Abstract
Background
The AmpliVue™ Trichomonas Assay (Quidel) is a new FDA cleared rapid test for qualitative detection of Trichomonas vaginalis (TV) DNA in female vaginal specimens. The assay is based on BioHelix’s Helicase-Dependent Amplification (HDA) isothermal technology in conjunction with a disposable lateral-flow detection device, with a total turn-around time of approximately 45 minutes.
Objective
The objective of this study was to compare the performance of this new assay to wet preparation and culture, as well as to another FDA cleared nucleic acid amplification assay.
Methods
Four clinician collected vaginal swabs were obtained from women attending STD, family planning, and OB/GYN clinics and tested by AmpliVue™ Trichomonas Assay and comparator tests: saline microscopy, TV culture (InPouch™), and Aptima® TV (ATV). AmpliVue™ Trichomonas Assay results were compared to a composite positive comparator (CPC) as determined by the results from culture and/or wet mount microscopic examination. At least one of either the wet preparation or culture reference test results was required to be positive to establish CPC.
Results
A total of 992 patients, 342 symptomatic and 650 asymptomatic patients, were included in the study. Results for AmpliVue for all women combined compared to saline microscopy and culture as a composite positive comparator yielded a sensitivity of 100%. Specificity for all women was 98.2%. Overall percent agreement versus Aptima® TV was 97.8%. Sensitivity for AmpliVue compared to Aptima® was 90.7% %, while specificity was 98.9%.
Conclusions
The rapid AmpliVue™ Trichomonas Assay performed as well as microscopy and culture, and had comparable sensitivity and specificity to another NAAT for the detection of TV. This study provided evidence of new diagnostic options and indicated very good performance of amplified testing for detection of TV in symptomatic and asymptomatic women.
Keywords: Trichomonas vaginalis, trichomoniasis, Point-of Care Test, Rapid amplified diagnostic test, AmpliVue Trichomonas test
Introduction
Trichomoniasis is an infection caused by Trichomonas vaginalis (TV), a motile protozoan parasite. The World Health Organization estimates that there are 276.4 million new cases of trichomonas per year in adults between the ages of 15 and 49 in 2008.1 This sexually transmitted infection is the most prevalent nonviral sexually transmitted infection (STI), and is estimated to infect 3.7 million people in the United States.2 As such, these infections represent the most common curable STI in young, sexually active men and women.2
TV infections in women have been associated with poor reproductive outcomes such as low birth weight (LBW) and premature birth.3–5 In a cohort of over 13,000 women, there was an attributable risk of trichomonas associated with LBW in Blacks of 11% vs. 1.6% in Hispanics, and 1.5% in Whites.3 The National Health and Nutrition Examination Survey (NHANES) 2001–2004 estimated that 3.1% of women in the United States have TV.6 Data from the NHANES also demonstrated that TV was associated with other STIs among women in the U.S. population in a sample of 3,648 women, which represented a weighted sample of the experience of 65,563,298 women between the ages of 14 and 49 years.7 The prevalence of trichomoniasis in these women was 3.2% with over 80% of cases being asymptomatic. T. vaginalis infection is also associated with significantly increased risks of HIV acquisition (two to three-fold), and pelvic inflammatory disease (PID) among HIV-infected women.8–11 Older age and health disparities are also prominent features in trichomonas epidemiology, affecting over 11% of women age ≥40 years and 13% of black women in the United States.12,13 T. vaginalis infection is not a notifiable infection to the Centers for Disease Control and Prevention in the United States.
Because this infection is very common, and can be associated with such serious adverse events, diagnostic testing for TV and treatment of TV infections are recommended for symptomatic women and men. For asymptomatic individuals, screening is only encouraged for all HIV positive women (symptomatic an asymptomatic), women attending HIV clinics, and persons in such locations as sexually transmitted disease (STD) clinics and correctional facilities.14
The conventional methods to detect T. vaginalis in vaginal swabs are wet mount microscopy and culture techniques. Wet mount microscopy is the most common method of T. vaginalis detection, and although this technique is rapid and inexpensive, it is only about 36 to 75% sensitive compared to culture even in the hands of trained microscopists.15 This sensitivity of culture method is less in studies than what can be achieved by nucleic acid amplification tests (NAATs).16 Thus, the use of more highly sensitive molecular tests is recommended for T. vaginalis detection in symptomatic women and men, since they have higher sensitivity than culture. Among women, NAATs may detect a prevalence three to five-fold higher than wet preparation microscopy.4 Presently, there are two FDA cleared NAATs for the detection of trichomonas in women, the Aptima Trichomonas vaginalis assay (Hologic Gen-Probe, San Diego, CA)12 and the BD ProbeTec Qx Assay on the BD Viper System (Becton Dickinson, Sparks, Maryland).17
The objective of this study was to evaluate the performance of a new amplified molecular assay for the detection of T. vaginalis, the Isothermal Helicase-Dependent AmpliVue® Assay (Quidel, San Diego, CA).
Methods
Symptomatic women and asymptomatic women who were 14 years or older were enrolled from high and low prevalence clinics from five geographically diverse areas. The study was performed April to November 2014 at four locations in the United States and one location in Canada. Specimens were obtained from each subject after written informed consent was obtained. Inclusion Criteria included women who presented with symptoms of T. vaginalis and asymptomatic women who were scheduled for a pelvic examination or screening for T. vaginalis. Patient age or date of birth was obtained.
The study was conducted in accord with the Health Insurance Portability and Accountability Act (HIPAA) and approved by the associated Institutional Review Boards.
Laboratory Studies
Four clinician collected vaginal swabs were obtained and tested from women attending STD, family planning, and OB/GYN clinics. Swabs 1 and 2 were randomized for saline microscopy (wet mount) and culture (InPouch™ TV, BioMed Diagnostics, Inc., White City, Oregon). The saline microscopy was performed by the clinician immediately after the swab collection to examine for motile trichomonads under the microscopy as prescribed by local clinic protocols. Culture pouches were observed by laboratorians daily for 5 reads. Swab 3 was tested by the AmpliVue Trichomonas assay in participating laboratories. Swab 4 was tested by a FDA cleared NAAT according to manufacturer’s instructions (Aptima TV, Gen-Probe/Hologic, San Diego, CA). The AmpliVue Trichomonas Assay results were first compared to a composite positive comparator (CPC) as determined by the results from saline microscopic examination and/or culture comparator tests. At least one of these two assays was required to be positive to establish a CPC. Results of the AmpliVue Trichomonas were also compared to the NAAT.
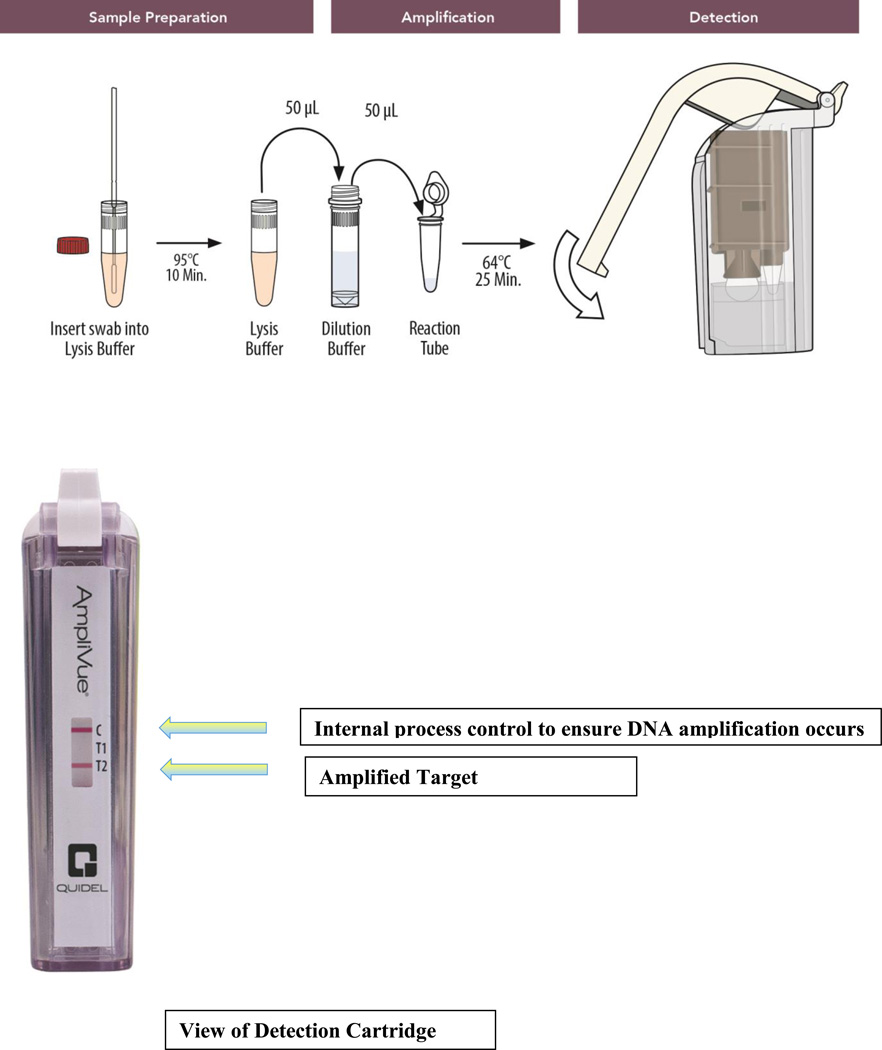
To detect T. vaginalis directly from vaginal swab specimens in symptomatic and asymptomatic women, the assay targets a conserved repeat sequence of the T. vaginalis DNA. The technology utilized isothermal helicase dependent amplification (HDA), which uses a helicase to separate DNA. With a total turn-around time of approximately 45 minutes, the assay combines three steps: 1) sample preparation with one-step dilution/heating in a small heat block for lysis for 10 min at 95°C (Figure 1); 2) isothermal DNA amplification of target sequences specific to T. vaginalis in a small heat block for 25 minutes at 64°C by HDA; and 3) lateral-flow strip based colorimetric detection in a self-contained, disposable device (Figure 1).
Figure 1.
Schematic of the AmpliVue Trichomonas vaginalis assay.
Quality Control
The AmpliVue™ Trichomonas Assay incorporated several controls to monitor assay performance: 1) The internal control was used to detect HDA inhibitory specimens and to confirm the integrity of assay reagents and cassette detection. The internal control was included in the reaction tubes. 2) The External assay positive control, provided separately, was used to confirm the ability of the assay to detect T. vaginalis DNA and was intended to monitor substantial reagent and cassette failure. 3) The external assay negative control was used to detect reagent or environment contamination or carry-over by either T. vaginalis DNA or amplicons. Laboratory-grade water was used to set up the external negative control assays and was treated as a patient specimen.
Quality Control testing was conducted in accordance with the Instructions for Use. The reactivity of each new lot and each new shipment of the AmpliVue™ Trichomonas Assay was verified on receipt and before use. On each testing day, at least one study technician from each laboratory tested all lots of AmpliVue™ Trichomonas Assays to be used in that day by setting up positive and negative control assays with AmpliVue™ Trichomonas external positive controls and laboratory-grade water.
Statistical analysis was performed by SAS version 9, (Cary, NC). We calculated sensitivity, specificity, positive predictive and negative predictive values, percent agreement, and a kappa statistic.
Results
Clinician-collected vaginal swab specimens were obtained from asymptomatic (n=650) or symptomatic (n=342) women (total 992) These were tested by the composite positive comparator methods and the AmpliVue® Trichomonas Assay, as well as the Aptima assay. Table 1 shows the ages and the numbers of asymptomatic and symptomatic women enrolled by site. The prevalence of T. vaginalis by absence or presence of symptoms was asymptomatic - 9.4%, symptomatic - 17.3% and for all women - 12.1%. Of the patients enrolled, 8 specimens generated invalid results upon initial testing with the AmpliVue® Trichomonas Assay (0.8%). These specimens were re-tested according to the instructions provided in the package insert. Six (6) of these specimens generated valid results upon re-testing (5 negative and 1 positive result), and two (2) specimens generated a second invalid result (0.2%). Table 2 shows the sensitivity, specificity, PPV, and NPV (100%, 98.2%, 88.2%, and 100%, respectively) of the AmpliVue® Trichomonas Assay compared to the composite reference methods of saline microscopy/culture (CPC).
Table 1.
Age and symptomatic status of women in the AmpliVue trial for the detection of Trichomonas vaginalis in vaginal specimens.
| Age | Total | Asymptomatic | Symptomatic |
|---|---|---|---|
| 14–19 | 64 (6.5%) | 38 (5.9%) | 26 (7.6%) |
| 20–24 | 263 (26.6%) | 157 (24.2%) | 106 (31.0%) |
| 25–29 | 179 (18.1%) | 104 (16.0%) | 75 (31.9%) |
| ≥30 | 484 (48.9%) | 349 (53.9%) | 135 (39.5%) |
| Total women | 990 | 648 | 342 |
| UAB | 296 (29.9%) | 133 (44.9%) | 163 (55.1%) |
| JHU | 115 (11.6%) | 46 (40%) | 69 (60%) |
| UW | 248 (25.1%) | 207 (83.5%) | 41 (16.5%) |
| MU | 295 (29.8%) | 260 (88.1%) | 35 (11.9%) |
| UNC | 36 (3.6%) | 2 (5.6%) | 34 (94.4%) |
UAB, University of Alabama at Birmingham; JHU, Johns Hopkins University, UW, University of Washington, MU, McMaster University; UNC, University of North Carolina.
Table 2.
Comparison of Amplivue results in symptomatic and asymptomatic women to composite reference method of wet preparation and cullture. (TP, true positive; FP, false positive; TN, true negative; FN, false negative).
| Symptom Status |
N | TP | FP | TN | FN | Prevalence % |
Sensitivity% (95% CI) |
Specificity% (95% CI) |
PPV % (95% CI) |
NPV % (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Asymptomatic women |
648 | 61 | 10 | 577 | 0 | 9.4 | 100 (94.1 to 100) |
98.3 (96.9 to 99.1) |
85.9 (76.0 to 92.2) |
100 (99.3 to 100) |
| Symptomatic women |
342 | 59 | 6 | 277 | 0 | 17.3 | 100 (93.9 to 100) |
97.9 (95.5 to 99.0) |
90.8 (81.3 to 95.7) |
100 (98.6 to 100) |
| All women | 990 | 120 | 16 | 854 | 0 | 12.1 | 100 (96.9 to 100) |
98.2 (97.0 to 98.9) |
88.2 (81.7 to 92.6) |
100 (99.6 to 100) |
Overall percent agreement versus Aptima TV was 97.8% (Cohen’s kappa= 90.7); 97.1% for symptomatic women; 97.7% for asymptomatic women. There were 16 discordant specimens (AmpliVue Positive/Composite Reference Method Negative), eight (8) of these specimens were positive by the FDA-cleared Aptima TV molecular assay. Sensitivity for AmpliVue compared to Aptima was 90.7% overall (90.1% for symptomatic and 87.2% for asymptomatic women) (Table 3).
Table 3.
Comparison of Amplivue results compared to individual comparator methods of wet preparation, culture, and Aptima TV.
| Saline Microscopy (wet mount) | TV culture (InPouch™) | Aptima TV (ATV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | # positive/ # total |
95% CI | % | # positive/ # total |
95% CI | % | # positive/ # total |
95% CI | ||||
| Sensitivity | 100 | (65/65) | 94.4 | 100 | 100 | (120/120) | 96.9 | 100 | 90.7 | (127/140) | 84.8 | 94.5 |
| Specificity | 92.3 | (854/925) | 90.4 | 93.9 | 98.2 | (854/870) | 97 | 98.9 | 98.9 | (841/850) | 98.0 | 99.4 |
| PPV | 47.8 | (65/135) | 39.6 | 56.1 | 88.2 | (120/136) | 81.7 | 92.6 | 93.4 | (127/136) | 87.9 | 96.5 |
| NPV | 100 | (854/854) | 99.6 | 100 | 100 | (854/854) | 99.6 | 100 | 98.5 | (841/854) | 97.4 | 99.1 |
| % agreement |
92.8 | (919/990) | 91.1 | 94.3 | 98.4 | (974/990) | 97.4 | 99.0 | 97.8 | (968/990) | 96.7 | 98.5 |
| Cohen's Kappa |
61.2 | 92.8 | 90.7 | |||||||||
Discussion
AmpliVue TV showed high sensitivity (100%) and specificity (98.2%) compared to the standard tests of saline microscopy and culture, regardless of symptoms. Additionally, AmpliVue TV demonstrated high agreement compared to Aptima TV with an overall agreement of 97.8%. Although there are now two FDA cleared NAAT assays for trichomonas, and it is generally accepted that NAATs for TV are more sensitive than culture, the reference standard set by the FDA for this study trial was saline microscopy and culture, which we refer to as the composite positive comparator (CPC). The single NAAT selected for additional comparison was performed over and above the input from the FDA with this fact in mind. Thus the study deign and the CPC used may have impacted the original point estimates. By also comparing the results of the AmpliVue to another NAAT assay, we believe we have also compared the assay to a higher standard than that of culture and saline microscopy. However, we were not able to ascertain whether the AmpliVue assay would be more accurate than the OSOM assay16 since it was studied in this trial.
Although the rapid AmpliVue assay appeared to perform better than saline microscopy/culture by detecting more positive results than the defined CPC, only 8 of the 16 discordant samples were confirmed as positive when a “tie-breaker” NAAT assay was performed, suggesting that half of the discordant AmpliVue specimens may have been false positives. Alternatively, the 8 of the 16 discordant samples that were positive by a NAAT assay would improve the specificity, if these data were included in the calculation of the specificity of the AmpliVue test. However, a limitation of the study design was that the swab for the Aptima TV was obtained as the 4th swab. Randomization of the last two swabs was not performed and may have contributed to the inability to confirm the AmpliVue positive results for the 8 samples that were Aptima TV negative.
There are additional limitations to the interpretation of our study with regard to interpretation of results. The use of saline microscopy and culture as the reference standard may have artificially inflated the point estimates of sensitivity and specificity; thus they may have been lower if the NAAT assay was also used in the composite reference methods. There may also have been delays in incubating cultures and reading saline microscopy tests, transportation delays or variability in interpretation of the results of these comparator assays, both of which are subjective in nature. The possibility of false positives by the NAAT test cannot be ruled out, but they are highly unlikely since the platform is a closed and robotic platform, which can eliminate cross contamination by a laboratorian.
This rapid assay demonstrated that an amplified molecular assay can perform as well as saline microscopy/culture and provided comparable results as another FDA cleared NAAT assay while yielding results in real time. This study provided evidence of new diagnostic options and indicates the performance of a new amplified testing method for detection of trichomoniasis in symptomatic and asymptomatic women in 45 minutes, demonstrating rapid turn-around-time. The AmpliVue Trichomonas Assay identified all of the culture and saline microscopy positive cases of trichomonas infections and substantially more than culture saline microscopy, as shown by detecting additional confirmed positives and the strong agreement with Aptima TV NAAT assay. It is now FDA cleared as moderately complex, but its potential to be CLIA waived in the future may increase its usefulness as a point-of-care (POC) test that can be used for treatment of patients before they leave the clinic. It appeared to yield a similar sensitivity in this study to another rapid, commercially available unamplified antigen trichomonas assay, when the confidence intervals are considered.16 The sensitivity for trichomonas compared to what is achieved in the trichomonas component of the Affirm vaginitis test is also much higher.18–19
As more data accumulate for the association of trichomonas infections with adverse events, such as HIV transmission and perinatal morbidity, and as new testing methods become more widely available, it seems prudent to advocate for more public health recognition with more testing for trichomonas and further modeling studies of its potential treatment impact to improve health outcomes.11,20–25
In summary, the rapid AmpliVue® Trichomonas Assay showed a high sensitivity and specificity in both asymptomatic (100%/98.3%) and symptomatic (100%/97.9%) women in 45 minutes of test time, when compared to the composite reference method that included saline microscopy and InPouch TV culture.
Acknowledgments
This study was funded by Quidel, San Diego, CA; CG also received funding from U54EB007958, NIBIB, NIH; U-01068613-01, NIH, NIAID
References
- 1.World Health Organization DoRhaR. Geneva: WHO; 2012. [accessed 09-28-15]. Global Incidence and prevalence of selected curabe sexually transmitted infections-2008; pp. 1–20. [Google Scholar]
- 2.Satterwhite CL, Torrone E, Meites E, Dunn EF, Mahajan R, Ocfemia NC, Su J, Zu F, Weinstock H. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2008. Sex Transmit Dis. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 3.Cotch MF, Pastorek JG, Nugent RP, et al. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex Tansmit Dis. 1997;24:353–360. doi: 10.1097/00007435-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Review. 2004;17:794–803. doi: 10.1128/CMR.17.4.794-803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silver BJ, Guy R, Kaldor JM, Jamil MS, Rumbold AR. Trichomonas vaginalis as a causse of perinatal morbidity: A systematic review and meta-analysis. Sex Transmit Dis. 2014;41:369–376. doi: 10.1097/OLQ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 6.Sutton M, Sternberg M, EH K, McQuillan G, Berman S, Markowitz L. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Inf Dis. 2007;45:1319–1326. doi: 10.1086/522532. [DOI] [PubMed] [Google Scholar]
- 7.Allsworth JE, Ratner JA, Peipert JF. Trichomonas and other sexually transmitted infections: Results from the 2001–2004 National Health and Nutrition Examination Surveys. Sex Tansmit Dis. 2009;36:738–744. doi: 10.1097/OLQ.0b013e3181b38a4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Pol B, Kwok C, Pierre-Louis B, et al. Trichomonas vaginalis Infection and Human Immunodeficiency Virus Acquisition in African Women. J Infect Dis. 2008;197:548–554. doi: 10.1086/526496. [DOI] [PubMed] [Google Scholar]
- 9.Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with Human Immunodeficiency Virus. Clin Infect Dis. 2002;34:519–522. doi: 10.1086/338399. [DOI] [PubMed] [Google Scholar]
- 10.Hughes J, Baeten J, Lingappa J, et al. Baeten, JMDLingappa, JReterminants of per-coital act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissinger P, Adamski A. Trichomoniasis and HIV interactions: a review. Sex Transmit. 2013;89:426–433. doi: 10.1136/sextrans-2012-051005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwebke JR, Hobbs M, Taylor SN, Sena AC, Catania MG, Weinbaum BS, Johnson AD, Getman DH, Gaydos CA. Molecular testing for Trichomonas vaginalis in women: Results of a pivotal US clinical trial. J Clin Microbiol. 2011;49:4106–4111. doi: 10.1128/JCM.01291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginocchio CC, Chapin K, Smith JS, Aslanzadeh J, Snook J, Hill CS, Gaydos CA. Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J Clin Microbiol. 2012;50:2601–2608. doi: 10.1128/JCM.00748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Workowski KA, Bolan A. Sexually Transmitted Diseases Treatment Guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-3):1–137. [PMC free article] [PubMed] [Google Scholar]
- 15.Nye MB, Schwebke JR, Body BA. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am J Obstet Gyneco. 2009;200:188.e1–188.e7. doi: 10.1016/j.ajog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Huppert JS, Mortensen JE, Reed JL, et al. Rapid Antigen Testing Compares Favorably with Transcription-Mediated Amplification Assay for the Detection of Trichomonas vaginalis in Young Women. Clin Infect Dis. 2007;45:194–198. doi: 10.1086/518851. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Pol B, Williams JA, Eddleman L, Fuller D, Taylor S, Schwebke J, Lebed J, Smith B, Nye M, Gaydos C. Evaluation of a New Amplified DNA Assay on the Becton Dickinson Viper System in Extracted Mode for the Detection of Trichomonas vaginalis from Vaginal Specimens. Sex Transmit Infect. 2013;89S:A464. [Google Scholar]
- 18.Briselden AM, Hillier SL. Evaluation of Affirm VP microbial identification test for Gardnerella vaginalis and Trichomonas vaginalis. J Clin Microbiol. 1994;32:148–152. doi: 10.1128/jcm.32.1.148-152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cartwright CP, Lambke BD, Ramachandran K, et al. Comparison of nucleic acid amplification assays with BD Affirm VPIII for diagnosis of vaginitis in symptomatic women. J Clin Microbiol. 2013;51:3694–3699. doi: 10.1128/JCM.01537-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silver BJ, Guy RJ, Kaldor JM, Jamil M, Rumbold AR. Trichomonas vaginalis as a Cause of Perinatal Morbidity: A Systematic Review and Meta-Analysis. Sex Transmit Dis. 2015;41:369,376. doi: 10.1097/OLQ.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 21.Anderson BL, Firnhaber C, Liu T, et al. Effect of trichomoniasis therapy on genital HIV burden among African women. Sex Transm Dis. 2012;39:638–642. doi: 10.1097/OLQ.0b013e31825725ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesson HW, Blandford JM, Pinkerton SD. Estimates of the annual number and cost of new HIV infections among women attributable to trichomoniasis in the United States. Sex Transm Dis. 2004;31:547–551. doi: 10.1097/01.olq.0000137900.63660.98. [DOI] [PubMed] [Google Scholar]
- 23.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36:11–16. doi: 10.1097/OLQ.0b013e318186decf. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlivan EB, Patel SN, Grodensky CA, Golin CE, Tien HC, Hobbs MM. Modeling the impact of Trichomonas vaginalis on HIV transmission in HIV-infected individuals in medical care. Sex Transm Dis. 2012;39:671–677. doi: 10.1097/OLQ.0b013e3182593839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trintis J, Epie N, Boss R, Riedel S. Neonatal Trichomonas vaginalis infection: a case report and review of literature. International Journal of STD & AIDS. 2010;21:606–607. doi: 10.1258/ijsa.2010.010174. [DOI] [PubMed] [Google Scholar]