Abstract
The aim of this study was to standardize a diagnosis procedure to detect Mycobacterium avium subsp. paratuberculosis (Map) DNA in raw cow milk samples under field conditions. A procedure that combines both immunomagnetic separation and IS900-PCR detection (IMS-IS1 PCR) was employed on milk samples from 265 lactating Holstein cows from Map infected and uninfected herds in Argentina. IMS-IS1 PCR results were analyzed and compared with those obtained from milk and fecal culture and serum ELISA. The extent of agreement between both tests was determined by the Kappa test. IMS-IS1 PCR showed a detection limit of 101 CFU of Map/mL of milk, when 50:50 mix of monoclonal and polyclonal antibodies were used to coat magnetic beads. All of the 118 samples from the Map uninfected herds were negative for the set of the tests. In Map infected herds, 80 out of 147 cows tested positive by milk IMS-IS1 PCR (55%), of which 2 (1.4%) were also positive by milk culture, 15 (10%) by fecal culture, and 20 (14%) by serum ELISA. Kappa statistics (95% CI) showed a slight agreement between the different tests (<0.20), and the proportions of agreement were ≤0.55. The IMS-IS1 PCR method detected Map in milk of the cows that were not positive in other techniques. This is the first report dealing with the application of IMS-IS1 PCR in the detection of Map in raw milk samples under field conditions in Argentina.
Keywords: Mycobacterium avium subsp. paratuberculosis, Immunomagnetic separation, PCR, Cow's milk
Introduction
Paratuberculosis (PTB) or Johne's disease is a chronic granulomatous enteritis of ruminants, caused by Mycobacterium avium subsp. paratuberculosis (Map). It is characterized by a long incubation period, weight loss, diarrhea, progressive cachexia, and death. PTB has a worldwide distribution and causes economic loss in dairy cattle due to reduced milk production and premature culling.1 The infected animals start to excrete Map in feces, milk, and colostrums, before the appearance of clinical symptoms2 resulting in contamination of the environment and subsequent infection of susceptible animals, primarily in calf hood.3 Unweaned calves, which are more susceptible than the adults, can be infected because Map is excreted through milk or the milk might be contaminated by feces.4 The presence of Map has been found in commercial cow's milk destined for human consumption all over the world5, 6, 7, 8, 9 including Argentina,10 and might imply a potential risk to public health due to its possible relationship with Crohn's disease. Paratuberculosis is listed in the World Organization for Animal Health's (OIE) Manual of Diagnostic Tests and Vaccines for Terrestrial Animals and classified under Risk Group 2 for human infections.11
There are several strategies to control Map dissemination within a herd that include vaccination, changes in management practices, and early detection and culling of the cows with subclinical infection, but the currently available diagnostic tests do not possess enough sensitivity (Se) to detect infected animals during the early stages of the infection. The fecal culture is considered as the reference test for detecting Map in live animals, but this method is labor intensive and requires long incubation periods and a minimum number of viable bacteria in the sample. The Se of this method in subclinically infected cows is low (23–29%), while its specificity (Sp) could reach up to 100%, if the culture isolates could be confirmed by PCR.12 The serum ELISA has commonly been used as a fast and low-cost serology test, but the Se of this method is also low (15%) in animals at subclinical stage with a low or moderate fecal shedding.13
In recent years, PCR has been the most widely used technique for detection of Map, though the Se of this method when applied directly to milk is low (23%) due to the presence of PCR inhibitory substances present in milk. Consequently, a suitable sample preparation prior to the PCR detection of Map is necessary in order to maximize the sensitivity of this method. The use of immunomagnetic separation (IMS) using magnetic nanoparticles coupled to polyclonal anti-Map antibodies is an effective procedure to capture Map from a heterogeneous and large volume sample, and to reduce the interferences of PCR inhibitory substances.2, 14, 15, 16, 17
In accordance with this approach, a diagnostic procedure was standardized to detect Map in raw cow milk samples. This method combines the use of immunomagnetic beads coupled to Map-specific polyclonal and monoclonal antibodies to isolate Map and modified IS900 PCR to detect Map DNA (IS1 PCR). The results were compared with those obtained through routine tests such as milk and fecal cultures and serum ELISA from the field samples.
Materials and methods
Bacterial strains
The reference Map strain ATCC 19698 and the field Map strain Malele 35 (M35; Bacteriology Laboratory Collection, EEA-INTA Balcarce) were used as positive controls. M35 was isolated from cattle and typed by IS900 PCR and RFLP.18 Both the Map strains were cultured in Middlebrook 7H10 medium (Difco Laboratories Inc., Becton, Dickinson and Company, Franklin Lakes, NJ, USA), supplemented with oleic acid, bovine albumin, dextrose and catalase (OADC, Difco), 2 mg/L mycobactin J (Allied Monitor, Fayette, USA), and 4.1 g/L sodium pyruvate (Sigma–Aldrich, St. Louis, MO, USA).
Mouse anti-Map antibodies
A monoclonal antibody (mAb) specific to Map-membrane protein p34 (clone 1A6E10)19 as well as a polyclonal antiserum (pAb) specific to whole Map were developed earlier in our laboratory. Ascitic fluid and mouse serum were semipurified by precipitation with ammonium sulfate and further dialyzed against PBS.
Standardization of IMS-IS1 PCR procedure
Coating of immunomagnetic beads
Goat anti-mouse IgG magnetic beads (New England BioLabs Inc., Ipswich, MA, USA) were mixed for 1 h at 4 °C under constant shaking. The beads (3.65 × 108) in a volume of 10 μL were coated with 10 μg of either anti-Map 1A6E10 mAb or anti-Map pAb. For negative controls, immunomagnetic beads were coated with an identical quantity of either a monoclonal antibody or a mouse polyclonal antiserum of a non-related specificity: anti-N-6-methyl adenine.17 After 1 h at 4 °C under constant shaking, each set of antibody-coated beads was separated for 10 min using a magnetic rack, washed three times in PBS, resuspended in 100 μL of PBS, and stored at 4 °C until further use.
Capture of Map by coated beads
A series of 10-fold dilution of milk samples (1 mL aliquots) initially spiked with 1012 CFU/mL of Map strains, ATCC 19698 or M35 was prepared after breaking the bacterial clumps by passing through a 25-gauge needle. In order to improve the amount of Map that could be obtained in the pellet, the samples were heated for 15 min at 50 °C, centrifuged at 6000 × g for 20 min at 4 °C, and the pellets were resuspended in 1 mL of PBS. This suspension was used for immunocapturing. Each set of the coated bead in a volume of 10 μL was added to each sample and incubated for 1 h at 4 °C under constant shaking to enable Map immunocapture. Then, the beads were separated for 10 min with a magnetic rack (immunomagnetic separation: IMS), and washed thrice with PBS. Both the supernatants from each coated beads and original samples were 10-fold serially diluted and cultured for three months at 37 °C in Middlebrook 7H10 medium supplemented as described above. Map bound to each type of the coated beads (bacterial recovery) was determined by subtracting the CFUs remaining on the respective supernatant after immunocapturing, from those that are originally present on the sample. The assay was repeated four times for each type of coated beads.
IS900 PCR detection of Map captured by coated beads
After IMS, the beads were boiled at 100 °C for 30 min and centrifuged at 7000 × g for 15 min, then 5 μL of supernatant was used as template for the PCR mixture of 50 μL reaction. IS900 PCR was performed according to the protocol of Mundo et al.17 to amplify IS1 fragment (nucleotides 1129–1283; 155 bp) in the IS900 sequence using primers IS1-F (5′-ACCCGCTGCGAGAGCAATCGCTGC-3′) and IS1-R (5′-ACGTCGGCGTGGTCGTCTGCTGGG-3′). Amplification was performed in 0.2 mL microtubes using a PTC-100TM Programmable Thermal Controller (MJ Research Inc., Waltham, MA, USA). The DNA from Map ATCC 19698 was used as the positive control and sterile water as negative control for each of the PCR assay. The products were electrophoretically separated at 50 V for 2 h in a 2% (w/v) agarose gel, stained with ethidium bromide (0.5 μg/mL), and observed under an ultraviolet transilluminator. The procedure that includes PCR detection of Map captured by coated beads, hereinafter, is referred to as IMS-IS1 PCR.
Application of IMS-IS1 PCR to field samples
Milk, feces, and blood samples were individually collected from 265 Holstein dairy cows: 147 from 5 Map infected herds and 118 from 4 Map uninfected herds, from Buenos Aires province, Argentina. All the animals were selected under no formal randomization; the criteria for inclusion in the study being age (4–10 years old) and biological and health status (cows should be lactating and healthy, without any clinical signs of PTB) at the time of collection of samples. The herds were considered Map infected when serum ELISA positive titers or Map isolation from fecal or milk culture was obtained, or there was a history of clinical PTB. The herds were considered Map uninfected, when none of these tests were positive during the previous five years. The milk samples (50 mL) were manually collected in sterile containers from cranial right and caudal left quarters after cleaning the udder and nipple with an iodide solution and foremilk stripping. The feces (10 g) was collected from the rectum of each cow using disposable examination gloves; 10 mL of whole blood was obtained by venipuncture of ventral median coccygeal vein. Fecal and milk samples from infected herds were individually processed, while those from uninfected herds were pooled in groups of five samples each. All samples were transported to the laboratory at 4 °C and stored at −20 °C without the addition of chemical preservatives until they were processed within two weeks. The milk samples were distributed into two parts, one for bacterial culture and the other for IMS-IS1 PCR. The results obtained by IMS-IS1 PCR procedure were compared with those obtained by routine diagnostic tests (milk and fecal culture and serum ELISA) from samples collected simultaneously.
IMS-IS1 PCR, F57 PCR, and PCR amplification check test
IMS-IS1 PCR was performed on 10 mL of milk samples as described above. To ensure that the PCR amplification products obtained from milk samples were not due to IS900-like sequences, amplification of F57 insertion element20 was performed in all positive samples according to the protocol described by Vansnick et al.21 using the F57 sequence as the reference (GenBank accession no. X70277). Briefly, the F57 PCR mixture was made-up into a final volume of 25 μL containing 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 1.6 mM MgCl2, 200 μM of each of the four dNTPs, 20 pM of each primer, 0.5 U of Taq polymerase (Taq DNA Polymerase Recombinant, Invitrogen, Sao Paulo, Brasil), and 5 μL of the bacterial DNA extract. The amplification reactions were performed in a PTC-100TM thermocycler (MJ Research) as follows: one cycle of denaturation at 94 °C for 4 min, 40 cycles of denaturation at 94 °C for 45 s, annealing at 68 °C for 45 s, extension at 72 °C for 45 s, and a final extension at 72 °C for 10 min. The PCR products were analyzed by electrophoresis at 50 V for 2 h on a 2% (w/v) agarose gel, stained with ethidium bromide, and observed under an ultraviolet transilluminator. In order to ensure that the negative results of IMS-IS1 PCR were not due to the presence of inhibitory substances, a PCR amplification check test was performed on each negative milk sample as follows: a duplicate of each milk sample was spiked with 105 CFU/mL of M35, 10-fold serially diluted to obtain a set of five milk samples containing between 105 and 101 CFU/mL, and IMS-IS1 PCR was performed.
Milk culture
The milk samples were cultured following the protocol described by Paolicchi et al.22 Briefly, 40 mL of milk was centrifuged at 2400 × g for 30 min; the pellet was resuspended in 60 mL of 0.75% hexadecylpyridinium chloride (HPC, Sigma–Aldrich), allowed to stand for 5 h, centrifuged at 2000 × g for 15 min, and finally resuspended in 1 mL of PBS. Four drops of the sample were cultured on three slopes of HEYM and observed as described above.
Fecal culture
The fecal samples were cultured following the protocol described by Paolicchi et al.22 Briefly, 10 g of feces were decontaminated with 100 mL of 0.75% HPC for 30 min under stirring, and then allowed to stand for a further 30 min. The supernatant (40 mL) was transferred to a clean tube, allowed to stand overnight at room temperature, centrifuged at 2000 × g for 15 min, and the pellet was resuspended in 1 mL of PBS and used as the inoculum. Four drops of this inoculum were cultured on three different slopes of Herrold's egg yolk medium (HEYM) alone, HEYM supplemented with 2.0 mg/L of mycobactin, and 4.1 g/L of sodium pyruvate (supplemented HEYM); and supplemented HEYM with an antibiotic mixture (100 μg/mL of vancomycin, 2.0 mg/mL of amphotericin B, 3.0 mg/mL nalidixic acid, and 100 μg/mL nystatin). The slopes were visually observed every 15 days for a total of four months; developing colonies were identified by Ziehl Neelsen staining, and confirmed by traditional IS900 PCR.23
ELISA
The assay was performed following the procedure described by Paolicchi et al.22 Briefly, Paratuberculosis Protoplasmic Antigen (PPA-3, Allied Monitor, Fayette, MO, USA) was used to sensitize 96-well polystyrene microplates (Immulon I, Dynatech, Arlington, TX, USA). The serum samples were pre-adsorbed with Mycobacterium phlei and tested at a 1:100 dilution; an HRP-conjugated anti-bovine IgG (Sigma–Aldrich) at a 1:4000 dilution was used as secondary antibody. The reaction was developed with 2,2′-azino-bis(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS, Sigma–Aldrich) and read at 405 nm. The Absorbance Indices (AI) were calculated as the ratio between the mean optical density (O.D.) of each serum sample and the mean O.D. of the negative control serum. Bovine sera were classified as negative (AI ≤ 1.5) or positive (AI > 1.5).
Statistical analysis
Kappa coefficient was calculated using WinEpiscope software version 2.0, 2000 (http://ww.clive.ed.ac.uk/winepiscope) to measure the agreement between different pairs of tests (milk culture, fecal culture, and ELISA versus IMS-IS1 PCR) performed on the 147 cows that belonged to infected herds. The results were ranked as described by Landis and Koch24: <0.00, poor agreement; 0.00–0.20, slight agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, substantial agreement; 0.81–1.00, almost agreement. The difference in the significance of the tests (p) was determined by the Wilcoxon signed-rank test.
Results
Standardization of IMS-IS1 PCR
The results of the comparison of recovery of Map from spiked raw milk samples obtained using different antibodies to coat immunomagnetic beads are shown in Table 1. The maximum recovery of Map (≈5 × 107 CFU) was obtained when a mixture of 5 μL each of mAb-beads and pAb-beads was applied to milk samples spiked with 108 CFU of Map. No further increase in recovery was obtained at higher spiking levels; however, nearly 100% recovery was obtained when milk samples were spiked with ≤107 CFU of Map. Hence, this mixture was used for further studies on field samples. The use of monoclonal antibodies or polyclonal antiserum of an unrelated specificity allowed a recovery of up to 9 × 101 CFU when milk was spiked with ≥108 CFU of Map, while no bacterium was retrieved when milk samples were spiked with ≤108 CFU. No statistically significant differences were detected between the recovery of Map strains, ATCC 19698 and M35.
Table 1.
Map capture (CFU) by beads coated with different antibodies from milk spiked with 108 CFU.
| Coated bead type | Map capture (CFU ± SD) |
p | |
|---|---|---|---|
| Reference strain ATCC 19698 | Field strain M35 | ||
| 10 μL mAb-beadsa | 3 × 104 ± 2 × 104 | 5 × 104 ± 3 × 104 | 0.46 |
| 10 μL pAb-beadsb | 6 × 103 ± 3 × 103 | 6 × 103 ± 2 × 103 | 1.00 |
| 5 μL mAb-beads + 5 μL pAb-beadsc | 4 × 107 ± 2 × 107 | 5 × 107 ± 3 × 107 | 0.66 |
| 10 μL c.mAb-beadsd | 5 × 101 ± 3 × 101 | 6 × 101 ± 1 × 101 | 0.71 |
| 10 μL c.pAb-beadsd | 8 × 101 ± 1 × 101 | 6 × 101 ± 3 × 101 | 0.34 |
SD, standard deviation; mAb, anti-Map monoclonal antibody 1A6E10; pAb, anti-Map polyclonal serum; c.mAb, monoclonal antibody of non-related specificity; c.pAb, polyclonal serum of non-related specificity. Within each strain, values with different letters mean significant differences by Wilcoxon rank sum test (a vs. c: p = 0.03).
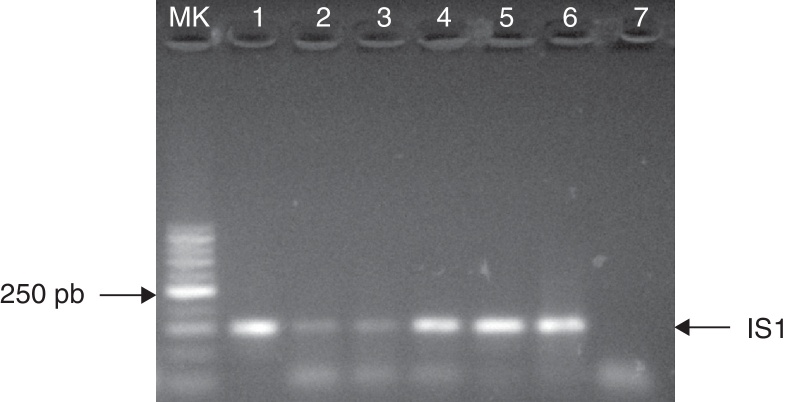
The IMS-IS1 PCR procedure enabled the detection of as little as 101 CFU of Map when the mixture of 5 μL each of mAb-beads and pAb-beads was used (Fig. 1). PCRs were always negative when beads coated with non-specific antibodies were analyzed.
Fig. 1.
Detection of Map (M35) by IMS-IS1 PCR procedure. Samples electrophoresed in 2% agarose. MK: 50 bp marker; Lane 1: positive control; Lane 2: 101 CFU/mL, Lane 3: 102 CFU/mL, Lane 4: 103 CFU/mL, Lane 5: 104 CFU/mL, Lane 6: 105 CFU/mL, Lane 7: negative control.
Application of IMS-IS1 PCR to field samples
A total of 265 Holstein dairy cows were analyzed by IMS-IS1 PCR using milk, fecal and milk culture, and serum ELISA. When IMS-IS1 PCR was applied to diagnose the 147 animals that belonged to Map infected herds, 80 were found to be Map-positive; among them, 31 were also tested positive with at least one of the other techniques performed (Table 2), whereas 49 were positive only to IMS-IS1 PCR. All of the 80 samples, deemed positive by IMS-IS1 PCR, were confirmed by the F57 PCR, while all the 67 samples that were found negative by IMS-IS1 PCR were confirmed negative for Map by performing the PCR amplification check test. The pooled samples from the 118 cows belonging to the uninfected herds and showing a negative test in IMS-IS1 PCR were further confirmed with the PCR amplification check test; and they also showed negative results to all the other techniques performed.
Table 2.
IMS-IS1 PCR results for the studied animals in those Map or anti-Map antibodies were detected.
| Cow ID | Map culture |
ELISA | IMS-IS1 PCR | |
|---|---|---|---|---|
| Fecal culture | Milk culture | |||
| 5788 | + | − | + | + |
| 6616 | + | − | + | + |
| 1595 | + | − | + | + |
| 2185 | + | − | + | + |
| 2390 | + | − | + | + |
| 2408 | + | − | + | − |
| 2106 | − | + | + | + |
| 2197 | − | + | − | + |
| 5984 | + | − | − | + |
| 6652 | + | − | − | + |
| 6669 | + | − | − | + |
| 6673 | + | − | − | + |
| 6873 | + | − | − | + |
| 7008 | + | − | − | + |
| 2008 | + | − | − | + |
| 2032 | + | − | − | + |
| 2218 | + | − | − | + |
| 1977 | + | − | − | + |
| 2417 | + | − | − | − |
| 6084 | − | − | + | + |
| 7006 | − | − | + | + |
| 7596 | − | − | + | + |
| 7509 | − | − | + | + |
| 7002 | − | − | + | + |
| 1758 | − | − | + | + |
| 1887 | − | − | + | + |
| 2184 | − | − | + | + |
| 2442 | − | − | + | + |
| 2473 | − | − | + | + |
| 2480 | − | − | + | + |
| 2482 | − | − | + | + |
| 2622 | − | − | + | + |
| 1784 | − | − | + | + |
| 7160 | − | − | + | − |
| 7501 | − | − | + | − |
| 1688 | − | − | + | − |
| 1950 | − | − | + | − |
| 2566 | − | − | + | − |
| Total | 17 | 2 | 26 | 31 |
Statistical analysis
The results for the different pairs of diagnostic methods are presented in Table 3a. The Kappa coefficient (95% CI) showed a slight agreement according to the ranking of Landis and Koch,24 and the proportions of agreement were ≤0.55 (Table 3b).
Table 3.
Kappa test analysis between results obtained in different sets of diagnostic methods for the 147 cows belonging to infected herds.
| a. Contingency table | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| IMS-IS1 PCR | Fecal culture |
Milk culture |
ELISA |
||||||
| + | − | + | − | + | − | ||||
| + | 15 | 65 | 80 | 2 | 78 | 80 | 20 | 60 | 80 |
| − | 2 | 65 | 67 | 0 | 67 | 67 | 6 | 61 | 67 |
| 17 | 130 | 147 | 2 | 145 | 147 | 26 | 121 | 147 | |
| b. Kappa coefficient and proportion of agreement | |||
|---|---|---|---|
| IMS-IS1 PCR | Fecal culture | Milk culture | ELISA |
| Kappa (95% CI) | 0.15 (0.05–0.24) | 0.02 (0.01–0.05) | 0.15 (0.03–0.27) |
| Concordant results | 80 | 69 | 81 |
| Proportion of agreement | 0.54 | 0.47 | 0.55 |
Discussion
Paratuberculosis has generated great interest in recent years because of its increasing worldwide prevalence leading to severe economic losses and health concerns caused by its zoonotic potential. In cattle, milk herds are mainly affected, as milking cows can remain in the farms for many years under an intensive production system.3 Fecal culture and serum ELISA are the techniques routinely used for the diagnosis of PTB, but their results are reliable only in the later stages of the disease.25, 26, 27 The detection of Map in milk to detect and remove infected animals has an economic impact in dairy industry and could be of great use in relation to food safety.
In this study, a diagnostic procedure known as IMS-IS1 PCR that involved the separation of Map by immunomagnetic beads coated with Map-specific antibodies followed by a modified IS900 PCR was standardized. An immunomagnetic separation technique was used in order to capture Map from milk samples, to remove the PCR-inhibitory substances, and to avoid the harmful effects of chemical decontamination. It was demonstrated that the use of a mixture of 5 μL each of mAb-beads and pAb-beads showed higher capture of Map than either of 10 μL of mAb-beads or 10 μL of pAb-beads used separately to capture Map, which in turn increased the quantity of DNA that was used for PCR. As a result, the detection limit of the IMS-IS1 PCR procedure reached 101 CFU/mL that is comparable to that described by Mundo et al.17 and Khare et al.2 by radiometric detection and real-time PCR, respectively. This limit of detection is higher than those described by Grant et al.,14 Metzger-Boddien et al.,28 and Stratmann et al.15 but slightly lower than those described by Foddai et al.29 who used specific antibodies or peptides to detect Map by immunomagnetic separation techniques.
IMS-IS1 PCR procedure was assessed under field conditions, and the results were compared with those obtained by milk culture, fecal culture, and a serum in-house ELISA. A slight level of agreement was observed by Kappa test between this procedure and routine diagnostic techniques, probably because each technique detects different targets. In this study, more positive results were obtained through IMS-IS1 PCR than through any other technique. This could be due to the effects of concentration of Map and removal of milk inhibitors of PCR achieved by immunomagnetic beads, as well as the possibility that PCR will detect viable as well as non-viable Map. On the other hand, the bacteria carrying IS900-like sequences30 were not amplified; all the samples that showed IS1 amplification gave negative results with F57 PCR, confirming the fact that they were true positive samples. In Map infected herds, the proportion of positive results obtained by fecal and milk culture was low (11.6% and 1.4%, respectively). This could be explained by the fact that fecal as well as milk shedding of Map can be low and intermittent in subclinically infected cows, a significant fraction of Map can be killed during the decontamination procedure, and also not all of the Map that is present in the samples can be cultured successfully.27, 31 Therefore, fecal and milk culture results are likely to be underestimates.
It has been described that the measurable humoral immune response to Map in subclinical cows can vary widely over time, even from day to day,32 while it is typically related to the later stages of the disease. This could possibly be the reason behind the low number of positive animals that were detected by the in-house ELISA (17.7%). The pre-absorption of each serum with M. phlei reduces the number of false positives results, but may also reduce the sensitivity.33 In this study, some animals (12.9%) were identified as positive by ELISA but negative by fecal or milk culture. This is in agreement with Nielsen and Toft12 who found that only approximately 10–30% of ELISA-positive cows could be confirmed by the culture, as some cows could be Map infected, but not yet Map-shedders.
The absence of clinical signs in the animals evaluated in this study indicates that the animals must be identified through laboratory diagnostic tests that currently suffer from low sensitivity. The apparently higher performance shown by the IMS-IS1 PCR procedure confirms the disadvantage of performing the diagnosis of PTB only by means of bacterial culture34 or serum ELISA.35, 36 Under field conditions, the methodology described in this study permitted identification of milking cows in subclinical stage from Map infected herds that were elusive to other techniques. We recognized that the confirmation of true infection status can only be obtained by histopathology and culture of intestinal tissues, but post mortem evaluation is beyond the scope of this study.
In summary, this report is the first one in which IMS-IS1 PCR procedure has been applied for the detection of Map in raw cow milk samples from Argentina under field conditions. The combined use of IMS-IS1 PCR, bacterial culture, and ELISA could increase the overall detection rate for the diagnosis of paratuberculosis.
Conclusions
The aim of this study was to standardize a procedure that could be applied for the detection of Map in cow milk samples based on immunomagnetic separation and IS1 PCR detection. Further in-depth studies are necessary in order to establish the true proportion of infected animals that can be detected by this method and the stages of the disease when it is present.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by Grants from Agencia Nacional de Promoción Científica y Técnica, Argentina (BIDPICT 2010-2672), Universidad de Buenos Aires, Argentina (UBACyT 20020100100912), and Instituto Nacional de Tecnología Agropecuaria, Argentina (INTA #202831 AESA).
Associate Editor: Jorge Luiz Mello Sampaio
References
- 1.McKenna S.L., Keefe G.P., Tiwari A., VanLeeuwen J., Barkema H.W. Johne's disease in Canada. Part II: disease impacts, risk factors, and control programs for dairy producers. Can Vet J. 2006;47:1089–1099. [PMC free article] [PubMed] [Google Scholar]
- 2.Khare S., Ficht T.A., Santos R.L. Rapid and sensitive detection of Mycobacterium avium subsp. paratuberculosis in bovine milk and feces by a combination of immunomagnetic bead separation-conventional PCR and real-time PCR. J Clin Microbiol. 2004;42:1075–1081. doi: 10.1128/JCM.42.3.1075-1081.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barkema H.W., Hesselink J.W., McKenna S.L.B., Benedictus G., Groenendaal H. Global prevalence and economics of infection with Mycobacterium avium ssp. paratuberculosis in ruminants. In: Behr M.A., Collins D.M., editors. Paratuberculosis. Organism, Disease, Control. CABI; 2010. pp. 10–17. ISBN: 9781845936136. [Google Scholar]
- 4.Radia D., Bond K., Limon G., van Winden S., Guitian J. Relationship between periparturient management, prevalence of MAP and preventable economic losses in UK dairy herds. Vet Rec. 2013;14:173–343. doi: 10.1136/vr.101408. [DOI] [PubMed] [Google Scholar]
- 5.Grant I.R., Ball H.J., Rowe M.T. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurised cows’ milk from approved dairy processing establishments in the United Kingdom. Appl Environ Microbiol. 2002;68:2428–2435. doi: 10.1128/AEM.68.5.2428-2435.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayele W.Y., Svastova P., Roubal P., Bartos M., Pavlik I. Mycobacterium avium subspecies paratuberculosis cultured from locally and commercially pasteurized cow's milk in the Czech Republic. Appl Environ Microbiol. 2005;71:1210–1214. doi: 10.1128/AEM.71.3.1210-1214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellingson J.L., Anderson J.L., Koziczkowski J.J. Detection of viable Mycobacterium avium ssp. paratuberculosis in retail pasteurized whole milk by two culture methods and PCR. J Food Prot. 2005;68:966–972. doi: 10.4315/0362-028x-68.5.966. [DOI] [PubMed] [Google Scholar]
- 8.Shankar H., Singh S., Singh P., Singh A., Sohal J., Greenstein R. Presence, characterization and genotype profiles of Mycobacterium avium subsp. paratuberculosis from unpasteurized individual and pooled milk, commercial pasteurized milk and milk production products in India by culture, PCR and PCR REA methods. Int J Infect Dis. 2009;14:121–126. doi: 10.1016/j.ijid.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 9.Carvalho I.A., Pietralonga P.A.G., Schwarz D.G.G., Faria A.C.S., Moreira M.A.S. Short communication: recovery of viable Mycobacterium avium subspecies paratuberculosis from retail pasteurized whole milk in Brazil. J Dairy Sci. 2012;95:6946–6948. doi: 10.3168/jds.2012-5657. [DOI] [PubMed] [Google Scholar]
- 10.Paolicchi F.A., Cirone K., Morsella C., Gioffré A. First isolation of Mycobacterium avium subsp. paratuberculosis from commercial pasteurized milk in Argentina. Braz J Microbiol. 2012;43:1034–1037. doi: 10.1590/S1517-838220120003000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2014 Chapter. 2.1.11, Paratuberculosis (Enfermedad de Johne). http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.11_PARATB.pdf (accessed 26 May 2014).
- 12.Nielsen S.S., Toft N. Ante mortem diagnosis of paratuberculosis: a review of accuracies of ELISA, interferon-γ assay and fecal culture techniques. Vet Microbiol. 2008;129:217–235. doi: 10.1016/j.vetmic.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Chui L.W., King R., Sim J. Development of an immunocapture-polymerase chain reaction assay using IgY to detect Mycobacterium avium ssp. paratuberculosis. Can J Vet Res. 2010;74:102–107. [PMC free article] [PubMed] [Google Scholar]
- 14.Grant I.R., Pope C.M., O’Riordan L.M., Ball H.J., Rowe M.T. Improved detection of Mycobacterium avium subsp. paratuberculosis in milk by immunomagnetic PCR. Vet Microbiol. 2000;77:369–378. doi: 10.1016/s0378-1135(00)00322-9. [DOI] [PubMed] [Google Scholar]
- 15.Stratmann J., Dohmann K., Heinzmann J., Gerlach G.F. Peptide aMptD-mediated capture PCR for detection of Mycobacterium avium subsp. paratuberculosis in bulk milk samples. Appl Environ Microbiol. 2006;72:5150–5158. doi: 10.1128/AEM.00590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logar K., Kopin R., Bandelj P., Starič J., Lapanje A., Ocepek M. Evaluation of combined high-efficiency DNA extraction and real-time PCR for detection of Mycobacterium avium subsp. paratuberculosis in subclinically infected dairy cattle: comparison with faecal culture, milk real-time PCR and milk ELISA. BMC Vet Res. 2012;8:49. doi: 10.1186/1746-6148-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundo S.L., Gilardoni L.R., Hoffman F.J., Lopez O.J. Rapid and sensitive method to identify Mycobacterium avium subsp. paratuberculosis in cow's milk by DNA methylase genotyping. Appl Environ Microbiol. 2013;79:1612–1618. doi: 10.1128/AEM.02719-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreira A.R., Paolicchi F.A., Morsella C. Distribution of IS900 restriction fragment length polymorphism types among animal Mycobacterium avium subsp. paratuberculosis isolates from Argentina and Europe. Vet Microbiol. 1999;70:251–259. doi: 10.1016/s0378-1135(99)00144-3. [DOI] [PubMed] [Google Scholar]
- 19.Stempler A., Jolly A., Colavecchia S., Soba M., Fontanals A., Mundo S.L. Development and evaluation of a monoclonal antibody against Mycobacterium avium subsp. paratuberculosis. Vet Immunol Immunopathol. 2009;128:335–336. [Google Scholar]
- 20.Poupart P., Coene M., Van Heuverswyn H., Cocito C. Preparation of a specific RNA probe for detection of Mycobacterium paratuberculosis and diagnosis of Johne's disease. J Clin Microbiol. 1993;31:1601–1605. doi: 10.1128/jcm.31.6.1601-1605.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vansnick E., de Rijk P., Vercammen F., Geysen D., Rigouts L., Portaels F. Newly developed primers for the detection of Mycobacterium avium subsp. paratuberculosis. Vet Microbiol. 2004;100:197–204. doi: 10.1016/j.vetmic.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Paolicchi F.A., Zumarraga M.J., Gioffre A. Application of different methods for the diagnosis of paratuberculosis in a dairy cattle herd in Argentina. J Vet Med B. 2003;50:20–26. doi: 10.1046/j.1439-0450.2003.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Collins D.M., Stephens D.M., de Lisle G.W. Comparison of polymerase chain reaction test and faecal culture for detecting Mycobacterium paratuberculosis in bovine faeces. Vet Microbiol. 1993;36:289–299. doi: 10.1016/0378-1135(93)90095-o. [DOI] [PubMed] [Google Scholar]
- 24.Landis J.R., Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 25.Bakker D. Paratuberculosis control measures in Europe. In: Behr M.A., Collins D.M., editors. CABI; 2010. pp. 306–315. (Paratuberculosis. Organism, Disease, Control). ISBN: 9781845936136. [Google Scholar]
- 26.Nielsen S.S. Immune-based diagnosis of paratuberculosis. In: Behr M.A., Collins D.M., editors. Paratuberculosis. Organism, Disease, Control. CABI; 2010. pp. 284–291. ISBN: 9781845936136. [Google Scholar]
- 27.Whittington R.J. Cultivation of Mycobacterium avium subsp. paratuberculosis. In: Behr M.A., Collins D.M., editors. Paratuberculosis. Organism, Disease, Control. CABI; 2010. pp. 244–260. ISBN: 9781845936136. [Google Scholar]
- 28.Metzger-Boddien C., Khaschabi D., Schönbaur M., Boddien S., Schederer T., Kehle J. Automated high-throughput immunomagnetic separation for detection of Mycobacterium avium subsp. paratuberculosis in bovine milk. Int J Food Microbiol. 2006;110:201–208. doi: 10.1016/j.ijfoodmicro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Foddai A., Elliott C.T., Grant I.R. Maximizing capture efficiency and specificity of magnetic separation for Mycobacterium avium subsp. paratuberculosis cells. Appl Environ Microbiol. 2010;76:7550–7558. doi: 10.1128/AEM.01432-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Möbius P., Hotzel H., Rassbach A., Köhler H. Comparison of 13 single-rounds and nested PCR assays targeting IS900, ISMav2, ISF57 and locus 255 for detection of Mycobacterium avium subsp. paratuberculosis. Vet Microbiol. 2008;126:324–333. doi: 10.1016/j.vetmic.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Grant I.R., Rowe M.T. Effect of chemical decontamination and refrigerated storage on the isolation of Mycobacterium avium subsp. paratuberculosis from heat-treated milk. Lett Appl Microbiol. 2004;38:283–288. doi: 10.1111/j.1472-765x.2004.01498.x. [DOI] [PubMed] [Google Scholar]
- 32.Barrington G.M., Gay J.M., Eriks I.S. Temporal patterns of diagnostic results in serial samples from cattle with advanced paratuberculosis infection. J Vet Diagn Invest. 2003;15:195–220. doi: 10.1177/104063870301500219. [DOI] [PubMed] [Google Scholar]
- 33.McKenna S.L., Sockett D.C., Keefe G.P., McClure J., Van Leeuwen J.A., Barkema H.W. Comparison of two enzyme-linked immunosorbent assays for diagnosis of Mycobacterium avium subsp. paratuberculosis. J Vet Diagn Invest. 2005;17:463–466. doi: 10.1177/104063870501700510. [DOI] [PubMed] [Google Scholar]
- 34.Alinovi C.A., Ward M.P., Lin T.L., Moore G.E., Wu C.C. Real time PCR compared to liquid and solid culture media and ELISA, for the detection of Mycobacterium avium ssp. paratuberculosis. Vet Microbiol. 2009;14:177–179. doi: 10.1016/j.vetmic.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Pinedo P.J., Williams J.E., Monif G.R.G., Rae D.O., Buergelt C.D. Mycobacterium paratuberculosis shedding into milk: association of ELISA seroreactivity with DNA detection in milk. Int J Appl Res Vet Med. 2008;6:137–144. [Google Scholar]
- 36.Badi F.A., Al Haroon A.I., Alluwaimi A.M. The gamma delta cells as marker of non-seroconverted cattle naturally infected with Mycobacterium avium ssp. paratuberculosis. Res Vet Sci. 2010;88:72–76. doi: 10.1016/j.rvsc.2009.06.004. [DOI] [PubMed] [Google Scholar]