Abstract
Escherichia coli is the major causative agent of human cystitis. In this study, a preliminary molecular analysis carried out by PCR (polymerase chain reaction) demonstrated that 100% of 31 E. coli strains isolated from patients with recurrent UTIs (urinary tract infections) showed the presence of the curli fimbria gene (csgA). Curli fimbria is known to be associated with bacterial biofilm formation but not with the adhesion of human cystitis-associated E. coli. Therefore, this work aimed to study how curli fimbria is associated with uropathogenic E. coli (UPEC) as an adhesion factor. For this purpose, the csgA gene was deleted from strain UPEC-4, which carries three adhesion factor genes (csgA, fimH and ompA). The wild-type UPEC-4 strain and its mutant (ΔcsgA) were analyzed for their adhesion ability over HTB-9 (human bladder carcinoma), Vero (kidney cells of African green monkey) and HUVEC (human umbilical vein) cells in the presence of α-d-mannose. All the wild-type UPEC strains tested (100%) were able to adhere to all three cell types, while the UPEC-4 ΔcsgA mutant lost its adherence to HTB-9 but continued to adhere to the HUVEC and Vero cells. The results suggest that curli fimbria has an important role in the adhesion processes associated with human UPEC-induced cystitis.
Keywords: Curli fimbria, Adhesion, Escherichia coli, UPEC
Urinary tract infections (UTIs) are among the most frequent human extraintestinal infections. Escherichia coli is the primary agent of UTIs, and the bladder and periuretral tissues represent 95% of all primary UTI sites. E. coli belongs to the Enterobacteriaceae family and is present in the natural microbiota of humans and other homoeothermic animals. The E. coli associated with UTIs are denoted uropathogenic E. coli (UPEC).1, 2, 3
The adherence of UPEC to uroepithelium cells establishes the initial occurrence of the pathogenesis of a urinary tract infection (UTI).4, 5 Adhesion is generally mediated by fimbriae, which are filamentous appendices localized on the bacterial surface, generally of protein nature, which promote specific binding to receptors on host eukaryotic cells (uroepithelial cells, erythrocytes and urinary glycocalyx), being also capable of biofilm formation over abiotic surfaces such as plastic catheters.4
Thirty-one E. coli isolates from patients with recurrent cystitis, kindly supplied by Dr. Ulysses de Oliveira (FCM, UNICAMP), were analyzed for a number of genes associated with UPEC adherence through polymerase chain reaction (PCR) following the methodology described by Blanco and colleagues,6 such as type 1 fimbriae (fimH), curli (csgA), P (papC), antigen 43 (flu), F9 (f9) and protein membrane A (ompA). It was observed that all the UPEC strains analyzed in the study presented the curli fimbria gene (csgA), and also the genes OmpA (93.3%), fimH (87.1%), f9 (32.3%), papC (25.8%) and flu (19.4%).
Then, after the molecular characterization, we attempted to verify whether curli fimbria acts as an adhesion factor associated with human cystitis. Type 1 fimbriae and P fimbriae are known as the main UPEC adhesins,7, 8 however the role of curli fimbria among UPEC remains unclear.
Curli fimbria is a fibrous surface protein that is important for biofilm development by E. coli and Salmonella, and its presence is associated with severe human infections.9 Fimbria interacts specifically with host matrix proteins such as fibronectin, laminin and plasminogen to initiate adherence to and colonization of the host.10, 11, 12
Curli fimbria presents chemical affinity to Congo red dye according to Hammar et al.,13 and this property is used to phenotypically characterize curli fimbria presence in the adhesion test14.
The ability of these UPEC to form biofilm on abiotic surfaces was assessed by the methodology previously described by Wakimoto et al.15 using 96-well polystyrene microplates (Falcon Becton Dickinson, USA). Briefly, 5 μL of UPEC cultures was inoculated into 195 μL of DMEM containing 0.45% glucose and applied to 96-well polystyrene plates (BD Falcon, USA), being incubated for 24 h at 37 °C. Each sample was stained for five minutes with 0.5% crystal violet and washed four times with PBS (0.01 M, pH 7.4), and 200 μL of 95% (v/v) ethanol was added. Quantification was measured at 570 nm using an automated plate reader (Biotek). Of the 31 strains tested, 28 (90.3%) were considered able to form biofilm in vitro by presenting an OD570 > 0.2.15
UPEC adherence assays were performed over confluent monolayers of 105 HTB-9 (human bladder carcinoma), Vero (African green monkey kidney cells) and HUVEC (human umbilical vein) cells as previously described by Scalestsky et al.16 These cell cultures were grown in Dulbecco's modified Eagle medium (DMEM; Gibco-BRL) containing 10% bovine calf serum (BCS) using 24-well plates with coverslips (Falcon Becton Dickinson, USA). The UPEC strains were grown in 3 mL of TSB for 18 h at 37 °C. Monolayers of Vero, HTB-9 and HEp-2 cells were inoculated with approximately 3 × 108 bacteria/mL (Tube 1 of MacFarland's scale) and were incubated at 37 °C for 3 h. Then, the assay supernatant was diluted to 10−6 and applied over MacConkey agar plates. After overnight incubation, the plates were used for colony counting and for colony forming unit (CFU) determination.17 All the strains analyzed in this study were able to promote cell adhesion over the HTB9, Vero and HUVEC cell lineages.
Thus, to verify if curli fimbria is an adhesin of UPEC associated with human cystitis, UPEC-4 (csgA, fimH, ompA), among 31 UPEC strains, was selected to obtain a null mutant for this gene using the technique described by Datsenko and Wanner.18
We designed the primers CsgA_F: 5′-ACAACGTTAATTTCCATTCGACTTTTAAATCAAT CCGATGGGGGTTTTACGTGTAG-3′ and CsgA_R: 5′-CCCGAAAAAAAACAGGGCTTGC GCCCTGTTTCTTTAATACAGATGATGTAATGGGAATTAGCCATGGTCC-3′ to amplify a chloramphenicol resistance cassette from the pKD3 plasmid. The PCR product was inserted into a UPEC strain containing the pKD46 plasmid. Mutants were selected on MacConkey agar supplemented with chloramphenicol and confirmed by PCR using the detection primers CsgADT_F: 5′-TGCCAGTATTTCGCAAGGTG-3′ and CsgADT_R: 5′-TTGCTTCGTCTGACTTTGCC-3′. Further, the UPEC-4 csgA null mutant was phenotypically confirmed on Congo red agar plates,19 as the mutant cells did not present red color, a characteristic of the wild-type strain. So, the deletion of the curli gene in UPEC-4 was confirmed.
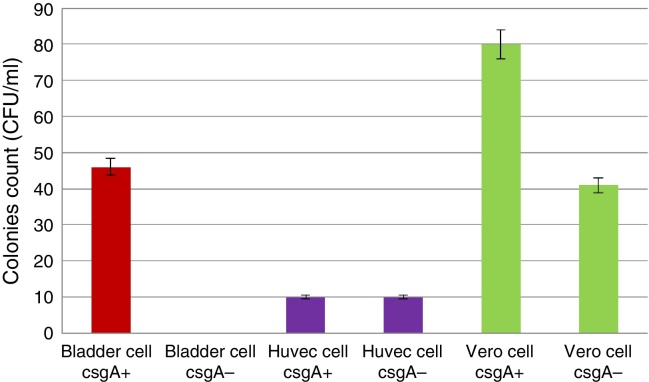
The wild-type UPEC-4 was able to promote its adhesion over HTB-9, HUVEC and Vero cells in the presence of d-mannose. However, the UPEC 4 mutant did not adhere to the HTB-9 cells but continued to adhere to the HUVEC and Vero cells in the presence of d-mannose (Fig. 1). These results suggest that the adherence observed was induced by curli fimbria and/or ompA, as d-mannose inhibits type 1 fimbriae. However, there are no literature reports of ompA being able to promote adhesion to HTB-9 cells or to HUVEC cells, but ompA does adhere to VERO cells.20 As HUVEC and Vero cells do not present receptors to curli fimbria, this adhesion of UPEC-4 in particular must have been promoted independently of curli fimbria, as it was genetically suppressed.
Fig. 1.
Quantitative adhesion of wild-type UPEC-4 (csgA+) and the mutant (csgA−) to HTB-9, HUVEC and Vero cells. Dilution 10−2.
These results suggest that curli fimbria is an adhesin factor of uropathogenic E. coli (UPEC) responsible for human cystitis.
Conflicts of interest
No authors have any conflicts of interest to declare.
Acknowledgments
The authors wish to thank Ana Stella Menegon Degrossoli for technical assistance and Luciano Moura Martins for helping with the English review. We are also grateful to CNPq (Conselho Nacional de Desenvolvimento e Tecnologico) for providing financial support.
Associate Editor: Waldir Pereira Elias Junior
References
- 1.Mol O., Oudega B. Molecular and structural aspects of fimbriae biosynthesis and assembly in Escherichia coli. FEMS Microbiol Rev. 1996;19:25–52. doi: 10.1111/j.1574-6976.1996.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 2.Féria C., Machado J., Correia J.D. Distribution of papG alleles among uropathogenic Escherichia coli isolated from different species. FEMS Microbiol Lett. 2001;202:205–208. doi: 10.1111/j.1574-6968.2001.tb10804.x. [DOI] [PubMed] [Google Scholar]
- 3.Mulvey M.A., Schilling J.D., Martinez J.J. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia M.I., Le Bouguénec C. Role of adhesion in pathogenicity of human uropathogenic and diarrhoeogenic Escherichia coli. Bull Inst Pasteur. 1996;94:201–236. [Google Scholar]
- 5.Mulvey M.A. Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol. 2002;4:257–271. doi: 10.1046/j.1462-5822.2002.00193.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanco M., Blanco J.E., Alonso M.P. Detection of pap, sfa and afa adhesin-encoding operons in uropathogenic Escherichia coli strains: relationship with expression of adhesins and production of toxins. Res Microbiol. 1997;148:745–755. doi: 10.1016/s0923-2508(97)82450-3. [DOI] [PubMed] [Google Scholar]
- 7.Connel I., Agace W., Klemm P. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wullt B., Bergsten G., Connell H. P fimbriae enhance the early establishment of Escherichia coli in the human urinary tract. Mol Microbiol. 2000;38:456–464. doi: 10.1046/j.1365-2958.2000.02165.x. [DOI] [PubMed] [Google Scholar]
- 9.Barnhart M.M., Chapman M.R. Curli biogenesis and function. Rev Microbiol. 2006;60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnqvist A., Olsén A., Pfeifer J. The Curli protein activates cryptic genes for curli formation and fibronectin binding in Escherichia coli HB101. Mol Microbiol. 1992;6:2443–2452. doi: 10.1111/j.1365-2958.1992.tb01420.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsén A., Arnqvist A., Hammar M. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993;7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 12.Sjöbring U., Pohl G., Olsén A. Plasminogen, absorbed by Escherichia coli expressing curli or by Salmonella enteritidis expressing thin aggregative fimbriae, can be activated by simultaneously captured tissue-type plasminogen activator (t-PA) Mol Microbiol. 1994;14:443–452. doi: 10.1111/j.1365-2958.1994.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 13.Hammar M., Arnqvist A., Bian Z. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol. 1995;18:661–670. doi: 10.1111/j.1365-2958.1995.mmi_18040661.x.. [DOI] [PubMed] [Google Scholar]
- 14.Olsén A., Jonsson A., Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 15.Wakimoto N., Nishi J., Sheikh J. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2004;71:687–690. [PubMed] [Google Scholar]
- 16.Scaletsky I.C.A., Silva M.L.M., Trabulsi L.R. Distinctive patterns of adherence of enterophatogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Döpfer D., Almeida R.A., Lam T.J. Adhesion and invasion of Escherichia coli from single and recurrent clinical cases of bovine mastitis in vitro. Vet Microbiol. 2000;74:331–343. doi: 10.1016/s0378-1135(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 18.Datsenko K.A., Wanner B.L. One-step inactivation of chromossomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma V.K., Bearson B.L. Hha controls Escherichia coli O157:H7 biofilm formation by differential regulation of global transcriptional regulators FlhDC and CsgD. Appl Environ Microbiol. 2013;79:2384–2396. doi: 10.1128/AEM.02998-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qinghai H., Xiangan H., Xiaojin Z. OmpA is a virulence factor of Riemerella anatipestifer. Vet Microbiol. 2011;150:278–283. doi: 10.1016/j.vetmic.2011.01.022. [DOI] [PubMed] [Google Scholar]