incontinence has been evaluated. Patients with SUI either have no LA defect or have a higher prevalence of mild LA defect as compared to moderate or severe LA defects. [13]. Additionally, LAD severity is associated with the anatomical position of the levator plate within the pelvic floor [14]; but its relation to pelvic floor function is unclear. It is to be expected that the severe deficiency should have an effect on pelvic floor contraction strength.
One of the basic forms of functional assessment of the levator ani muscle is digital palpation; however qualitative assessment based on palpation may have limited repeatability [15]. Messelink et al. recommended quantifying contractions by using the Modified Oxford Scale (MOS) to classify digital pelvic muscle strength into six categories ranging from absent to strong [15].
The aim of our study was to evaluate the performance of LAD evaluated by 3D EVUS to detect pelvic floor muscle function compared to that assessed by MOS digital examination.
Methods
Approval of this study was obtained from the Institutional Review Board at our institution. This cross-sectional study includes women who were referred to our urogynecology clinic because of different pelvic floor dysfunction symptoms including pelvic organ prolapse, urinary incontinence, anal incontinence, pelvic pain, and mesh related complications during January 2013–January 2014. The study time frame was selected because it corresponded with the dates in which we started performing and documenting MOS on all our patients. Of the 149 potentially eligible patients, 77 women had complete chart with needed data during January 2013 and January 2014 and were included in this study. Patients completed standardized questionnaires including PFDI-20 and PISQ and received a standard examination; assessment of pelvic floor by 3D EVUS, and digital assessment of pelvic floor function using MOS in their initial visit. Women with a history of prior pelvic floor reconstructive surgery and CNS or peripheral neurology diseases were excluded.
Ultrasound protocol
Imaging was obtained at the time of the primary visit using the BK Medical Ultrafocus (Peabody, MA, USA) and a 2052/8838 12 MHz transducer. All ultrasound exams were performed in the office setting, with the patient in dorsal lithotomy position, with hips flexed and abducted. No preparation was required and the patient was recommended to have a comfortable volume of urine in the bladder. No rectal or vaginal contrast was used. To avoid excessive pressure on surrounding structures that might distort the anatomy, the probe was inserted into the vagina in a neutral position with no pressure on vaginal walls. EVUS volumes were digitally stored for further analysis.
EVUS volumes were evaluated blinded to patient symptoms, and muscles were scored according to LAD scoring system, which has been previously validated. The levator muscle was divided into three subgroups; the puboperinealis/puboanalis [PA], puborectalis [PR], and iliococcygeus/pubococcygeus [PV][10]. Subgroups were evaluated and scored (0=no defect, 1=minimal defect with < 50% muscle loss, 2=major defect with >50% muscle loss, 3=total absence of the muscle) on each side based on thickness and detachment from the pubic bone. Scores were categorized as mild (scores 0–10) and significant (scores 11–18) levator ani deficiency (LAD) (Figure 1).
Figure 1.
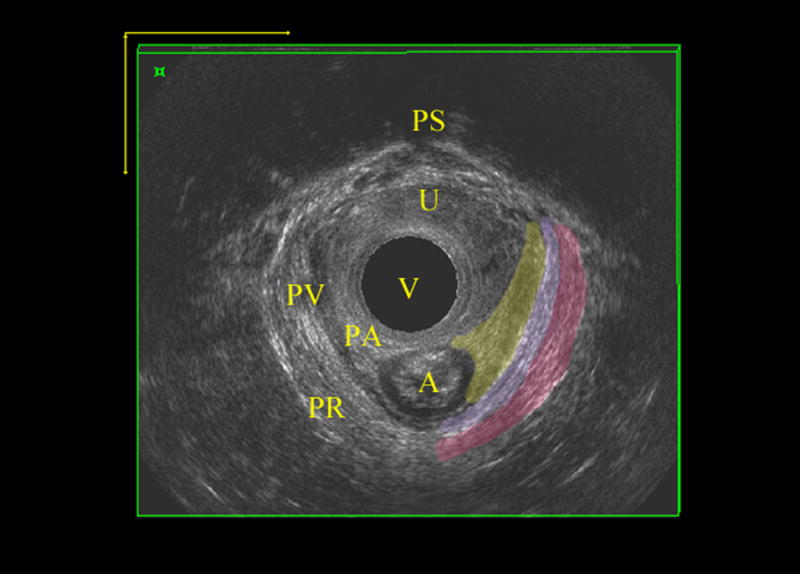
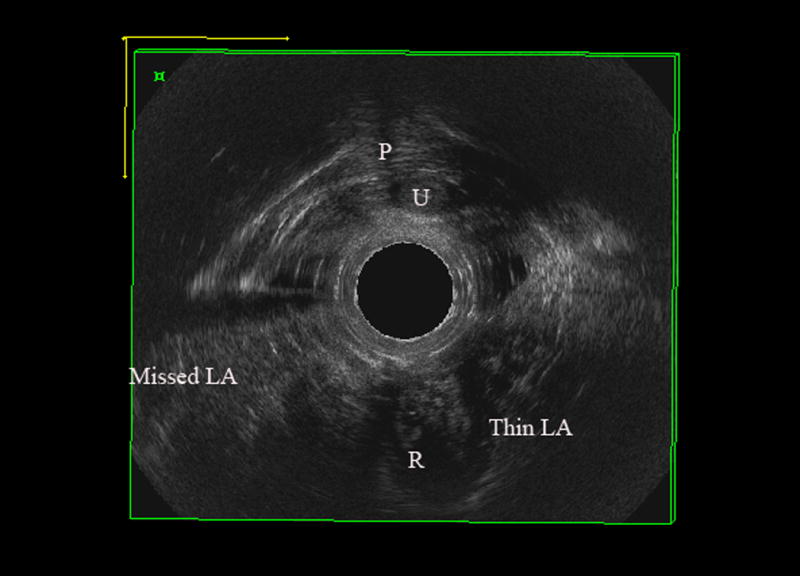
Levator ani deficiency detected by 3D EVUS, A) normal levator ani muscles B) Significant levator ani deficiency with total score of 14.
P; pubic symphysis, R; Rectum, U; urethra, Missed LA; complete avulsion of levator ani muscle on right, Thin LA; partial avulsion of iliococcygeus on left with thinning of puborectalis
MOS Digital Palpation Protocol
Examiners were blinded to ultrasound finding while performing muscle function digital assessment. Pelvic floor muscle function was assessed subjectively by digital palpation while inserting a lubricated gloved index finger approximately 4 cm into vagina [17,18]. All women were instructed to squeeze their levator ani muscles without activation of other groups of muscles, abdominal, gluteal and adductor muscles. Examiner instructed the patient to contract correct muscles. Muscle strength was graded using the six-point MOS; 0, no contraction; 1, minor muscle flicker; 2, weak muscle contraction; 3, moderate muscle contraction, 4; good and 5, strong muscle contraction against resistance by the examining finger [15,18]. A score was used for both left and right side and the lower score was used for analysis. To compare LAD performance to digital palpation in this study, we categorized the MOS as non-functional (scores 0–1) and functional (scores 2–5).
Statistical methods
Statistical analysis was performed using SAS v9.2 (SAS Institute, Cary, NC). The distribution of patient characteristics were compared between those with mild and significant LAD and between those with functional and non-functional pelvic floor muscles using chi square tests, fishers exact tests, t-tests and Wilcoxon rank sum tests, as appropriate. The Spearman correlation coefficient was calculated to assess the association between the LAD and MOS scales. The sensitivity and specificity of ultrasound-detected LAD to identify poor muscle function was assessed using MOS as the gold standard. The kappa coefficient was calculated to evaluate agreement between two tests. A receiver operating characteristic (ROC) curve was estimated to evaluate the ability of LAD to discriminate between patients with and without poor muscle function.
Results
Mean age of the 77 participants was 56 (SD± 12.5) and approximately 71% were post-menopausal. Median body mass index and parity were 28.2 (range 20.0–49.3) and 3 (range 0–6), respectively. Pelvic organ prolapse was observed in 53.5% of patients. Among those with data available for these characteristics, 70% (51/73) had a history of prior hysterectomy and 21% (14/66) reported smoking. Women with significant LAD were more likely to be older and more likely to be menopausal compared to women with mild LAD. Similarly, women with nonfunctional MOS were also more likely to be older and more likely to be menopausal compared to those with functional MOS. Patient characteristics by LAD and MOS categories are summarized in Table 1.
Table 1.
Distribution of subject characteristics by levator ani deficiency (LAD) status and pelvic muscle strength as evaluation by the Modified Oxford Scale (MOS)
| Mild LAD (n=43) |
Significant LAD (n=34) |
P value | |||
|---|---|---|---|---|---|
|
| |||||
| nˆ | Mean ± sd | nˆ | Mean ± sd | ||
| Age (years) | 43 | 51.9±12.7 | 34 | 62.4±9.5 | 0.0001a |
|
| |||||
| Median (range) | Median (range) | ||||
|
| |||||
| Parity | 25 | 3 (0–6) | 22 | 3 (1–5) | 0.51b |
| BMI | 29 | 27.8 (20.0–49.3) | 23 | 28.7 (21.9–44.7) | 0.38b |
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| Pelvic Organ Prolapse | 38 | 17 (44.7) | 33 | 21 (63.6) | 0.11c |
| Menopause | 30 | 17 (56.7) | 21 | 19 (90.5) | 0.009c |
| Prior Hysterectomy | 41 | 25 (61.0) | 32 | 26 (81.3) | 0.06c |
| Smoking | 37 | 9 (24.3) | 29 | 5 (17.2) | 0.48c |
|
| |||||
|
Functional MOS (n=52) |
Non-functional MOS (n=25) |
||||
|
| |||||
| nˆ | Mean ± sd | nˆ | Mean ± sd | ||
|
| |||||
| Age (years) | 52 | 53.6±12.6 | 25 | 62.6±9.8 | 0.003a |
|
| |||||
| Median (range) | Median (range) | ||||
|
| |||||
| Parity | 29 | 3 (1–6) | 18 | 3 (0–5) | 0.77b |
| BMI | 35 | 27.3 (20.0–49.3) | 17 | 30.8 (22.5–44.7) | 0.05b |
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| Pelvic Organ Prolapse | 48 | 22 (45.8) | 23 | 16 (69.6) | 0.06c |
| Menopause | 35 | 21 (60.0) | 16 | 15 (93.8) | 0.02d |
| Prior Hysterectomy | 50 | 36 (72.0) | 23 | 15 (65.2) | 0.56c |
| Smoking | 46 | 10 (21.7) | 20 | 4 (20.0) | 1.00d |
Sample size for LAD or MOS strata with data available for the listed characteristic.
Student’s t-test;
Wilcoxon rank sum test;
Chi-square test;
Fishers exact test
Overall, the prevalence of non-functional pelvic floor was 32.5%. A total of 44.2% were classified as having significant LAD. The LAD and MOS scales were moderately negatively correlated (Spearman correlation coefficient=−0.40, p=0.0003). A scatterplot of the association is displayed in Figure 2. LAD as defined by ultrasound had a sensitivity of 60% (95% CI 41%–79%) for detecting non-functional muscle status and a specificity of 63% (95% CI 50%–77%) for detecting functional muscle status (Table 2).
Figure 2.
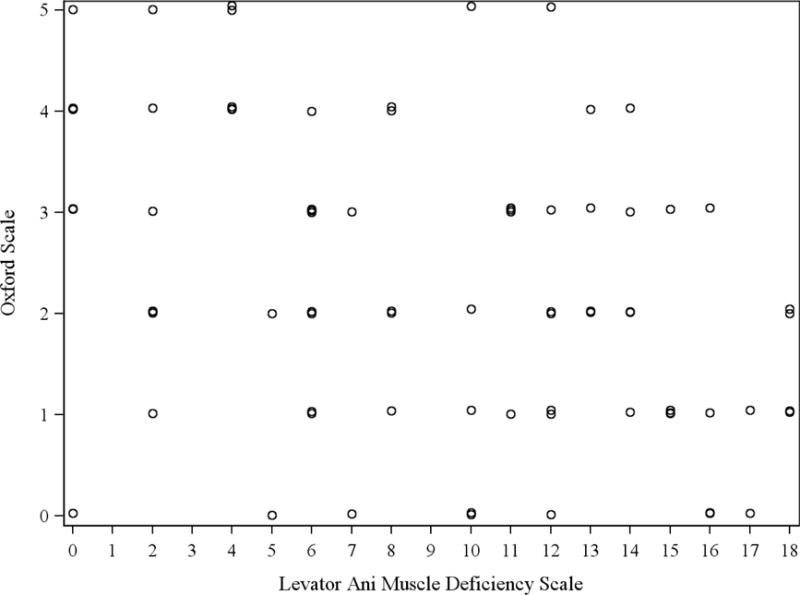
Scatter plot comparing LAD and MOS measurements
Table 2.
Comparison of LAD and Modified Oxford Scale categories
| Nonfunctional MOS (Scores 0–1) n (%) |
Functional MOS (Scores 2–5) n (%) |
Total n (%) |
|
|---|---|---|---|
|
Significant LAD (Score11–18) |
15 (60.0) |
19 (36.5) |
34 (44.2) |
|
Normal/Mild LAD (Score 0–10) |
10 (40.0) | 33 (63.5) | 43 (55.8) |
| Total* | 25 (32.5) | 52 (67.5) | 77 (100.0) |
Simple Kappa coefficient = 0.21
Row percentage
The area under the ROC curve was 0.70 (95% CI 0.58–0.83) (Figure 3), indicating LAD had fair ability to distinguish between patients with and without poor muscle function. The positive predictive value (PPV) of significant LAD for predicting non-functional MOS was 44.1% (95% CI 27%–61%) and the negative predictive value (NPV) of normal/mild LAD for predicting functional MOS was 76.7% (95% CI 64%–89%). However, when examining the extremes of the LAD score distribution, 70% of women with severe LAD scores of 16–18 (i.e., 7 of the 10 patients with almost total muscle loss) were classified as having non-functional muscle status. Among patients with normal to minimal LAD scores of 0–4, 17/19 (89.5%) were classified as having functional muscle status.
Figure 3.
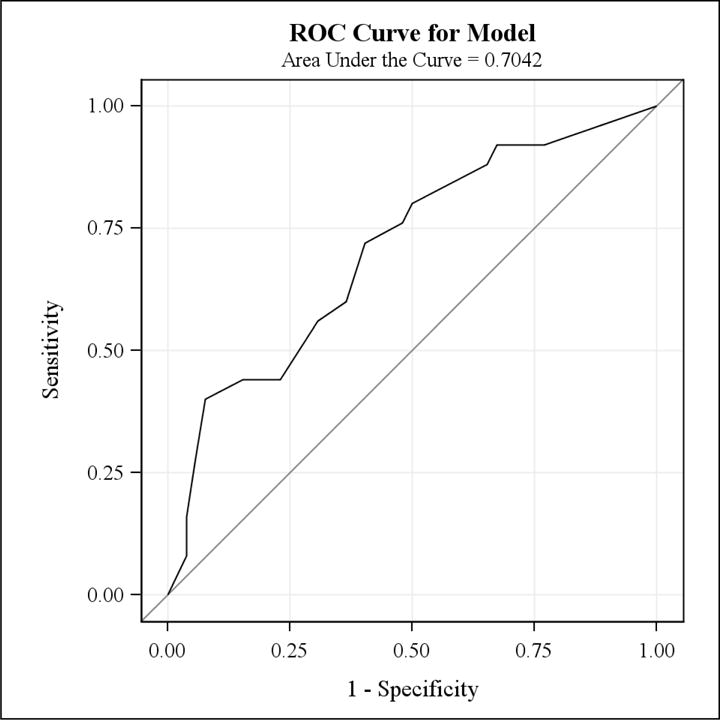
ROC Curve for the model
Discussion
Our study indicates that the morphology of levator ani muscle as visualized on EVUS is moderately predictive of the function of levator ani muscles. Sixty percent of women with non-functional muscle status were classified as having significant LAD. Of women with functional muscle status, 63% were classified as mild LAD. Additionally, 70% of women with severe LAD had non-functional levator muscle, which was present in only 10.5% of women with normal morphology of levator muscles on EVUS of the pelvic floor.
DeLancey et al. compared the levator ani muscle defect and function in women with and without prolapse [19]. They used MRI for visualization and grading of levator ani muscle morphology. In their study, levator function was assessed with a vaginal speculum specially designed to record the isometric force acting on anterior and posterior bills of the speculum at rest and with pelvic floor contraction. The study quantified how vaginal closure force is affected by the presence of pelvic organ prolapse and levator defect status. Women with muscle defects were unable to augment vaginal closure force as well as those with normal muscles regardless of prolapse status. Dietz et al. studied the levator avulsion and grading of pelvic floor strength [20]. The levator avulsion was diagnosed on transperineal ultrasound and muscle strength was investigated by digital palpation based on MOS. The presence of avulsion was associated with a significant reduction in the mean overall MOS grading (1.9 vs 2.78, p<0,001). Additionally, Steensma et al. found that underactive pelvic floor muscles, which was defined as absent, or weak pelvic floor contraction on ultrasonography resulting in no or minimal reduction of the levator hiatus dimensions on contraction, occurred more often in patients with an avulsion injury [21]. Levator muscle injuries were present in 53.8% with underactive pelvic floor muscle compared to 16.1% with normal pelvic floor muscle contraction. Our study findings are in agreement with other researches results. Levator ani muscle morphology is not the only predictor of muscle function but to some extent there is a relation between impaired morphology and impaired function. The predictive value of function by EVUS was described in this study.
Our study is limited by the relatively small sample size. Additionally, three clinicians performed MOS digital examination for pelvic floor function assessment; thus, inter-rater variability could have introduced some error in the assessment of muscle function. Although the examiner instructed the patient to contract the correct muscles, the measured strength of the muscle contraction by MOS digital examination could also be limited by the patient’s knowledge of how to isolate these pelvic muscles MOS is widely used in clinical practice, as it is easy to perform, inexpensive, and requires no special equipment. Furthermore, vaginal palpation is an effective aid in providing feedback to patients when they perform a pelvic floor muscle contraction [16]. The feasibility of this technique, however, is a strong point and allows for comparison with an examination technique that is a standard component of routine evaluation.
Ultrasound evaluation of the morphology of levator ani muscle is becoming more popular in urogynecology practice. The predictive value of morphology for functional assessment is a holds critical value in advancing knowledge in this field.
Acknowledgments
This research was supported in part by a grant from the National Institutes of Health, National Institute of General Medical Sciences, grant 1 U54 GM104938-01A1
Financial Disclaimers: None
Footnotes
Conflict of interest: None
Author’s participation in manuscript:
GR; project development, data collection, manuscript writing
JDP; statistical analysis, manuscript editing
LHQ; project development, manuscript editing
SAS; project development, manuscript editing
References
- 1.Ashton-Miller JA, Delancey JOL. On the biomechanics of vaginal birth and common sequelae. Annual review of biomedical engineering. 2009;11:163–176. doi: 10.1146/annurev-bioeng-061008-124823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG: an international journal of obstetrics and gynaecology. 2010;117(12):1485–1492. doi: 10.1111/j.1471-0528.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 3.Kearney R, Miller JM, Ashton-Miller JA, DeLancey JO. Obstetric factors associated with levator ani muscle injury after vaginal birth. Obstetrics and gynecology. 2006;107(1):144–149. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyte L, Jakab M, Warfield SK, Shott S, Flesh G, Fielding JR. Levator ani thickness variations in symptomatic and asymptomatic women using magnetic resonance-based 3-dimensional color mapping. American journal of obstetrics and gynecology. 2004;191(3):856–861. doi: 10.1016/j.ajog.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 5.Yousuf AA, DeLancey JO, Brandon CJ, Miller JM. Pelvic structure and function at 1 month compared to 7 months by dynamic magnetic resonance after vaginal birth. American journal of obstetrics and gynecology. 2009;201(5):514, e511–517. doi: 10.1016/j.ajog.2009.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weemhoff M, Shek KL, Dietz HP. Effects of age on levator function and morphometry of the levator hiatus in women with pelvic floor disorders. International urogynecology journal. 2010;21(9):1137–1142. doi: 10.1007/s00192-010-1150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Delft K, Sultan A, Thakar R, Schwertner-Tiepelmann N, Kluivers K. The relationship between postpartum levator ani muscle avulsion and signs and symptoms of pelvic floor dysfunction. BJOG: an international journal of obstetrics and gynaecology. 2014 doi: 10.1111/1471-0528.12666. [DOI] [PubMed] [Google Scholar]
- 8.Santoro GAWA, Dietz HP, Mellgren A, Sultan AH, Shobeiri SA, et al. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound in Obstetrics & Gynecology. 2011 doi: 10.1002/uog.8816. [DOI] [PubMed] [Google Scholar]
- 9.Rostaminia G, White D, Hegde A, Quiroz LH, Davila GW, Shobeiri SA. Levator ani deficiency and pelvic organ prolapse severity. Obstetrics and gynecology. 2013;121(5):1017–1024. doi: 10.1097/AOG.0b013e31828ce97d. [DOI] [PubMed] [Google Scholar]
- 10.Shobeiri SA, Leclaire E, Nihira MA, Quiroz LH, O’Donoghue D. Appearance of the levator ani muscle subdivisions in endovaginal three-dimensional ultrasonography. Obstetrics & Gynecology. 2009;114:66–72. doi: 10.1097/AOG.0b013e3181aa2c89. [DOI] [PubMed] [Google Scholar]
- 11.Rostaminia G, Manonai J, Leclaire E, Omoumi F, Marchiorlatti M, Quiroz LH, Shobeiri SA. Interrater reliability of assessing levator ani deficiency with 360 degrees 3D endovaginal ultrasound. International urogynecology journal. 2013 doi: 10.1007/s00192-013-2286-5. [DOI] [PubMed] [Google Scholar]
- 12.Rostaminia G, White D, Quiroz L, Shobeiri S. Levator ani role in anal incontinence. International urogynecology journal. 2013;24(Suppl 1):S1–152. [Google Scholar]
- 13.Hegde A, Augilar V. Davila G levator ani muscle deficiency and stress urinary incontinence. International urogynecology journal. 24(Suppl 1):S90. doi: 10.1007/s00192-016-3068-7. abstract#112. [DOI] [PubMed] [Google Scholar]
- 14.Rostaminia G, White DE, Quiroz LH, Shobeiri SA. Levator plate descent correlates with levator ani muscle deficiency. Neurourology and urodynamics. 2013 doi: 10.1002/nau.22509. [DOI] [PubMed] [Google Scholar]
- 15.Messelink B, Benson T, Berghmans B, Bo K, Corcos J, Fowler C, Laycock J, Lim PH, van Lunsen R, a Nijeholt GL, Pemberton J, Wang A, Watier A, Van Kerrebroeck P. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourology and urodynamics. 2005;24(4):374–380. doi: 10.1002/nau.20144. [DOI] [PubMed] [Google Scholar]
- 16.Bo K, Finckenhagen HB. Vaginal palpation of pelvic floor muscle strength: inter-test reproducibility and comparison between palpation and vaginal squeeze pressure. Acta obstetricia et gynecologica Scandinavica. 2001;80(10):883–887. doi: 10.1034/j.1600-0412.2001.801003.x. [DOI] [PubMed] [Google Scholar]
- 17.van Delft K, Shobeiri SA, Thakar R, Schwertner-Tiepelmann N, Sultan AH. Intra- and inter-rater reliability of levator ani muscle biometry and avulsion using three-dimensional endovaginal sonography. Ultrasound in obstetrics & gynecology: the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2013 doi: 10.1002/uog.13193. [DOI] [PubMed] [Google Scholar]
- 18.Laycock J. Clinical evaluation of the pelvic floor. In pelvic floor re-education: Principles and practice. 1994:42–48. [Google Scholar]
- 19.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 20.Dietz HP, Shek C. Levator avulsion and grading of pelvic floor muscle strength. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):633–636. doi: 10.1007/s00192-007-0491-9. [DOI] [PubMed] [Google Scholar]
- 21.Steensma AB, Konstantinovic ML, Burger CW, de Ridder D, Timmerman D, Deprest J. Prevalence of major levator abnormalities in symptomatic patients with an underactive pelvic floor contraction. Int Urogynecol J. 2010;21(7):861–867. doi: 10.1007/s00192-010-1111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


