Abstract
Colorectal cancer had a low incidence several decades ago. However, it has become a predominant cancer and now accounts for approximately 10% of cancer-related mortality in western countries. The ‘rise’ of colorectal cancer in developed countries can be attributed to the increasingly ageing population, unfavourable modern dietary habits and an increase in risk factors such as smoking, low physical exercise and obesity. New treatments for primary and metastatic colorectal cancer have emerged, providing additional options for patients; these treatments include laparoscopic surgery for primary disease, more-aggressive resection of metastatic disease (such as liver and pulmonary metastases), radiotherapy for rectal cancer and neoadjuvant and palliative chemotherapies. However, these new treatment options have had limited impact on cure rates and long-term survival. For these reasons, and the recognition that colorectal cancer is long preceded by a polypoid precursor, screening programmes have gained momentum. This Primer provides an overview of the current state of art knowledge on the epidemiology and mechanisms of colorectal cancer, as well as on diagnosis and treatment.
Introduction
We live in an era with improved worldwide average living standards and increased access to adequate healthcare that has considerably improved the diagnosis and treatment of diseases. These measures have had an impact on average life expectancy in most regions of the world. However, although death rates from communicable diseases have improved globally as a result of these medical improvements, cancer-related mortality has increased by almost 40% over the past 40 years. A further 60% increase is expected in the coming 15 years, with 13 million people estimated to die of cancer in 2030 1. The main causes of cancer-related mortality have also changed, attributable to alterations in disease incidence, introduction of screening programmes and therapeutic improvements. Colorectal cancer was rather rare in 1950, but has become a predominant cancer in Western countries, now accounting for approximately 10% of cancer-related mortality. Reasons explaining this increased incidence include population ageing and the preponderance of poor dietary habits, smoking, low physical activity and obesity in western countries. The change in incidence is not only apparent in the rates of sporadic disease, but also in some familial cancer syndromes. Indeed, given that rates of Helicobacter pylori infection (a causative factor of gastric cancer) have fallen dramatically, colorectal cancer is now the predominant presentation of Lynch syndrome (a hereditary non-polyposis type of colorectal cancer), whereas carriers of this syndrome used to be predominantly affected by gastric cancer 2–4.
New treatments for primary and metastatic colorectal cancer have been developed and include laparoscopic surgery for primary disease; resection of metastatic disease affecting, for example, the liver and lungs; radiotherapy for rectal cancer and some forms of metastatic disease; and neoadjuvant and palliative chemotherapy5–7. Despite advances in surgical and medical therapies, cure rates and long-term survival have changed little in the past several decades. Against this background, and given that colorectal cancer is preceded by a polypoid precursor (Figure 1), screening programmes for early detection have gained momentum.
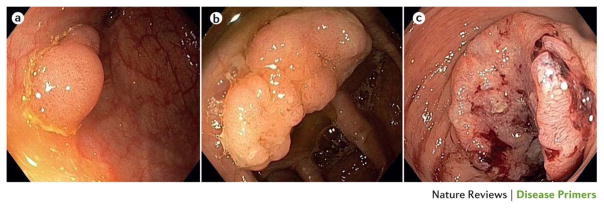
Figure 1. Colorectal neoplasia at different stages.
(a) A small sessile adenoma. (b) An advanced, larger sessile adenoma. (c) A large, dish-shaped, ulcerating sigmoid carcinoma. The tumour covers most of the circumference, but has not yet led to substantial obstruction of the lumen.
Indeed, screening is expected to have a major impact on colorectal cancer incidence and mortality in the next 15 years, an effect that is unlikely to come from lifestyle interventions or from new therapeutics. Screening will only make these improvements with high uptake; accordingly, major improvements in noninvasive screening (for example, faecal immunochemical testing and faecal DNA testing) are being investigated as alternatives to the current gold standard, but invasive, screening methodology — colonoscopy. Alongside these advances, the quality of screening colonoscopy has undergone substantial improvement in terms of technical changes and training, and quality assurance8,9.
In this Primer, we provide an overview of the current knowledge on epidemiology and mechanisms underlying colorectal cancer, as well as on diagnosis and treatment, including surgical and medical approaches.
Epidemiology
Colorectal cancer is the second- and third-most common cancer in women and men, respectively. In 2012, 614,000 women (9.2% of all new cancer cases) and 746,000 men (10.0% of new cancer cases) were diagnosed with colorectal cancer worldwide10. Combined, in both sexes, colorectal cancer is the third-most common cancer and accounts for 9.7% of all cancers excluding non-melanoma skin cancer. More than half of the cases occur in more-developed regions of world. The age-standardized incidence rate (ASRi) of colorectal cancer is higher in men (20.6 per 100,000 individuals) than in women (14.3 per 100,000). The majority of patients with sporadic cancer are >50 years of age, with 75% of patients with rectal cancer and 80% of patients with colon cancer patients being ≥60 years of age at the time of diagnosis.
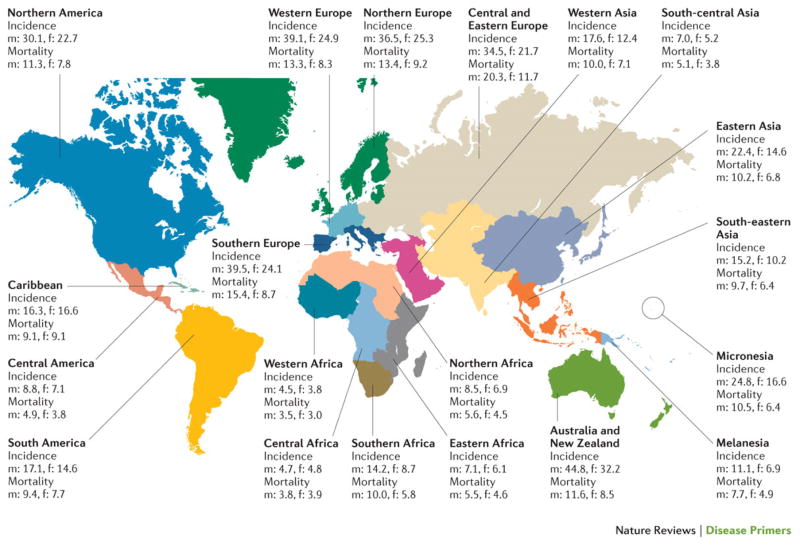
Incidence varies geographically, with the highest incidence in Australia and New Zealand (ASRi 44.8 and 32.2 per 100,000 men and women, respectively), whereas Western Africa (ASRi 4.5 and 3.8 per 100,000) has the lowest incidence (Figure 2). More-developed regions (Europe, Northern America, Australia, New Zealand and Japan; combined ASRi 29.2 per 100,000) have a higher incidence than less-developed regions (all regions of Africa, Asia (excluding Japan), Latin America and the Caribbean, Melanesia, Micronesia and Polynesia; ASRi 11.7 per 100,000) 10. The seven world regions can be ranked according to increasing ASRi, from Africa (6.3 per 100,000), Asia (13.7 per 100,000), Latin America and Caribbean (14.0 per 100,000), Micronesia/Polynesia (15.0 per 100,000), North America (26.1 per 100,000), Europe (29.5 per 100,000), to Oceania (34.8 per 100,000) 10. Within each of these regions, the ASRi can show marked variation. In Europe, Albania (8.4 per 100,000) and Ukraine (23.4 per 100,000) have a lower incidence, whereas Slovakia (42.7 per 100,000), Hungary (42.3 per 100,000) and Denmark (40.5 per 100,000) have a high incidence. Asia has the greatest diversity with regard to the ASRi of colorectal cancer. The incidence is high in Korea (45.0 per 100 000), Singapore (33.7 per 100,000) and Japan (32.2 per 100,000), but much lower in Nepal (3.2 per 100,000), Bhutan (3.5 per 100,000) and India (6.1 per 100,000). These variations are associated with different socioeconomic levels11.
Figure 2. The age-standardized incidence and mortality rates in men (m) and women (f) (per 100.000 people) across geographic zones10.
Rates are consistently higher in men than in women, and vary considerably between regions. Highest rates occur in Australia and New Zealand, Europe and North America.
In 2013, 771,000 people died as a result of colorectal cancer globally12, making the disease the fourth most common cause of cancer-related death worldwide after lung, liver and stomach cancer12. The age-standardized mortality rate (ASRm) of colorectal cancer in different countries reflects disease incidence, which explains why the ASRm is higher in men (10.0 per 100,000) than in women (6.9 per 100,000). Mortality also depends on the stage distribution at diagnosis, which is influenced by the availability of a population-screening programme and by the level of care in each country. The ASRm is almost two-fold higher in more-developed regions (11.6 per 100,000) than in less-developed regions (6.6 per 100,000). The ASRm in both sexes ranged from 3.3 per 100,000 people in Western Africa to 14.9 per 100,000 people in Central and Eastern Europe; in men, this value ranged from 3.5 per 100,000 people in Western Africa to 20.3 in Central and Eastern Europe, whereas in women, ASRm ranged from 3.0 per 100,000 people in Western Africa to 11.7 per 100,000 people in Central and Eastern Europe. That is, Western Africa showed the lowest age-standardized mortality in the world and Central and Eastern Europe exhibited the highest mortality in the world, in both men and women. Worldwide, mortality due to colorectal cancer has increased with 57% between 1990 and 2013 12. Since the 1980s, in several countries in Europe, North America and Asia, mortality has tended to decrease. This decrease might be attributable to the introduction of colonoscopy, which has improved detection and treatment of early lesions.
Risk factors
Both genetic and environmental factors play an important part in the aetiology of colorectal cancer. The majority of colorectal cancers are sporadic; approximately three-quarters of patients have a negative family history. In most Western populations, the average lifetime risk for colorectal cancer is in the range of 3–5%. However, this risk almost doubles in individuals with a first-degree family member with colorectal cancer who was diagnosed at 50–70 years of age; the risk triples if the first-degree relative was <50 years of age at diagnosis. Risk further increases in individuals who have two or more affected family members. For sporadic colorectal cancer, this increased risk in the presence of affected family at least in part reflects low-penetrance genetic factors. Accordingly, positive family history has a role in approximately 15–20% of patients with colorectal cancer.
Indeed, a specific subgroup of the patient population is formed by those affected by a hereditary colorectal cancer syndrome, accounting for 5–10% of all patients. The most common syndrome in this category is Lynch syndrome. This syndrome is caused by a mutation in one of the DNA mismatch-repair genes: MLH1, MSH2, MSH6, PMS2 or EPCAM. Impaired mismatch repair during replication gives rise to accumulation of DNA mutations, which occur, in particular, in microsatellite DNA fragments with repetitive nucleotide sequence. This microsatellite instability (MSI) can be identified by means of polymerase chain reaction (PCR) testing, which compares normal and tumour DNA of the same patient. Patients with Lynch syndrome used to be identified by means of clinicopathological criteria, such as the Amsterdam and Bethesda criteria4,13. However, clinical practice is shifting towards unrestricted testing of tumour material of all patients diagnosed before the age of 70 years by means of MSI PCR and immunohistochemistry for lack of expression of specific mismatch-repair proteins14,15.
The second most common hereditary colorectal cancer syndrome is familial adenomatous polyposis. This syndrome is caused by mutations in the adenomatous polyposis coli (APC) gene, which controls activity of the Wnt signalling pathway4. Most patients with familial adenomatous polyposis develop very large numbers of colorectal adenomas and subsequent colorectal cancer at a young age. Other hereditary colorectal cancer syndromes are polyposis associated with mutations in the mutY DNA glycosylase (MUTYH) gene, Peutz Jeghers syndrome, serrated polyposis and juvenile polyposis; the diagnosis and management of which have been discussed elsewhere4.
Chronic colitis due to inflammatory bowel disease (IBD) is also associated with increased risk of colorectal cancer. This risk increases with longer duration of IBD16. IBD explains only 1% of colorectal cancers in western populations, and a range of studies suggest that the incidence of colorectal cancer in those with IBD is decreasing because of effective anti-inflammatory treatments and improved surveillance17,18, although this observation is not yet unanimous19.
A range of environmental — largely modifiable — lifestyle factors influence the risk of developing colorectal cancer. The risk is increased by smoking, alcohol intake and increased body weight. With each unit increase of the body mass index, the risk for colorectal cancer increases by 2–3% 20. In close conjunction, patients with type 2 diabetes mellitus also have an increased risk for colorectal cancer21. Moderate alcohol consumption (2–3 units per day) has been estimated to increase risk by 20%, whereas even higher alcohol consumption is associated with an up to 50% increased risk22. Prolonged heavy smoking has an effect of similar magnitude23,24. Intake of red meat and processed meat increases colorectal cancer risk by an estimated 1.16-fold per 100 g increase of daily intake25. By contrast, consumption of milk, whole grains, fresh fruits and vegetables, as well as intake of calcium, fibre, multivitamins and vitamin D, decrease risk. The decrease of risk is estimated to approximate 10% per daily intake of every 10 g fiber, 300 mg calcium or 200 ml milk 25,26. Daily physical activity for 30 minutes has a similar magnitude of effect 20,27. Low-dose aspirin has also been associated with decreased risk of colorectal cancer28.
The prevalence of these modifiable lifestyle factors can explain, to a considerable extent, the geographic and socioeconomic differences in colorectal cancer incidence29. Several studies have estimated that 16–71% of colorectal cancers in Europe and the United States are attributable to lifestyle factors30–32. Any benefit from lifestyle changes can be augmented by regular intake of aspirin and other nonsteroidal anti-inflammatory drugs33; however, this effect seems to depend on host genotype34,35. Statin use might have a small preventive effect on colorectal cancer incidence36,37, as does hormone therapy in post-menopausal women38.
The variety of environmental factors that influence colorectal carcinogenesis is likely reflected in the heterogeneity of colorectal cancer, and has stimulated research into the so-called field of ‘molecular pathological epidemiology’, which focuses on the correlation between environmental and genetic factors, and between molecular tumour characteristics and disease progression39. Further research into the correlation between colonic microbiota and colorectal cancer will likely provide further insights (see below).
Mechanisms/pathophysiology
The environmental and genetic factors that cause colorectal cancer do so by promoting the acquisition of hallmark behaviours of cancer (Box 1) in colon epithelial cells40,41. One way these hallmark cancer traits are acquired is through the progressive accumulation of genetic and epigenetic alterations that activate oncogenes and inactivate tumour suppressor genes. The loss of genomic and/or epigenomic stability has been observed in the majority of early neoplastic lesions in the colon (namely, aberrant crypt foci, adenomas and serrated polyps) and is likely a central molecular and pathophysiological event in the initiation and formation of colorectal cancer42,43. The loss of genomic and epigenomic stability accelerates the accumulation of mutations and epigenetic alterations in tumour suppressor genes and oncogenes, which drive the malignant transformation of colon cells through rounds of clonal expansion that select for those cells with the most aggressive and malignant behaviour44–46. A prevailing paradigm is that the cell of origin of most colorectal cancers is a stem cell or stem cell-like cell that resides in the base of the colon crypts47. In this model, mutations in oncogenes and tumour suppressor genes in these cells lead to the formation of cancer stem cells, which are essential for the initiation and maintenance of a tumour.
Box 1. The hallmarks of cancer40,41.
Avoiding immune destruction: immune suppression in tumour microenvironment by induction of local cytokines
Evading growth suppressors: mutation and downregulation of growth-inhibiting factors and their receptors
Genome instability and mutation: inactivation of DNA repair mechanisms
Enabling replicative immortality: inhibition of mechanisms that induce senescence and induction of telomerase activity
Deregulating cellular energetics: aerobic glycolysis (Warburg phenomenon) and glutaminolysis
Tumour-promoting inflammation: induction of growth-promoting and angiogenesis-promoting factors by secreted proteins made by local inflammatory cells
Inducing angiogenesis: induction of the formation of new blood vessels
Resisting cell death: escape from autonomous and paracrine mediators of apoptosis and other forms of cell death (necrosis, necroptosis)
Activating invasion and metastasis: remodelling of extracellular matrix to promote cell motility and induction of epithelial–mesenchymal transition
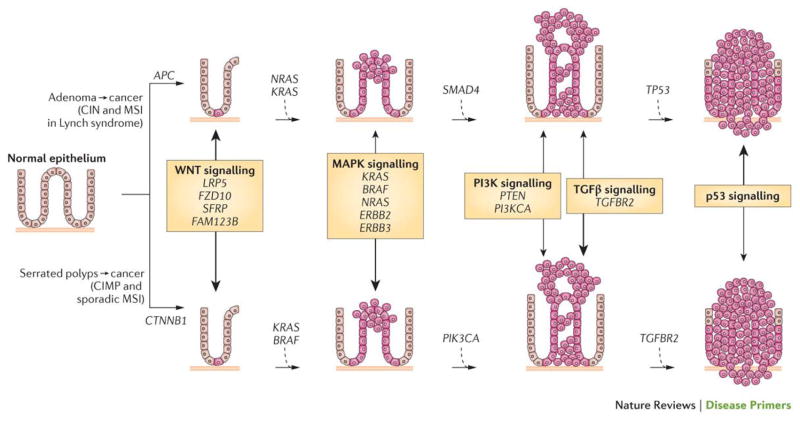
In the colon, the evolution of normal epithelial cells to adenocarcinoma by and large follows a predictable progression of histological and concurrent epigenetic and genetic changes (Figure 3). In the ‘classic’ colorectal cancer formation model, the vast majority of cancers arise from a polyp beginning with an aberrant crypt, which then evolves into an early adenoma (<1 cm in size, with tubular or tubulovillous histology). The adenoma then progresses to an advanced adenoma (>1cm in size, and/or with villous histology) before finally becoming a colorectal cancer. This process is driven by accumulation of mutations and epigenetic alterations and takes 10–15 years to occur but can progress more rapidly in certain settings (for example, in patients with Lynch syndrome)48. Notably, although the histology of conventional tubular adenomas is fairly homogeneous, the molecular biology of these polyps are heterogeneous, which might explain why some adenomas progress to colorectal cancer (approximately 10% of polyps) and some do not49,50.
Figure 3. The polyp to colorectal cancer sequences.
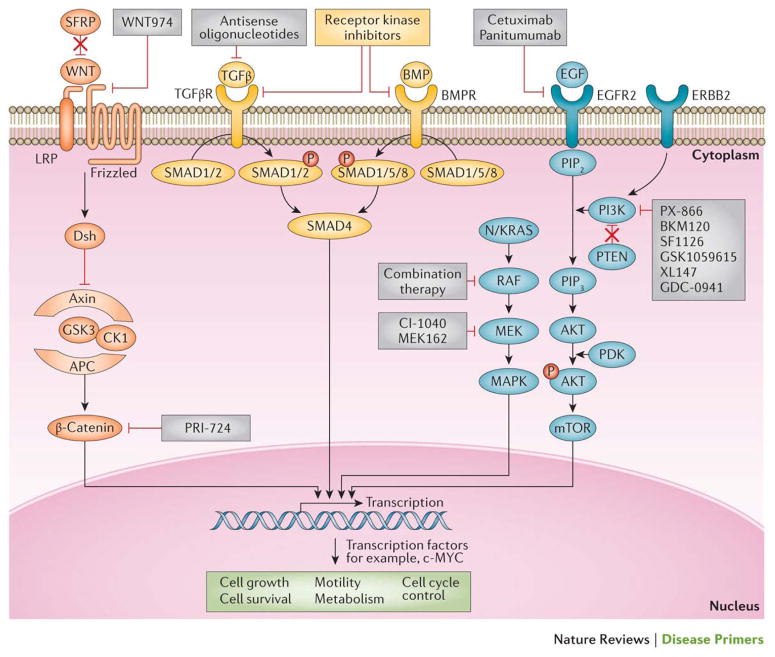
Currently, two discrete normal colon to colorectal cancer sequences have been identified. Both sequences involve the progression of normal colon epithelial cells to aberrant crypt foci, followed by early and advanced polyps with subsequent progression to early cancer and then advanced cancer. The ‘classic’ or traditional pathway (top) involves the development of tubular adenomas that can progress to adenocarcinomas. An alternate pathway (bottom) involves serrated polyps and their progression to serrated colorectal cancer has been described in the last 5–10 years. The genes mutated or epigenetically altered are indicated in each sequence; some genes are shared between the two pathways whereas others are unique (for example, BRAF mutations and CpG Island Methylator Phenotype (CIMP) only occur in the serrated pathway). The signalling pathways deregulated during the progression sequence are also shown, with the width of the arrow reflecting the significance of the signalling pathway in tumour formation.
APC, adenomatous polyposis coli; CIN, chromosomal instability; CTNNB1, catenin-β1; FAM123B, family with sequence similarity 123B (also known as AMER1); FZD10, frizzled class receptor 10; LRP5, low-density lipoprotein receptor-related protein 5; MAPK, mitogen-activated protein kinase; MSI, microsatellite instability; PI3K, phosphatidylinositol 3-kinase; PI3KCA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α; PTEN, phosphatase and tensin homologue; SFRP, secreted frizzled-related protein; SMAD4, SMAD family member 4; TGFβ, transforming growth factor-β; TGFBR2, TGFβ _receptor 2.
Figure adapted from 229, Nature Publishing Group.
Until 5–10 years ago tubular and tubulovillous adenomatous polyps were thought to be the only lesions capable of progressing to cancer. However, some colorectal cancers have been shown to evolve from a subset of polyps called sessile serrated polyps, which account for roughly 5–10% of all polyps. These serrated polyps arise by molecular and histological events that are distinct from tubular adenomas51–53 and are classified into three categories: hyperplastic polyps, sessile serrated adenomas and traditional serrated adenomas54. The sessile serrated polyps have the potential to transform into colorectal cancers through the following sequence: hyperplastic polyp to sessile serrated polyp to adenocarcinoma51,55. Furthermore, serrated polyps that arise in the right colon (which includes the cecum, ascending colon and transverse colon) commonly show MSI and a form of epigenetic instability characterized by excessive aberrant CpG island DNA methylation, termed the CpG Island Methylator Phenotype (CIMP). By contrast, polyps that arise in the left colon (which includes the descending colon, sigmoid colon and rectum) are typically microsatellite stable but frequently carry mutations in KRAS and a subset of these polyps have an attenuated form of the CIMP52,53,56.
Given these molecular differences in the polyps and cancers they evolve into, a classification system for colorectal cancer has been proposed, with four subgroups of differing molecular features: hypermutable/microsatellite unstable (Hyp-MSI), hypermutable-microsatellite stable (Hyp-MSS), microsatellite stable (MSS) or chromosome unstable (CIN) and CIMP cancers43,57. The frequency of specific mutations can vary dramatically between the molecular subclasses, suggesting each has its own set of cooperating drivers57. However, the specific mutations and epigenetic alterations that define these molecular subgroups are still being determined. Some mutations, such as those in APC and SMAD family member 4 (SMAD4), are common among all the molecular subgroups — suggesting a central role in colorectal cancer in general — whereas others are restricted to one subgroup (for example, BRAF in CIMP colorectal cancers)58.
In colorectal cancer, substantial heterogeneity in the specific mutations is evident between tumours, although the mutations seem to cluster in epistatically related groups (for example, genes involved in a certain signalling pathway) 59–61. The most common alterations seen in colorectal cancer include those in APC, catenin-beta1 (CTNNB1), KRAS, BRAF, SMAD4, transforming-growth factor-beta receptor 2 (TGFBR2), TP53, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-alpha (PIK3CA), AT-rich interactive domain 1A (ARID1A), SRY (sex-determining region Y) box 9 (SOX9), family with sequence similarity 123B (FAM123B; also known as AMER1) and ERBB2, which promote tumorigenesis by perturbing the function of key signalling pathways, including the Wnt–β-catenin, epidermal growth factor (EGF)-mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K) and TGF-β signalling pathways, or by affecting genes that regulate central behaviours of cells, such as DNA repair and proliferation62,63 (Table 1). Colorectal cancer is frequently initiated by alterations that affect the Wnt signalling pathway, and the ensuing neoplastic cells then progress upon deregulation of other signalling pathways, including the RAS–RAF–MAPK, TGF-β, and the PI3K–AKT pathways 61,64.
Table 1.
Common genetic and epigenetic alterations in colorectal cancer*
| Gene or biomarker | Chromosome | Function | Molecular lesion | Frequency (%) | Predictive? | Prognostic? | Diagnostic? |
|---|---|---|---|---|---|---|---|
| Tumour suppressors | |||||||
| APC | 5 | Regulates Wnt signalling pathway | Inactivating mutations | 40–70 | No | No | Familial Adenomatous Polyposis |
| ARID1A | 1 | Member of SWI/SNF family, regulates chromatin structure and gene transcription | Inactivating mutations | 15 | No | No | NA |
| CTNNB1 | 3 | Regulates Wnt signalling pathway | Activating mutations | 1 | No | No | No |
| DCC | 18 | Netrin receptor; regulates apoptosis, deleted but not mutated in colorectal cancer, role in primary cancer still unclear | Deletion/LOH | 9 (mutations)/ 70(LOH) | No | Possible | No |
| FAM123B | X | Involved in Wnt signalling pathway | Inactivating mutations [ | 10 | No | No | No |
| FBXW7 | 4 | Regulates proteasome mediated protein degradation | Inactivating mutations | 20 | No | No | No |
| PTEN | 10 | Regulates PI3K–AKT pathway | Inactivating mutations, loss of protein by immunohistochemistry | 10(mutation) 30 (loss of expression) |
Possible | No | Cowdens syndrome‡ |
| RET | 10 | Regulates GDNF signalling pathway | Inactivating mutations, aberrant DNA methylation | 7 (mutation); 60(methylation) | No | No | No |
| SMAD4 | 18 | Regulates TGF-β and BMP pathways | Inactivating mutations, deletion | 25 | Possible | Possible | Juvenile Polypsis |
| TGFBR2 | 3 | Regulates TGF-β pathway | Inactivating mutations | 20 | No | No | No |
| TP53 | 17 | Regulates expression of target genes involved in cell-cycle progression, DNA repair and apoptosis | Inactivating mutations | 50 | Possible | Possible | Li Fraumeni Syndrome |
| Proto-oncogenes | |||||||
| BRAF | 7 | Involved in MAPK signalling pathway | V600E activating mutation | 8–28 | Probable | Probable | Lynch syndrome |
| ERBB2 | 17 | Involved in EGF–MAPK signalling pathway | Amplification | 35 | No | No | No |
| GNAS | 20 | Regulates G-protein signalling | Mutation | 20 | No | No | No |
| IGF2 | 11 | Regulates IGF signalling pathway | Copy number gain, loss of imprinting | 7(mutations)/ 10(methylation) | No | No | No |
| KRAS | Regulates intracellular signalling via the MAPK pathway | Activating mutations in codon 12 or 13 but rarely in codons 61, 117 and 146 | 40 | Yes | Possible | NA | |
| MYC | 8 | Regulates proliferation and differentiation | Amplification | 2(mutations)/ 10 (CNV- gain) | No | No | No |
| NRAS | 1 | Regulates the MAPK pathway | Mutation in codon 12 or 13 | 2 | Yes | No | No |
| PIK3CA | 3 | Regulates PI3K–AKT pathway | al and kina20Mutase mutations in kinase (exon 20) and helical(exon 9) domain | 20 | Probable | Possible | No |
| RSPO2 and RSPO3 | 1 | Ligand for LGR family receptors, and activate Wnt signalling | Gene fusion translocation and | 10 | No | No | No |
| SOX9 | 17 | Regulates apoptosis | Copy number gain | 9(mutations)/ <5 (CNV gain) | No | No | No |
| TCF7L2 | 10 | Regulates Wnt signalling | Gene fusion and translocation | 10 | No | No | No |
| Other molecular alterations | |||||||
| Chromosome Instability (CIN) | N/A | NA | Aneuploidy | 70 | Probable | Probable | No |
| CpG Island Methylator Phenotype (CIMP | N/A | NA | Methylation of >20% loci from a selected panel of markers | 15 | Probable | Probable | No |
| Microsatellite Instability (MSI) | N/A | NA | Unstable microsatellite repeats in consensus panel | 15 | Probable | Yes | Lynch syndrome |
| Mismatch Repair Genes | N/A | Regulate DNA mismatch repair | Loss of protein by immunohistochemistry; methylation; inactivating mutations | 1–15 | Possible | Probable | Lynch Syndrome |
| SEPT9 | 17 | NA | Methylation | >90 | No | No | Serum based assay for cancer detection |
|
VIM NDRG4, BMP3 |
10, 16 and 4, respectively | NA | Methylation | 75 | No | No | Stool based test for early detection |
| 18qLOH | 18 | NA | Deletion of the long arm of chromosome 18 | 50 | Probable | Probable | No |
APC, adenomatous polyposis coli; ARID1A, AT-rich interactive domain 1A; BMP, bone morphogenetic protein; CNV, copy number variation; CTNNB1, catenin-β1; DCC, DCC netrin 1 receptor; EGF, epidermal growth factor; FAM123B, family with sequence similarity 123B; FBXW7, F-box and WD repeat domain-containing 7, E3 ubiquitin protein ligase; GDNF, glial cell-derived neurotrophic factor; GNAS, guanine nucleotide-binding protein, α-stimulating complex locus; IGF, insulin-like growth factor; LGR, leucine-rich repeat-containing G protein-coupled receptor; LOH, loss of heterozygosity; MAPK, mitogen-activated protein kinase; N/A, not applicable; NDRG4, NDRG family member 4; PI3K, phosphatidylinositol 3-kinase; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit-α; PTEN, phosphatase and tensin homologue; RSPO, R-spondin; SEPT9, septin 9; SMAD4, SMAD family member 4; SOX9, SRY (sex-determining region Y) box 9; TCF7L2, transcription factor 7-like 2; TGFβ, transforming growth factor-β; TGFBR2, TGFβ _receptor 2; VIM, vimentin.
Includes alterations in gene expression, gene deletions and amplifications, somatic mutations and aberrant promoter methylation.
Germline mutation, not somatic.
In addition to gene mutations, epigenetic alterations commonly occur in polyps and colorectal cancers and seem to cooperate with gene mutations to drive the polyp to cancer progression59,65,66. DNA methylation affects CpG-rich regions (CpG islands), which are often located in the 5′ region of genes and can result in transcriptional silencing through effects on transcription factor binding and changes in chromatin structure67. Modifications in DNA methylation related to the development of cancer (in general) include two fundamental changes: hypermethylation of CpG islands in gene promoters, which can silence tumour suppressor genes, and hypomethylation of repetitive genetic elements, which can lead to genomic instability or oncogene activation68. Hypermethylation, such as of the septin 9 (SEPT9) gene promotor, is also used for screening purposes (see below).
Importantly, the frequencies of many of these molecular features vary depending on the location of the tumour in the gut (from the ascending colon to the rectum)69,70. Some studies support a gradual gradient in change in frequency of the molecular alterations, whereas others suggest a more abrupt dichotomy. This has led to the traditional dichotomy of ‘proximal’ and ‘distal’ colorectal cancer versus adoption of a continuum model. Both models support the notion that the tumor microenvironment (the gut microbiome and inflammatory state of adjacent tissue) modulates the way these mutations affect cancer formation and disease progression. Thus, our current understanding of the pathogenesis of colorectal cancer is that the disease results from the accumulation of alterations in genes that then drive the formation of the tumour in the context of tumour-promoting factors derived from the adjacent tissue. This paradigm formed the basis for recent recommendation to determine the in situ immune cell infiltrate of the tumour as a prognostic marker alongside its (standard) TNM stage71. In close conjunction with these data, recent research has focused on the role of the gut microbiota in colorectal carcinogenesis. Indeed, studies have shown the enriched presence of Fusobacteria72, in particular in cancers with CIMP status73, which might be inversely related to the CD3+ T cells in colorectal cancers74. Together, these data form a basis for further research into the role of the colon microbiota and colon carcinogenesis.
Diagnosis, screening and prevention
Diagnosis
A diagnosis of colorectal cancer either results from an assessment of a patient presenting with symptoms, or as a result of screening. The disease can be associated with spectrum of symptoms, including blood in stools, change in bowel habits and abdominal pain. Other symptoms include fatigue, anaemia-related symptoms such as pale appearance and shortness of breath, and weight loss. The predictive value of these symptoms for the presence of colorectal cancer in an elderly patient is limited, but they do warrant further clinical evaluation. With the widespread introduction of population screening for colorectal cancer, many individuals are diagnosed at a pre-clinical stage. In symptomatic patients, colonoscopy is the preferred method of investigation, but other endoscopic methods are also available or being developed (Box 2). For population screening, a range of other methods can be used for primary assessment, followed by colonoscopy in case of a positive test.
Box 2. Endoscopic techniques for the diagnosis of colorectal cancer.
High-definition white-light endoscopy
Current standard for colonoscopy, combining high-definition video endoscopes with high-resolution videoscreens
Provides detailed image of the gastrointestinal mucosa
Advantage of routine endoscopy, disadvantage that it provides no specific contrast for detection of neoplastic lesions
Chromoendoscopy
The use of a dye spray during gastrointestinal endoscopy to improve visualization
Improves detection of neoplastic lesions
Time-consuming to spray the complete colon
A new technique with dye incorporated into colon preparation is under investigation
Magnification endoscopy
Endoscope with zoom-lens in tip, which enables 6–150-fold enlargement of the mucosa
Can characterize and determine the extension of neoplastic lesions
Not suitable for screening of the complete colon
Can be combined with other methods
Narrow band imaging
Technique that can be built into white-light endoscopes
Filters light to two bands, with a wave length of respectively 415 nm (blue) and 540 nm (green)
Longer wavelength light is less scattered and, therefore, penetrates deeper into the mucosa
Blue light enhances superficial capillaries, whereas the green light displays deeper, subepithelial vessels
Can characterize and determine the extension of neoplastic lesions
Does not increase neoplasia detection rates
Intelligent colour enhancement (FICE) imaging (Fujinon) and iScan imaging (Pentax)
Similar techniques as narrow band imaging, but no filtering of the outgoing light
Instead, processes the reflected light
Autofluorescence endoscopy
Technique that can also be built into white-light endoscopes
Based on the principle that illumination with a specific blue wavelength light can lead to excitation of tissue, which then emits light with longer wavelength
Wavelength of the emitted light is longer for neoplastic tissue
Can be used to search for neoplastic lesions
Endomicroscopy
Technique of extreme magnification endoscopy
Enables in vivo visualization of individual glands and cellular structures
Can evaluate neoplastic lesions
Not suitable for scanning larger mucosal surfaces
Colonoscopy
Colonoscopy is the gold standard for diagnosis of colorectal cancer. It has a high diagnostic accuracy and can assess the location of the tumour. Importantly, the technique can enable simultaneous biopsy sampling and, hence, histological confirmation of the diagnosis and material for molecular profiling. Colonoscopy is also the only screening technique that provides both a diagnostic and therapeutic effect. Removal of adenomas using endoscopic polypectomy can reduce cancer incidence and mortality9,75–78. Indeed, the efficacy of colonoscopy for reduction of colorectal cancer incidence and mortality was well demonstrated by the US National Polyp Study77,79. Recent 20-year follow-up data from this study showed a reduction in colorectal cancer-related mortality of 53%77, an encouraging result that has been echoed by a more-recent study80. The quality of colonoscopy is a determining factor in the diagnostic yield of cancer and adenoma, which is the most certain way of avoiding interval cancers (that is, a tumour arising in between screening visits)9,76,81,82.
The image-quality of colonoscopy has markedly improved over the past 20 years, from original fibre-optic to videochip endoscopes. Videochip endoscopes were further improved over the years, leading to higher resolution and wider angle of view. The current standard combines high-power endoscopes with high-resolution videoscreens to yield high-definition white light endoscopy (hWLE). Although various technologies for further image enhancement in colonoscopy have been introduced over the past decade, none of them has been shown to improve the diagnosis of polyps and colorectal cancer compared with white light colonoscopy83. Only chromoendoscopy (Box 2), has proven to be superior to hWLE in identifying adenomas84. Narrow band imaging, imaging with the Fujinon Intelligent Color Enhancement system (Fujinon Corporation, Saitama, Japan) and autofluorescence endoscopy are not advantageous over hWLE in detecting adenomas or carcinomas85. The Third Eye Retroscope® device (Avantis Medical Systems, California, United States) was designed to address the fact that lesions behind mucosal folds in the gut are often missed. This endoscope provides a simultaneous retrograde view of the colon that complements the forward view of a standard colonoscope. Several pilot studies have indicated that it might be useful, but more data are needed86–88. The invasive nature of colonoscopy poses a burden to screenees and patients, which might affect participation in screening programmes. In recent years, several alternative diagnostic methods have been introduced, such as capsule endoscopy and biomarker tests.
Capsule endoscopy
Capsule endoscopy uses a wireless capsule device swallowed by the screenee, and enables examination of almost the entire gastrointestinal tract without the use of conventional endoscopy89– 92. Capsule endoscopy is useful in diagnosing adenomas and colorectal cancer. The first-generation capsule endoscopy was found to be able to detect polyps ≥6 mm in size with a sensitivity of approximately 60% and specificity of >80%89. Cancer detection was achieved in 74% patients with colorectal cancer89. With the development of the second-generation capsule endoscopy for the colon (PillCam® Colon2 (Given Imaging Ltd, Yokne’am Illit, Israel), the frame speed was increased from a fixed speed of four pictures per second to a variable 4–35 pictures per second depending on capsule movement. The angle of view was widened from 156° to 172° on both ends of the capsule, providing a 344° view. A large trial in the United States and Israel assessed the accuracy of this new capsule to diagnose colorectal neoplasia. With 884 patients included, sensitivity was shown to be 88% and specificity 82% for detection of adenomas >6 mm in size93.
The European Society for Gastrointestinal Endoscopy Guideline for Colon Capsule Endoscopy recommends capsule endoscopy as a feasible and safe tool for visualization of the colonic mucosa in patients, who have undergone no or incomplete colonoscopies92. This recommendation was then also incorporated in the Asia-Pacific guidelines on colorectal cancer screening94. The indications for capsule endoscopy are at this moment limited to patients who refuse conventional colonoscopy and to those in whom a complete colonoscopy is not possible for anatomical reasons. The presence of a stenosis is a contraindication for capsule endoscopy as it could lead to capsule retention.
CT colonography
CT colonography uses low-dose CT scanning to obtain an interior view of the colon. The technique is well established as a diagnostic modality for colorectal cancer95. In a systematic review and meta-analysis that included >11,000 people from 49 centres, CT colonography was shown to have a sensitivity of 96% for colorectal cancer detection96. This performance is similar to that of conventional colonoscopy. A recent study reported similar performance of CT colonography and capsule endoscopy in patients with previous incomplete colonoscopy97. A large trial in 411 patients with obstructive cancers showed excellent performance of CT colonography for evaluation of proximal synchronous lesions98. An observational study based on data from England of 2,731 people with a positive guaiac faecal occult blood test (gFOBT, see below) showed that the detection rate of advanced neoplasia was significantly lower for subsequent CT colonography than for subsequent colonoscopy99. Furthermore, the detection and accuracy rates for advanced neoplasia were better in high-volume centres. These findings underline the need for adequate quality assurance similar to measures implemented for colonoscopy screening.
CT colonography requires full bowel preparation (clearance of the bowel), air inflation and change in position of the patients during the examination. The discomfort to the screenee of CT colonography is similar to colonoscopy in experienced hands, in particular because of the need of significant bowel insufflation100, but it has the advantage of obviating the use of sedation and can be used as part of the staging procedure in a confirmed case of colorectal cancer. However, CT colonography has low sensitivity for small (6–9mm) and flat lesions101. The technique is associated with high colonoscopy referral rates (up to 30%), and high rates of extra-colonic findings in non-cancer cases, which translate to unnecessary investigations and increased anxiety for individuals102,103. The costs of CT colonography, and the need for further investigation in a subset of screenees limit the usefulness of this method for population screening in most countries.
CT colonography has been recommended as one of the options for colorectal cancer screening in guidelines in the United State and Europe 104,105. In many countries, CT colonography has replaced double-contrast barium enema (the conventional X-ray-based imaging modality for the colon) examination and is increasingly being used as an alternative to conventional colonoscopy. However, CT colonography has not readily been accepted in Europe because of radiation exposure, costs, burden to patients and high colonoscopy referral rates. In the Asia–Pacific region, CT colonography is not recommended for colorectal cancer screening unless in those for whom total colonoscopy is not possible94.
Biomarkers of colorectal cancer
Molecular detection of colorectal cancer offers a noninvasive test that is appealing to patients and clinicians as samples of multiple patients can be analysed in batch. The ideal molecular marker should be highly discriminating between cancer and advanced adenomas from other lesions, be continuously released into the bowel lumen or circulation, and disappear or reduce after the lesion is removed or treated. Indeed, assays using proteins, RNA and DNA in the blood, stool and urine have been developed but with varying degrees of success (Table 1). Stool tests are based on the fact that early cancers as well as advanced premalignant lesions can bleed and shed cells into the bowel lumen, which can be detected. Blood tests obviate the handling of stool and urine and can be performed alongside routine checking of blood sugar and cholesterol in the elderly population.
The gene SEPT9 belongs to a class of GTPases, and hypermethylation of its promoter region is associated with colorectal cancer; aberrant methylation of SEPT9 at the tissue level discriminates colorectal neoplasia from normal mucosa. Early case–control studies from referral centres showed that SEPT9 methylation testing yielded a moderate sensitivity of 50–70% for colorectal cancer, with a specificity of 85–90%106. However, a more-recent larger scale study in population with average risk of developing the disease suggested a colorectal cancer detection rate of <50% when using SEPT9 methylation testing107. The reported detection of advanced colonic adenoma by SEPT9 methylation status is only approximately 10%. As such, SEPT9 assays are outperformed by current quantitative faecal immunochemical tests (FITs).
Mutation of APC and KRAS has been tested in DNA shed by epithelial cells and isolated from stool samples. The first-generation faecal DNA tests only gave satisfactory results with fair sensitivity for the detection of colorectal cancer but low sensitivity for the detection of advanced colonic adenomas108. Since then, several technological improvements have been made, including the use of a stabilizing buffer, the addition of other more-discriminating markers (KRAS mutations, aberrant NDRG family member 4 (NDRG4), bone morphogenetic protein 3 (BMP3) methylation and presence of β-actin), the use of more-sensitive analytical methods and the optimization of the determining algorithm — all of which have improved the accuracy of the assay (see further description below)109. Other potentially useful markers under investigation include circulating tumour mRNA, microRNA and circulating cytokeratins110.
Screening and prevention
Colorectal cancer is more suitable for population screening than any other malignancy owing to a combination of factors1. Firstly, the incidence of the disease is high and outcome for a significant proportion of affected patients is poor despite intense, burdensome and often very costly treatments111. Colorectal cancer also has a long preclinical stage. For instance, 7,151 Dutch citizens aged 55–75 years were newly diagnosed with colorectal cancer in 2012 112, which corresponds to approximately 0.2% of the 3.5 million people in that age group. Such an incidence is in line with similar annual incidences in other Western European countries. However, colonoscopy screening studies generally tend to find prevalent colorectal cancer in 0.5–0.9% of the participants in the same age group54,63,64. Although an increased willingness of symptomatic screenees might confound this difference, these data suggest that colorectal cancer on average progresses for several years before becoming symptomatic. Furthermore, colorectal cancer is preceded by colorectal adenoma. In individuals with sporadic (non-hereditary) disease, the progression from adenoma to cancer takes at least 5–10 years113. The long preclinical stage of disease offers a large window of opportunity for screening.
Second, colorectal cancer is also suitable for screening because adenomas and early cancers are detectable and treatable entities, which is in contrast to precursors of other highly common cancers of the breast, prostate and lung.
Last, both endoscopic removal of adenomas as well as treatment of early stage cancer have a profound impact on colorectal cancer mortality. After 20-year follow-up of the US National Polyp Study cohort, colorectal cancer-specific mortality was approximately 50% lower among subjects who at baseline had undergone endoscopic removal of adenomas than in an unscreened control cohort77. Furthermore, the 5-year survival rates for patients with early stage cancer are approximately 90%, compared with 10% for patients diagnosed with advanced-stage metastatic disease. Together, these factors form the background for various international guidelines on colorectal cancer screening. Screening in most countries aims to capture men and women aged 50–75 years, although different age ranges are being used in various programmes depending on the available resources114. Adoption of lifestyle measures can also significantly impact colorectal cancer incidence.
Endoscopy
Given that imaging of the colon can confirm a diagnosis or exclude colorectal neoplasia, clinicians often favour these methods for screening purposes. Colorectal adenomas and early stage cancers can directly be visualized by endoscopy, CT colonography or capsule endoscopy77,90,96,103. A randomized comparison between CT colonography and colonoscopy for primary population screening showed a slightly higher uptake of the former, counterbalanced by a slightly lower sensitivity for advanced neoplasia103. Capsule endoscopy screening might in the near future provide an alternative visualization method for primary screening90. Overall, colonoscopy has the highest accuracy and is generally considered the gold standard for screening and is associated with a number of advantages (Table 2). Recent large observational studies showed that screening colonoscopy reduced the risk for colorectal cancer by approximately 80%, and had a similar effect on related mortality115,116. This preventive effect of colonoscopy strongly depends on procedural quality, which can be measured in terms of adenoma detection rate of the performing endoscopist76. Other measures for procedural quality include the level of bowel preparation, caecal intubation rates, complication rates, average sedative medication dose and patient burden scores9. In a study from the United States, adenoma detection rates per colonoscopist ranged from 7% in the lowest quintile of detection to 50% in the highest quintile — a difference that is associated with an almost two-fold risk in interval cancer81. The correlation between risk of post-colonoscopy cancer and adenoma detection rates was also reported in a study from Poland76. Training and quality assurance measures, and adherence to surveillance guidelines also have an impact on the rate of post-colonoscopy cancers75,117.
Table 2.
Key performance indicators for organized screening with different modalities
| Test | Advantages | Disadvantages | Refs |
|---|---|---|---|
| gFOBT | Cheap Low screenee burden Reasonable uptake |
Limited sensitivity for advanced neoplasia Need for short screening intervals No effect on colorectal cancer incidence Qualitative, not automated Multiple sampling Moderate positive predictive value |
126,127,230 |
| FIT | Cheap Low screenee burden Quantitative, automated Single sample Sensitive for colorectal cancer Highest uptake Effect on incidence and mortality |
Limited sensitivity for advanced adenoma Moderate positive predictive value Repeated screening needed (interval can likely be longer than for gFOBT) Temperature-dependent performance* |
127,129,130,135,231 |
| Sigmoidoscopy | Sensitive for distal advanced neoplasia Long screening interval Effect on incidence and mortality |
Low uptake Moderately sensitive for proximal advanced neoplasia Expensive |
118,120,232,233 |
| Colonoscopy | Sensitive and specific Long screening interval Effect on incidence and mortality |
Low uptake Expensive Burdensome Associated with complications |
76,77,81,234,235 |
| CT colonography | Sensitive and specific Long screening interval Likely effect on incidence and mortality |
Low uptake Expensive Need for repeated lavage in case of advanced neoplasia Radiation exposure Burdensome |
95,96,98–100,103,105,236 |
| Multi-target faecal DNA test | Sensitive and specific | Uptake unknown Expensive Lack of prospective data |
110,134 |
Less problematic with newer generation tests. FIT fecal immunochemical test; gFOBT, guaiac fecal occult blood test.
Sigmoidoscopy, which images the rectum and sigmoid colon and can include the descending colon, has been shown in several randomized prospective trials to reduce the incidence of colorectal cancer by approximately 33%, and reduce related mortality by 38–59%1,118–120. This effect was obtained by single sigmoidoscopy screening with further colonoscopy in those with signs of advanced polyps — a finding that formed the basis for the current roll-out of nationwide primary sigmoidoscopy screening in England. The wide use of colonoscopy and sigmoidoscopy for primary screening in various countries supports the introduction of non-physician endoscopists who can perform diagnostic endoscopy according to international standards121. Further studies are needed to assess performance and cost efficacy122.
Population screening
Given the considerable rise in treatment costs, colorectal cancer screening is in many countries a cost-saving exercise123. Screening can be done with a range of methods, both invasive and non-invasive (Table 2). Most programmes are based on a single primary screening test, followed by colonoscopy in those who test positive114. In other settings, screenees are offered a choice between different screening methods, which might increase or decrease participation rates depending on the local setting124,125.
Population screening must consider more than just test accuracy, but should take test uptake and demand on resources into account. Accordingly, screening results must be reported in terms of identification of subjects with advanced neoplasia per 1,000 invited and in numbers needed to scope. A very accurate test by definition has no impact on cancer incidence and mortality in a population if not widely applied1,111. Similarly, limitations in endoscopy capacity preclude the use of colonoscopy for primary screening. For these reasons, many countries prefer a two-step approach in population screening, first using noninvasive screening test to select a subgroup of screenees who are at high risk of cancer for subsequent colonoscopy. Typically, faecal occult blood test is this primary screen1, either using gFOBTs or FITs. FITs are now more widely used than gFOBTs because of easier handling, resulting on average in approximately 10% higher uptake, higher sensitivity for advanced neoplasia and automated analysis126,127. Indeed, quantitative FITs offer the additional advantage that their cut-off points can be adjusted to match colonoscopy capacity128. For an optimal impact on the population level, adequate quality assurance is needed over the full range of the screening programme, as is organized active call–recall screening1.
The effect of uptake on the yield of screening was shown by a randomized study comparing primary colonoscopy and FIT screening in Spain129. The cancer detection rate was similar in both groups, but a considerable proportion of cancers in the colonoscopy group were actually detected by primary FIT after screenees first refused primary colonoscopy. Similarly, in a range of screening trials in the Rotterdam area, the highest detection rate was observed with repeated FIT screening1,130. This detection rate can be further increased with the use of two samples per screening round, especially in the first screening round131, although this approach is less cost-effective than screening with one sample132. gFOBT screening routinely makes use of a 1–2-year interval, the higher accuracy of FIT can allow for extension of the screening interval to 3 years133.
The performance of the aforementioned multi-target faecal DNA plus FIT testing was compared with FIT alone for detection of colorectal neoplasia134. All participants in the study underwent each of the ‘experimental’ screening methods and a confirmatory colonoscopy. The combined tests identified 60 of 65 patients (92%) with colorectal cancer and 321 of 757 patients (42%) with advanced adenomas; FIT alone detected 48 patients with colorectal cancer (74%, P = 0.002) and 180 patients with advanced adenomas (24% P <0.001)134. These results provide evidence for the accuracy of the DNA test in asymptomatic average-risk individuals, and led to FDA approval of the multi-target faecal DNA test plus FIT. However, the positive predictive value of the multi-target faecal DNA test was low (24%) for a non-invasive test, and the DNA test plus FIT yielded a 16.1% positivity rate versus 7.0% for FIT alone, thus necessitating 2.3-fold more colonoscopies in the DNA test plus FIT arm. If both tests were compared at the same positivity rate, a crucial determinant in countries with limited colonoscopy resources, the actual diagnostic yield and positive predictive value could have been approximated. This assumption is supported by previous studies that reported a similar number needed to screen to detect advanced neoplasia135. Finally, study design did not include a component to examine uptake of either test. For these reasons, further studies are needed to position the DNA test as a population screening method.
Surveillance after resection
Patients who have adenomatous polyps or colorectal cancer continue to be at risk for new neoplastic lesions after these have initially been removed — either because of biological or environmental factors, or both136. These patients could benefit from surveillance to detect and remove new lesions. Most evidence supporting this hypothesis is based on surveillance studies that have documented higher rates of tubular adenomas >10mm, adenomas with villous histology, high-grade dysplasia or cancer in patients with neoplasia at the baseline colonoscopy exam; the risk of developing subsequent tumours also depends on the size and histology of polyps at the index exam136–138. Furthermore, there is a relationship between the index lesion and subsequent risk of death from colorectal cancer139. Together, this body of data provides a strong justification for surveillance, but does not prove with certainty that surveillance will actually prevent recurrent cancer or reduce mortality.
Guidelines for surveillance in patients without hereditary syndromes vary in the United States and Europe137,140,141. The underlying premise of all such recommendations is that the baseline exam must be complete (including the caecum), with adequate bowel preparation, and that any detected lesions are removed completely. If the completeness of the resection or quality of the exam comes into question, early re-examination is recommended. The guidelines stratify risk based on the findings of the index examination (Box 3). The US guidelines endorse a 10-year interval if the baseline exam is negative or if the patient only has hyperplastic polyps in the rectum or sigmoid colon. New evidence adds further support for this recommendation80,142. Interval faecal blood testing is generally not recommended, owing to a lack of evidence of benefit137,140.
Box 3. Risk-stratified guidelines for surveillance after removal of adenomatous polyps or colorectal cancer.
Colorectal cancer
Patients with colorectal cancer should have intensive follow-up care
If a complete colonoscopy was not possible prior to surgical resection, colonoscopy should be offered within 3–6 months to detect synchronous lesions
If a complete colonoscopy was performed at baseline, patients with cancer should have colonoscopy at 1 year; if negative, every 3–5 years thereafter
High-risk adenoma
High-risk features include adenomas with high-grade dysplasia, villous histology, tubular adenoma ≥10mm in size, serrated lesions ≥10mm in size, serrated lesions with dysplasia or ≥3 adenomas
The risk of advanced neoplasia during surveillance is 15–20%, which is roughly 2–3-fold higher than individuals with 1–2 small (<10mm) tubular adenomas and 5–6-fold higher than individuals with no polyps at baseline colonoscopy137
The US Multi-Society Task Force on Colorectal Cancer (USMSTF) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines recommend a 3-year interval for surveillance137,140
The UK guidelines define highest-risk features as ≥5 small adenomas or ≥3 adenomas where at least one is >10 mm in size and recommends annual surveillance141 based on data indicating a high likelihood of finding additional high-risk adenomas at 1 year226
The UK guidelines define intermediate-risk features as 3–4 small (<10mm) adenomas or ≥1 large (≥10mm) adenomas, irrespective of histology, and suggest a 3-year screening interval
Low-risk adenoma
Individuals with 1–2 tubular adenomas <10mm in size represent a low-risk group
A statistically insignificant increase in risk, relative to patients with no polyps at baseline colonoscopy, are attributed to these patients
The UK guidelines recommend no specific follow-up141; the ESGE guidelines recommend follow-up at 10 years140; the USMSTF guidelines recommend surveillance at 5–10 years, with evidence supporting the 10-year interval if the index exam preparation was adequate137
Serrated lesions <10mm in size with no dysplasia might also represent a low-risk lesion, but evidence is weak; the USMSTF recommends a 5-year interval for surveillance and the ESGE recommends a 10-year interval
Several longitudinal studies of patients after adenoma removal have provided some guidance for the optimal intervals for surveillance examinations136,138. Surveillance intervals are based on the findings at last colonoscopy (Box 3). If the patient has an adenoma with high-risk features at baseline, but no polyp or an adenoma with low-risk features at surveillance, the next exam is recommended at 5 years. If the patient has an adenoma with low-risk features at baseline and at surveillance, the next exam interval is recommended at 5 years; if there is no polyp at surveillance, the next exam interval is 10 years. Finally, if a high-risk adenoma is found at surveillance, the next exam is recommended at 3 years. These recommendations are designed to reduce the frequency of surveillance for many individuals with low-risk lesions and are based on findings using high-quality colonoscopy. Complete examinations with good bowel preparation9 are required, but the role of other mitigating factors during surveillance such as lifestyle, sex and race are unknown. Surveillance should be discontinued when the risks of performing the bowel preparation and/or colonoscopy could outweigh any potential benefit. These factors should also be considered in elderly patients with comorbid conditions that might limit life expectancy, diminish any potential benefit of polyp removal and increase risk of complications during the colonoscopy procedure143,144.
How to conduct surveillance of patients with serrated lesions is under debate. Understanding the natural history of these lesions requires accurate histological definition, endoscopic detection and longitudinal follow-up145. Furthermore, inter-observer variability in histological interpretation, wide variation in detection rates and virtually no longitudinal follow-up study of these patients have hindered surveillance assessment146. Nevertheless, some evidence suggests that this pathway accounts >20% of colorectal cancers and patients may be at risk for recurrent disease and, therefore, require surveillance after resection. Further studies have to substantiate the risk for recurrent polyps and define optimal surveillance schedules.
In addition to endoscopic surveillance after cancer resection, follow-up surveillance by measuring carcinoembryonic antigen (CEA) levels in the plasma and/or CT imaging might detect curatively treatable metastatic recurrence147. There have been concerns about the cost, benefit and number needed to test to achieve a survival benefit. A randomized study found that CEA testing resulted in 6.7% of patients receiving treatment with curative intent and CT resulted in 8.0% receiving treatment, which was significantly more than a group receiving minimum follow-up care that involved only targeted diagnostic assessment if symptomatic148. The actual survival benefit was probably small. The cost-effectiveness is also uncertain, but CEA testing is likely to be more cost-effective than CT, depending on the cost in different countries.
Management
Although the molecular drivers of colorectal cancer have been described, where in the gut a tumour occurs has implications for treatment. That is, colon cancer and rectal cancer are two distinct cancers requiring different approaches, also depending on their stage. Cancer registries from different countries show huge differences in outcomes after treatment for colorectal cancer, although a trend for improvement is emerging149. Fortunately, increasing attention is being paid to quality assurance in cancer care150. Indeed, unravelling the effects of treatment on outcome is of utmost importance and, for this, population-based registries and audits are used to critically assess practice.
Surgery
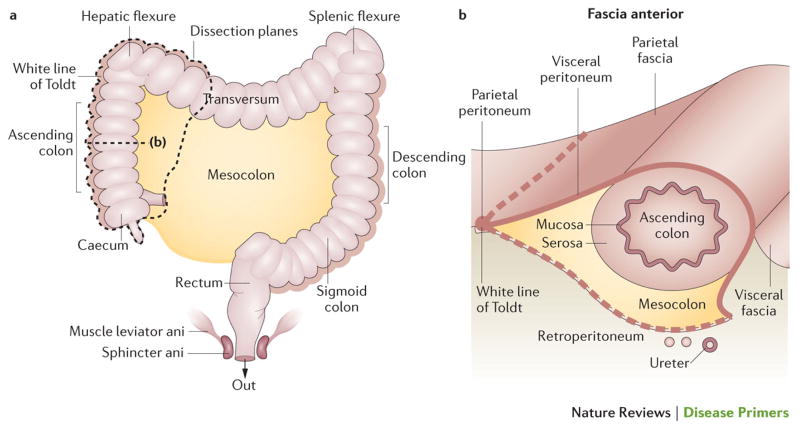
Surgery is the mainstay curative treatment for patients with non-metastasized colorectal cancer. However, outcome is strongly related to the quality of surgery151,152, the quality of pre-operative staging and treatment selection. The dissection should ideally follow the embryological anatomical planes to ensure that the tumour and its principle zone of lymphatic spread are removed. Special attention should be given to the circumferential surgical resection margins 152,153 (Figure 4). In more-advanced cases of rectal cancer, neoadjuvant treatment (for example, preoperative chemotherapy for T4 colon cancer, and (chemo)radiotherapy for locally advanced cancer) can reduce tumour load and even tumour stage, and might be necessary to optimize the chances for a successful resection150,152,154. Thus, a multidisciplinary approach before beginning treatment, based on adequate staging information, is mandatory 151,153,155,156.
Figure 4. Surgical planes for right colon surgery.
The mesocolon harbours the major blood vessels and draining lymph nodes; surgical planning involves considering the large blood vessels and the resection lines. For the caecum and the ascending colon (before the hepatic flexure), the main vessels are the ileocolic and right colic artery. The transverse colon begins at the hepatic flexure and ends at the splenic flexure; important vessels to consider in this region are the middle colic artery (via the superior mesenteric artery) arcading on the left side, with branches of the left colic artery (inferior mesenteric artery). The descending colon ‘bends’ at the sigmoid colon (at the left iliac crest) before continuing to the rectum. In the paracolic grooves, the parietal peritoneum is attached to the lateral border of the visceral peritoneum that overlies the colon and forms the surgical planes referred to as White Line of Toldt, which gives access to the avascular plane above Gerota’s fascia — the fascia on top of the retroperitoneum covering the kidney and ureter — without interfering with peri-renal space or ureters.
Preoperative assessment
When considering a patient for surgery, several factors such as their age, fitness, the perioperative management plan, tumour staging, type of surgery (including resection planes and reconstruction) and quality assurance are important. In terms of age, elderly patients with colorectal cancer have lower overall survival rates than their younger counterparts149. Indeed, postoperative mortality rates increase in elderly in the immediate postoperative period (first 30 days) and can double in the first 6–12 postoperative months157–160. However, ‘elderly patients’ as a group are heterogeneous, with varying comorbidities, degrees of fitness for surgery and risks for postoperative complications. Accordingly, age alone should not be a reason not to operate.
Before surgery of colorectal cancer, it is important to be informed about the whole colon to rule out synchronous cancers, which occur in some 4% of patients161. If preoperative endoscopy was incomplete owing to tumour obstruction, visualization of the colon should either be completed prior to surgery by CT colonography, or endoscopy should be performed in the 3 months following surgical resection161,162. Active search for distant metastases in the lungs and liver by means of chest and abdominal CT is also recommended before surgery155. CEA is preferably obtained before colorectal cancer surgery to provide a baseline value for postoperative surveillance. Genetic counselling is advised in young patients with a positive family history of colorectal cancer. Fast track protocols and laparoscopy should be considered to minimize the surgical trauma. In those with obstructive colorectal disease, abdominal CT imaging can also assess for T4 or stage IV disease. In patients with rectal cancer, preoperative MRI imaging of the pelvis is further recommended for planning purposes, as well as to distinguish the tumour in relation to the mesorectal fascia, and to assess T stage163. This information is necessary to select patients with T3c, T3d and T4 tumours for preoperative (chemo)radiotherapy.
Colon surgery
Laparoscopic resection of colorectal cancer (Figure 5) has been shown to be as safe as open surgery164–166. As with any surgical procedure, the team needs to be skilled in laparoscopic colorectal surgery and adequately select patients. Contraindications for laparoscopic approach are obesity, previous abdominal surgeries and advanced-stage disease151,152,165. If, during the laparoscopic procedure, conversion to open surgery is necessary, the earlier this is done the better the outcomes.
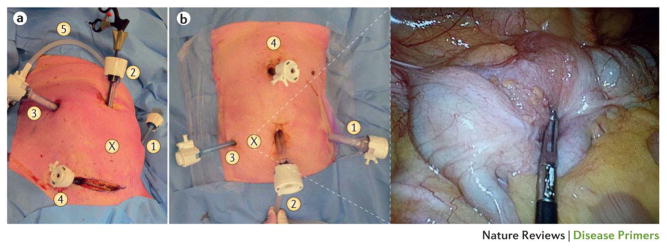
Figure 5. Laparoscopic surgery for colorectal cancer.
(a) A sigmoidectomy can be performed using three to six trocars. The laparoscopic exploration via the supraumbilical trocar (position 2) is a guide for the location of the other operating trocars. (X) The tumour location. (1) A 5 mm trocar in the left hypochondrium, for retracting the descending colon. (2) The first trocar to be introduced is a 12 mm trocar through the umbilical port. (3) A 12 mm trocar is used as an optical and operating port. (4) A 5 mm trocar is used for retracting tissue. (5) Carbon dioxide insufflation: pneumoperitoneum.
(b) The number of trocar ports for right colectomy varies from depending on the surgeon and operative difficulties. Trocar positioning is also variable, but our standard for a tumour in the caecum (shown in insert, position X) approach is to place (1) a 12 mm trocar in left hypochondrium as an optical or operating port. (2) The umbilical port side can be extended to a small laparotomy to extract the dissected colon and perform the extracorporeal anastomosis. (3) A 5 mm trocar is placed for operating and retracting the tissue (ascending colon or caecum). (4) A 5 mm trocar is used to retract the hepatic flexure, to expose ileocolic and right colic vessels, and perform the division. In both images, the patient’s head is at the top, their feet at the bottom.
In colon surgery, anatomical planes of the mesocolon with the parietal cavity wall and retroperitoneum should be followed to avoid damage of the ureters, duodenum, pancreas and spleen. Moreover the mesenteric margins are planned accurately, ensuring proficient vascularization of the remnant bowel loops for the anastomosis. A tension-free and torsion-free anastomosis must be created to avoid the feared complication of an anastomotic leakage.
Some patients might require perioperative placement of a stoma, in which the faeces are diverted into a bag on the outside of the body. Loop ileostomy or loop colostomy (Figure 6), or permanent colostomies, are an essential part of surgery for rectal and sigmoid cancer, either to protect the anastomosis or when the distal rectum is resected. In cases of a rectal obstruction, a loop colostomy is placed on the right (ascending) side; a permanent stoma is placed in cases an abdominoperineal excision (APE; that is removal of the anus, rectum and part of the sigmoid colon along with the associated lymph nodes). Each stoma has its advantages and disadvantages; there is no strong argument for superiority of one over the other167. Complications of stomas are numerous and cumbersome for the patient, and include prolapse, retraction, dermatitis, leakage, para-stomal hernia, obstruction and anastomotic leakage after stoma closure.
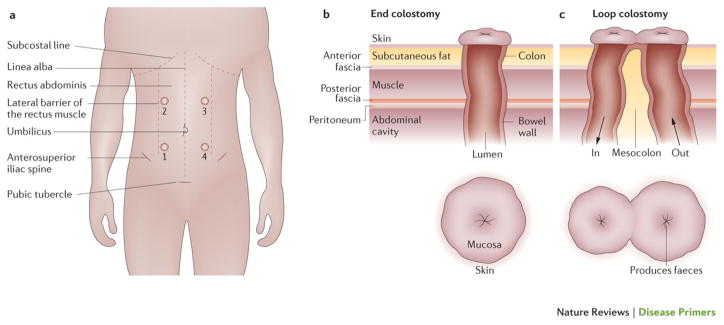
Figure 6. Stoma surgery for colorectal cancer.
A colostomy is a surgical procedure in which a stoma (from the Greek for ‘mouth’ or ’opening’) is formed by drawing the healthy end of the large intestine (colon) through an incision in the anterior abdominal wall and suturing it into place. (a) For stoma positioning (sites 1–4), the subcostal line, lateral border of the rectal abdominus muscle, anterosuperior spine of the ilium, shape of the abdomen and abdominal creases (for example, when trousers and belt are worn, and while sitting) are considered. Ill-placed ostomies result in invalidating leakage and dermatitis. The position of an end ileostomy or a loop ileostomy is preferable in the right hypochondria (position 1); a loop transversostomy is preferred in the right upper quadrant (position 2) to preserve the left side upper and lower quadrants (positions 3 and 4, respectively) for a definitive end colostomy if necessary. (b) In end stoma formation, the inside of the intestinal loop with the mucosa is placed at the abdominal wall. End stomas provide only one lumen, commonly formed to stay. A well-placed ostomy is about 2–3 cm above the skin, which ensures that the faeces are not in contact with the skin. (c) In loop stoma formation, two openings are sewn into the skin: efferent and afferent. The afferent (in) limb produces the stool and the efferent (out) limb allows passage of flatus from the distal portion of the bowel.
In patients presenting with (sub)total obstruction due to a left-sided (descending) tumour, temporary pre-operative stenting can be considered to reduce perioperative morbidity and risks of surgery, but the risk of perforation must be considered151,152,168. Colostomy versus stent for palliation could be considered in patients presenting with obstruction and multiple distant metastases151,152,169.
Rectal surgery
There are several surgical approaches for patients with rectal cancer, depending on tumour stage. Each technique aims for adequate oncological treatment with complete tumour and local node resection to minimize locoregional and distant recurrence and optimize disease-free and overall survival. In addition, sphincter preservation and avoidance of a permanent stoma are important additional goals of rectal cancer treatment. Accordingly, a careful, balanced choice of treatment is needed for each individual patient.
For early stage rectal cancer, advances in minimally invasive techniques have reduced the number of open rectal resections and have improved functional outcome dramatically. Transanal endoscopic microsurgery (TEM) is just such a minimally invasive technique for local tumour excision of well-differentiated T1N0 tumors170–172. TEM is associated with better functional outcomes and is performed through the anus (and, therefore, does not leave an abdominal scar or require a stoma), but has the trade-off of higher local recurrences. Thus, TEM is not recommended for tumours that are unlikely to be completely resected, as well as for poorly differentiated tumours given their high risk of local recurrence. The technical complexity of TEM and the high costs of the apparatus led to the introduction of new transanal techniques, in particular transanal minimally invasive surgery (TAMIS). This technique makes use of a disposable multichannel port that is positioned transanally and provides access for conventional laparoscopic equipment173.
Total mesorectal excision (TME) is the gold standard surgical technique for rectal tumours staged T1, T2, and favorable T3 (T3 with negative nodal status (T3N0M0) and excluding low-seated rectal cancers, and T3c and T3d disease). In patients with unfavourable rectal tumors, TME surgery is only recommended after neoadjuvant therapy to reduce the risk of local recurrences. For tumour resection, the anatomical plane is the mesorectal fascia and the circumferential resection margin is just outside of this fascia (Figure 7)174–176. The intact mesorectum, the fatty envelope that surrounds the rectal bowel wall, includes the draining lymph nodes. Complete resection involves removal of the bowel wall and these nodes. TME can be performed by open approach as well as laparoscopically; both have similar rates of locoregional recurrence, and disease-free and overall survival165. Rectal cancer surgery in locally advanced stages is associated with more blood loss; longer operation duration; more concomitant organ resections; and more postoperative complications such as anastomotic leakage, pelvic floor dysfunction, incontinence and genitourinary problems. However, robotic rectal resection may improve perioperative outcomes, such as reduction of perioperative blood loss, and is being explored177.
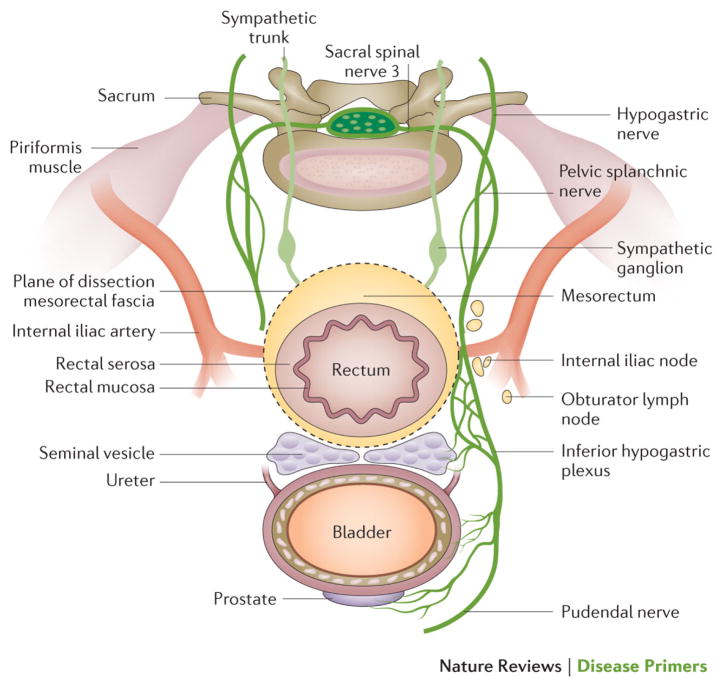
Figure 7. Surgical planes for rectal surgery.
The plane between the urogenitoury structures (prostate, urethra and seminal vesicle in men, and the vagina, uterus and ovaries in women) and the rectum is called Denonvilliers’ fascia. The dissection plane of the total mesorectal excision is sharp around the mesorectal fascia and surrounds the mesorectal fat, in which the draining lymph nodes and the rectum are located. The plane is avascular, and avoids the parasympatethic and sympathetic nerves in the pelvic lateral space, which coordinate sexual and urinary function. The superior hypogastric plexus is formed at the level of the sacral promontory, distally dividing in the hypogastric nerves. Together with the parasympathetic erigentes nerves, these form the inferior hypogastric (pelvic) plexus, which should not to be clamped during surgery to avoid damage. The pudendal nerve innervates the external sphincter, puborectalis muscle and external genitalia, among other structures.
Local recurrences after rectal surgery can be minimized using short-course radiotherapy178–180, although long-term data (12-year follow-up) showed no effect on overall survival for this approach 181. The timing of surgery after short-course radiotherapy is important. Surgery after a longer waiting period is associated with fewer complications than immediate surgery after radiotherapy182. Importantly, neoadjuvant radiotherapy (that is, before surgery) is associated with an increased risk for low anterior syndrome (a complex of symptoms that include frequent and urgent stools, numerous bowel movements over a few hours, stool incontinence and sexual dysfunction)183.
Neoadjuvant radiotherapy (or chemoradiotherapy) can be proposed for patients with unfavourable T3 (upper and mid T3c, T3d and low T3b) rectal tumours: those that invade >5 mm into the mesorectal fat and/or approach within 2 mm of the mesorectal fascia as visualized on MRI. T4 and lymph node-positive rectal cancer need short-course fractionated radiotherapy or chemoradiotherapy depending on the patient and tumour characteristics184. After the primary radiotherapy or chemoradiotherapy, restaging by means of endoscopy and MRI is recommended for these patients. TME surgery can be possible when the tumour has been downsized sufficiently. In patients with advanced and recurrent rectal cancer, surgery should aim for complete resection and conventional surgical planes may not be adhered to185. In some patients, a clinical complete response can be achieved after chemoradiation alone. This raises the question whether surgery can be omitted in these patients. In the largest series of patients treated nonsurgically, high response rates were reported186. Other series had lower response rates187,188. Prospective research will be necessary for this group of patients. Indeed, in 2015, the prospective International Watch & Wait Database for rectal cancer was launched (http://www.iwwd.org); this initiative aims to produce assess whether nonsurgical approaches are valuable alternatives to surgery.
Finally, a prospective multicentre randomized trial in Japan comparing TME alone versus TME with dissection of lateral nodes was recently completed189. In this study, approximately 10% of patients had pathological pelvic sidewall lymph nodes. Given that preoperative radiotherapy on lateral nodes might not completely eradicate nodal metastases, TME surgery with lateral lymph node clearance might be justified.
Quality assurance
The resected tumour specimen can be used to judge the quality of surgery; if the margin around the specimen is free of cancer cells in both colon and rectal cancer, the surgery is considered high quality174,175. The removal and assessment of the lymph nodes is another guide for determining whether the mesocolic or mesorectal resection is adequate 153. Internationally, removal of 12 lymph nodes is viewed as the cut-off value needed to provide adequate histopathological staging; the lymph nodes can also be used to prognosticate patients. However, the role of procedures to remove the sentinel node (the first lymph node or group of nodes draining the cancer) in colorectal cancer is still unclear.
Furthermore, quality assurance in colorectal cancer care has been defined for several aspects of the care continuum: performing trials, working in multidisciplinary teams, integrated care pathways, shared decision-making, auditing cancer care, centralization of complex procedures and international comparison of cancer outcomes. Auditing is a powerful instrument to improve cancer care. Especially for rectal cancer, survival and local recurrences have been shown to drastically improve with national auditing initiatives150,190,191. To reduce the differences in Europe, an international, multidisciplinary, outcome-based quality improvement project, European Registration of Cancer Care (EURECCA), was launched in 2007 156. EURECCA aims to capture the best practices and promote uniform structured data collection and analysis to study outcomes of all patients with cancer. Although these analyses are used to feedback surgeons on the best techniques at hand, volume is another issue that has been shown to improve patient outcome in colorectal cancer management192.
Recovery after surgery
Perioperative protocols such as fast track and Enhanced Recovery After Surgery (ERAS) have been designed to minimize surgical complications193,194. The protocol describes the perioperative care pathway and lists elements of care for patients at various steps in the perioperative process. Considering these elements are supported by evidence to improve recovery time after surgery, ERAS was first implemented for patients undergoing colectomy195 and includes elements such as preoperative counselling and bowel preparation, perioperative fluid management and prevention of ileus (obstipation and intolerance to oral intake) and postoperative glucose control and early mobilization. Indeed, for patients at high risk of postoperative ileus, enteral nutrition should be anticipated even before surgery196.
Systemic treatments for primary disease
The systemic treatment of patients with colorectal cancer has substantially developed over the past two decades, with major improvements in the neoadjuvant setting for rectal cancer, and adjuvant settings for cancer of the colon.
Neoadjuvant treatment
There is no accepted neoadjuvant treatment for colon cancer. However, for rectal cancer, neoadjuvant radiotherapy or chemoradiotherapy are recommended for intermediate-stage and advanced-stage cancer (for example, very low-tract anteriorly located cT2 lesions, most T3 lesions, some T4a lesions with limited peritoneal involvement, N+ lesions, cT3 lesions that invade the mesorectal fascia, and cT4a and cT4b lesions with positive lateral nodes) to reduce the rate of local recurrence. The neoadjuvant treatment can either be given as short-course radiotherapy followed by surgery or as chemoradiotherapy with 5-fluorouracil or capecitabine (an oral fluoropyrimidine). Although preoperative (chemo-)radiotherapy is more effective than postoperative treatment in reducing local recurrence, it does not improve overall survival181,197. Strategies that aimed to improve neoadjuvant treatment by intensifying the chemoradiotherapy regimen (for example, by combining 5-fluorouracil and oxaliplatin with radiotherapy instead of using 5-fluorouracil with radiotherapy) did not exhibit clear survival benefit, but increased toxicity198; more research is needed.
Adjuvant treatment
The cure rate by surgery alone for T3, T4a, T4b and N0M0 colon cancers (Union for International Cancer Control (UICC) stage II) is high and only approximately 5% of patients benefit from adjuvant chemotherapy. However, guidelines endorsed by European and Japanese societies recommend considering adjuvant therapy in high-risk cases (that is, poorly differentiated tumours; when <12 lymph nodes were resected; in cases with vascular, lymphatic or perineural tumour invasion; in cases with obstructive or perforated tumours; or tumours with pT4 stage)199. By contrast, adjuvant treatment is standard for UICC stage III tumours (any T, N1–2 (3 or more positive nodes), M0); a combination of 5-fluorouracil (orally, as in the XELOX protocol, or intravenously as in the FOLFOX4 protocol) plus oxaliplatin is used. Currently, no data support that the addition of targeted therapies (such as epidermal growth factor receptor (EGFR)-specific or vascular endothelial growth factor (VEGF)-specific monoclonal antibodies) improves the outcome for patients in the adjuvant setting199. Data from pooled analyses suggest that patients >70 years of age might not benefit profoundly from oxaliplatin-based chemotherapy combinations in the adjuvant setting. These patients may benefit from fluoropyrimidine chemotherapy, similar to younger patients200. For rectal cancer, postoperative chemoradiotherapy can be applied if no preoperative treatment was given and if certain risk factors (including positive resection margins, perforation in the tumour area or defects in the mesorectum) are present; adjuvant chemotherapy typically uses fluoropyrimidines.
Metastatic disease
The survival of patients with metastatic disease has substantially improved over the past two decades and a median overall survival of 30 months has been achieved in clinical trials. This improvement in survival can be attributed to use of chemotherapeutics such as oxaliplatin and irinotecan, the introduction of targeted therapies that address specific properties of the tumour or its microenvironment and the incorporation of multidisciplinary approaches, including surgical resection of liver metastases.
Chemotherapy combinations
The chemotherapy backbone for first-line treatment of metastatic disease is typically a combination of 5-fluorouracil, leucovorin and either oxaliplatin (FOLFOX protocol) or irinotecan (FOLFIRI protocol). 5-Fluorouracil in the FOLFOX regimen can be replaced by capecitabine, but combination of capecitabine with irinotecan is more toxic than FOLFIRI. Doublet (two chemotherapeutic agents) and triplet (three chemotherapeutic agents) chemotherapy regimens consisting of 5-fluorouracil, leucovorin, oxaliplatin and irinotecan (the FOLFOXIRI protocol) have been shown to be efficacious201. As compared with single-agent fluoropyrimidine, combination chemotherapy achieves better tumour growth control. However, elderly and frail patients in particular might benefit from a sequential approach with initial single-agent fluoropyrimidine chemotherapy or combined fluoropyrimidine with VEGF-A-targeted therapy (bevacizumab; see below).
Targeted therapies
Alongside these combined chemotherapy regimens, targeted agents are used for metastatic colorectal cancer treatment. In particular, these include three major groups of drugs: monoclonal antibodies against EGFR (cetuximab and panitumumab), monoclonal antibodies against VEGF-A (bevacizumab), and fusion proteins that target multiple proangiogenic growth factors (for example, aflibercept) and small molecule-based multikinase inhibitors (for example, regorafenib).
Approximately 80% of all colorectal cancers express or overexpress EGFR; overexpression correlates with reduced survival and increased risk of metastases. The EGFR tyrosine kinase can be blocked by monocloncal antibodies specific to the extracellular domain of the receptor, decoy receptors that bind and block the soluble ligand, or small molecules that inhibit receptor dimerization or fit into the ATP binding pocket of its cytoplasmic tyrosine kinase domain. Most clinical data in colorectal cancer are available for receptor-blocking antibodies, such as cetuximab, which is a recombinant chimeric monoclonal IgG1 antibody, and panitumumab, which is a human EGFR-specific antibody. These antibodies show efficacy in chemotherapy-naive patients as well as in patients whose tumours are refractory to chemotherapy by improving the overall response rate of the tumours. These strategies also improve progression-free survival (PFS) and even overall survival in patients with metastatic colorectal cancer. However, a prerequisite for the efficacy of these agents is that the tumours do not harbour activating mutations in KRAS and NRAS 202,203.
RAS is mutated in about half of all colorectal cancers, with codons 12 and 13 being most commonly affected; codons 61 and 146 of KRAS and codons 12, 13 and 61 of NRAS are affected to a lesser extent. HRAS mutations have so far not been described in colorectal cancer. The mutations render the Ras GTPase constitutively active; active Ras induces a plethora of tumorigenic intracellular signalling pathways. Thus, the Ras status of the tumour must be examined before treatment with EGFR-specific antibodies.
Tumours establish a vascular network of their own once they reach a critical size204. Accordingly, a major effector of tumour angiogenesis is the secreted glycoprotein VEGF-A, which binds to VEGFR-1 and VEGFR-2. VEGF-A is produced by many tumour and stromal cells, promotes proliferation and migration of endothelial cells and increases vessel permeability. VEGF is also a growth factor for various tumour cells. VEGF-specific therapies are used in metastatic colorectal cancer, but the precise mechanisms of action are not fully understood. These compounds might act by normalizing the dysregulated tumour vasculature, which would lead to improved tumour oxygenation and delivery of chemotherapy205. There are as yet no predictive biomarkers for anti-angiogenic agents.
Bevacizumab has demonstrated efficacy in combination with chemotherapy in the metastatic setting; combined with 5-fluorouracil and irinotecan, bevacizumab significantly improved median PFS and median overall survival of patients in a Phase III trial compared with chemotherapy alone6. The addition of bevacizumab also significantly improved median PFS in patients receiving a combination of fluoropyrimidine and oxaliplatin. Interestingly, the combination of bevacizumab and 5-fluorouracil/oxaliplatin also yielded a significant improvement in tumour response, median PFS and median overall survival compared to chemotherapy alone in patients with chemorefractory metastatic disease206. Bevacizumab is also one of the few compounds that confer a survival benefit to patients when treatment is continued even after disease progression207.
Aflibercept also targets angiogenesis. This drug is a recombinant fusion protein that consists of the VEGF-binding portions from the extracellular domains of human VEGFR-1 and VEGFR-2 fused to the Fc portion of the human IgG1 immunoglobulin. Aflibercept also binds the placenta growth factor (PLGF) and, therefore, has a somewhat broader antiangiogenic activity than bevcacizumab. Aflibercept has been shown to improve PFS and overall survival when used in combination with FOLFIRI in the second-line setting of treatment for metastatic disease208.
Metastatic resection
For patients with colorectal cancer who have isolated liver and/or lung metastases that are technically R0 resectable, surgery should be considered — particularly when the metastases are limited in number and size. The 5-year overall survival rate in this group is about 20%209,210, an impressive figure for metastatic disease. One clinical trial has used a perioperative FOLFOX protocol in this group of patients and showed an improvement in PFS, but no significant difference in overall survival compared with surgery alone 201.
In the majority of patients with isolated liver and/or lung metastases, a R0 resection cannot be primarily achieved. However, if the metastases can be downsized and combined with adjuvant chemotherapy, the 5-year overall survival rate is similar to R0 resections 211. In this situation, the most active chemotherapy should be employed to ‘convert’ the disease to a resectable state; FOLFOXIRI triplet chemotherapy regimen confers high response rate (approximately 60%)212. In a RAS wild-type population, chemotherapy doublets plus EGFR-specific treatment also result in high response rates. According to the data of the FIRE3 study, EGFR-specific antibodies in combination with FOLFIRI seem to induce more pronounced tumour shrinkage than FOLFIRI plus bevacizumab. Thus, this combination is an option if the tumour is RAS wild-type213.
If a more-active treatment with the intent to downsize metastases for secondary resectability is used, it is important to ensure that the tumour is regularly re-evaluated by a multidisciplinary team and resection of metastases is performed at the earliest time point when an R0 resection is possible. In doing so, chemotherapy toxicity is reduced and perioperative morbidity is minimized. The ‘disappearance’ of the metastases on CT imaging does not necessarily indicate a complete destruction of the metastases in most patients, and makes it difficult for the surgeon to completely resect all lesions214.
Further considerations
Patients with symptomatic or more-aggressive metastatic disease without chance of secondary metastatic resection benefit from active first-line treatment to achieve optimal tumour control. This can generally be achieved using doublet chemotherapy in combination with a targeted agent such as bevacizumab. In patients with RAS wild-type tumours, doublet chemotherapy together with an EGFR-specific antibody treatment can also be used. For those who respond to this ‘induction’ treatment, or who have a stable disease after 4–6 months of the treatment, the intensity of the treatment should be reduced to avoid excessive toxicity. This is particularly important if a FOLFOX protocol is used to avoid the cumulative neurotoxicity of oxaliplatin. A Phase III trial showed that after FOLFOX plus bevacizumab induction therapy, a maintenance strategy with fluoropyrimidine chemotherapy plus bevacizumab prolonged PFS without significantly improving overall survival compared to a complete treatment break215. Thus, active maintenance, but also treatment discontinuation, can be considered when tumours respond or are stable during a 4–6 months induction treatment and the tumour burden is not high.
In the palliative setting, a less-aggressive approach with monotherapy with fluoropyrimidine chemotherapy or a combination of fluoropyrimidine chemotherapy with bevacizumab is possible. Such a strategy requires the patient to be at low risk for rapid deterioration. Upon disease progression, treatment should be escalated and combination chemotherapy (together with bevacizumab) should be used. However, recent data from the FIRE3 and CALGB trials suggest that using a more-intensive treatment in the first-line setting can achieve a median overall survival of about 30 months in a RAS wild-type population. Such survival rates have so far not been reported in a sequential setting when treatment starts with just fluoropyrimidine chemotherapy with or without bevacizumab201.
In the second-line palliative setting, upon further disease progression, chemotherapy should be changed to a regimen not used in the first line (either FOLFOX/XELOX or FOLFIRI). A recent study showed that bevacizumab can be given after disease progression and improves overall survival in the second-line setting207. Apart from bevacizumab, aflibercept can be used in the second-line setting (in combination with FOLFIRI). Cetuximab or panitumumab can also be used if not previously used and if the tumour is RAS wild-type. For these compounds, efficacy beyond progression has not been demonstrated.
In cancers that are refractory to two lines of chemotherapy, EGFR-specific antibodies can be used if the tumour is RAS wild-type and an EGFR-specific antibody has not been used previously202. Regorafenib is an orally available multikinase inhibitor that has shown efficacy in patients who had previously been treated with all available therapies. Accordingly, it has become the standard in pre-treated patients216.
Quality of life
Colorectal cancer can manifestly impair quality of life through, for example, direct consequences of the disease, such as abdominal pain, change in bowel movements, blood loss and anaemia, fatigue, and weight loss. Furthermore, treatment incurs a burden to quality of life by means of surgery, chemotherapy and radiotherapy, which can be associated in the short-term with impaired nutrient intake and physical activity217. Indeed, weight loss and reduced physical condition is particularly relevant for elderly patients and those with co-morbidities, and should be adequately monitored during treatment and follow-up care.
Each treatment modality can be associated with further specific adverse effects and complications. One of the most feared surgical complications is the occurrence of leakage of the anastomosis, at the suture-line of the intestinal loops after removal of the tumour. This event usually requires further surgical or radiological intervention and is associated with significant morbidity and lengthening of hospital stay and mortality. Other more common complications of surgery are wound dehiscence (rupture of the wound along a surgical suture), and abdominal scar herniation. Overall, the impact on quality of life does not differ between open and laparoscopic surgery133. A range of stoma-related complications can also significantly impair social functioning and impair quality of life; these can sometimes be managed conservatively, but may require surgical revision of the stoma.
Treatment for rectal cancer is frequently associated with long-term complications. These include faecal incontinence and increased numbers of stools. These complications are well defined in the validated low anterior resection syndrome (LARS) score218. Pelvic floor problems are more frequent in rectal cancer patients receiving neoadjuvant (chemo) radiotherapy219. Toxicity is higher after chemoradiotherapy in comparison to radiotherapy alone 220. Moreover erectile dysfunction in men and dyspareunia in women are common after rectal cancer treatment 221,222.
With respect to chemotherapy, 5-fluorouracil is usually well tolerated, but oxaliplatin or irinotecan more often give rise to adverse effects such as neutropenia and diarrhoea. Targeted therapies have important adverse effects that must be considered. For the EGFR-specific antibodies, papulopustulous rash and paronychia (infection of the nail) occur within days of treatment, followed by skin atrophy after several weeks and alopecia that occurs within a few months. High-grade skin toxicity can involve pain and secondary infections. Antiangiogenic agents cause bleeding, arterial thromboembolic events, impaired wound healing, hypertension and proteinuria6. Aflibercept increases (to some extent) chemotherapy- induced adverse events such as diarrhoea, neutropenia and asthenia208.
Metastatic disease can give rise to a range of additional symptoms that affect quality of life, such as cachexia, loss of appetite, anaemia, liver failure, biliary obstruction and impaired pulmonary function223. These symptoms relate to duration of survival to some extent223. A range of interventions, with focus on management of pain, improvement of food intake and maintenance of physical activity benefit individual patient groups. For example, a systematic review of three studies reported that increased physical activity improved quality of life in patients with colorectal cancer224. Clinicians are aware of the potential major impact of colorectal cancer on many aspects of quality of life, and individualized options to improve this should be given225 (Box 4).
Box 4. Supportive palliative care for patients with colorectal cancer.
Maintenance of adequate nutrient intake
Surgery and chemoradiotherapy can temporarily or for prolonged periods impair energy intake. Nutritional counselling and dietary monitoring can improve nutritional status, which benefits physical condition.
Pain relief
A substantial proportion of patients with advanced-stage disease require opioid treatment in the last months of their life. In a large UK study, approximately 20% of patients received intense opioid combination therapy. Such pain relief requires adequate patient monitoring, physician training and access to a dedicated pain treatment team227.
Physical condition maintenance
Various studies focusing on patients with advanced-stage colorectal as well as other cancers reported that exercise programmes can improve the patient’s physical condition, mobility and sleep, and reduce fatigue27,228.
Prevention of avoidable hospital admission
A considerable proportion of hospital admissions in patients with advanced-stage colorectal cancer are potentially avoidable by adequate support at home and hospice access. Potentially avoidable admissions seem to occur more often in elderly patients and those with end-stage disease.
Psychosocial support
Routine assessment at outpatient clinic visits or visits at home can help to identify patients who need specific psychosocial support in a timely manner.
Outlook
Colorectal cancer has over the past several decades become one of the most common cancers, and its incidence is expected to continue to increase in coming years. Despite major advances in treatment, mortality from colorectal cancer remains high and 40–50% of patients eventually die because of their disease. As discussed above, colorectal cancer arises as a result of environmental factors and genetic factors cooperating to generate colon polyps that progress to colorectal cancer. The polyp to cancer progression sequence is primarily driven at the cellular level by gene mutations and epigenetic alterations and is now recognized to be a heterogeneous process. It is widely anticipated that insights into the unique gene alterations will lead to more-precise and individualized care for people with polyps and cancers, which will be guided by the molecular characterization of the individual’s colon tumour.
The future of cancer surgery for colorectal disease is aimed at minimizing surgical trauma and preserving organ function. Population-based studies to unravel the effects of multimodal strategies for elderly patients and those with comorbidities need to be undertaken. High-precision imaging will lead to image-guided techniques. Each patient is unique and surgery needs to be tailor-made, aimed at complete removal for cure. Feedback on performance is required to keep on improving our efforts.
Chemotherapy has made substantial progress in recent years. We can now individualize the treatment according to the type of metastases (isolated liver/lung metastases, resectable or primarily not resectable), the RAS mutation state of the tumour and the response to a given treatment (for maintenance strategies or therapeutic breaks). The Human Cancer Genome Atlas and various other genomic projects have identified a number of novel potential molecular targets and markers for colorectal cancer that might be used to guide more-specific treatments for subgroups of patients (Figure 8).
Figure 8. Emerging drug targets and drug candidates in colorectal cancer.
The Cancer Genome Atlas and various other genomics projects have identified several novel potential molecular targets and markers in colorectal cancer that might be used to guide specific treatments for subgroups of patients. These targets include the Wnt, transforming growth factor (TGF)-β and epidermal growth factor (EGF) receptor signaling pathways. Experimental agents targeting these molecules are included in grey boxes. APC, adenomatosis polyposis coli; BMP, Bone morphogenetic protein; BMPR, BMP receptor; CK1, casein kinase 1; Dsh, Dishevelled; GSK3, glycogen synthase kinase 3; LRP, low-density lipoprotein receptor-related protein; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; P (in a red circle), phosphate; PDK, 3-phosphoinositide-dependent protein kinase; PI3K, phosphatidylinositol 3-kinase; PIP2, phosphatidylinositol-(4,5)-bisphosphate; PIP3, phosphatidylinositol-(3,4,5)-trisphosphate; PTEN, phosphatase and tensin homologue; SFRP, Secreted frizzled-related protein 1; SMAD, SMAD family member; TGFβR, TGF)-β receptor.
These developments in surgery and chemo(radio)therapy will improve and further individualize treatment in the near future, which should prolong survival of patients. The largest impact on incidence and mortality will, however, come from widespread organized population screening. Screening programmes should aim for optimal uptake and smart use of available resources. Opportunistic screening programmes must be replaced by organized screening, together with incorporation of strict quality assurance measures. With such an approach, the foreseen rapid rise in colorectal cancer incidence and mortality could be reversed in the coming decade.
Footnotes
Author contributions
Introduction (E.J.K.); Epidemiology (T.W., E.J.K.); Mechanisms/pathophysiology (W.M.G.); Diagnosis, screening and prevention (J.J.S., E.J.K., D.L.); Management (P.G.B., C.vd.V., T.S., E.J.K.); Quality of life (E.J.K.); Outlook (All authors); Overview of the Primer (E.J.K).
Competing interests
T.S. has received honoraria for lectures or advisory boards from Roche, Merck-Serono, Amgen and Bayer. All other authors declare no competing interests.
References
- 1.Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer screening--optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013;10:130–42. doi: 10.1038/nrclinonc.2013.12. Review of current state of art of colorectal cancer screening. [DOI] [PubMed] [Google Scholar]
- 2.Warthin AS. Heredity with reference to carcinoma: as shown by the study of the cases examined in the pathological laboratory of the University of Michigan. Arch Intern Med. 1913;12:546–55. [Google Scholar]
- 3.Capelle LG, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487–92. doi: 10.1053/j.gastro.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 4.Vasen HFA, Tomlinson I, Castells A. Clinical management of hereditary colorectal cancer syndromes. Nat Rev Gastroenterol Hepatol. 2015;12:88–97. doi: 10.1038/nrgastro.2014.229. [DOI] [PubMed] [Google Scholar]
- 5.Papamichael D, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol. 2014;26:463–476. doi: 10.1093/annonc/mdu253. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Heemskerk-Gerritsen BAM, et al. Improved overall survival after contralateral risk-reducing mastectomy in BRCA1/2 mutation carriers with a history of unilateral breast cancer: a prospective analysis. Int J Cancer. 2015;136:668–77. doi: 10.1002/ijc.29032. [DOI] [PubMed] [Google Scholar]
- 8.De Jonge V, et al. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75:98–106. doi: 10.1016/j.gie.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Valori R, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Quality assurance in endoscopy in colorectal cancer screening and diagnosis. Endoscopy. 2012;44(Suppl 3):SE88–105. doi: 10.1055/s-0032-1309795. Result of extensive, international consensus on quality measures for colorectal cancer screening. [DOI] [PubMed] [Google Scholar]
- 10.Globocan. 2012 at < http://globocan.iarc.fr/Default.aspx>.
- 11.Rohani-Rasaf M, Abdollahi M, Jazayeri S, Kalantari N, Asadi-Lari M. Correlation of cancer incidence with diet, smoking and socio- economic position across 22 districts of Tehran in 2008. Asian Pac J Cancer Prev. 2013;14:1669–76. doi: 10.7314/apjcp.2013.14.3.1669. [DOI] [PubMed] [Google Scholar]
- 12.Draft C, et al. Global, regional and national levels of age-specific mortality and 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:1990–2013. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tiwari AK, Roy HK, Lynch HT. Lynch Syndrome in the 21st Century: Clinical Perspectives. QJM. 2015 doi: 10.1093/qjmed/hcv137. at < http://www.ncbi.nlm.nih.gov/pubmed/26224055>. [DOI] [PubMed]
- 14.Pérez-Carbonell L, et al. Comparison between universal molecular screening for Lynch syndrome and revised Bethesda guidelines in a large population-based cohort of patients with colorectal cancer. Gut. 2012;61:865–72. doi: 10.1136/gutjnl-2011-300041. [DOI] [PubMed] [Google Scholar]
- 15.Van Lier MGF, et al. Yield of routine molecular analyses in colorectal cancer patients ≤70 years to detect underlying Lynch syndrome. J Pathol. 2012;226:764–74. doi: 10.1002/path.3963. [DOI] [PubMed] [Google Scholar]
- 16.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Jess T, et al. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–81.e1. doi: 10.1053/j.gastro.2012.04.016. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 18.Castaño-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther. 2014;39:645–59. doi: 10.1111/apt.12651. [DOI] [PubMed] [Google Scholar]
- 19.Herrinton LJ, et al. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 20.World Cancer Research Fund / American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011 at < http://www.dietandcancerreport.org/cancer_resource_center/downloads/cu/Colorectal-Cancer-2011-Report.pdf>.
- 21.Guraya SY. Association of type 2 diabetes mellitus and the risk of colorectal cancer: A meta-analysis and systematic review. World J Gastroenterol. 2015;21:6026–31. doi: 10.3748/wjg.v21.i19.6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedirko V, et al. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1958–72. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 23.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–15. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 24.Botteri E, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 25.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–60.e16. doi: 10.1053/j.gastro.2014.12.035. Extensive review of current knowledge on nutrients and colorectal risk as well as prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahm CC, et al. Dietary Fiber and Colorectal Cancer Risk: A Nested Case – Control Study Using Food Diaries. 2010;102 doi: 10.1093/jnci/djq092. [DOI] [PubMed] [Google Scholar]
- 27.Arem H, et al. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer. 2014;135:423–431. doi: 10.1002/ijc.28659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 29.Doubeni CA, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst. 2012;104:1353–62. doi: 10.1093/jnci/djs346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erdrich J, Zhang X, Giovannucci E, Willett W. Proportion of colon cancer attributable to lifestyle in a cohort of US women. Cancer Causes Control. 2015 doi: 10.1007/s10552-015-0619-z. at < http://www.ncbi.nlm.nih.gov/pubmed/26092381>. [DOI] [PMC free article] [PubMed]
- 31.Platz EA, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579–88. doi: 10.1023/a:1008999232442. [DOI] [PubMed] [Google Scholar]
- 32.Aleksandrova K, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168. doi: 10.1186/s12916-014-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 34.Andersen V, Vogel U. Systematic review: interactions between aspirin, and other nonsteroidal anti-inflammatory drugs, and polymorphisms in relation to colorectal cancer. Aliment Pharmacol Ther. 2014;40:147–59. doi: 10.1111/apt.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nan H, et al. Association of Aspirin and NSAID Use With Risk of Colorectal Cancer According to Genetic Variants. JAMA. 2015;313:1133–1142. doi: 10.1001/jama.2015.1815. Large study combining data from multiple case-control and cohort studies looking at preventive effect of aspirin and NSAID use on risk of colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010;59:1572–85. doi: 10.1136/gut.2009.190900. Review on preventive effects of statin therapy on colorectal cancer development. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, et al. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014;25:237–49. doi: 10.1007/s10552-013-0326-6. [DOI] [PubMed] [Google Scholar]
- 38.Limsui D, et al. Postmenopausal hormone therapy and colorectal cancer risk by molecularly defined subtypes among older women. Gut. 2012;61:1299–305. doi: 10.1136/gutjnl-2011-300719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogino S, Chan AT, Fuchs CS, Giovannucci E. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Colussi D, Brandi G, Bazzoli F, Ricciardiello L. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–85. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grady WM, Carethers JM. Genomic and epigenetic instability in colorectal cancer pathogenesis. Gastroenterology. 2008;135:1079–99. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 45.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 46.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 47.Zeki SS, Graham TA, Wright NA. Stem cells and their implications for colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:90–100. doi: 10.1038/nrgastro.2010.211. [DOI] [PubMed] [Google Scholar]
- 48.Jones S, et al. Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci. 2008;105:4283–4288. doi: 10.1073/pnas.0712345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo Y, et al. Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer. Gastroenterology. 2014;147:418–29.e8. doi: 10.1053/j.gastro.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Engeland M, Derks S, Smits KM, Meijer GA, Herman JG. Colorectal Cancer Epigenetics: Complex Simplicity. J Clin Oncol. 2011;29:1382–1391. doi: 10.1200/JCO.2010.28.2319. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein NS. Serrated pathway and APC (conventional)-type colorectal polyps: molecular-morphologic correlations, genetic pathways, and implications for classification. Am J Clin Pathol. 2006;125:146–53. [PubMed] [Google Scholar]
- 52.Jass JR. Hyperplastic polyps and colorectal cancer: is there a link? Clin Gastroenterol Hepatol. 2004;2:1–8. doi: 10.1016/s1542-3565(03)00284-2. [DOI] [PubMed] [Google Scholar]
- 53.Bettington M, et al. The serrated pathway to colorectal carcinoma: current concepts and challenges. Histopathology. 2013;62:367–86. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 54.Rex DK, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–29. doi: 10.1038/ajg.2012.161. quiz 1314, 1330. Expert panel recommendations for detection and management of serrated colorectal polyps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kambara T, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–44. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noffsinger AE. Serrated polyps and colorectal cancer: new pathway to malignancy. Annu Rev Pathol. 2009;4:343–64. doi: 10.1146/annurev.pathol.4.110807.092317. [DOI] [PubMed] [Google Scholar]
- 57.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chittenden TW, et al. Functional classification analysis of somatically mutated genes in human breast and colorectal cancers. Genomics. 2008;91:508–11. doi: 10.1016/j.ygeno.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jubb AM, Bell SM, Quirke P. Methylation and colorectal cancer. J Pathol. 2001;195:111–34. doi: 10.1002/path.923. [DOI] [PubMed] [Google Scholar]
- 60.Starr TK, et al. A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science. 2009;323:1747–50. doi: 10.1126/science.1163040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parsons DW, et al. Colorectal cancer: mutations in a signalling pathway. Nature. 2005;436:792. doi: 10.1038/436792a. [DOI] [PubMed] [Google Scholar]
- 62.Brennan CW, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grady WM, Pritchard CC. Molecular alterations and biomarkers in colorectal cancer. Toxicol Pathol. 2014;42:124–39. doi: 10.1177/0192623313505155. Review on molecular pathways leading to colorectal cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bardelli A. Mutational Analysis of the Tyrosine Kinome in Colorectal Cancers. Science (80-) 2003;300:949–949. doi: 10.1126/science.1082596. [DOI] [PubMed] [Google Scholar]
- 65.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim YH, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006;45:781–9. doi: 10.1002/gcc.20341. [DOI] [PubMed] [Google Scholar]
- 67.Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- 68.Worthley DL, et al. DNA methylation within the normal colorectal mucosa is associated with pathway-specific predisposition to cancer. Oncogene. 2010;29:1653–62. doi: 10.1038/onc.2009.449. [DOI] [PubMed] [Google Scholar]
- 69.Yamauchi M, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. Correlation molecular features of CRC with bowel location. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosty C, et al. PIK3CA activating mutation in colorectal carcinoma: associations with molecular features and survival. PLoS One. 2013;8:e65479. doi: 10.1371/journal.pone.0065479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galon J, et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kostic AD, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tahara T, et al. Fusobacterium in colonic flora and molecular features of colorectal carcinoma. Cancer Res. 2014;74:1311–8. doi: 10.1158/0008-5472.CAN-13-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mima K, et al. Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA Oncol. 2015;1:E1–9. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Morris EJA, Rutter MD, Finan PJ, Thomas JD, Valori R. Post-colonoscopy colorectal cancer (PCCRC) rates vary considerably depending on the method used to calculate them: a retrospective observational population-based study of PCCRC in the English National Health Service. Gut. 2014 doi: 10.1136/gutjnl-2014-308362. at < http://www.ncbi.nlm.nih.gov/pubmed/25416064>. [DOI] [PMC free article] [PubMed]
- 76.Kaminski MF, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. First study to show that the risk of post-colonoscopy cancer is higher when the colonoscopy was performed by an endoscopist with low adenoma detection rates. [DOI] [PubMed] [Google Scholar]
- 77.Zauber AG, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. Long-term follow-up of the National Polyp Study, showing the persistently lower mortality of colorectal cancer after colonoscopy with adenoma removal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pox CP, et al. Efficacy of a Nationwide Screening Colonoscopy Program for Colorectal Cancer. Gastroenterology. 2012;142:1460–1467.e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Winawer SJ, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 80.Nishihara R, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corley Da, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. Important confirmation that the risk of colorectal cancer and death with long-term follow-up after colonoscopy depends relates to adenoma detection rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sanduleanu S, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257–67. doi: 10.1136/gutjnl-2014-307992. [DOI] [PubMed] [Google Scholar]
- 83.Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. doi: 10.1002/14651858.CD008361.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pohl J, et al. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2010;60:485–490. doi: 10.1136/gut.2010.229534. [DOI] [PubMed] [Google Scholar]
- 85.Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane database Syst Rev. 2012;1:CD008361. doi: 10.1002/14651858.CD008361.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waye JD, et al. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos) Gastrointest Endosc. 2010;71:551–6. doi: 10.1016/j.gie.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 87.Leufkens AM, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: The TERRACE study. Gastrointest Endosc. 2011;73:480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 88.DeMarco DC, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest Endosc. 2010;71:542–550. doi: 10.1016/j.gie.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 89.Van Gossum A, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–70. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 90.Spada C, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581–589.e1. doi: 10.1016/j.gie.2011.03.1125. [DOI] [PubMed] [Google Scholar]
- 91.Spada C, et al. Meta-analysis Shows Colon Capsule Endoscopy Is Effective in Detecting Colorectal Polyps. Clin Gastroenterol Hepatol. 2010;8:516–522.e8. doi: 10.1016/j.cgh.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 92.Spada C, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527–36. doi: 10.1055/s-0031-1291717. [DOI] [PubMed] [Google Scholar]
- 93.Rex DK, et al. Accuracy of Capsule Colonoscopy in Detecting Colorectal Polyps in a Screening Population. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 94.Sung JJY, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. 2014;64:121–132. doi: 10.1136/gutjnl-2013-306503. [DOI] [PubMed] [Google Scholar]
- 95.Kim DH, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403–12. doi: 10.1056/NEJMoa070543. [DOI] [PubMed] [Google Scholar]
- 96.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393–405. doi: 10.1148/radiol.11101887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spada C, et al. Colon capsule versus CT colonography in patients with incomplete colonoscopy: a prospective, comparative trial. Gut. 2014:1–10. doi: 10.1136/gutjnl-2013-306550. [DOI] [PubMed] [Google Scholar]
- 98.Park SH, et al. CT colonography for detection and characterisation of synchronous proximal colonic lesions in patients with stenosing colorectal cancer. Gut. 2011:1716–1722. doi: 10.1136/gutjnl-2011-301135. [DOI] [PubMed] [Google Scholar]
- 99.Plumb aa, et al. CT colonography in the English Bowel Cancer Screening Programme: National survey of current practice. Clin Radiol. 2013;68:479–487. doi: 10.1016/j.crad.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 100.De Wijkerslooth TR, et al. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: randomised controlled trial. Gut. 2012;61:1552–1559. doi: 10.1136/gutjnl-2011-301308. [DOI] [PubMed] [Google Scholar]
- 101.De Haan MC, van Gelder RE, Graser A, Bipat S, Stoker J. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: a meta-analysis. Eur Radiol. 2011;21:1747–63. doi: 10.1007/s00330-011-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pooler BD, Kim DH, Lam VP, Burnside ES, Pickhardt PJ. CT Colonography Reporting and Data System (C-RADS): Benchmark Values From a Clinical Screening Program. Am J Roentgenol. 2014;202:1232–1237. doi: 10.2214/AJR.13.11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stoop EM, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13:55–64. doi: 10.1016/S1470-2045(11)70283-2. [DOI] [PubMed] [Google Scholar]
- 104.Yee J, et al. ACR Appropriateness Criteria colorectal cancer screening. J Am Coll Radiol. 2014;11:543–51. doi: 10.1016/j.jacr.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Spada C, et al. Clinical indications for computed tomographic colonography: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastrointestinal and Abdominal Radiology (ESGAR) Guideline. Endoscopy. 2014;46:897–915. doi: 10.1055/s-0034-1378092. [DOI] [PubMed] [Google Scholar]
- 106.Grützmann R, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Church TR, et al. Prospective evaluation of methylated SEPT9 in plasma for detection of asymptomatic colorectal cancer. Gut. 2014;63:317–25. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahlquist DA, et al. Stool DNA and occult blood testing for screen detection of colorectal neoplasia. Ann Intern Med. 2008;149:441–50. W81. doi: 10.7326/0003-4819-149-7-200810070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Imperiale TF, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 110.Bosch LJW, et al. Molecular tests for colorectal cancer screening. Clin Colorectal Cancer. 2011;10:8–23. doi: 10.3816/CCC.2011.n.002. [DOI] [PubMed] [Google Scholar]
- 111.Kuipers EJ. Colorectal cancer: screening-one small step for mankind, one giant leap for man. Nat Rev Clin Oncol. 2014;11:5–6. doi: 10.1038/nrclinonc.2013.213. [DOI] [PubMed] [Google Scholar]
- 112.The Dutch Cancer Registry. www.cijfersoverkanker.nl.
- 113.Brenner H, Altenhofen L, Stock C, Hoffmeister M. Natural history of colorectal adenomas: birth cohort analysis among 3.6 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev. 2013;22:1043–51. doi: 10.1158/1055-9965.EPI-13-0162. [DOI] [PubMed] [Google Scholar]
- 114.Schreuders EH, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015 doi: 10.1136/gutjnl-2014-309086. at < http://www.ncbi.nlm.nih.gov/pubmed/26041752> Review of existing colorectal cancer programmes worldwide. [DOI] [PubMed]
- 115.Singh H, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 116.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. Br Med J. 2014;2467:1–12. doi: 10.1136/bmj.g2467. Systematic review of long-term impact of sigmoidoscopy and colonoscopy on colorectal cancer incidence and mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Clercq CMC, et al. Metachronous colorectal cancers result from missed lesions and non-compliance with surveillance. Gastrointest Endosc. 2015 doi: 10.1016/j.gie.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 118.Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. doi: 10.1136/bmj.b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Atkin WS, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 120.Segnan N, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310–22. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 121.Van Putten PG, et al. Nurse endoscopists perform colonoscopies according to the international standard and with high patient satisfaction. Endoscopy. 2012;44:1127–32. doi: 10.1055/s-0032-1310154. [DOI] [PubMed] [Google Scholar]
- 122.Stephens M, et al. Non-physician endoscopists: A systematic review. World J Gastroenterol. 2015;21:5056–71. doi: 10.3748/wjg.v21.i16.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG, Habbema JDF, Kuipers EJ. Effect of Rising Chemotherapy Costs on the Cost Savings of Colorectal Cancer Screening. JNCI J Natl Cancer Inst. 2009;101:1412–1422. doi: 10.1093/jnci/djp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inadomi JM, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575–82. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Dam L, Kuipers EJ, Steyerberg EW, van Leerdam ME, de Beaufort ID. The price of autonomy: should we offer individuals a choice of colorectal cancer screening strategies? Lancet Oncol. 2013;14:e38–46. doi: 10.1016/S1470-2045(12)70455-2. [DOI] [PubMed] [Google Scholar]
- 126.Hol L, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2009;59:62–68. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 127.Van Rossum LG, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 128.Wilschut JA, et al. Fecal occult blood testing when colonoscopy capacity is limited. J Natl Cancer Inst. 2011;103:1741–51. doi: 10.1093/jnci/djr385. [DOI] [PubMed] [Google Scholar]
- 129.Quintero E, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 130.Kapidzic A, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014;109:1257–64. doi: 10.1038/ajg.2014.168. [DOI] [PubMed] [Google Scholar]
- 131.Van Roon AHC, et al. Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clin Gastroenterol Hepatol. 2011;9:333–9. doi: 10.1016/j.cgh.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 132.Goede SL, et al. Cost-effectiveness of one versus two sample faecal immunochemical testing for colorectal cancer screening. Gut. 2012;62:727–734. doi: 10.1136/gutjnl-2011-301917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Van Roon AHC, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut. 2012;62:409–415. doi: 10.1136/gutjnl-2011-301583. [DOI] [PubMed] [Google Scholar]
- 134.Imperiale TF, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370:1287–97. doi: 10.1056/NEJMoa1311194. [DOI] [PubMed] [Google Scholar]
- 135.Hol L, et al. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer. 2009;100:1103–1110. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Van Heijningen EMB, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410–1418. doi: 10.1053/j.gastro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 137.Lieberman DA, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 138.Martínez ME, et al. A Pooled Analysis of Advanced Colorectal Neoplasia Diagnoses After Colonoscopic Polypectomy. Gastroenterology. 2009;136:832–841. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Løberg M, et al. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807. doi: 10.1056/NEJMoa1315870. [DOI] [PubMed] [Google Scholar]
- 140.Hassan C, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–51. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 141.Cairns SR, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 142.Lieberman DA, et al. Low Rate of Large Polyps (>9 mm) Within 10 Years After an Adequate Baseline Colonoscopy With No Polyps. Gastroenterology. 2014;147:343–350. doi: 10.1053/j.gastro.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lansdorp-Vogelaar I, et al. Personalizing age of cancer screening cessation based on comorbid conditions: Model estimates of harms and benefits. Ann Intern Med. 2014;161:104–112. doi: 10.7326/M13-2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hees F, Van Habbema JDF, Meester RG, Lansdorp-vogelaar I, Van Ballegooijen M. Annals of Internal Medicine Should Colorectal Cancer Screening Be Considered in Elderly Persons Without Previous Screening? Ann Intern Med. 2014 doi: 10.7326/M13-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hazewinkel Y, et al. Prevalence of serrated polyps and association with synchronous advanced neoplasia in screening colonoscopy. Endoscopy. 2014;46:219–224. doi: 10.1055/s-0033-1358800. [DOI] [PubMed] [Google Scholar]
- 146.Jspeert IJEG, et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Gut. 2015:1–8. doi: 10.1136/gutjnl-2014-308411. [DOI] [PubMed] [Google Scholar]
- 147.Jeffery M, Hickey BE, Hider PN. Cochrane Database of Systematic Reviews. The Cochrane database of systematic reviews. John Wiley & Sons, Ltd; 1996. [DOI] [Google Scholar]
- 148.Primrose JN, et al. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–70. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 149.Brenner H, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer. 2012;131:1649–58. doi: 10.1002/ijc.26192. [DOI] [PubMed] [Google Scholar]
- 150.Breugom AJ, et al. Quality assurance in the treatment of colorectal cancer: the EURECCA initiative. Ann Oncol. 2014;25:1485–92. doi: 10.1093/annonc/mdu039. [DOI] [PubMed] [Google Scholar]
- 151.Van de Velde CJH, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon & rectum. Eur J Cancer. 2014;50:1.e1–1.e34. doi: 10.1016/j.ejca.2013.06.048. [DOI] [PubMed] [Google Scholar]
- 152.Van de Velde CJH, et al. Experts reviews of the multidisciplinary consensus conference colon and rectal cancer 2012: science, opinions and experiences from the experts of surgery. Eur J Surg Oncol. 2014;40:454–68. doi: 10.1016/j.ejso.2013.10.013. Review of the EURECCA consensus on colorectal cancer surgery. [DOI] [PubMed] [Google Scholar]
- 153.Quirke P, West NP, Nagtegaal ID. EURECCA consensus conference highlights about colorectal cancer clinical management: the pathologists expert review. Virchows Arch. 2014;464:129–34. doi: 10.1007/s00428-013-1534-x. [DOI] [PubMed] [Google Scholar]
- 154.Valentini V, et al. EURECCA consensus conference highlights about rectal cancer clinical management: The radiation oncologist’s expert review. Radiother Oncol. 2014;110:195–198. doi: 10.1016/j.radonc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 155.Tudyka V, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol. 2014;40:469–75. doi: 10.1016/j.ejso.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 156.Van de Velde CJH, et al. EURECCA colorectal: multidisciplinary mission statement on better care for patients with colon and rectal cancer in Europe. Eur J Cancer. 2013;49:2784–90. doi: 10.1016/j.ejca.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 157.Rutten HJT, den Dulk M, Lemmens VEPP, van de Velde CJH, Marijnen CAM. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol. 2008;9:494–501. doi: 10.1016/S1470-2045(08)70129-3. [DOI] [PubMed] [Google Scholar]
- 158.Gooiker GA, et al. Risk Factors for Excess Mortality in the First Year After Curative Surgery for Colorectal Cancer. Ann Surg Oncol. 2012;19:2428–2434. doi: 10.1245/s10434-012-2294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Van den Broek CBM, et al. The survival gap between middle-aged and elderly colon cancer patients. Time trends in treatment and survival. Eur J Surg Oncol. 2011;37:904–912. doi: 10.1016/j.ejso.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 160.Dekker JWT, et al. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol. 2014;40:1481–7. doi: 10.1016/j.ejso.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 161.Mulder SA, et al. Prevalence and prognosis of synchronous colorectal cancer: a Dutch population-based study. Cancer Epidemiol. 2011;35:442–7. doi: 10.1016/j.canep.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 162.Park SH, et al. CT colonography for detection and characterisation of synchronous proximal colonic lesions in patients with stenosing colorectal cancer. Gut. 2012;61:1716–22. doi: 10.1136/gutjnl-2011-301135. [DOI] [PubMed] [Google Scholar]
- 163.Taylor FGM, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34–43. doi: 10.1200/JCO.2012.45.3258. [DOI] [PubMed] [Google Scholar]
- 164.Veldkamp R, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 165.Bonjer HJ, et al. A Randomized Trial of Laparoscopic versus Open Surgery for Rectal Cancer. N Engl J Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. Randomized trial with long-term follow-up showing similar results for open and laparoscopic rectal cancer surgery. [DOI] [PubMed] [Google Scholar]
- 166.Colon T, Laparoscopic C, Study R. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 167.Chen J, et al. Meta-analysis of temporary ileostomy versus colostomy for colorectal anastomoses. Acta Chir Belg. 113:330–9. [PubMed] [Google Scholar]
- 168.Van Hooft JE, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46:990–1053. doi: 10.1055/s-0034-1390700. [DOI] [PubMed] [Google Scholar]
- 169.Fiori E, et al. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg. 2012;204:321–6. doi: 10.1016/j.amjsurg.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 170.De Graaf EJR, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol. 2009;35:1280–5. doi: 10.1016/j.ejso.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 171.Doornebosch PG, et al. Transanal endoscopic microsurgery for T1 rectal cancer: size matters! Surg Endosc. 2011;26:551–557. doi: 10.1007/s00464-011-1918-4. [DOI] [PubMed] [Google Scholar]
- 172.Lezoche E, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211–1218. doi: 10.1002/bjs.8821. [DOI] [PubMed] [Google Scholar]
- 173.Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775–88. doi: 10.1007/s10151-014-1148-6. [DOI] [PubMed] [Google Scholar]
- 174.Nagtegaal ID, Marijnen CAM, Kranenbarg EK, van de Velde CJH, van Krieken JHJM. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350–7. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 175.Quirke P, et al. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–8. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 177.Ramji KM, et al. Comparison of clinical and economic outcomes between robotic, laparoscopic, and open rectal cancer surgery: early experience at a tertiary care center. Surg Endosc. 2015 doi: 10.1007/s00464-015-4390-8. at < http://www.ncbi.nlm.nih.gov/pubmed/26173546>. [DOI] [PubMed]
- 178.Kapiteijn E, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 179.Folkesson J, et al. Swedish rectal cancer trial: Long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644–5650. doi: 10.1200/JCO.2005.08.144. [DOI] [PubMed] [Google Scholar]
- 180.Sebag-Montefiore D, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–20. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Van Gijn W, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575–82. doi: 10.1016/S1470-2045(11)70097-3. Randomized trial showing that preoperative radiotherapy reduces local recurrence rates after TME surgery. [DOI] [PubMed] [Google Scholar]
- 182.Pettersson D, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010;97:580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 183.Juul T, et al. Low Anterior Resection Syndrome and Quality of Life. Dis Colon Rectum. 2014;57:585–591. doi: 10.1097/DCR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 184.García M, et al. Phase II study of preoperative bevacizumab, capecitabine and radiotherapy for resectable locally-advanced rectal cancer. BMC Cancer. 2015;15:59. doi: 10.1186/s12885-015-1052-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg. 2013;100:E1–33. doi: 10.1002/bjs.9192_1. [DOI] [PubMed] [Google Scholar]
- 186.Habr-Gama A, Perez RO, São Julião GP, Proscurshim I, Gama-Rodrigues J. Nonoperative approaches to rectal cancer: a critical evaluation. Semin Radiat Oncol. 2011;21:234–9. doi: 10.1016/j.semradonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 187.Maas M, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29:4633–4640. doi: 10.1200/JCO.2011.37.7176. [DOI] [PubMed] [Google Scholar]
- 188.Dalton RSJ, et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis. 2012;14:567–71. doi: 10.1111/j.1463-1318.2011.02752.x. [DOI] [PubMed] [Google Scholar]
- 189.Fujita S, et al. Postoperative morbidity and mortality after mesorectal excision with and without lateral lymph node dissection for clinical stage II or stage III lower rectal cancer (JCOG0212): results from a multicentre, randomised controlled, non-inferiority trial. Lancet Oncol. 2012;13:616–621. doi: 10.1016/S1470-2045(12)70158-4. [DOI] [PubMed] [Google Scholar]
- 190.Påhlman L, et al. The Swedish rectal cancer registry. Br J Surg. 2007;94:1285–92. doi: 10.1002/bjs.5679. [DOI] [PubMed] [Google Scholar]
- 191.Wibe A, et al. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg. 2005;92:217–24. doi: 10.1002/bjs.4821. [DOI] [PubMed] [Google Scholar]
- 192.Birkmeyer JD, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 193.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–17. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 194.Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86:227–30. doi: 10.1046/j.1365-2168.1999.01023.x. [DOI] [PubMed] [Google Scholar]
- 195.Lassen K, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–9. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 196.Boelens PG, et al. Reduction of Postoperative Ileus by Early Enteral Nutrition in Patients Undergoing Major Rectal Surgery. Ann Surg. 2014;259:649–655. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 197.Sauer R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 198.Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi81–vi88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 199.Labianca R, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 200.McCleary NJ, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol. 2013;31:2600–6. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2014;25(Suppl 3):iii1–9. doi: 10.1093/annonc/mdu260. European Society of Medical Oncology guidelines for management of metastatic colorectal cancer. [DOI] [PubMed] [Google Scholar]
- 202.Amado RG, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 203.Douillard JY, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 204.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 205.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Giantonio BJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 207.Kubicka S, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24:2342–9. doi: 10.1093/annonc/mdt231. [DOI] [PubMed] [Google Scholar]
- 208.Van Cutsem E, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 209.Tomlinson JS, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 210.Evrard S, et al. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PLoS One. 2014;9:e114404. doi: 10.1371/journal.pone.0114404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ayez N, et al. Outcome of microscopic incomplete resection (R1) of colorectal liver metastases in the era of neoadjuvant chemotherapy. Ann Surg Oncol. 2012;19:1618–27. doi: 10.1245/s10434-011-2114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Falcone A, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nor. J Clin Oncol. 2007;25:1670–6. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 213.Heinemann V, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 214.Benoist S, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–45. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 215.Simkens LHJ, Koopman M, Punt CJA. Optimal duration of systemic treatment in metastatic colorectal cancer. Curr Opin Oncol. 2014;26:448–453. doi: 10.1097/CCO.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 216.Grothey A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 217.Smith JJ, Weiser MR. Outcomes in non-metastatic colorectal cancer. J Surg Oncol. 2014:518–526. doi: 10.1002/jso.23696. [DOI] [PubMed] [Google Scholar]
- 218.Juul T, et al. International validation of the low anterior resection syndrome score. Ann Surg. 2014;259:728–34. doi: 10.1097/SLA.0b013e31828fac0b. [DOI] [PubMed] [Google Scholar]
- 219.Feddern ML, Jensen TS, Laurberg S. Chronic pain in the pelvic area or lower extremities after rectal cancer treatment and its impact on quality of life: a population-based cross-sectional study. Pain. 2015;156:1765–71. doi: 10.1097/j.pain.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 220.De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane database Syst Rev. 2013;2:CD006041. doi: 10.1002/14651858.CD006041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bregendahl S, Emmertsen KJ, Lindegaard JC, Laurberg S. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Color Dis. 2015;17:26–37. doi: 10.1111/codi.12758. [DOI] [PubMed] [Google Scholar]
- 222.Gilbert A, et al. Systematic Review of Radiation Therapy Toxicity Reporting in Randomized Controlled Trials of Rectal Cancer: A Comparison of Patient-Reported Outcomes and Clinician Toxicity Reporting. Int J Radiat Oncol Biol Phys. 2015;92:555–67. doi: 10.1016/j.ijrobp.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 223.Diouf M, et al. Could baseline health-related quality of life (QoL) predict overall survival in metastatic colorectal cancer? The results of the GERCOR OPTIMOX 1 study. Health Qual Life Outcomes. 2014;12:69. doi: 10.1186/1477-7525-12-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Otto SJ, et al. Association of change in physical activity and body weight with quality of life and mortality in colorectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2015;23:1237–50. doi: 10.1007/s00520-014-2480-0. [DOI] [PubMed] [Google Scholar]
- 225.Averyt JC, Nishimoto PW. Psychosocial issues in colorectal cancer survivorship: the top ten questions patients may not be asking. J Gastrointest Oncol. 2014;5:395–400. doi: 10.3978/j.issn.2078-6891.2014.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Martínez ME, et al. One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines. Ann Intern Med. 2012;157:856–64. doi: 10.7326/0003-4819-157-12-201212180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gao W, Gulliford M, Bennett MI, Murtagh FEM, Higginson IJ. Managing cancer pain at the end of life with multiple strong opioids: A population-based retrospective cohort study in primary care. PLoS One. 2014;9 doi: 10.1371/journal.pone.0079266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhong S, et al. Association between physical activity and mortality in breast cancer: A meta-analysis of cohort studies. Eur J Epidemiol. 2014;29:391–404. doi: 10.1007/s10654-014-9916-1. [DOI] [PubMed] [Google Scholar]
- 229.Davies RJ, Miller R, Coleman N. Colorectal cancer screening: prospects for molecular stool analysis. Nat Rev Cancer. 2005;5:199–209. doi: 10.1038/nrc1569. [DOI] [PubMed] [Google Scholar]
- 230.Towler BP, Irwig L, Glasziou P, Weller D, Kewenter J. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev. 2000:CD001216. doi: 10.1002/14651858.CD001216. [DOI] [PubMed] [Google Scholar]
- 231.Brenner H, Tao S. Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer. 2013;49:3049–3054. doi: 10.1016/j.ejca.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 232.Atkin WS, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 233.Schoen RE, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 235.Løberg M, et al. Long-Term Colorectal-Cancer Mortality after Adenoma Removal. N Engl J Med. 2014;371:799–807. doi: 10.1056/NEJMoa1315870. [DOI] [PubMed] [Google Scholar]
- 236.Cash BD, et al. CT colonography of a medicare-aged population: Outcomes observed in an analysis of more than 1400 patients. Am J Roentgenol. 2012;199:27–34. doi: 10.2214/AJR.11.7729. [DOI] [PubMed] [Google Scholar]