Abstract
A high concentration of histamine, one of the biogenic amines (BAs) usually found in fermented foods, can cause undesirable physiological side effects in sensitive humans. The objective of this study is to isolate indigenous Acetobacter strains from naturally fermented Bokbunja vinegar in Korea with reduced histamine production during starter fermentation. Further, we examined its physiological and biochemical properties, including BA synthesis. The obtained strain MBA-77, identified as Acetobacter aceti by 16S rDNA homology and biochemical analysis and named A. aceti MBA-77. A. aceti MBA-77 showed optimal acidity % production at pH 5; the optimal temperature was 25 °C. When we prepared and examined the BAs synthesis spectrum during the fermentation process, Bokbunja wine fermented with Saccharomyces cerevisiae showed that the histamine concentration increased from 2.72 of Bokbunja extract to 5.29 mg/L and cadaverine and dopamine was decreased to 2.6 and 10.12 mg/L, respectively. Bokbunja vinegar prepared by A. aceti MBA-77 as the starter, the histamine concentration of the vinegar preparation step was decreased up to 3.66 mg/L from 5.29 mg/L in the wine preparation step. To our knowledge, this is the first report to demonstrate acetic acid bacteria isolated from Bokbunja seed vinegar with low spectrum BA and would be useful for wellbeing vinegar preparation.
Keywords: Biogenic amines, Histamine, Acetic acid bacteria, Vinegar, Wine
Introduction
Biogenic amines (BAs) are organic bases that primarily occur in fermented foods due to the decarboxylation of amino acids by certain microorganisms with biological activity. Due to the undesirable physiological effects caused by a high concentration of BAs in sensitive humans, many efforts have been performed in order to reduce their concentration in various foods. In particular, the consumption of foods with high-level concentration of histamine can have vasodilation effects1 in humans as well as cause headaches, heart palpitations, edema, vomiting, diarrhea and other symptoms2; thus, there are great demands to reduce the histamine concentration in the preparation of fermented foods.
In fermented foods, several species of histamine-producing lactic acid bacteria (LAB) and yeast belonging to the Lactobacillus, Leuconostoc, Pediococcus genera,3 Oenococcus oeni,4 Staphylococcus spp., Enterobacter cloacae, and Candida spp.5 have been determined in a wide range of food products, not only fermented from fish, meat, vegetables and dairy products, but also from other types of foods, such as beer and wine. Although many other BAs, except histamines such as putrescine, spermidine, methylamine, ethylamine, phenylethylamine, isoamylamine and cadaverine, are usually derived from the raw materials of grapes and grape must themselves, most of the BAs can be synthesized or degraded during fermentation.6 With regard to wine fermentation, BAs, especially through fermentation processes, are well related with the malolactic fermentation step. In this step, a large amount of BAs are formed in wine by the decarboxylation of Lactobacilli.7 LABs isolated from wine, such as P. parvulus, L. mali, Leu. Mesenteroids, L. brevis, O. oeni and L. hilgardii, were shown to produce various BAs with different BA spectrum.8 It has been also demonstrated that many yeast strains present in wines (Saccharomyces cerevisiae, Brettanomyces bruxellensis, Kloeckera apiculata, among others) can produce histamine, phenylethylamine and cadaverine.9, 10 These results clearly indicate that microorganisms, such as fermentation factors, are critical for controlling BA synthesis during fermentation.
Vinegar fermentation usually occurred after alcohol fermentation under absolutely aerobic condition during little bit long time fermentation through the oxidation of the ethanol by AAB. When the total BAs contents in various vinegars were examined, interestingly, Balsamic and “Pedro Ximénez” Sherry vinegars showed the highest amounts of BAs; however, apple, white and Sherry wine vinegars had the lowest concentrations, thereby indicating that biogenic contents significantly remained after vinegar fermentation, although the amount of BAs were different depending on the vinegars.11 Although the produced BAs after vinegar fermentation usually originate from yeasts or LABs during wine fermentation, the final products of various vinegars still contains significant amounts of BAs, which might cause significant health problems because BAs levels are increased during malolactic fermentation.12 Hence, there are numerous LABs that are isolated in order to control BAs during fermentation; furthermore, they are examined for their BA reduction ability, as well as convey a potential possibility for BAs reduction in the final wine product.13 However, there has been no study yet on AAB for BA reduction ability during the fermentation process.8 Only minimal knowledge on the BA synthesis ability of AABs is present.
Black raspberry (Rubus coreanus Miquel), which is well known as ‘Bokbunja’ in Korea, is mainly cultivated in the southern parts of Korea, China and Japan, and has been increased in cultivation area due to the expanded usage as a traditional herbal medicine.14 Because Bokbunja contains rich ingredients such as flavonoids, tannins, triterpenosides and phenolic compounds, various functional aspects, such as cell proliferation inhibition and apoptosis stimulation in HT-29 human colon cancer cells15 and anti-fatigue, anti-gastropathic, anti-inflammatory, anti-rheumatic and antioxidant activities, have been reported. Due to its extraordinary functionality, at the present, various types of processed products, such as sugar extracts, wine and vinegars, have been actively produced to create high-value added Bokbunja food products. The traditional Bokbunja vinegar is made from the fermentation of fully ripened fruits through a two-step fermentation of alcohol and vinegar fermentation steps with the sugar-soaking step prior to fermentation. Many studies indicated that the chemical constituents change the raw Bokbunja fruit and the fermented wine and beverage change enhance the functional aspects, such as the antioxidant activity. Nevertheless, a high concentration of BAs might also be formed during the ethanol and vinegar fermentation through the decarboxylation of amino acids.
In this study, our purpose was to isolate indigenous AAB strains which possess the biogenic amine reduction ability especially for histamine production from naturally fermented Bokbunja vinegar, and examined their usefulness for preparation of fermented Bokbunja vinegar showing reduced BA concentration with especially histamine.
Materials and methods
Bacteria and culture conditions
AAB were isolated from naturally fermented Bokbunja vinegar in the Muju area. Samples were serial diluted and cultivated in a GYEC agar medium (5% glucose, 1% yeast extract, ethanol 3%, 1.5% calcium carbonate, 0.8% agar) for 3 days at 29° C. Isolated AAB colonies showed a clear zone on the GYEC agar plate, which was cultivated in GYE broth (5% glucose, 1% yeast extract, 3% ethanol) for 3 days at 29° C, 160 rpm. Culture broth was collected every day for analyzing the pH, °Brix, acidity (%) and cell growth.
Identification by 16S rDNA sequencing
Bacterial cells were grown aerobically in GYE broth at 26° C. The bacterial sets of two primers 27f and 1490r were applied in order to amplify the V3 variable region of the eubacterial 16S rDNA fragments. The DNA sequences of primers 27f (5′-AGTTTGATCCTGGCTCAG-3′) and 1490r (5′-GTTACCTTGTTACGACTTC-3′) were used. PCRs were performed in a Bio-Rad thermocycler (MyCycler, Bio-Rad Laboratories, Hercules, CA, USA) as described by Kopermsub and Yunchalard.16 The PCR products were separated in 1.0% agarose gel in a 1× TBE buffer. After electrophoresis, the gels were stained with ethidium bromide and documented by the GelDoc XR+ using the Image Lab Software (Bio-Rad Laboratories, USA) imaging System 2000 (Bio-Rad Laboratories, USA). DNA sequencing was performed with the ABI-Prism Big Dye Determinator Cycle Sequencing Ready Reaction kit and ABI-Prism 377 Sequencer (Applied Biosystems Japan, Tokyo, Japan). Sequence similarity searches were carried out using the Basic local alignment search (BLAST) on the EMBL/GenBank databases; phylogenetic trees were constructed using the neighbor-joining method. Finally, API 20 NE Kit (bioMerieux, France) was also used for the identification of non-enteric Gram negative rods.
SEM analysis
Cell morphology was observed by FE-SEM (Field Emission Scanning Electron Microscope). The bacterial cells growing on GYE broth for 3 days at 26° C were fixed in 2.5% glutaraldehyde in distilled water for 30 min, post-fixed in 1% osmium tetroxide in distilled water for 20 min, dehydrated through graded ethanol; the pellet was dried for 24 h before the observation and gold-coated by sputtering. The cells were coated with gold by a coater (SC502, Polaron, West Sussex, UK) and observed under a scanning electron microscope (SEM 515, Philips, Netherlands).
Phenotypic and technological features of strains
Cell shape was observed after incubating the cultures in GYE broth at 28° C for 3 days. Oxidation of ethanol to acetic acid was tested in the GYE broth containing glucose 10 g, yeast extract 10 g, ethanol 30 mL (per liter) after 3 days of incubation at 28° C. Growth on 30% d-glucose; 0.5% yeast extract and 30% glucose incubation 7 days 28° C and tolerance to ethanol (5, 10, 15 and 20%, v/v) were tested; a gradient volume of absolute ethanol was added to each flask containing the same medium.17 Growth on single carbon sources was carried out with fructose, glycerol, raffinose, sucrose, mannose and sorbitol. The carbon sources were sterilized by filtration (0.2 μm) and added to the sterile basal medium (yeast extract, 0.5 g; vitamin-free casamino acid, 3 g) to a final concentration of 0.3% by 2% pre-culture inoculation.18 The control was used as a culture broth without the carbon source. The optical density was measured at 600 nm using spectrophotometer (Beckman Spectrophotometer, USA). Catalase activity was performed by adding young cells to a drop of a 10% H2O2 solution and observing the production of O2, as described previously.19 Catalase optimum culture condition was also demonstrated. Five factors of culture broth pH were considered for the culture conditions. Five hundred μL of pre-culture was inoculated into 50 mL of each of the GYE broth. The broth cultures were then incubated at 29 °C at 160 rpm. The acidity of the culture media was measured by titrating 10 mL samples with 0.1 N NaOH using phenolphthalein as a pH indicator.
Wine and vinegar fermentation with Bokbunja
Organically grown black raspberry was harvested from Muju province in South Korea and quickly cooled in the freezer at −20° C before use. The cooled black raspberry was mixed with organic sugar (Goiasa Goiatuba Alcool Ltda, Brazil) at a ratio of 6:4, and then ripened for 40 days at 20° C. The pressed crude extract was filtered, sterilized at 85° C for 15–20 min diluted to 20–25 °Brix with distilled water. Ethanol fermentation was carried out by starter yeast, S. cerevisiae no.7013 (Fermivin, DSM Food Specialties, France). Dry yeast was re-hydrated to 10% (w/v) by distilled water at 37° C and swelled for 30 min before inoculation in the Bokbunja crude extract; ethanol fermentation was started with the inoculation of 0.02% (v/v) re-hydrated starter yeast and fermented at 23° C for 5 days. Static vinegar fermentation by the traditional method was performed directly inoculate 10% of starter culture into the obtained Bokbunja wine. The acetic acid fermentation was performed in a crock at 23° C without agitation for 14 days.
Analysis of BAs produced by microorganism in culture broth by HPLC
The determination of BA was carried out by high pressure liquid chromatography. Analysis was performed with HPLC 1200 series (Agilent Technologies, Santa Clara, CA, USA). The 5 mL samples and 5 mL of 0.1 N HCl were mixed and 1 mL of mixed solution were derivatized with 0.5 mL internal standard (1,7-diaminoheptane, 100 mg/L) using saturated Na2CO3 0.5 mL and 1% dansyl chloride acetone solution 1 mL at 45° C for 1 h. After derivatization, 10% proline solution 1 mL was added in order to remove the remained derivatization reagents. 5 mL of ether was mixed with shaking for 3 min and a supernatant was dried using N2 gas. Samples were injected in triplicate onto the column after adding 2 mL of acetonitrile to the dried samples, followed by being filtered through a 0.45 μm filter (Minisart NY 25, Sartorius-stedim biotech). All separations were performed on a CapcellPak C18 Column (4.6 mm × 250 mm, 5 μm, Shiseido Co., Tokyo, Japan); the fluorescence wavelength for detection was 254 nm. The column was set at 40° C, flow rate 1 mL/min. As the mobile phases, acetonitrile was used. The program used was as follows: linear gradient elution from 55% for 10 min; 65% for 5 min; 80% for 10 min followed by 90% for 15 min; the total time was 40 min. Eleven amines (tryptamine, 2-phenylethylamine, putescine, cadaverine, histamine, serotonin, trymine, spermidine, spermine, l-noradrenaline, and dopamine) were injected as the standard solution.
Statistics
All analyses were performed in triplicate. The data were analyzed by a one-way ANOVA using the SPSS version 16.0 program. The results on the analyses are expressed as mean ± standard deviation (SD). The differences among groups were assessed by using Duncan's multiple range tests. Statistical significance was considered at p < 0.05.
Results and discussion
Isolation, screening and identification of AAB from naturally fermented Korean black raspberry vinegar
Bokbunja vinegars, as the screening source, was obtained from various areas in Muju, Korea (127°44′, 35°54′), which fermented Bokbunja naturally by using organically grown Bokbunja. During the acetic acid fermentation over a course of 144 days, AAB continuously increased up to log 5.8 CFU/mL. The most high-level ethanol concentration was 12% at the initial fermentation stage of 3 days with a concomitant growth of yeast. Then, the acidity gradually increased from 1.06% (w/v) to 2% (w/v) after 45 days of fermentation, and then finally achieved 4.4% (w/v) acidity after vinegar fermentation for 144 days.
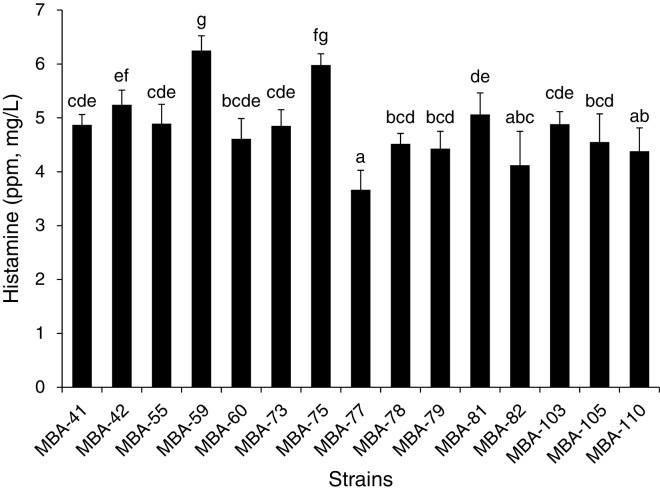
From a total of 147 AAB-like strains isolated from naturally fermented Bokbunja vinegar on the GYEC medium, fifteen strains, displaying both a high-level acetic acid producing activities as well as a high growth rate, were selected. Then, the fifteen strains were examined for BA spectrum at a modified condition after cultivation on the GYEC medium. As shown in Fig. 1, most of the examined strains showed an identical BA profile, showing only the histamine detected. Cadaverin, spermidine, spermine, tyramine, serotonin and dopamine were not detected in our analysis. When we compared the histamine concentration, strain MBA-77 showed the significantly lowest concentration (3.05 mg/L) of histamine (p < 0.05) with the highest acetic acid in the medium, as shown in Fig. 1. However, strain MBA-59 showed a significantly higher histamine production (p < 0.05).
Fig. 1.
Production of histamine (ppm, mg/L) by MBA-77 isolated from naturally fermented Bokbunja vinegar. The determination of biogenic amine was carried out by HPLC. Eleven amines: tryptamine, 2-phenylethylamine, putescine, cadaverine, histamine, serotonin, trymine, spermidine, spermine, l-noradrenaline, dopamine were derivatized with dansyl chloride with internal standard (1,7-diaminoheptane) and injected as standard solution. Values represent means of triplicate determination ± standard deviation. Different letters (e.g. a–g) were assigned to significantly different groups and the same letters were assigned to significantly similar groups (p < 0.05).
Identification of the selected AAB from naturally fermented Korean black raspberry vinegar
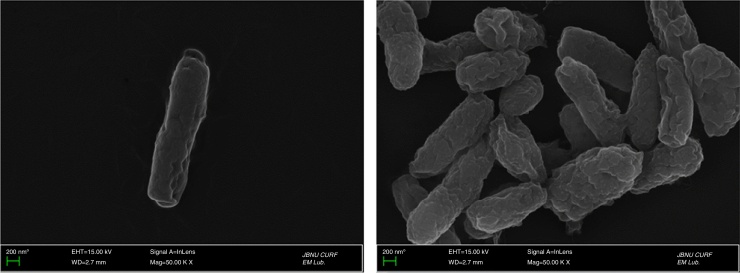
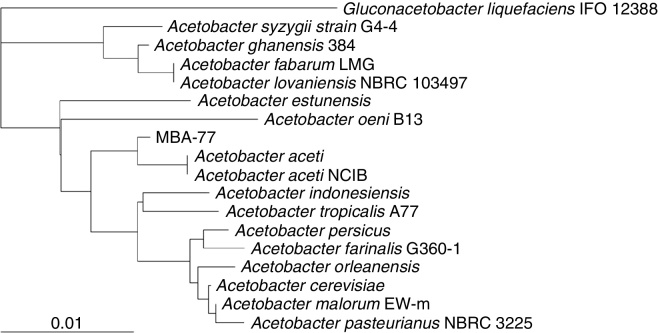
As shown in Fig. 2, the isolate was rod-shaped and regular short length size of about 4 μm length. When the selected fifteen strains were identified by 16S rDNA sequence, all the strains showed high similarity with A. aceti strain NCBI 8621 with 99–100% homology. In particular, the strain MBA-77 showing the lowest histamine production showed high similarity with the A. aceti strain NCBI 8621 with 99% homology (Fig. 3). Moreover, in the biochemical test results using API 20 NE Kit, the selected strain MBA-77 showed a typical characteristic of AAB, thereby indicating almost identical chemical metabolic properties with A. aceti (data not shown). As shown in Table 1, the selected strain MBA-77 was revealed as Gram negative, catalase positive and oxidized ethanol to acetic acid. All together, we were able to identify the selected strain MBA-77 as Acetobacter aceti, and naming it A. aceti MBA-77.
Fig. 2.
Field emission-scanning electron microscope (FE-SEM) of cells of acetic acid bacteria isolate (MBA-77) grown in GYE broth (50,000×).
Fig. 3.
Phylogenetic tree of 16S rDNA sequences of MBA-77 isolated from natural fermented Bokbunja vinegar. Sequence similarity searches were carried out using Basic local alignment search (BLAST) on the EMBL/GenBank databases and the phylogenetic trees were constructed using the neighbor-joining method.
Table 1.
Phenotypic and technological features of acetic acid bacteria isolated from traditional Korean fermented vinegar.
| Strain | Ethanol (%) |
d-Glucose (%) | Growth on single carbon source (%) |
Catalase activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 30 | Fructose | Glycerol | Raffinose | Sucrose | Mannose | Methanol | ||
| A. aceti MBA-77 | + | − | − | − | − | + | ++ | Wa | + | ++ | W | + |
w: weak.
Physiological, biochemical and growth properties
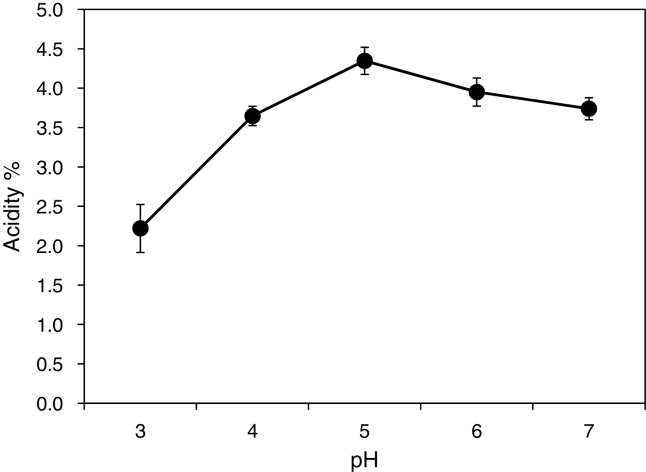
As shown in Table 1, A. aceti MBA-77 were tolerant at 5% ethanol concentration and showed better growth and availability at 5% ethanol. This ability to grow from 5% (v/v) of ethanol is one of the main phenotypic traits for selecting the AAB starter for vinegar production.20 When the fermentation profile was examined by using various single carbon sources, the A. aceti MBA-77 showed weak growth in the present methanol as a carbon source as well as a lower growth rate in the raffinose containing medium. However, the A. aceti MBA-77 was able to ferment raffinose and mannose more efficiently. According to Wu et al.,18 Acetobacter pasteurianus species showed a high degree of phenotypic variability, such as ethanol tolerance, growth on 30% of d-glucose and single carbon sources, which are in accordance with the phenotypic variability of A. aceti and A. aceti MBA-77. Some of these features were previously used as the discriminative tools for taxonomic purposes in order to differentiate Acetobacter from Gluconobacter and Gluconacetobacter genera, because the growth on d-mannitol and 30% of d-glucose were different.18 However, in our result, the same A. aceti species showed similar phenotypic variability, except for the slight difference raffinose and mannose fermentability (data not shown). The optimal acetic acid production of A. aceti MBA-77 was shown at pH 5 after 192 h fermentation, as shown in Fig. 4. Compared to A. aceti MBA-77, the optimal growth of AAB was generally observed at pH 4–5 for optimal acetic acid production due to the fast decline of viable cells of A. aceti by a low oxygen concentration when the pH value was lower than 3.4.21 AAB is also able to grow at lower pH values where bacterial activity has been detected for pH values under 3, even though the optimal pH growth of AAB is between 5.0 and 6.5.17 Thus, the observed optimal condition of the A. aceti MBA-77 for growth and acetic acid production appears to be at a very unusually high level. These results might be explained by the properties of the original source we used in this study for screening because the pH of Bokbunja seed vinegar maintained a very low pH of 2.4–3.0 during vinegar fermentation. Thus, the A. aceti MBA-77 might be adapted to such low pH environment. Moreover, the A. aceti MBA-77 showed the highest acetic acid production at pH 5, which corresponds to most of the AABs have been known for optimal acetic acid production at pH 5 during fermentation.
Fig. 4.
Optimal pH of A. aceti MBA-77 isolated from traditional Korean fermented vinegar. The acidity (%) was measured under various conditions. The pH experiments were performed under five factors of culture broth pH (3, 4, 5, 6 and 7) incubated at 160 rpm. Each experiment was performed in triplicate.
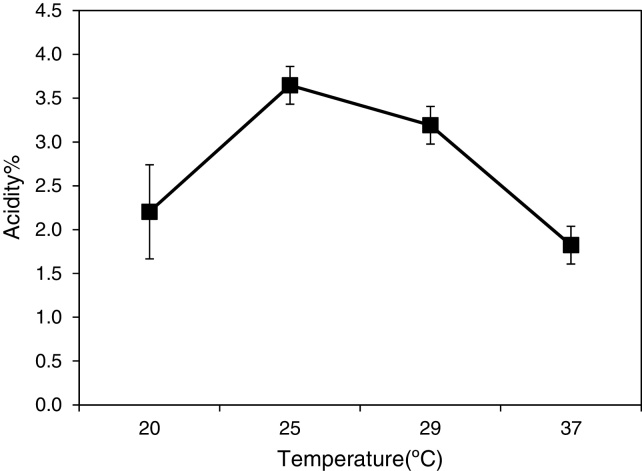
When we examined the temperature for optimal acetic acid fermentation, the A. aceti MBA-77 showed a higher acidity (%) at 25° C after 192 h fermentation, as shown in Fig. 5, which is relatively lower than the generally known AABs. In the above optimum temperature (25–30° C), AABs were deactivated by the denature of enzymes, membrane damage and sensitivity to the toxic effect of acetic acid.22
Fig. 5.
Optimal temperature of A. aceti MBA-77 isolated from traditional Korean fermented vinegar. The acidity (%) was measured under various conditions. The temperature experiments were performed 20, 25, 29 and 37 °C, incubated at 160 rpm. Each experiment was performed in triplicate.
BA analysis for Bokbunja extract
To analyze the BAs production during Bokbunja vinegar fermentation, vinegar fermentation in a lab scale was performed and sampling was carried out at each fermentation step of the Bokbunja extract, wine fermentation and acetic acid fermentation with a single culture inoculation of A. aceti MBA-77. As shown in Table 2, BA spectrum of cadaverine, histamine and spermidine was already observed in the Bokbunja extract; 5.09, 2.72 and 1.21 mg/L, respectively. García-Marino et al.23 reported that grape must before winemaking contains BAs (8.35 mg/L); histamine 3.26 mg/L, ethylamine 1.03 mg/L, putrescine 3.21 mg/L and cadaverine 0.85 mg/L. Their BA spectrums were slightly different but consistent with our result that total BA content tends to increase throughout winemaking (13.17 mg/L). Surprisingly, serotonin and dopamine were detected as major BAs in Bokbunja extract. We could not detect other BAs of tyramine and putrescine in the Bokbunja extract, which already has been identified as being dominant in raw materials.6 It has been known that the BAs of putrescine, spermidine and histamine are normal constituents of the raw materials in wine fermentation; further, a diverse high concentration of BAs might occur during the fermentation processes of the wine-making process through the decarboxylation of amino acids.24
Table 2.
Production of biogenic amines (mg/L) in Bokbunja extract, wine and vinegar fermented by acetic acid bacteria isolated in Bokbunja vinegar.
| Bokbunja extract | Bokbunja wine | Bokbunja vinegara | |
|---|---|---|---|
| Tryptamine | nd | nd | nd |
| 2-Phenylethylamine | nd | nd | nd |
| Putescine | nd | nd | nd |
| Cadaverine | 5.09 ± 0.08a | 2.6 ± 0.16b | nd |
| Histamine | 2.72 ± 0.03c | 5.29 ± 0.05a | 3.66 ± 0.11b |
| Serotonin | 70.25 ± 1.24b | 80.98 ± 1.74a | nd |
| Tyramine | nd | nd | nd |
| Spermidine | 1.21 ± 0.03b | 4.77 ± 0.09a | nd |
| l-Noradrenaine | nd | nd | nd |
| Dopamine | 11.43 ± 0.35a | 10.12 ± 0.07b | nd |
| Spermine | nd | nd | nd |
| Total amines | 90.7 ± 1.73b | 104.06 ± 2.11a | 3.66 ± 0.11c |
Bokbunja vinegar fermented with A. aceti MBA-77.
nd: not detected.
Data are the mean ± SD of the samples analyzed in triplicate.
Different lower letters (e.g. a–c) were assigned to significantly different between samples for each biogenic amine (p < 0.05).
Change of BAs during ethanol fermentation by S. cerevisiae
During Bokbunja wine fermentation processes by S. cerevisiae as the starter, 20 °Brix of initial sugar was reduced to a final 10 °Brix, accumulating approximately 12% ethanol after 5 days with continuous increasing yeast until log 7.0 CFU/mL. When we analyzed Bokbunja wine after 5 days of alcoholic fermentation, the BAs of cadaverine, histamine, serotonin, spermidine and dopamine were detected. The histamine concentration increased from 2.72 of Bokbunja extract to 5.29 mg/L of wine by S. cerevisiae during wine fermentation. Similar observations have been reported by other authors9, 10 that this result generally due to the yeasts metabolite various amino acids during alcohol fermentation. We did not control the wine fermentation process by S. cerevisiae in order to understand the effect of AABs we used in this study. When we compared the obtained BAs profile with several commercial Bokbunja wines purchased from different regions in South Korea,25 our results were slightly different. Compared to all the samples in which putrescine and tyramine exist as the most dominant amines detected 100% in the samples, and tryptamine, cadaverine and spermidine were found in 83%, 78% and 83% of the samples; the least common amines were 2-phenylethylamine and histamine, which were detected in 44% and 50% of the samples, respectively. However, in our result, putrescine and tyramine were not detected, and the most prevalent BAs of spermidine and dopamine were detected in 88%; cadaverine, histamine and spermine were found in 2.6, 5.3 and 4.8%, respectively, in Bokbunja wine fermented in the Muju province. These results clearly indicated that BA spectra were significantly expanded after the Bokbunja wine fermentation step by using S. cerevisiae no.7013 as a starter culture. As shown in Table 2, ethanol fermentation by S. cerevisiae no.7013 resulted in significant changes on BAs. During fermentation, cadaverine (5.09 mg/L) and dopamine (11.43 mg/L), which were originally presented in the Bokbunja extract, was decreased to 2.6 and 10.12 mg/L. However, other BAs, such as histamine, serotonin and spermidine, were produced 2.57, 10.73 and 3.56 mg/L, respectively, during alcohol fermentation. It is not unusual for the new synthesis of other BAs during ethanol fermentation because generally, four BAs, such as histamine, tyramine, putrescine and cadaverine, have been found in a higher concentration in wine. Furthermore, most of the BA synthesis is responsible for the microbial fermentation of yeast and LAB during wine fermentation. It has been demonstrated that many strains of yeasts present in wines (S. cerevisiae, B. bruxellensis, K. apiculata, etc.) can produce histamine, ethanolamine, agmatine, phenylethylamine and cadaverine. Caruso et al.9 reported that B. bruxellensis produced the highest total BAs (15 mg/L), followed by S. cerevisiae (12.14 mg/L) and histamine; cadaverine, putrescine and tryptamine were all produced by strains less than 4 mg/L. Also, they reported that the highest fermentative yeast (S. cerevisiae) showed the highest amount of agmatine, and middle fermentative yeast Candida stellata also showed a capacity to produce cadaverine. Generally, in alcoholic beverages, the toxic dose is considered to be between 8 and 20 mg/L for histamine, 25 and 40 mg/L for tyramine, whereas as little as 3 mg/L of phenylethylamine can cause negative physiological effects.26 Although the BA contents in Bokbunja wine in this study did not exceed the toxic levels (especially for histamine of 5.29 mg/L), which are the highest histamine concentration all through the fermented wine at present, it strongly indicates that there must be a novel method to decrease those BAs, including histamine.
Change of BAs during acetic acid fermentation by A. aceti MBA-77 after ethanol fermentation
Acetic acid fermentation was started by inoculating starter culture after 5 days of alcohol fermentation with increasing AAB increased up to log 5.8 CFU/mL during 14 days and finally converted to acetic acid up to 6% during fermentation (data not shown). Lots of AAB from various vinegars and wine based on different raw materials such as grape, strawberry or persimmon has been isolated and examined for their acetification as a starter.27, 28, 29, 30 When Acetobacter malorum recovered from strawberry wine were used as native starters for acetification, the acetic acid concentration reached was 5.5–6.6% (w/v) which almost two times higher than spontaneous acidification on strawberry.30 Similarly, our selected strain A. aceti MBA-77 as the natural starter was also showed good fermentation profile; on Bokbunja as raw materials, indicating that the use of indigenous strain for acetification must be applicable for efficient fermentation acetification process. Moreover, when we examined the BAs of Bokbunja vinegar after acetic acid fermentation by using our selected A. aceti MBA-77 as the starter during the 14 days, we found that the histamine concentration was detected in the Bokbunja vinegar sample as 3.66 mg/L, which is a 0.7-times reduced level compared to Bokbunja wine (5.29 mg/L), as shown in Table 2. Moreover, in addition to a significant decrease of histamine concentration after acetic acid fermentation, the expanded BA synthesis spectrum after ethanol fermentation significantly decreased by A. aceti MBA-77 as well. Even though the reducing rate of histamine concentration did not increase as high as we expected in this experiment, however, other BAs occurred in Bokbunja wine can be reduced significantly, which clearly indicates that the introduction of A. aceti MBA-77 as the starter for acetic acid fermentation was a very effective tool for reducing the BAs contents, including histamine, in the preparation of Bokbunja vinegar. Generally, a high concentration of BAs can be formed during the ethanol fermentation through the decarboxylation of various amino acids.23, 31 In addition, BAs are physiologically degraded through oxidative deamination catalyzed by amines oxidase. Some amines degrading bacteria have been studied.32, 33 However, due to significant inhibition activity by ethanol for specific enzymes, such as monoamine oxidase, diamine oxidase and histamine-N-methyltransferase, which are responsible for the neutralization of BAs to non-toxic products under normal conditions after wine fermentation, the fermented wine containing BAs might cause unfavorable side effects even if the concentration of each individual BAs did not reach up to the high toxic level.6 Namely, it would be desirable to reduce ethanol concentration or BAs concentration in the fermented foods in order to prevent those unfavorable side effects. Interestingly, vinification itself may not reduce the already formed BAs, including histamine. Rather, a more high content of BA concentration is found after vinification.34 This result might be caused by tedious and long-term vinegar fermentation in unstable environments. Compared to BAs in balsamic vinegar resulting in 51.5–525 μg/L of putrescine, 23.1–60.4 μg/L of cadaverine and 101–223.3 μg/L of spermidine by Ordóñez et al.,11 however, those BAs and as well other BAs, such as cadaverine, serotonin, spermidine and dopamine in Bokbunja wine, were not detected after acetic acid fermentation in Bokbunja vinegar. Previously, it was also reported that the most abundantly remained BAs after vinegar fermentation were putrescine and histamine, which were produced after wine fermentation and transferred to vinegar.11 The toxic effects of histamine among the BAs are enhanced in the presence of cadaverine and putrescine by inhibiting the histamine metabolizing enzymes of diamine oxidase and histamine methyl transferase.1 When we examined the putrescine concentration of Bokbunja vinegar, we found that putrescine was not detected all through the Bokbunja vinegar fermentation, thereby indicating that vinegar fermentation by using Bokbunja must be useful to control the BAs, although Bokbunja also contains numerous functional components, such as phenolic compounds, like other materials. During vinegar fermentation, the actual total content of BAs in vinegars are very diverse depending on the used law materials ranging from 23.35 to 1445.2 μg/L; moreover its contents generally decreased after wine fermentation (130 mg/L) perhaps due to the degradation by some bacterial strains present in wine or vinegar.11 However, most of the studies on bacterial reduction in vinegar fermentation have been focused mainly on LAB. Previously, Landete et al.8 reported that none of the AABs among the 40 strains and 36 of yeast strains which isolated from wine or grape must produce BAs whereas LAB isolated from wine, Pediococcus sp., Lactobacillus sp., L. mesenteroids and O. ovni can produce histamine, tyramine, phenylethylamine and putrescine. Also, P. parbulus and L. hilgardii have been detected as high histamine-producing bacteria.7 In our studies, the total BA concentration significantly decreased after vinegar fermentation (3.66 ± 0.11 mg/L, only histamine) compared to wine (104.06 ± 2.11 mg/L) and extract (90.7 ± 1.73 mg/L), even though we tried vinegar fermentation by using A. aceti MBA-77. Because A. aceti MBA-77 was never identified as BA-degrading AAB, this is the first report ever published to indicate that an acetic acid bacterium is able to decrease histamine.
In this study, we were able to isolate the MBA-77 strain, which showed higher acetic acid synthesis and low BA synthesis ability as well. The selected strain was identified as A. aceti by a biochemical and 16S rRNA sequencing analysis. The optimal condition for A. aceti MBA-77 was pH 7 and 25° C. Moreover, A. aceti MBA-77 was tolerant at the 5% ethanol concentration along with a lower growth rate in a raffinose containing medium. When the selected was used as the starter for acetic acid fermentation, BAs spectrum and concentration present in Bokbunja extract were increased during alcoholic fermentation. However, those increased BAs spectrum and concentration were decreased after vinegar fermentation by A. aceti MBA-77. The AAB isolated herein is the first application as a starter culture for BA analysis in vinegar; furthermore, it might be helpful to manufacture vinegar with a low BA synthesis.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by High value-added Food Technology Development Program, of the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET), and the Ministry for Food, Agriculture, Forestry, and Fisheries of Republic of Korea (313037-03).
Associate Editor: Solange Ines Mussatto
References
- 1.Lehane L., Olley J. Histamine fish poisoning revisited. Int J Food Microbiol. 2000;58:1–37. doi: 10.1016/s0168-1605(00)00296-8. [DOI] [PubMed] [Google Scholar]
- 2.Taylor S.L., Eitenmiller R.R. Histamine food poisoning: toxicology and clinical aspects. Crit Rev Toxicol. 1986;17:91–128. doi: 10.3109/10408448609023767. [DOI] [PubMed] [Google Scholar]
- 3.Landete J.M., Ferrer S., Polo L., Pardo I. Biogenic amines in wines from three Spanish regions. J Agric Food Chem. 2005;53:1119–1124. doi: 10.1021/jf049340k. [DOI] [PubMed] [Google Scholar]
- 4.Lonvaud-Funel A., Joyeux A. Histamine production by wine lactic acid bacteria: isolation of a histamine-producing strain of Leuconostoc oenos. J Appl Bacteriol. 1994;77:401–407. doi: 10.1111/j.1365-2672.1994.tb03441.x. [DOI] [PubMed] [Google Scholar]
- 5.Kung H.F., Lee Y.H., Teng D.F., Hsieh P.C., Wei C.I., Tsai Y.H. Histamine formation by histamine-forming bacteria and yeast in mustard pickle products in Taiwan. Food Chem. 2006;99:579–585. [Google Scholar]
- 6.Lonvaud-Funel A. Biogenic amines in wines: role of lactic acid bacteria. FEMS Microbiol Lett. 2001;199:9–13. doi: 10.1111/j.1574-6968.2001.tb10643.x. [DOI] [PubMed] [Google Scholar]
- 7.Landete J.M., Ferrer S., Pardo I. Which lactic acid bacteria are responsible for histamine production in wine? J Appl Microbiol. 2005;99:580–586. doi: 10.1111/j.1365-2672.2005.02633.x. [DOI] [PubMed] [Google Scholar]
- 8.Landete J.M., Ferrer S., Pardo I. Biogenic amine production by lactic acid bacteria, acetic bacteria and yeast isolated from wine. Food Control. 2007;18:1569–1574. [Google Scholar]
- 9.Caruso M., Fiore C., Contursi M., Salzano G., Paparella A., Romano P. Formation of biogenic amines as criteria for the selection of wine yeasts. J Microbiol Biotechnol. 2002;18:159–163. [Google Scholar]
- 10.Torrea D., Ancín C. Content of biogenic amines in a Chardonnay wine obtained through spontaneous and inoculated fermentations. J Agric Food Chem. 2002;50:4895–4899. doi: 10.1021/jf011653h. [DOI] [PubMed] [Google Scholar]
- 11.Ordóñez J.L., Callejón R.M., Morales M.L., García-Parrilla M.C. A survey of biogenic amines in vinegars. Food Chem. 2013;141:2713–2719. doi: 10.1016/j.foodchem.2013.05.087. [DOI] [PubMed] [Google Scholar]
- 12.mo Dugo G., Vilasi F., La Torre G.K., Pellicano T.M. Reverse phase HPLC/DAD determination of biogenic amines as dansyl derivatives in experimental red wines. Food Chem. 2006;95:672–676. [Google Scholar]
- 13.Capozzi V., Russo P., Ladero V. Biogenic amines degradation by Lactobacillus plantarum: toward a potential application in wine. Front Microbiol. 2012;3:122. doi: 10.3389/fmicb.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim T.J. Department of Seoul University; Seoul, Korea: 1996. Korean Resources Plants II. [Google Scholar]
- 15.Kim E.J., Lee Y.J., Shin H.K., Park J.H.Y. Induction of apoptosis by the aqueous extract of Rubus coreanum in HT-29 human colon cancer cells. Nutrition. 2005;21:1141–1148. doi: 10.1016/j.nut.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Kopermsub P., Yunchalard S. Identification of lactic acid bacteria associated with the production of plaa-som, a traditional fermented fish product of Thailand. Int J Food Microbiol. 2010;138:200–204. doi: 10.1016/j.ijfoodmicro.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Sievers M., Swings J. Family Acetobacteraceae. In: Garrity G.M., editor. Vol. 2. Springer; New York: 2005. pp. 41–95. (Bergey's Manual of Systematic Bacteriology). [Google Scholar]
- 18.Wu J.J., Gullo M., Chen F.S. Diversity of Acetobacter pasteurianus strains isolated from solid-state fermentation of cereal vinegars. Curr Microbiol. 2010;60:280–286. doi: 10.1007/s00284-009-9538-0. [DOI] [PubMed] [Google Scholar]
- 19.Navarro R.R., Komagata K. Differentiation of Gluconacetobacter liquefaciens and Gluconacetobacter xylinus on the basis of DNA base composition. J Gen Appl Microbiol. 1999;45:7–15. doi: 10.2323/jgam.45.7. [DOI] [PubMed] [Google Scholar]
- 20.Gullo M., Giudici P. Acetic acid bacteria in traditional balsamic vinegar: phenotypic traits relevant for starter cultures selection. Int J Food Microbiol. 2008;125:46–53. doi: 10.1016/j.ijfoodmicro.2007.11.076. [DOI] [PubMed] [Google Scholar]
- 21.Joyeux A., Lafon-Lafourcade S., Ribereau-Gayon P. Evolution of acetic acid bacteria during fermentation and storage of wine. Appl Environ Microbiol. 1984;48:153–156. doi: 10.1128/aem.48.1.153-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Ory I., Romero L.E., Cantero D. Modelling the kinetics of growth of Acetobacter aceti in discontinuous culture: influence of the temperature of operation. Appl Microbiol Biotechnol. 1998;49:189–193. [Google Scholar]
- 23.García-Marino M., Trigueros Á., Escribano-Bailón T. Influence of oenological practices on the formation of biogenic amines in quality red wines. J Food Comp Anal. 2010;23:455–462. [Google Scholar]
- 24.Hajós G., Sass-Kiss A., Szerdahelyi E., Bardocz S. Changes in biogenic amine content of Tokaj grapes, wines and Aszu-wines. J Food Sci. 2000;65:1142–1144. [Google Scholar]
- 25.Jia S., Kang Y.P., Park J.H., Lee J., Kwon S.W. Determination of biogenic amines in Bokbunja (Rubus coreanus Miq.) wines using a novel ultra-performance liquid chromatography coupled with quadrupole-time of flight mass spectrometry. Food Chem. 2012;132:1185–1190. doi: 10.1016/j.foodchem.2011.11.069. [DOI] [PubMed] [Google Scholar]
- 26.Soufleros E., Barrios M.L., Bertrand A. Correlation between the content of biogenic amines and other wine compounds. Am J Enol Vitic. 1998;49:266–269. [Google Scholar]
- 27.Hidalgo C., Mateo E., Cerezo A.B., Torija M.J., Mas A. Technological process for production of persimmon and strawberry vinegars. Int J Wine Res. 2010;2:55–61. [Google Scholar]
- 28.Hidalgo C., Vegas C., Mateo E. Effect of barrel design and the inoculation of Acetobacter pasteurianus in wine vinegar production. Int J Food Microbiol. 2010;141:56–62. doi: 10.1016/j.ijfoodmicro.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo C., García D., Romero J., Mas A., Torija M.J., Mateo E. Acetobacter strains isolated during the acetification of blueberry (Vaccinium corymbosum L.) wine. Lett Appl Microbiol. 2013;57:227–232. doi: 10.1111/lam.12104. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo C., Torija M.J., Mas A., Mateo E. Effect of inoculation on strawberry fermentation and acetification processes using native strains of yeast and acetic acid bacteria. Food Microbiol. 2013;34:88–94. doi: 10.1016/j.fm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Moreno-Arribas M.V., Polo M.C. Occurrence of lactic acid bacteria and biogenic amines in biologically aged wines. Food Microbiol. 2008;25:875–881. doi: 10.1016/j.fm.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Callejón S., Sendra R., Ferrer S., Pardo I. Identification of a novel enzymatic activity from lactic acid bacteria able to degrade biogenic amines in wine. Appl Microbiol Biotechnol. 2014;98:185–198. doi: 10.1007/s00253-013-4829-6. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez M.A., Moreno-Arribas M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci Technol. 2014;39:146–155. [Google Scholar]
- 34.Chang S.C., Lin C.W., Jiang C.M. Histamine production by bacilli bacteria, acetic bacteria and yeast isolated from fruit wines. LWT-Food Sci Technol. 2009;42:280–285. [Google Scholar]