Abstract
Ungulate tetraparvovirus 2 (UTV2), formerly known as porcine hokovirus due to its discovery in Hong Kong, is closely related to a Primate tetraparvovirus (human PARV-4) and Ungulate tetraparvovirus 1 (bovine hokovirus). Until now, UTV2 was detected in European, Asian and North American countries, but its occurrence in Latin America is still unknown. This study describes the first report of UTV2 in Brazil, as well as its phylogenetic characterization. Tissue samples (lymph node, lung, liver, spleen and kidney) of 240 piglets from eight different herds (30 animals each herd) were processed for DNA extraction. UTV2 DNA was detected by PCR and the entire VP1/VP2 gene was sequenced for phylogenetic analysis. All pigs from this study displayed postweaning multisystemic wasting syndrome (PMWS). UTV2 was detected in 55.3% of the samples distributed in the variety of porcine tissues investigated, as well as detected in almost all herds, with one exception. The phylogenetic analysis demonstrated that Brazilian UTV2 sequences were more closely related to sequences from Europe and United States.
Keywords: Porcine, Tetraparvovirus, Phylogeny, Hokovirus, Detection, Ungulate
Introduction
Porcine hokovirus, recently classified as Ungulate tetraparvovirus 2 (UTV2) belongs to the Parvoviridae family and is related to Primate tetraparvovirus (formerly known as human PARV-4) and Ungulate tetraparvovirus 1(UTV1) (formerly known as bovine hokovirus).1, 2 The UTV2 genome organization contains two major open reading frames (ORFs), named ORF1 and ORF2, separated by a small non-coding region. ORF1 encodes non-structural proteins (NS) and ORF2 encodes the two overlapping capsid proteins (VP1/VP2) and a small conserved putative protein.1 Studies demonstrated high prevalence rates of UTV2 in domestic pigs and wild boar populations ranging from 22.8% to 50.5%.1, 3, 4 UTV2 can infect pigs of different ages but higher prevalence was found in older pigs (grow-finishing),5 as well as in adult wild boars.3 UTV2 has been detected in domestic pigs in many countries as Hong Kong,1 United States,5 China,6 Cameroon,7 as well as wild boars from Germany3 and Romania,4 indicating that the virus can be endemic worldwide in both domestic and wild pig population.
UTV2 has been detected in a variety of porcine tissues, fecal and serum samples from healthy or sick pigs, but no clinical symptoms have been linked to infection or viral persistence.1, 3, 8 However, one study observed that pigs displaying respiratory disease had higher levels of UTV2 compared to those with enteric disease or abortion.5 In addition, some studies demonstrated concurrent infection between emerging parvovirus with the porcine circovirus 2 (PCV2).9, 10 Therefore, the aim of this study was to detect and analyze phylogenetically UTV2 in pigs suffering from postweaning multisystemic wasting syndrome (PMWS).
Material and methods
Samples
In 2005, during a PMWS outbreak, tissue samples were submitted to the Department of Pathology at Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil, for PMWS diagnosis and research purposes. The samples (lymph node, lung, spleen, liver and kidney) were collected from 240 animals, approximately 12–15 weeks of age, from eight different herds (30 animals each herd) from Rio Grande do Sul State, Brazil. The inclusion criteria adopted to fulfill the triad of requisites to define PMWS disease was followed as proposed by Sorden (2000)11: (i) presence of clinical signs, such as lessened weight gain, diarrhea and wasting; (ii) histopathological evidence of lymphocytic depletion, multinucleated giant cell formation in lymph nodes, histiocytic replacement of follicles in lymphoid tissues, and multifocal lymphohistiocytic interstitial pneumonia and positive presence of PCV2 antigen in the lungs by immunohistochemistry (IHC) associated with microscopic lesions; (iii) PCV2 DNA confirmation by PCR. Sections of swine lymph nodes and lungs were submitted for histopathological investigation consisting of the hematoxylin–eosin standard treatment, with samples embedded in paraffin blocks. To identify PCV2 antigen in the lesion site, sections of the organs presenting pathological changes were submitted to IHC analysis using the anti-PCV2 polyclonal antibody (Department of Veterinary Diagnostic and Production Animal Medicine, Ames, IA, USA) diluted to 1:1000, followed by streptavidin–biotin–peroxidase complex technique (Dako Cytomation Inc., Carpinteria, CA, USA), and revealed using diaminobenzidine method (Dako Cytomation Inc., Carpinteria, CA, USA).
Tissue samples (approximately 1 g) were minced and homogenized in 1 mL of phosphate buffered saline (PBS, pH 7.4). The homogenized tissues were three times frozen and thawed, and then clarified by centrifugation at 6160 × g. Total nucleic acids were extracted from 100 μL of each tissue homogenate, using a commercial extraction kit based on silica (NewGene kit, Simbios Biotecnologia, Brazil) as recommended by the manufacturer.
PCR detection and sequencing
PCV2 was detected using a previously described PCR protocol.12 Primers for UTV2 were selected using the Vector NTI Advance 10 Software (sense: 5′-GTG GCA GTG ATA TTG CAT CG-3′ and antisense: 5′-TGG CAG TCA TTG AAT GGA AA-3′). PCR mix conditions were the following: 2.5 μL of 10× buffer, 1.5 mM of MgCl2, 200 μM of dNTPs, 20 pmol of each primer, 1 U of Taq DNA polymerase (Ludwig Biotecnologia, Brazil), 2 μL of DNA sample and water up to 25 μL. PCR thermal cycle was performed with an initial cycle of 95 °C for 2 min, 35 cycles at 95 °C for 30 s, 50 °C for 1 min, 72 °C for 1 min and a final extension at 72 °C for 5 min, which amplified a product of 250 base pairs (bp). For the phylogenetic analysis, primers were selected in order to cover the entire VP gene using Vector NTI Advance 10 software (Table 1). PCR mix and thermal conditions for each PCR were the same as described above. The sequences were randomly selected from distinct herds and the amplified DNA samples (30–45 ng) were purified with NucleoSpin® II kit, (Macherey-Nagel, Germany), labeled with 3.2 pmol of each primer (Table 1) and 2 μL of BigDye Terminator v3.1 Cycle Sequencing RR-100 (Applied Biosystems, USA) using the automatic sequencer ABI-PRISM 3100 Genetic Analyzer, armed with 50 cm capillaries and POP6 polymer (Applied Biosystems, USA).
Table 1.
Primers selected to sequence VP gene of UTV2.
| Primer | Sequence (5′–3′) | Positiona | bpb |
|---|---|---|---|
| P1 | GCA CTG AGG GCT ACG TTC GTT CTC | 1985–2009 | 503 |
| P2 | TGA CCA GGT CCA TGA AAA ATC TCC C | 2487–2464 | |
| P3 | GGA GTA GAC GTA TGG GTG GGG GTT A | 2285–2309 | 405 |
| P4 | GGA ATA TTT CCT GGA ACT GCC CTT C | 2734–2710 | |
| P5 | CTT AGG TGA TTT TCA CCG GCC GC | 2550–2572 | 562 |
| P6 | CTC CTT TGC CTC CCA GAT ACC CC | 3111–3089 | |
| P7 | GGT GAT TTG CCA TAC ATC CAT GGG | 2968–2991 | 530 |
| P8 | GTC CGC ATA CCC ATA ACA GGC TGC | 3497–3474 | |
| P9 | GTT GGG GGG CAC TCA TTT CTC TG | 3354–3376 | 550 |
| P10 | CCC CAG ACA CTC TGC ATC ATG ATG | 3903–3380 | |
| P11 | GCC CGG GGA GAA TTA TGT TCT TCC T | 3762–3786 | 487 |
| P12 | GCG GAT ACT CCA GGG TTG ATG TAC C | 4248–4224 | |
| P13 | CGG CAT AAT CCC ATT CCT CCG TC | 3991–4013 | 494 |
| P14 | CTT CCA CTC CCA CTC CCA TCC ACT C | 4484–4460 | |
| P15 | GCC GTC ACG AGG AAA GTT ATA CCG G | 4322–4346 | 594 |
| P16 | CGT ACA CAG GTA CAT TGG GGT CCC | 4915–4892 | |
Nucleotide position according to HK1 sequence (GenBank accession no.: EU200671).
Sequence length in base pairs (bp) of each PCR.
Phylogenetic analysis
The VP data set was composed by assembled sequences (DNA Baser version 3.0 Software) from this study (JQ700067–JQ700072) and sequences retrieved from Genbank EU200671–EU200677, JN990266–JN990269 from China, FJ982246–FJ982255 from Great Britain, JQ425257–JQ425259 from United States, JF738350, JF738351, JF738357, JF738362, JF738364, JF738366–JF738368 from Romania and GQ869539, GQ869540, GQ869542, GQ869543 from Germany. An UTV1 sequence (EU200668) was used as outgroup in the phylogenetic analysis. The VP data set was aligned using MEGA Software version 4.0.13 The phylogenetic analysis was performed using two methods: a heuristic search using PAUP 4.0.b software14 with a support of 1000 bootstrap repetitions and a Bayesian Inference (BI) was conducted with MrBayes 3.1.2 software.15 The substitution models for these approaches were found using MrMODELTEST with the AIC criterion. For the BI method, four Markov chains, one cold and three heated were used and the run was set for 2 × 106 generations, with trees sampled every 100 generations. Trees generated prior to stationary phase were discarded as “burn-in”.
Results
All pigs from the present study fulfilled the requisites for the case definition of PMWS as previously proposed by Sorden.11 In the histopathology analysis, all of the lymph node samples displayed lymphocytic depletion, multinucleated giant cell formation and histiocytic replacement of follicles. In addition, the lung samples demonstrated multifocal lymphohistiocytic interstitial pneumonia. PCV2 antigen was detected by IHC in all lung and lymph node samples and all the samples were confirmed to be PCV2 positive in the PCR assay. A total of 55.3% samples were UTV2 PCR-positive distributed in all herds, with exception of one herd, which UTV2 was not detected. In the evaluation of porcine tissues, the highest positivity of UTV2 was found in spleen (68.5%) followed by the lung (65%), lymph node (57.5%), kidney (57.5%) and liver (54.3%).
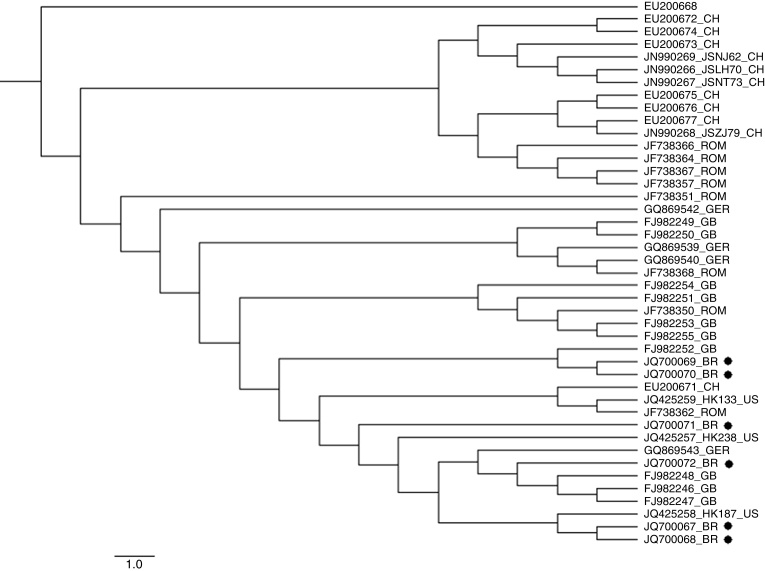
In total, six sequences covering all VP1/VP2 region (with length of approximately 2696 bp) were obtained. The sequences came from four spleen samples (accession number: JQ700067, JQ700069, JQ700072 and JQ700070), one lung sample (accession number: JQ700068) and one lymph node sample (accession number: JQ700071). Both phylogenetic tree construction methods displayed similar consensus trees demonstrating that Brazilian sequences were clustered mostly with sequences from Great Britain, United States and Germany. Most of the Chinese sequences clustered together with exception of sequence EU200671 (Fig. 1).
Fig. 1.
Phylogenetic tree constructed by Bayesian Inference based on complete nucleotide sequences of the VP gene of UTV2. The acronym refers to country of each sequence: BR, Brazil; CH, China; GB, Great Britain; GER, Germany; ROM, Romania; US, United States. All the sequences are represented with the accession number from Genbank. The sequences from this study are marked with a black dot. The trees were statistically evaluated with the posterior probability confidence. An UTV1 sequence (EU200668) was used as outgroup.
Discussion
A high number of UTV2 positive samples in pigs displaying PMWS was observed and represents the first report of UTV2 in Brazil. Since the samples were collected during a PMWS outbreak in 2005, our data indicate that UTV2 was present in Brazilian herds at least for more than ten years. UTV2 has been detected in several countries as Hong Kong,1 Germany,3 Romania,4 Hungary,9 United States,5 Canada,16 China6 and Cameroon7 in domestic and wild pigs. Nevertheless, until now there is no available data of this virus in Latin America.
Several studies have demonstrated that pigs coinfected with PCV2 and Ungulate protoparvovirus 1 (UPV1), formerly known as porcine parvovirus 1, develop more severe disease and lesions than pigs infected with PCV2 alone.17, 18, 19 For UTV2, recent studies demonstrated concurrent infection with PCV2 in pigs.6, 9, 10 Our study demonstrated high positivity of UTV2 in pigs displaying PMWS as well, however it is not possible to associate with a role in pathogenesis or any impact in PCV2 associated disease since it was not a case–control or experimental infection study.
It is widely known that parvoviruses, as UPV1, have tropism for high mitotic rate tissue as reproductive organs and fetus tissues, which characterizes reproductive failure in pigs that can lead to significant economic losses in swine industry.20, 21 So far, previous studies showed that UTV2 has been preferentially detected in lymph nodes,1 although has been also detected in lung, liver, spleen, heart, tonsil and kidney.1, 3, 4, 8 In our study, a similar percentage of UTV2 detection in the tissues (spleen, lung, lymph node, kidney and liver) was found, suggesting that the virus can infect a wide variety of porcine tissues. In order to evaluate the pathogenicity, viral isolation in distinct cell lines was attempted in the present study without success.
Previous analysis of age distribution showed an increased prevalence rate of UTV2 in grow-finish pigs (8–25 weeks of age) compared to nursery pigs (3–7 weeks of age),5 similar to the age of pigs from our study (12–15 weeks of age). In wild boars was reported higher UTV2 prevalence in animals older than one year, but the highest proportion of animals with high virus loads was seen in animals younger than two years of age, suggesting that these animals were infected in an early stage of life.3
Phylogenetic studies demonstrated that UTV2 has a close relationship with Primate tetraparvovirus showing approximately 65% similarity at nucleotide or amino acid levels.1, 3, 4 Based on genome sequences analysis, it has been suggested that UTV2 strains circulate in both domestic and wild pig populations in Europe.4 However, due to the lack of UTV2 sequences available from wild boars from Brazil it is not possible to estimate if the Brazilian virus strains co-circulate in domestic and wild pigs populations. According to ORF2 phylogenetic analysis, a main cluster could be evidenced with most of Chinese and Romanian sequences, as previously reported.4, 5 Brazilian sequences were displaced outside this main cluster together with sequences from Germany, Great Britain, Romanian and United States. These sequences presented several sub-clusterizations, however a main cluster could not be observed. This closely relationship between U.S. sequences and those from Great Britain and Romania was previously observed with high sequence identities between sequences.5 The relatedness between Brazilian, North American and European sequences found in our study needs to be further investigated. Although it could be hypothesized that this phylogenetic relationship resulted from live pig genetic transfer from Europe and/or United States to Brazil. Furthermore, investigations in contaminated commercial biological products, as vaccines, may elucidate the origin and route of transmission of UTV2 across the countries.
Conclusion
In conclusion, our data demonstrated a high number of UTV2 positive samples in pigs displaying PMWS and represent the first report of UTV2 in pigs in Brazil. In addition, the Brazilian sequences demonstrated a close phylogenetic relationship with European and North American sequences.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Associate Editor: João Pessoa Araújo Junior
References
- 1.Lau S.K., Woo P.C., Tse H. Identification of novel porcine and bovine parvoviruses closely related to human parvovirus 4. J Gen Virol. 2008;89(8):1840–1848. doi: 10.1099/vir.0.2008/000380-0. [DOI] [PubMed] [Google Scholar]
- 2.Cotmore S.F., Agbandje-McKenna M., Chiorini J.A. The family Parvoviridae. Arch Virol. 2014;159(5):1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adlhoch C., Kaiser M., Ellerbrok H., Pauli G. High prevalence of porcine Hokovirus in German wild boar populations. Virol J. 2010;7:171. doi: 10.1186/1743-422X-7-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadar D., Cságola A., Lorincz M., Tombácz K., Spînu M., Tuboly T. Distribution and genetic diversity of porcine hokovirus in wild boars. Arch Virol. 2011;156(12):2233–2239. doi: 10.1007/s00705-011-1125-6. [DOI] [PubMed] [Google Scholar]
- 5.Xiao C.T., Giménez-Lirola L.G., Halbur P.G., Opriessnig T. Increasing porcine PARV4 prevalence with pig age in the U.S. pig population. Vet Microbiol. 2012;160(3–4):290–296. doi: 10.1016/j.vetmic.2012.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Li S., Wei Y., Liu J., Tang Q., Liu C. Prevalence of porcine hokovirus and its co-infection with porcine circovirus 2 in China. Arch Virol. 2013;158(9):1987–1991. doi: 10.1007/s00705-013-1690-y. [DOI] [PubMed] [Google Scholar]
- 7.Adlhoch C., Kaiser M., Kingsley M.T. Porcine hokovirus in domestic pigs, Cameroon. Emerg Infect Dis. 2013;19(12):2060–2062. doi: 10.3201/eid1912.130891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streck A.F., Homeier T., Foerster T., Fischer S., Truyen U. Analysis of porcine parvoviruses in tonsils and hearts from healthy pigs reveals high prevalence and genetic diversity in Germany. Arch Virol. 2013;158(6):1173–1180. doi: 10.1007/s00705-013-1603-0. [DOI] [PubMed] [Google Scholar]
- 9.Cságola A., Lőrincz M., Cadar D., Tombácz K., Biksi I., Tuboly T. Detection, prevalence and analysis of emerging porcine parvovirus infections. Arch Virol. 2012;157(6):1003–1010. doi: 10.1007/s00705-012-1257-3. [DOI] [PubMed] [Google Scholar]
- 10.Opriessnig T., Xiao C.T., Gerber P.F., Halbur P.G. Identification of recently described porcine parvoviruses in archived North American samples from 1996 and association with porcine circovirus associated disease. Vet Microbiol. 2014;173(1–2):9–16. doi: 10.1016/j.vetmic.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 11.Sorden S.D. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS) Swine Health Prod. 2000;8:133–136. [Google Scholar]
- 12.Larochelle R., Antaya M., Morin M., Magar R. Typing of porcine circovirus in clinical specimens by multiplex PCR. J Virol Methods. 1999;80(1):69–75. doi: 10.1016/s0166-0934(99)00032-4. [DOI] [PubMed] [Google Scholar]
- 13.Tamura K., Dudley J., Nei M., Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 14.Swofford D.L. Sinauer Associates; MA: 2002. PAUP* phylogenetic analysis using parsimony (*and other methods). Version 4.0b. [Google Scholar]
- 15.Huelsenbeck J.P., Ronquist F. MRBAYES: Bayesian inference of phylogeny. Bioinformatics. 2001;17(8):754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 16.Bellehumeur C., Boyle B., Mandeville I., Gagnon C.A. High-throughput sequencing revealed the presence of an unforeseen parvovirus species in Canadian swine: the porcine partetravirus. Can Vet J. 2013;54(8):787–789. [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis J., Clark E., Haines D. Porcine circovirus-2 and concurrent infections in the field. Vet Microbiol. 2004;98(2):159–163. doi: 10.1016/j.vetmic.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Sharma R., Saikumar G. Porcine parvovirus- and porcine circovirus 2-associated reproductive failure and neonatal mortality in crossbred Indian pigs. Trop Anim Health Prod. 2010;42(3):515–522. doi: 10.1007/s11250-009-9454-0. [DOI] [PubMed] [Google Scholar]
- 19.Opriessnig T., Halbur P.G. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 2012;164(1–2):20–32. doi: 10.1016/j.virusres.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo H.S., Donaldson-Wood C.R., Johnson R.H. Observations on the pathogenesis of porcine parvovirus infection. Arch Virol. 1976;51(1–2):123–129. doi: 10.1007/BF01317841. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson-Wood C.R., Joo H.S., Johnson R.H. The effect on reproductive performance of porcine parvovirus infection in a susceptible pig herd. Vet Rec. 1977;100(12):237–239. doi: 10.1136/vr.100.12.237. [DOI] [PubMed] [Google Scholar]